Abstract
Purpose
We aimed to determine the feasibility of targeting low-normal or high-normal mean arterial pressure (MAP) after out-of-hospital cardiac arrest (OHCA) and its effect on markers of neurological injury.
Methods
In the Carbon dioxide, Oxygen and Mean arterial pressure After Cardiac Arrest and REsuscitation (COMACARE) trial, we used a 23 factorial design to randomly assign patients after OHCA and resuscitation to low-normal or high-normal levels of arterial carbon dioxide tension, to normoxia or moderate hyperoxia, and to low-normal or high-normal MAP. In this paper we report the results of the low-normal (65–75 mmHg) vs. high-normal (80–100 mmHg) MAP comparison. The primary outcome was the serum concentration of neuron-specific enolase (NSE) at 48 h after cardiac arrest. The feasibility outcome was the difference in MAP between the groups. Secondary outcomes included S100B protein and cardiac troponin (TnT) concentrations, electroencephalography (EEG) findings, cerebral oxygenation and neurological outcome at 6 months after cardiac arrest.
Results
We recruited 123 patients and included 120 in the final analysis. We found a clear separation in MAP between the groups (p < 0.001). The median (interquartile range) NSE concentration at 48 h was 20.6 µg/L (15.2–34.9 µg/L) in the low-normal MAP group and 22.0 µg/L (13.6–30.9 µg/L) in the high-normal MAP group, p = 0.522. We found no differences in the secondary outcomes.
Conclusions
Targeting a specific range of MAP was feasible during post-resuscitation intensive care. However, the blood pressure level did not affect the NSE concentration at 48 h after cardiac arrest, nor any secondary outcomes.
Electronic supplementary material
The online version of this article (10.1007/s00134-018-5446-8) contains supplementary material, which is available to authorized users.
Keywords: Arterial pressure, Cardiac arrest, Intensive care, Neuron-specific enolase (NSE), Hypoxic ischemic encephalopathy, Mechanical ventilation
Introduction
Arterial hypotension is associated with increased mortality and poor neurological outcome after cardiac arrest and resuscitation [1]. The hemodynamic instability during the post-resuscitation period is thought to be caused by a global ischemia–reperfusion injury leading to myocardial stunning [2], a sepsis-like systemic inflammation [3] and adrenal axis suppression [4]. Because of the hypoxic ischaemic encephalopathy (HIE) developing early after the return of spontaneous circulation (ROSC), autoregulation of cerebral blood flow (CBF) may be impaired and cerebral perfusion may become directly dependent on blood pressure [5]. Thus, arterial hypotension after cardiac arrest may lead to cerebral hypoperfusion and increasing epileptic activity, which may aggravate the developing brain damage.
Targeting a specific blood pressure level has been proposed as a treatment strategy after cardiac arrest, but the optimal target remains unknown [6]. During intensive care, the blood pressure can be regulated with fluid infusions and vasoactive agents. In experimental studies, inducing hypertension with vasoactive agents during the post-resuscitation period has been associated with attenuation of HIE and better neurological outcomes after asphyxial cardiac arrest [7]. According to observational human data, higher mean arterial pressure (MAP) is associated with increased survival and better neurological outcomes [1, 8–10]. In addition, a MAP between 87 and 101 mmHg was associated with cerebral oxygenation values considered as optimal [11]. However, the benefit of using vasoactive agents to raise the MAP to a certain level remains controversial. Actually, both low blood pressures and high vasopressor doses have been associated with increased mortality [12]. Importantly, no randomised controlled trials comparing different levels of MAP after cardiac arrest have been performed [6].
Accordingly, we performed a multi-centre, randomised pilot trial to assess the feasibility and the effect on the serum concentration of neuron-specific enolase (NSE) of targeting low-normal or high-normal MAP after OHCA and successful resuscitation. In addition, we investigated the effect of low-normal vs. high-normal MAP on other markers of neurological and myocardial injury, electroencephalography (EEG) and cerebral oxygenation. Our hypothesis was that higher MAP would result in lower NSE concentrations and attenuation of HIE.
Methods
Trial design
We have published the protocol of the Carbon dioxide, Oxygen and Mean arterial pressure After Cardiac Arrest and REsuscitation (COMACARE) study previously [13]. Briefly, we performed a prospective, multi-centre, randomised trial with 23 factorial design. We randomly assigned 123 unconscious, mechanically ventilated patients resuscitated from OHCA to targeting low-normal or high-normal arterial carbon dioxide tension (PaCO2), normal or moderately elevated arterial oxygen tension (PaO2) and low-normal or high-normal MAP for the first 36 h at the intensive care unit (ICU). Accordingly, each patient was randomised into one of eight arms, each arm with a different combination of targets for PaCO2, PaO2 and MAP. We report the results of the low-normal vs. high-normal MAP comparison here, and the results of the other parts of the study elsewhere in this journal.
