Abstract
Purpose
Genetic factors have been implicated in the pathogenesis of renal cell carcinoma (RCC), with around 3% of cases having a family history. A greater knowledge of the genetics of inherited RCC has the potential to translate into novel therapeutic targets for sporadic RCC.
Methods
A literature review was performed summarising the current knowledge on hereditary RCC diagnosis, surveillance and management.
Results
Familial RCC is usually inherited in an autosomal dominant manner, although inherited RCC may present without a relevant family history. A number of familial RCC syndromes have been identified. Familial non-syndromic RCC is suspected when ≥ 2 relatives are affected in the absence of syndromic features, although clear diagnostic criteria are lacking. Young age at onset and bilateral/multicentric tumours are recognised characteristics which should prompt molecular genetic analysis. Surveillance in individuals at risk of inherited RCC aims to prevent morbidity and mortality via early detection of tumours. Though screening and management guidelines for some inherited RCC syndromes (e.g. von Hippel–Lindau disease, Birt–Hogg–Dube syndrome, hereditary leiomyomatosis) are well defined for rare cause of inherited RCC (e.g. germline BAP1 mutations), there is limited information regarding the lifetime RCC risks and the most appropriate screening modalities.
Conclusion
Increasing knowledge of the natural history and genetic basis has led to characterisation and tailored management of hereditary RCC syndromes. International data sharing of inherited RCC gene variant information may enable evidence-based improvements in the diagnosis, surveillance protocols and management of these rare conditions.
Keywords: Genetics, von Hippel–Lindau review, Familial syndromic renal cancer
Introduction
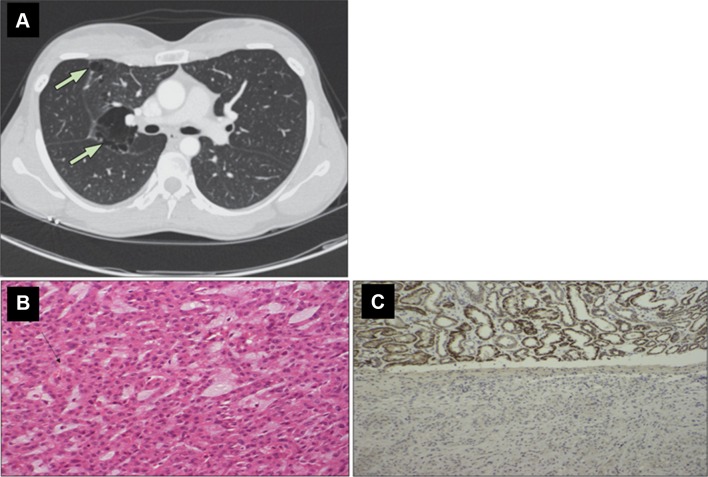
In addition to smoking, obesity and hypertension, genetic factors have been implicated in the pathogenesis of renal cell carcinoma (RCC) with around 3% of cases having a family history [1]. Familial RCC is usually inherited in an autosomal dominant manner (though there may be incomplete penetrance). However, patients harbouring a mutation in a gene predisposing to RCC do not necessarily have a family history of RCC (the mutation may have arisen de novo in the proband or the mutation may be non-penetrant in a carrier parent. If there is no family history, hereditary RCC might be suspected by the presence of bilateral/multicentric or young onset RCC and then confirmed by molecular genetic analysis. In addition, the presence of additional (non-RCC) clinical features in the proband or close relatives might suggest a specific multisystem hereditary RCC syndrome [e.g. pulmonary cysts in Birt–Hogg–Dube syndrome (see Fig. 1), cerebellar haemangioblastomas in von Hippel–Lindau disease; cutaneous or uterine leiomyomas in HLRCC, etc. (see later)]. Histopathological features or immunohistochemical investigations may also guide molecular genetic investigations (Table 1 and Fig. 1).
Fig. 1.
Examples of radiological, histological and immunohistochemical features that might suggest an inherited predisposition to renal cell carcinoma. Upper panel: a high-resolution CT thorax showing multiple basal cysts in a patient with Birt–Hogg–Dube syndrome (reprinted with permission from [52]). Lower panel: b the H + E-stained histological appearance of an SDHB-deficient RCC. There is evidence of intracytoplasmic vacuoles marked by the black arrow. c Loss of SDHB protein expression on immunostaining of the RCC tumour in the lower part of the image, with SDHB staining present in the adjacent normal renal tissue visible in the upper image.