Participants
The eligible participants included adults resuscitated from witnessed OHCA with ventricular fibrillation (VF) or ventricular tachycardia (VT) as the initial rhythm. In addition, all of the following inclusion criteria had to be met: (1) return of spontaneous circulation (ROSC) 10–45 min from the onset of cardiac arrest, (2) confirmed or suspected cardiac origin of the arrest, (3) mechanical ventilation upon ICU arrival, (4) markedly impaired level of consciousness defined as no response to verbal commands and Glasgow Coma Scale (GCS) motor score < 5 (withdrawal from painful stimuli at best), (5) deferred consent from next of kin possible or likely, and (6) active intensive care and targeted temperature management (TTM) initiated. Patients with confirmed or suspected acute or pre-existing intracranial pathology and/or suspicion of increased intracranial pressure were excluded from the study. In addition, patients with severe oxygenation failure (defined as PaO2/FiO2 (fraction of inspired oxygen) < 100 mmHg upon arrival at the ICU) or severe chronic obstructive pulmonary disease were excluded. Other exclusion criteria were age < 18 or > 80 years and pregnancy.
Six ICUs in Finland and one in Denmark participated in the trial. All patients who were resuscitated from OHCA and admitted to one of the participating ICUs were screened for eligibility. Because of the nature of the trial, the patients’ unconscious state and the need for a timely intervention, it was not possible to obtain prior informed consent from the participants at the time of randomisation. Therefore, the patients were randomised, and the intervention was initiated at the time of ICU admission. Deferred informed consent from the patients’ next of kin was obtained as soon as they were present at the hospital. After the intervention period, informed consent was also obtained from all patients who regained sufficient neurological function for independent decision-making [cerebral performance category (CPC) 1–2]. The study protocol and the deferred consent option were approved by the research ethics committees of the Northern Savo Hospital District, Finland (decision No. 295/2015) and the Midtjylland region, Denmark (decision No. 1-10-72-163-16). In addition, the trial protocol was approved by the institutional review board at each site.
Interventions
After ICU admission and randomisation, we guided the treating personnel to target low-normal (65–75 mmHg) or high-normal (80–100 mmHg) MAP by using a continuous infusion of noradrenaline as needed. We allowed fluid boluses to treat hypovolemia according to the treating clinicians’ preference. If low cardiac output was confirmed or suspected, the use of an inotrope such as dobutamine or levosimendan was allowed. There were no limits to the infusion rates of noradrenalin or inotropes. We did not lower the blood pressure by any means other than sedation and pain medication in order to meet the low-normal MAP target. In cases with MAP exceeding 140 mmHg or detected left ventricular systolic dysfunction, we allowed the use of vasodilating agents to lower the blood pressure according to the treating clinicians’ decisions.
In addition to verbal instructions, we used laminated signs designating the intervention targets at the patients’ bedside. The intervention was continued for 36 h from ICU admission or until the patient was extubated or ventilation was set to a spontaneous mode. All patients were treated with targeted temperature management (TTM) at 33 °C or 36 °C and sedated as needed. Standard monitoring, care and investigations according to the local protocol of the ICU were used for all patients, including direct blood pressure monitoring via an arterial catheter.
Outcomes
The primary outcome was the NSE serum concentration at 48 h after cardiac arrest. The main feasibility outcome was the difference in MAP between groups targeting low-normal (65–75 mmHg) and high-normal (80–100 mmHg) MAP. The pre-specified secondary outcomes were serum NSE concentrations at 24 h and 72 h after cardiac arrest; serum S100B protein (a biomarker of glial injury) concentrations at 24 h, 48 h, and 72 h after cardiac arrest; plasma cardiac troponin (TnT) concentrations at 24 h, 48 h, and 72 h after cardiac arrest; regional frontal cerebral oxygenation (rSO2) measured by continuous near-infrared spectroscopy (NIRS) monitoring during the first 48 h after admission to the ICU; results of continuous electroencephalography (EEG) monitoring for the first 48 h after admission to the ICU, interpreted by an experienced senior neurophysiologist blinded to study group allocation; CPC at 6 months after cardiac arrest (CPC 1–2 considered as good outcome and CPC 3–5 as poor outcome), determined by an experienced neurologist blinded to study group allocation; total duration of intensive care; total duration of mechanical ventilation; length of hospital stay; discharge destination and vital status at 30 days after cardiac arrest (dead or alive). Other feasibility outcomes included distribution of values for primary and secondary outcomes, randomised/screened patient ratio, consent rate, data completion rate and duration of recruitment. The pre-defined serious adverse events (SAE) that could be related to the interventions were severe hypercapnia and respiratory acidosis (PaCO2 > 10 kPa and pH < 7.15), unexplained brain edema on CT scanning and severe unexplained ARDS (PaO2/FiO2 ratio of < 100 mmHg).