(Reprinted with permission from [39])
Table 1.
Overview of major hereditary renal cell cancer syndromes.
Adapted from Menko and Maher [1]
| Syndrome | Inheritance | Gene | Estimated RCC risk | Renal tumour histological subtypes | Functional consequences of mutation |
|---|---|---|---|---|---|
| Von Hippel–Lindau disease | AD | VHL | 70% | Clear cell RCC | Activation of hypoxic response pathways |
| Birt–Hogg–Dubé syndrome | AD | FLCN | 25% | Various, but hybrid chromophobe/oncocytic RCC typical | Activation of the mTOR pathway |
| Hereditary type 1 papillary RCC | AD | MET | Increased | Papillary type 1 RCC | Activation of MET signalling pathway |
| Hereditary leiomyomatosis and renal cell cancer | AD | FH | 15% | Papillary type 2 RCC | Activation of hypoxic response pathways Epigenetic changes (e.g. DNA methylation) |
| Succinate dehydrogenase subunit-related RCC | AD |
SDHB
SDHD a SDHC SDHA |
Highest risk (up to 10–15% with SDHB | Various types, but specific features recognised | Activation of hypoxic response pathways Epigenetic changes (e.g. DNA methylation) |
| Chromosome 3 translocations | Chromosomal | Chromosome 3 | Increased (up to 70%) | Clear cell RCC | Loss of translocated chromosome 3p and somatic mutation of VHL leads to activation of hypoxic response pathways |
| PTEN hamartoma tumour syndrome | AD | PTEN | 5–35% | Mostly papillary RCC | Activation of phosphoinositide 3-kinase (PI3K) signalling pathway |
| Hereditary BAP1 tumour syndrome | AD | BAP1 | Increased | Clear cell | BAP1 inactivation associated with altered chromatin architecture, DNA damage response and cell cycle regulation |
AD autosomal dominant, RCC renal cel carcinoma
aInheritance is characterised by maternal imprinting
Methods
A non-systematic literature search was conducted using Medline, updated to December 2017. The reference lists of selected manuscripts were checked manually for eligible articles. The most relevant articles summarising existing knowledge on hereditary RCC syndromes, including diagnosis, management and surveillance, were selected for this review.
Results
Major inherited forms of RCC
von Hippel–Lindau disease
This rare autosomal dominantly inherited disorder has an incidence of approximately 1 in 30,000 and is caused by constitutional mutations in the VHL tumour suppressor gene (TSG) [2, 3]. In most cases, the lifetime risks of retinal and central nervous haemangioblastomas and clear cell RCC are over 70% each [4, 5]. Less frequent tumours include phaeochromocytoma/paraganglioma (approximately 20% of cases), pancreatic neuroendocrine tumours (approximately 10%) and endolymphatic sac tumours (approximately 5–10%). Multiple visceral cysts (renal, pancreatic and epididymal) are common and may help indicate the diagnosis. More than 95% of patients with VHL disease will have a detectable VHL gene mutation and well-defined genotype–phenotype mean that the nature of the mutation may predict likely tumour risks (e.g. risk of phaeochromocytoma is small with truncating mutations and exonic deletions) [3, 5]. The VHL gene product has a critical role in regulating the hypoxic gene response through stability of the α-subunits of the HIF-1 and HIF-2 transcription factors [6–8]. This knowledge provides the rationale for the use of antiangiogenic tyrosine kinase inhibitors (e.g. sunitinib/sorafenib) in sporadic RCC, as most clear cell RCC have somatic mutations in the VHL TSG [3]. Patients with VHL disease and asymptomatic family members found to carry the familial mutation are screened annually to detect asymptomatic tumours [full details of the surveillance programmes are published elsewhere (10, 11), but generally renal screening is by annual MRI from age 16 years] and enable early intervention (small RCC are usually removed when they reach 3 cm diameter) [9–11].