Data collection
Baseline data regarding the participants’ age, gender, prior health status, and functional capacity, as well as resuscitation details were saved in a web-based study database (Absolute Imaginary Software, Helsinki, Finland). For the first 48 h after ICU admission, all of the monitored vital parameters, including direct blood pressure measurements via arterial cannula, were saved in a medically approved tablet computer (Arbor M1040, Taiwan) connected to the patient monitor with the GE Healthcare S/5 collect software version 4.0. We obtained blood samples for ABG analysis via an arterial cannula and analysed them on-site as part of the routine ICU care. The results of the ABG analysis, corrected to the patient’s actual temperature, were entered manually into the web-based study database. The doses of sedative and vasoactive drug infusions were also entered manually into the database. The ventilator settings were exported directly from the ventilators and saved to a USB drive after the intervention or, if this was not possible, entered manually into the electronic database.
We obtained blood samples for the analyses of the NSE, S100B and TnT concentrations upon ICU admission and 24 h, 48 h and 72 h after cardiac arrest. In the Finnish centres, the samples were centrifuged (2000 G, 10 min) and frozen at − 70 °C at the hospital laboratory. Determination of NSE, S100B and TnT was performed using a COBAS e601 line (Hitachi High Technology Co, Tokyo, Japan) with an electrochemiluminescent immunoassay kit (Roche Diagnostics GmbH, Mannheim, Germany) in January 2018. Because of possible interference with the NSE results, all serum samples were tested for haemolysis using the Roche haemolysis index and all samples with a haemolysis index > 500 mg of free haemoglobin per litre (n = 7) were excluded from the NSE analyses. At the Aarhus University Hospital, Denmark, the samples were analysed immediately by the local laboratory using the same kits as those used by the ISLAB laboratory.
We measured the frontal rSO2 with a Covidien INVOS 5100C device (Covidien Company, USA) with two non-invasive skin sensors attached to both sides of the patient’s forehead by a study nurse. The rSO2 values (approximately 10 measurements per minute) from both sensors were saved to a USB memory stick attached to the device. We calculated the hourly medians of the rSO2 values of the left channel and used them in the analysis.
Upon the patient’s admission to the ICU, continuous four-channel EEG monitoring with a GE Carescape module connected to the monitor (Datex-Ohmeda S/5 or GE B650/B850 depending on the centre) was initiated and continued for 48 h after admission. We saved the EEG data in the tablet computer recording the vital parameters with the GE Healthcare collect software. One senior neurophysiologist blinded to the study group allocations later analysed all the EEG recordings. The EEG recordings were categorised into three groups according to the degree of abnormality (mild, moderate or severe) as proposed by Crepeau et al. [14] at the beginning and at the end of the intervention.
Randomisation
The study centres used a web-based randomisation system. A cryptographically strong random number generator with modulo bias eliminated was used to generate random numbers, and an unbiased Fisher-Yates (Durstenfeld) algorithm was used to shuffle the blocks. The randomisation was stratified according to the target temperature (33 °C or 36 °C). The participants were enrolled and assigned to study interventions by a study nurse or the treating clinician in the ICU. Because of the nature of the interventions, the treating personnel could not be blinded regarding the treatment targets. However, the NIRS monitor screens were not visible to the ICU staff, as NIRS monitoring was not part of the routine post-resuscitation care at the study centres. Moreover, the neurophysiologist analysing the EEG results and the neurologist evaluating the neurologic recovery of the participants were blinded to the study group allocations.
Sample size calculation
Based on a previous cohort of OHCA patients, we expected the mean NSE concentration at 48 h to be close to 17 µg/L, and the standard deviation near 20 µg/L [15]. A study with 39 patients in each arm would have a power of 80%, with the significance set at 0.05, to detect a 50% difference in NSE. Given the possibility of death prior to 48 h and loss of follow-up, we decided to recruit 50% more patients. Therefore, the sample size was set at 120 patients.
Statistical methods
We compared the categorical data by using the Chi-square test. We tested the normality of the continuous data and compared the data with a normal distribution with the Student’s t test and the data with a non-normal distribution with the Mann–Whitney U test. We divided the intervention time into 1 h periods starting at the time of the ICU admission, and for each patient, we calculated the median MAP for each period. We then compared these 1 h medians for the low-normal and high-normal MAP groups over time using a generalized mixed model with a compound-symmetry covariance matrix. For the low-normal and high-normal MAP groups, we compared the NSE serum concentrations at 48 h using the Mann–Whitney U test. We compared the NSE, S100B, TnT and rSO2 values over time with a generalised mixed model with a compound-symmetry covariance matrix. The distributions of the EEG abnormalities (mild, moderate, or severe), 30-day mortality rates and 6-month CPC results were compared by using the Chi-square test. The interaction effect of the PaCO2 target and the TTM, PaO2 and MAP targets on the CPC results at 6 months was analysed with a binary logistic regression model. The interaction of the TTM, PaO2 and MAP targets on the NSE results at 48 h was assessed with the univariate analysis of variance. We performed all statistical analyses with SPSS version 24.0.
Results
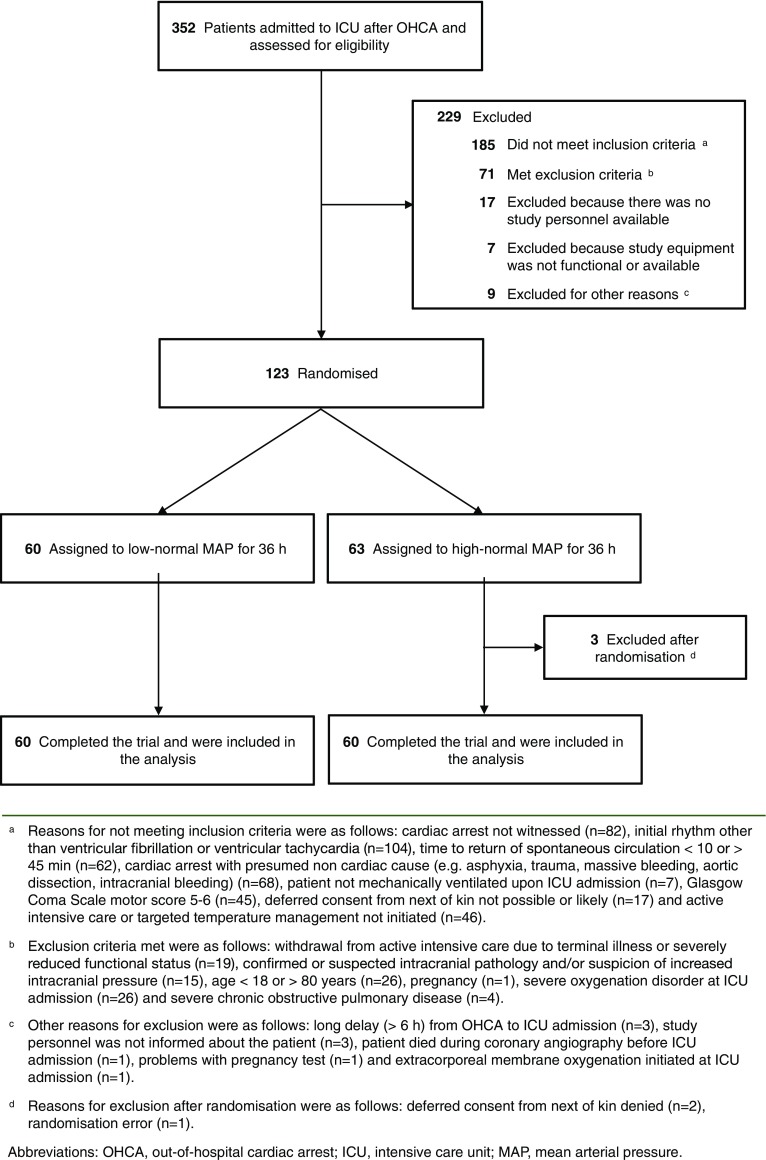
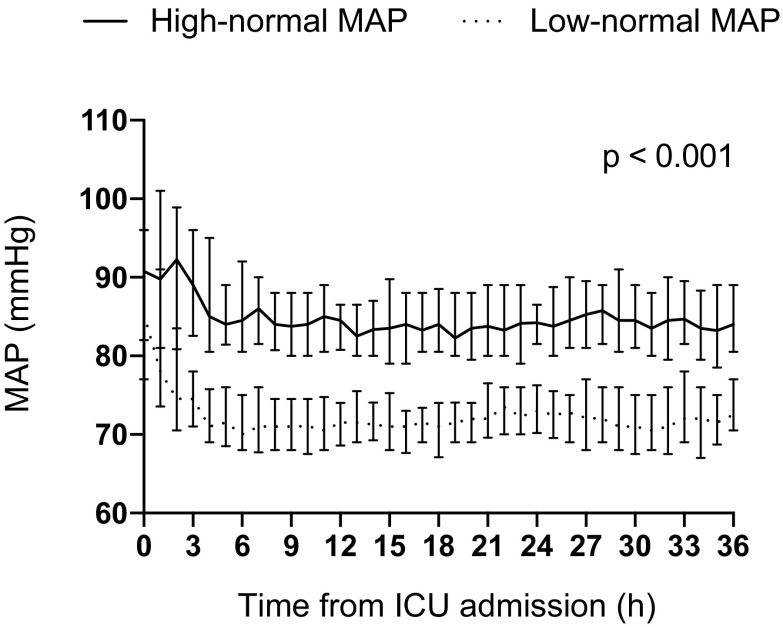
The flowchart demonstrating patient enrollment and group allocation is presented in Fig. 1. The patient recruitment began on 22 March 2016 and was completed by 3 November 2017. The 6-month follow-up of the last patient was completed by 3 May 2018. The baseline characteristics and resuscitation-associated factors were comparable between the groups (Table 1). We observed a clear separation in MAP between the groups, p < 0.001 (Fig. 2). The median (inter-quartile range, IQR) noradrenalin dose used was 0.05 µg/kg/min (IQR 0.02–0.11 µg/kg/min) in the low-normal MAP group and 0.13 µg/kg/min (IQR 0.08–0.20 µg/kg/min) in the high-normal MAP group, p < 0.001.