Birt–Hogg–Dubé syndrome
Birt–Hogg–Dubé (BHD) syndrome is characterised by an autosomal dominantly inherited predisposition to multiple fibrofolliculomas (characteristically on the face), lung cysts and pneumothorax, renal cell carcinoma and possibly colorectal tumours [12, 13]. The risk of RCC in BHD syndrome is significantly less than that in VHL disease (approximately 25%), but annual renal surveillance (usually by MRI or renal ultrasonography if MRI is unavailable or not tolerated) is offered to patients and mutation carriers from age 20 years [13]. Though the characteristic RCC histology contains chromophobe and oncocytic elements, other subtypes, including clear cell RCC, are well described [14, 15]. Germline inactivating mutations in the FLCN TSG can cause both BHD syndrome and non-syndromic familial pneumothorax. The function of the FLCN gene product has not been fully elucidated; however, inactivation leads to activation of the mTOR pathway [16, 17].
Hereditary leiomyomatosis and renal cell cancer
The presence of multiple cutaneous leiomyomas in a patient with RCC suggests a diagnosis of hereditary leiomyomatosis and renal cell cancer (HLRCC). This very rare disorder (incidence approximately 1 in 200,000) is caused by inactivating mutations in the FH gene which encodes fumarate hydratase, a key component of the tricarboxylic acid (Krebs) cycle [18]. Affected females may present with early-onset multiple uterine leiomyomas (fibroids) and FH mutations are a rare cause of inherited phaeochromocytoma/paraganglioma [19–21]. RCC in HLRCC is typically classified as Type 2 papillary RCC or collecting duct RCC [22]. Though the lifetime risk of RCC in this condition is around 15%, it is typically an aggressive early metastatic tumour that can occur at a young age (mean 41 years, earliest report at 11 years) and therefore annual surveillance (by MRI as tumours may not be visualised by ultrasonography) is offered [19].
Succinate dehydrogenase-related RCC
Succinate dehydrogenase (SDH) is a tetrameric enzyme (encoded by the SDHA, SDHB, SDHC and SDHD genes) that is upstream of fumarate hydratase in the tricarboxylic acid (Krebs) cycle. Germline mutations in these SDHx were initially described in association with phaeochromocytoma/paraganglioma and head and neck paraganglioma (HNPGL), but the tumour spectrum has since expanded to include gastrointestinal stromal tumours, pituitary tumours and RCC [23–27]. A variety of histopathological subtypes have been described in SDHx-associated RCC, but recently a distinctive histopathology that should prompt molecular genetic investigations has been defined [28]. Though RCC has been associated with mutations in each of the subunits, the most commonly associated gene is SDHB. Germline mutations in SDHB may present with a familial RCC-only phenotype [26]. The lifetime risk of RCC in SDHB mutation carriers is not well defined, but likely less than 10–15%; however, annual or biannual renal surveillance by MRI can be combined with screening for phaeochromocytoma/paraganglioma (which commences in older children).
Hereditary papillary RCC
Activating mutations in the MET proto-oncogene predispose to Type 1 hereditary papillary RCC (HPRC) [29]. This is an extremely rare disorder that is inherited as an autosomal dominant trait with incomplete penetrance. Annual renal surveillance by MRI should be offered to patients and at risk relatives, but if the patient presents with metastatic RCC then treatment with a Met-inhibitor can be considered [30].
Hereditary BAP1-associated RCC
Following reports of germline BAP1 mutations in familial uveal melanoma, cutaneous melanoma and mesothelioma [31, 32], it was recognised that RCCs are also part of the BAP1 tumour syndrome spectrum [33, 34]. Though germline BAP1 mutations have been described in patients with familial RCC and no other BAP1-related tumours, such cases are rare and BAP1 mutation analysis is not yet performed in all cases of inherited RCC. There are no generally agreed surveillance protocols for BAP1 mutation carriers, but, as with other inherited cancer predisposition syndromes, repeated irradiation should be avoided and therefore surveillance should be by MRI rather than CT scanning.
Constitutional chromosome 3 translocations
Constitutional chromosome 3 translocations are a rare, but well-validated cause of familial RCC. In patients with familial RCC, the detection of a constitutional chromosome 3 translocation is likely to be relevant (though the translocation breakpoints are variable), but if a chromosome 3 translocation is detected for another reason (e.g. prenatal diagnosis) and there is no personal or family history of RCC then the risk of RCC is very small [35].