Fig. 1.
Screened, excluded and included patients in the study
Table 1.
Baseline characteristics of the study population
| Low-normal MAP group (n = 60) | High-normal MAP group (n = 60) | |
|---|---|---|
| Demographic characteristics | ||
| Age, mean ± SD, years | 61 ± 11 | 58 ± 14 |
| Male sex, n (%) | 48 (80) | 50 (83) |
| Weight, mean ± SD, kg | 86 ± 19 | 83 ± 14 |
| Neurologic function before cardiac arrest | ||
| Normal, CPC score 1, n (%) | 57 (95) | 54 (90) |
| Some disability, CPC score 2, n (%) | 3 (5) | 6 (10) |
| Medical history | ||
| Antihypertensive medication, n (%) | 26 (43) | 34 (57) |
| Chronic heart failure (NYHA class IV), n (%)a | 0 | 2 (3) |
| Inhaled corticosteroids, n (%) | 4 (7) | 2 (3) |
| Inhaled bronchodilators, n (%) | 5 (8) | 3 (5) |
| Smoker, n (%)b | 20 (33) | 20 (33) |
| Cardiac arrest location | ||
| Home, n (%) | 32 (53) | 28 (47) |
| Public place, n (%) | 28 (47) | 32 (53) |
| Resuscitation factors | ||
| Bystander-initiated resuscitation, n (%) | 51 (85) | 47 (78) |
| Time to basic life supportd, median (IQR), min | 8 (6–10) | 7 (5–9) |
| Time to advanced life support, median (IQR), min | 10 (7–12) | 10 (7–12) |
| Time to ROSC, median (IQR), min | 22 (16–27) | 19 (15–25) |
| Intubated during resuscitation, n (%) | 26 (43) | 31 (52) |
| Immediate interventional cardiology | ||
| Pre-hospital thrombolysis, n (%) | 3 (5) | 1 (2) |
| Coronary angiography before ICU admission, n (%) | 35 (58) | 28 (47) |
| Clinical status on ICU admission | ||
| GCS after ROSC, median (IQR)c | 3 (3–3) | 3 (3–3) |
| APACHE II score, median (IQR) | 28 (24–32) | 27 (24–31) |
| Pre-hospital cooling, n (%) | 4 (7) | 6 (10) |
| Dose of norepinephrine, mean ± SD, μg/kg/min | 0.06 ± 0.08 | 0.08 ± 0.11 |
| Time from ROSC to randomisation, median (IQR), min | 171 (148–214) | 172 (128–204) |
| Targeted temperature management | ||
| 33 °C, n (%) | 42 (70) | 41 (68) |
| 36 °C, n (%) | 18 (30) | 19 (32) |
SD standard deviation, IQR inter-quartile range, CPC cerebral performance category [1 good cerebral performance (normal life); 2 moderate cerebral disability (disabled but independent); 3 severe cerebral disability (conscious but disabled and dependent); 4 coma or vegetative state (unconscious); 5 brain death]; NYHA New York Heart Association, CPR cardiopulmonary resuscitation, ICU intensive care unit, GCS Glasgow coma scale, ROSC return of spontaneous circulation, APACHE acute physiology and chronic health evaluation
aData missing for two patients
bData missing for 13 patients
cData missing for nine patients
dThe time for a paramedic unit with BLS equipment and skills to reach the patient
Fig. 2.
Median (inter-quartile range) MAP during the intervention in the study groups
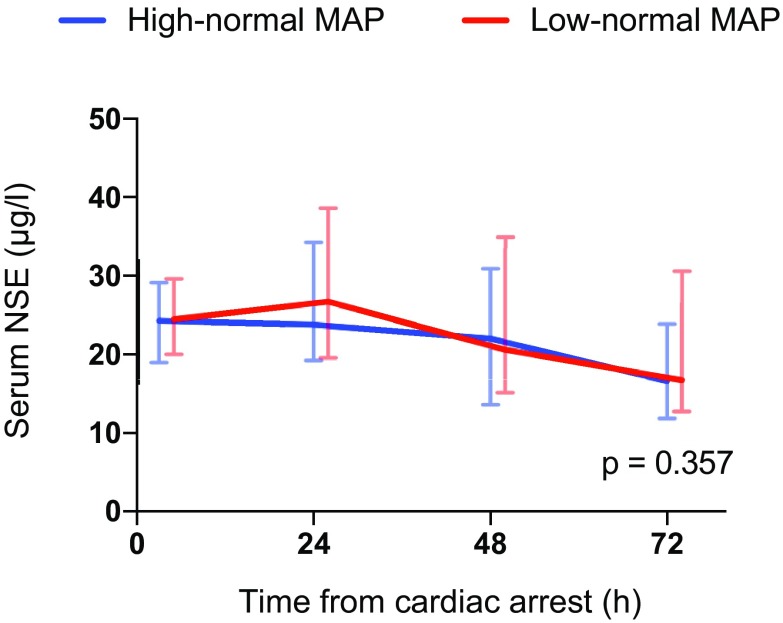
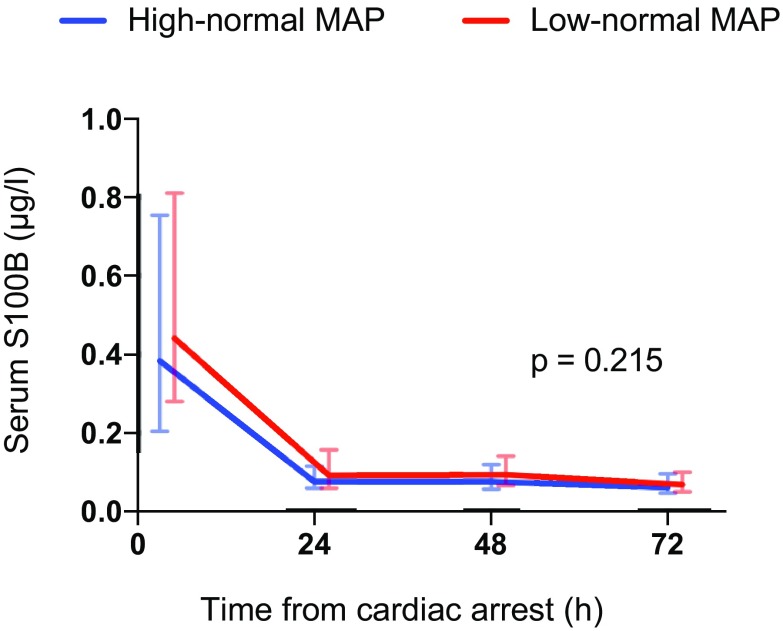
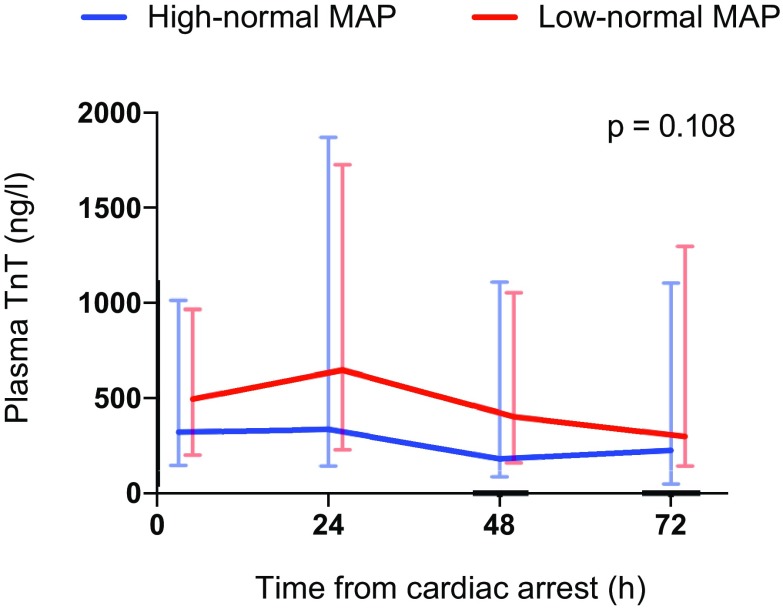
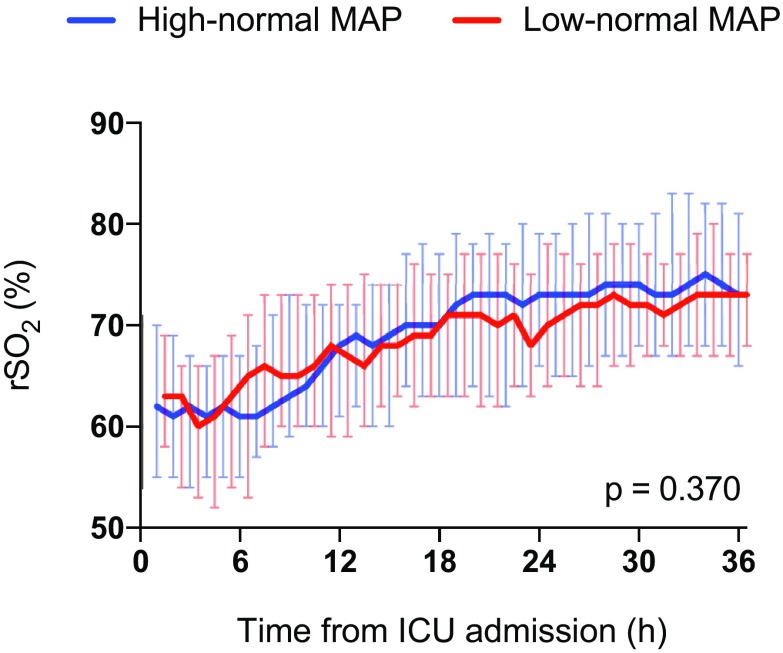
The median serum NSE concentration at 48 h after cardiac arrest was 20.6 µg/L (IQR 15.2–34.9 µg/L) in the low-normal MAP group and 22.0 µg/L (IQR 13.6–30.9 µg/L) in the high-normal MAP group, p = 0.522. The median NSE, S100B and TnT concentrations and frontal rSO2 values over time were comparable in the two groups (Figs. 3, 4, 5, 6). We found no significant differences in any of the following outcomes: mortality at 30 days after cardiac arrest, the proportion of patients with good neurological recovery (CPC 1–2) at 6 months after cardiac arrest, the duration of intensive care or mechanical ventilation (Table 2), EEG grading (Table 3) or the frequency of the pre-defined serious adverse events between the groups (Table 2). Regarding the NSE results at 48 h after cardiac arrest or good neurological outcomes at 6 months, we did not find any significant interactions between the PaCO2, PaO2, MAP or TTM targets (online Table 4).