Other rare conditions
A variety of other rare causes of RCC predisposition have been described. There has been increasing recognition of a significant risk of RCC in patients with Cowden/PTEN hamartoma tumour syndrome, but germline PTEN mutations are very rare in patients with non-syndromic inherited RCC [36]. Tuberose sclerosis may be associated with early-onset RCC, but RCC is rare in this condition and renal lesions are most commonly angiomyolipomas [37]. Germline VHL, SDHx and FH mutations may predispose to phaeochromocytoma/paraganglioma and RCC, and on rare occasions renal tumours have been reported in association with mutations in the phaeochromocytoma genes TMEM127 and MAX [38, 39]. Germline mutations in CDC73 are associated with hyperparathyroidism-jaw tumour syndrome, a very rare disorder that has been associated with Wilms tumour and, on one occasion, papillary RCC [40].
Familial non-syndromic RCC
There are no clear diagnostic criteria for this disorder, but broadly this condition is suspected when two or more relatives have RCC and there are no features to suggest an underlying “syndromic cause” (Table 1). The presence of early-onset tumours and/or multiple/bilateral tumours should further increase suspicion. Despite the absence of syndromic features, germline mutations in SDHB and FLCN are not uncommon in such cases and molecular genetic testing for an RCC gene panel [e.g. FLCN, FH, MET, SDHB, VHL (± BAP1)] and cytogenetic analysis is generally performed in suspected cases. However, in most cases a genetic cause is not identified. Some familial cases may result from chance or shared environmental factors or polygenic inheritance (genome-wide association studies have identified RCC susceptibility loci), but it is highly suspected that additional RCC predisposition genes remain to be identified [41, 42]. Familial non-syndromic RCC without an identifiable genetic cause is likely to be genetically heterogeneous and clinical studies suggest that autosomal dominant inheritance (in inherited cases) is the most likely form of transmission [15]. Germline SDHB and FLCN mutations have also been described in non-syndromic early-onset or bilateral RCC cases with no family history [15, 25].
Diagnosis of familial RCC
Young age at onset and bilateral/multicentric tumours are well recognised features of inherited syndromic RCC and often present in non-syndromic RCC, and these are therefore indications for genetic analysis [45]. However, testing of individuals at low prior risk for a mutation can lead to diagnostic uncertainties from the identification of rare variants of uncertain significance (VUSs). In cases of inherited RCC with syndromic features, molecular genetic testing can usually be expected to unequivocally confirm the diagnosis, particularly for well-characterised genes such as VHL in which a wide range of germline mutations have been described and VUSs are relatively infrequent. For less well studied genes such as BAP1 variant, interpretation can be more challenging. Therefore, as suggested for diagnostic testing of hereditary phaeochromocytoma and paraganglioma, the establishment of agreed gene panels and curated databases of inherited RCC-associated gene variants would facilitate expert genetic testing [46]. Another issue to be addressed is which groups of patients should be offered testing. The mean age at diagnosis of symptomatic RCC in VHL disease was around 45 years compared to an average age of > 60 years in sporadic cases [43], but there is not an agreed age threshold at which earlier-onset cases should be tested for RCC predisposition mutations. Schuh et al. [44] suggested that patients with sporadic RCC aged 46 years or younger should trigger consideration for germline mutation testing. However, the germline mutation detection rate will be low and for those that test negative the residual risk of an underlying genetic cause is unclear and so other centres have a lower threshold for testing (e.g. age 40 years). The low mutation detection rate in familial non-syndromic RCC suggests that there are further inherited RCC genes to be identified and achieving a comprehensive picture of the molecular architecture of inherited RCC is necessary for the development of optimal evidence-based guidelines for the molecular investigation of potential inherited RCC.
Management and surveillance of inherited RCC
Over the past 25 years, the identification and surveillance of large numbers of VHL mutation carriers has led to a broad consensus to how they should be investigated and managed. In particular, a consensus has developed that small (< 3 cm) screen-detected tumours should be managed by active surveillance and then nephron-sparing surgery performed when a solid lesion reaches 3 cm in diameter [47]. As an alternative to partial nephrectomy percutaneous radiofrequency ablation has been used to treat small renal lesions in VHL disease [51]. In general, the approach to the management of RCCs identified through surveillance in VHL disease has been extrapolated to individuals with Birt–Hogg–Dube syndrome with tumours followed by active surveillance until they reach a diameter of 3 cm and then nephron-sparing surgery is performed (alternatively, radiofrequency ablation may be used to treat smaller tumours) [13]. However, it is clear that the “3 cm rule” is not suitable for renal lesions in HLRCC, which can metastasise early and are not reliably detected by renal ultrasound. Consequently, individuals with germline FH mutations undergo annual MRI surveillance even though most will not develop a renal lesion and surgical intervention is indicated for small screen-detected lesions [19]. For individuals with a germline BAP1 mutation, there is very limited information on the lifetime risks of RCC and the most appropriate screening modalities. International data sharing of inherited RCC gene variant information and multicentre collaboration to pool results of natural history and screening protocols for mutation carriers are required to enable evidence-based surveillance programmes to be designed. For example, SDHx-associated RCC can be aggressive implying that surveillance for early detection is important. However, the tumour risks in SDHB mutation carriers are significantly less than originally thought and so there is (as in FH mutation carriers) a tension between over-investigation and early detection. This might be addressed by the development of biomarkers to identify the subset of individuals who will develop RCC and should be targeted for screening and/or novel early detection strategies (such as circulating tumour DNA biomarkers).
In some patients, particularly those with no previous family history, the diagnosis of inherited RCC disorder is only made after presentation with metastatic disease. Additionally, in VHL disease patients may develop multiple central nervous system haemangioblastomas that are not amenable to surgical treatment because of their critical location. Hence, there is a need to develop effective medical therapies for such cases. Most of the known inherited RCC genes encode tumour suppressor genes and biallelic inactivation of the relevant inherited RCC gene is present in all tumour cells. Therefore, in addition to standard therapies for metastatic RCC, targeted therapies with agents that exploit the specific molecular pathways provides a rational approach to therapy [48, 49]. The concept of synthetic lethality-based interventions is particularly interesting for inherited RCC (and haemangioblastomas) in disorders such as VHL disease, because the kidneys of VHL patients can harbour hundreds of small “tumourlets” with biallelic VHL inactivation, some of which will give rise to RCC years later [50]. Hence, it could be hypothesised that ablation of such tumourlets by administration of a synthetically lethal compound to young adults with VHL disease might reduce the risk of RCC at a later age. The development of novel therapeutic approaches to inherited RCC will require a deeper knowledge of the normal function of inherited RCC gene products and the consequences of mutations in the relevant pathways. However, a likely outcome of such research would be the potential for translating the knowledge of the pathogenesis of inherited RCC into novel treatments for sporadic RCC (as exemplified in VHL disease and the involvement of VHL inactivation in sporadic clear cell RCC).
Conclusion
A number of familial RCC syndromes and inherited non-syndromic RCC have been identified. The presence of bilateral/multicentric or young-onset RCC, syndromic and histopathological features should prompt and guide molecular genetic analysis. The principal strategy for preventing morbidity and mortality in individuals at risk of inherited RCC is detection of early stage tumours which can then be removed (e.g. HLRCC) or followed up to a safe size (e.g. 3 cm diameter in VHL disease and BHD syndrome) when they are removed or ablated. International data sharing of inherited RCC gene variant information may enable evidence-based improvements in the diagnosis and management of these rare conditions.
Author contributions
E R Maher: project development, data collection, data analysis, manuscript writing and editing.
Conflict of interest
The author declares no conflict of interest.
Research involving human participants and/or animals
The following manuscript is a review of existing data. Therefore, this article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study (review) formal consent is not required.