Fig. 3.
Baseline, 24 h, 48 h and 72 h median (inter-quartile range) serum neuron-specific enolase (NSE) concentrations for patients allocated to targeting low-normal and high-normal MAP
Fig. 4.
Baseline, 24 h, 48 h and 72 h median (inter-quartile range) serum S100B concentrations for patients allocated to targeting low-normal and high-normal MAP
Fig. 5.
Baseline, 24 h, 48 h and 72 h median (inter-quartile range) plasma cardiac troponin (TnT) concentrations for patients allocated to targeting low-normal and high-normal MAP
Fig. 6.
Median (inter-quartile range) regional cerebral oxygen saturation (rSO2) during the intervention in the study groups
Table 2.
Primary and secondary outcomes after the intervention
| Low-normal MAP group (n = 60) | High-normal MAP group (n = 60) | p value | |
|---|---|---|---|
| Primary outcome | |||
| Median (IQR) NSE at 48 h after cardiac arrest, μg/La | 21.2 (15.1–34.9) | 22.0 (13.6–30.9) | 0.392 |
| Secondary outcomes | |||
| Neurologic recovery at 6 months after cardiac arrest | |||
| Good, CPC score 1–2, n (%) | 37 (62) | 41 (68) | 0.444 |
| Mortality 30 days after cardiac arrest, n (%) | 20 (33) | 18 (30) | 0.695 |
| Median (IQR) duration of intensive care, hb | 107 (76–162) | 94 (75–136) | 0.283 |
| Median (IQR) duration of mechanical ventilation, hc | 82 (52–123) | 59 (49–88) | 0.074 |
| Severe adverse events | |||
| Severe hypercapnia and respiratory acidosis (PaCO2 > 10 kPa and pH < 7.15), n (%) | 1 (2) | 0 (0) | 0.315 |
| Unexplained brain edema on CT scanning, n (%) | 1 (2) | 0 (0) | 0.315 |
| Severe ARDS (PaO2/FiO2 < 100 mmHg), n (%) | 2 (3) | 0 (0) | 0.154 |
MAP mean arterial pressure, IQR inter-quartile range, NSE neuron-specific enolase, CPC cerebral performance category [1 good cerebral performance (normal life); 2 moderate cerebral disability (disabled but independent); 3 severe cerebral disability (conscious, but disabled and dependent); 4 coma or vegetative state (unconscious); 5 brain death], CT computed tomography, ARDS acute respiratory distress syndrome, FiO2 fraction of inspired oxygen
aData missing for one patient
bData missing for six patients
cData missing for three patients
Table 3.
EEG grading in the low-normal and high-normal MAP groups at ICU admission and at the end of the intervention
| N (%) of patients | ||||
|---|---|---|---|---|
| ICU admission | End of intervention | |||
| Low-normal MAP | High-normal MAP | Low-normal MAP | High-normal MAP | |
| EEG-gradea | ||||
| 1 | 12 (20) | 19 (32) | 39 (65) | 39 (65) |
| 2 | 2 (3) | 3 (5) | 3 (5) | 4 (7) |
| 3 | 45 (75) | 37 (62) | 17 (28) | 16 (27) |
| p value | 0.278 | 0.917 | ||
EEG electroencephalography, MAP mean arterial pressure, ICU intensive care unit
aEEG grading system for continuous EEG findings following cardiac arrest according to Crepeau et al.: mild (grade 1), moderate (grade 2) and severe (grade 3)
Discussion
We performed the first prospective, randomised clinical trial assessing the feasibility of targeting low-normal or high-normal MAP after OHCA and successful resuscitation. We compared the effects of low-normal vs. high-normal MAP on biomarkers of brain injury, myocardial injury, brain oxygenation and epileptic activity during the post-resuscitation intensive care. We found that targeting a specific level of blood pressure by using vasoactive infusions was feasible in comatose patients after cardiac arrest. However, a higher MAP level of 80–100 mmHg did not affect the serum concentration of NSE at 48 h after cardiac arrest, when compared with a lower MAP level of 65–75 mmHg. In addition, we found no effects of higher MAP on the secondary outcomes that included the serum concentration of S100B, the plasma concentration of TnT, brain oxygenation measured with NIRS and epileptic activity on the EEG.