References
- 1.Menko FH, Maher ER. Diagnosis and management of hereditary renal cell cancer. Recent Results Cancer Res. 2016;205:85–104. doi: 10.1007/978-3-319-29998-3_6. [DOI] [PubMed] [Google Scholar]
- 2.Maher ER, Iselius L, Yates JR, et al. Von Hippel–Lindau disease: a genetic study. J 662 Med Genet. 1991;28(7):443–447. doi: 10.1136/jmg.28.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15(1):55–64. doi: 10.1038/nrc3844. [DOI] [PubMed] [Google Scholar]
- 4.Maher ER, Yates JR, Harries R, et al. Clinical features and natural history of von Hippel–Lindau disease. Q J Med. 1990;77(283):1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 5.Ong KR, Woodward ER, Killick P, Lim C, Macdonald F, Maher ER. Genotype–phenotype correlations in von Hippel–Lindau disease. Hum Mutat. 2007;28:143–149. doi: 10.1002/humu.20385. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 7.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel–Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/S1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 8.Schödel J, Grampp S, Maher ER, Moch H, Ratcliffe PJ, Russo P, Mole DR. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur Urol. 2016;69:646–657. doi: 10.1016/j.eururo.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, Walther MM. The relationship between renal tumor size and metastases in patients with von Hippel–Lindau disease. J Urol. 2004;172:63–65. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 10.Maher ER, Neumann HP, Richard S. von Hippel–Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–623. doi: 10.1038/ejhg.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen SM, Rhodes L, Blanco I, Chung WK, Eng C, Maher ER, Richard S, Giles RH. Von Hippel–Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34:2172–2181. doi: 10.1200/JCO.2015.65.6140. [DOI] [PubMed] [Google Scholar]
- 12.Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt–Hogg–Dube syndrome. Cancer Cell. 2002;2(2):157–164. doi: 10.1016/S1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 13.Menko FH, van Steensel MAM, Giraud S, et al. Birt–Hogg–Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10(12):1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 14.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, Merino MJ. Renal tumors in the Birt–Hogg–Dubé syndrome. Am J Surg Pathol. 2002;26:1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Woodward ER, Ricketts C, Killick P, et al. Familial non-VHL clear cell (conventional) renal cell carcinoma: clinical features, segregation analysis, and mutation analysis of FLCN. Clin Cancer Res. 2008;14(18):5925–5930. doi: 10.1158/1078-0432.CCR-08-0608. [DOI] [PubMed] [Google Scholar]
- 16.Hasumi Y, Baba M, Ajima R, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc Natl Acad Sci. 2009;106(44):18722–18727. doi: 10.1073/pnas.0908853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartman TR, Nicolas E, Klein-Szanto A, Al-Saleem T, Cash TP, Simon MC, Henske EP. The role of the Birt–Hogg–Dubé protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28:1594–1604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA, Multiple Leiomyoma Consortium Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 19.Menko FH, Maher ER, Schmidt LS, Middelton LA, Aittomäki K, Tomlinson I, Richard S, Linehan WM. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637–644. doi: 10.1007/s10689-014-9735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toro JR, Nickerson ML, Wei MH, Warren MB, Glenn GM, Turner ML, Stewart L, Duray P, Tourre O, Sharma N, Choyke P, Stratton P, Merino M, Walther MM, Linehan WM, Schmidt LS, Zbar B. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark GR, Sciacovelli M, Gaude E, Walsh DM, Kirby G, Simpson MA, Trembath RC, Berg JN, Woodward ER, Kinning E, Morrison PJ, Frezza C, Maher ER. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab. 2014;99:E2046–E2050. doi: 10.1210/jc.2014-1659. [DOI] [PubMed] [Google Scholar]
- 22.Chen YB, Brannon AR, Toubaji A, Dudas ME, Won HH, Al-Ahmadie HA, Fine SW, Gopalan A, Frizzell N, Voss MH, Russo P, Berger MF, Tickoo SK, Reuter VE. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol. 2014;38:627–637. doi: 10.1097/PAS.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW, 3rd, Cornelisse CJ, Devilee P, Devlin B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 24.Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, Sköldberg F, Husebye ES, Eng C, Maher ER. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricketts C, Woodward ER, Killick P, et al. Germline SDHB mutations and familial renal cell carcinoma. J Nat Cancer Inst. 2008;100(17):1260–1262. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 26.Janeway KA, Kim SY, Lodish M, Nosé V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M, Lai AH, Kelly L, Hornick JL, NIH Pediatric and Wild-Type GIST Clinic. O’Sullivan M, de Krijger RR, Dinjens WN, Demetri GD, Antonescu CR, Fletcher JA, Helman L, Stratakis CA. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xekouki P, Szarek E, Bullova P, Giubellino A, Quezado M, Mastroyannis SA, Mastorakos P, Wassif CA, Raygada M, Rentia N, Dye L, Cougnoux A, Koziol D, Sierra Mde L, Lyssikatos C, Belyavskaya E, Malchoff C, Moline J, Eng C, Maher LJ, 3rd, Pacak K, Lodish M, Stratakis CA. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in humans and mice. J Clin Endocrinol Metab. 2015;100:E710–E719. doi: 10.1210/jc.2014-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill AJ, Hes O, Papathomas T, Šedivcová M, Tan PH, Agaimy A, Andresen PA, Kedziora A, Clarkson A, Toon CW, Sioson L, Watson N, Chou A, Paik J, Clifton-Bligh RJ, Robinson BG, Benn DE, Hills K, Maclean F, Niemeijer ND, Vlatkovic L, Hartmann A, Corssmit EP, van Leenders GJ, Przybycin C, McKenney JK, Magi-Galluzzi C, Yilmaz A, Yu D, Nicoll KD, Yong JL, Sibony M, Yakirevich E, Fleming S, Chow CW, Miettinen M, Michal M, Trpkov K. Succinate dehydrogenase (SDH)-deficient renal carcinoma: a morphologically distinct entity: a clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol. 2014;38:1588–1602. doi: 10.1097/PAS.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schimdt L, Duh FM, Chen F, Kishida T, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 30.Choueiri TK, Vaishampayan U, Rosenberg JE, Logan TF, Harzstark AL, Bukowski RM, Rini BI, Srinivas S, Stein MN, Adams LM, Ottesen LH, Laubscher KH, Sherman L, McDermott DF, Haas NB, Flaherty KT, Ross R, Eisenberg P, Meltzer PS, Merino MJ, Bottaro DP, Linehan WM, Srinivasan R. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol. 2013;31:181–186. doi: 10.1200/JCO.2012.43.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, Hesdorffer M, Nasu M, Powers A, Rivera Z, Comertpay S, Tanji M, Gaudino G, Yang H, Carbone M. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I, Viale A, Lash AE, Pirun M, Socci ND, Rütten A, Palmedo G, Abramson D, Offit K, Ott A, Becker JC, Cerroni L, Kutzner H, Bastian BC, Speicher MR. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popova T, Hebert L, Jacquemin V, Gad S, Caux-Moncoutier V, Dubois-d’Enghien C, Richaudeau B, Renaudin X, Sellers J, Nicolas A, Sastre-Garau X, Desjardins L, Gyapay G, Raynal V, Sinilnikova OM, Andrieu N, Manié E, de Pauw A, Gesta P, Bonadona V, Maugard CM, Penet C, Avril MF, Barillot E, Cabaret O, Delattre O, Richard S, Caron O, Benfodda M, Hu HH, Soufir N, Bressac-de Paillerets B, Stoppa-Lyonnet D, Stern MH. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92(6):974–980. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farley MN, Schmidt LS, Mester JL, Peña-Llopis S, Pavia-Jimenez A, Christie A, Vocke CD, Ricketts CJ, Peterson J, Middelton L, Kinch L, Grishin N, Merino MJ, Metwalli AR, Xing C, Xie XJ, Dahia PL, Eng C, Linehan WM, Brugarolas J. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res. 2013;11:1061–1071. doi: 10.1158/1541-7786.MCR-13-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodward ER, Skytte AB, Cruger DG, Maher ER. Population-based survey of cancer risks in chromosome 3 translocation carriers. Genes Chromosomes Cancer. 2010;49(1):52–58. doi: 10.1002/gcc.20718. [DOI] [PubMed] [Google Scholar]
- 36.Shuch B, Ricketts CJ, Vocke CD, Komiya T, Middelton LA, Kauffman EC, Merino MJ, Metwalli AR, Dennis P, Linehan WM. Germline PTEN mutation Cowden syndrome: an underappreciated form of hereditary kidney cancer. J Urol. 2013;190:1990–1998. doi: 10.1016/j.juro.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peron A, Vignoli A, La Briola F, Volpi A, Montanari E, Morenghi E, Ghelma F, Bulfamante G, Cefalo G, Canevini MP. Do patients with tuberous sclerosis complex have an increased risk for malignancies? Am J Med Genet A. 2016;170:1538–1544. doi: 10.1002/ajmg.a.37644. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez KG, Ezzat S, Morel CF, Swallow C, Otremba M, Dickson BC, Asa SL, Mete O. Familial pheochromocytoma and renal cell carcinoma syndrome: TMEM127 as a novel candidate gene for the association. Virchows Arch. 2015;466(6):727–732. doi: 10.1007/s00428-015-1755-2. [DOI] [PubMed] [Google Scholar]
- 39.Casey RT, Warren AY, Martin JE, Challis BG, Rattenberry E, Kithworth J, et al. JCEM. 2017;102(11):4013–4022. doi: 10.1210/jc.2017-00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haven CJ, Wong FK, van Dam EW, van der Juijt R, van Asperen C, Jansen J, Rosenberg C, de Wit M, Roijers J, Hoppener J, Lips CJ, Larsson C, Teh BT, Morreau H. A genotypic and histopathological study of a large Dutch kindred with hyperparathyroidism-jaw tumor syndrome. J Clin Endocrinol Metab. 2000;85:1449–1454. doi: 10.1210/jcem.85.4.6518. [DOI] [PubMed] [Google Scholar]
- 41.Schodel J, Bardella C, Sciesielski LK, et al. Common genetic variants at the 11q13.3 renal cancer susceptibility locus influence binding of HIF to an enhancer of cyclin D1 expression. Nat Genet. 2012;44(4):420–425. doi: 10.1038/ng.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grampp S, Platt JL, Lauer V, Salama R, Kranz F, Neumann VK, Wach S, Stöhr C, Hartmann A, Eckardt KU, Ratcliffe PJ, Mole DR, Schödel J. Genetic variation at the 8q24.21 renal cancer susceptibility locus affects HIF binding to a MYC enhancer. Nat Commun. 2016;7:13183. doi: 10.1038/ncomms13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maher ER, Yates JR, Ferguson-Smith MA. Statistical analysis of the two stage mutation model in von Hippel–Lindau disease, and in sporadic cerebellar haemangioblastoma and renal cell carcinoma. J Med Genet. 1990;27(5):311–314. doi: 10.1136/jmg.27.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shuch B, Vourganti S, Ricketts CJ, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J Clin Oncol. 2014;32:431–437. doi: 10.1200/JCO.2013.50.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reaume MN, Graham GE, Tomiak E, et al. Canadian guideline on genetic screening for hereditary renal cell cancers. Can Urol Assoc J. 2013;7:319–332. doi: 10.5489/cuaj.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NGS in PPGL (NGSnPPGL) Study Group. Toledo RA, Burnichon N, Cascon A, Benn DE, Bayley JP, Welander J, Tops CM, Firth H, Dwight T, Ercolino T, Mannelli M, Opocher G, Clifton-Bligh R, Gimm O, Maher ER, Robledo M, Gimenez-Roqueplo AP, Dahia PL. Consensus statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol. 2017;13:233–247. doi: 10.1038/nrendo.2016.185. [DOI] [PubMed] [Google Scholar]
- 47.Byler TK, Bratslavsky G. Hereditary renal cell carcinoma: genetics, clinical features, and surgical considerations. World J Urol. 2014;32:623–630. doi: 10.1007/s00345-014-1287-4. [DOI] [PubMed] [Google Scholar]
- 48.Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L, Micaroni M, Chaneton B, Adam J, Hedley A, Kalna G, Tomlinson IP, Pollard PJ, Watson DG, Deberardinis RJ, Shlomi T, Ruppin E, Gottlieb E. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225–228. doi: 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- 49.Sourbier C, Ricketts CJ, Matsumoto S, Crooks DR, Liao PJ, Mannes PZ, Yang Y, Wei MH, Srivastava G, Ghosh S, Chen V, Vocke CD, Merino M, Srinivasan R, Krishna MC, Mitchell JB, Pendergast AM, Rouault TA, Neckers L, Linehan WM. Targeting ABL1-mediated oxidative stress adaptation in fumarate hydratase-deficient cancer. Cancer Cell. 2014;26:840–850. doi: 10.1016/j.ccell.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, Maxwell PH. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. doi: 10.1016/S1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- 51.Park BK, Kim CK, Park SY, Shen SH. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease: indications, techniques, complications, and outcomes. Acta Radiol. 2013;54:418–427. doi: 10.1177/0284185113475441. [DOI] [PubMed] [Google Scholar]
- 52.Hopkins TG, Maher ER, Reid E, Marciniak SJ. Recurrent pneumothorax. Lancet. 2011;377(9777):1624. doi: 10.1016/S0140-6736(11)60072-X. [DOI] [PubMed] [Google Scholar]