According to observational studies, higher mean arterial pressures are associated with better outcomes, with thresholds of 70 mmHg [16] and 75 mmHg [17] presented. However, treatment recommendations cannot be given on the basis of observational studies alone, because high doses of vasopressors used to raise blood pressures may also be harmful [18]. Because of the lack of high-quality data from randomised trials, current guidelines do not give any specific target for the blood pressure after cardiac arrest, but instead recommend aiming for a blood pressure that is sufficient to achieve an adequate urine output (1 mL/kg/h) and normal or decreasing plasma lactate levels [6]. It is also unclear whether optimal MAP targets differ according to the target temperature, even though hypotension appears to be harmful irrespective of temperature [9]. In our randomised clinical trial, the results in the higher MAP (80–100 mmHg) group were comparable to those in the lower MAP (65–75 mmHg) group concerning all predefined study endpoints. Importantly, our study included patients treated in either 33 °C or 36 °C, and we found no interaction effect of the temperature target and the MAP level.
It has been suggested that the autoregulation of CBF is disturbed after cardiac arrest because of the developing brain damage and that cerebral perfusion may become directly dependent on cerebral perfusion pressure, which is dependent on MAP [5]. Thus, it is plausible that increasing the blood pressure might increase cerebral perfusion and improve oxygenation. However, the results of our trial do not support this hypothesis: cerebral oxygenation, as measured by NIRS, was unaffected by the MAP level. Our findings are in accordance with the results of a previous study in 10 cardiac arrest patients, where increasing MAP from 70 to 90 mmHg did not affect brain tissue oxygenation [19].
The rather small sample size in our trial limits the strength of conclusions that can be drawn. Nonetheless, the fact that we found no signals suggesting either benefits or harms from raising the MAP target from 65–75 to 80–100 mmHg gives little encouragement to the planning of larger trials using these MAP targets and these endpoints. However, it is possible that the optimal MAP may differ between patients, based on their previous blood pressure level and other health factors. Therefore, rather than using the same target for all patients, we may need to estimate the optimal MAP for each patient [20]. The best way to determine the adequate MAP target is unknown so far. Combining continuous transcranial Doppler ultrasound with NIRS might have provided additional information of the CBF, but for practical reasons it was impossible to implement in this multicentre trial.
Increased epileptiformic EEG activity is a predictor of poor outcome after cardiac arrest, but previous studies have not identified any association with blood pressure level and EEG activity [21]. We found that a higher MAP level of 80–100 mmHg did not affect the EEG findings when compared with a lower MAP level of 65–75 mmHg which supports the results of previous observational studies.
Myocardial damage caused by acute coronary syndrome and myocardial ischaemia–reperfusion injury is common after cardiac arrest and resuscitation. Excessive vasopressor use may lead to increased afterload and increased oxygen consumption of the heart, thereby aggravating the myocardial damage. On the other hand, hypotension or even low-normal blood pressure at hospital admission is associated with increased mortality in patients with acute myocardial infarction [22]. In addition, hypotension can decrease coronary perfusion pressure leading to myocardial hypoperfusion and ischaemia and, eventually, cardiovascular collapse [23]. In our study, the concentration of TnT was comparable in both intervention groups suggesting that there was no difference in the extent of myocardial damage between the groups despite the different blood pressure levels and significantly higher noradrenalin load in the high-normal MAP group.
This study has several strengths. First, we had published the study protocol and the plan for the statistical analysis in advance [13]. Second, we used relatively strict eligibility criteria to focus the trial on a homogenous group of OHCA patients in order to reduce the bias caused by possible differences in baseline characteristics and resuscitation-related factors. Third, we recorded the arterial pressure of the patients continuously for the whole intervention period and modified the infusion rates of vasoactive agents as needed to keep the blood pressure in the desired range. Fourth, we carried out post-resuscitation intensive care according to the current guidelines for all patients. Fifth, we studied patients in multiple centres and two different countries. Sixth, we included patients treated with TTM at both 33 °C and 36 °C. Seventh, we recorded frontal rSO2 and EEG continuously during the first 48 h of ICU care. Finally, we consider our results reliable because of a clear separation between the study groups in recorded MAP values that were close to the targeted levels.
We acknowledge that this study also has some limitations. First, despite multiple centres participating in the trial, a majority of the patients were recruited in one hospital. Second, as arterial pressure is an important monitored variable for all patients in the ICU, the study intervention could not be blinded, and the treating personnel were aware of the study group allocations. Third, for practical reasons, we decided to start the interventions at the hospital after ICU admission and not during pre-hospital care. We are aware that interventions aiming at affecting the course of HIE should be started as early as possible. However, we feel that the delay between the ROSC and the beginning of the interventions was acceptable for most participants (Table 1). Fourth, we used a four-channel technique for EEG monitoring and there is a risk that some focal epileptic discharges may have been undetected. Finally, the blood samples taken at the Danish site had to be analysed on-site for logistical reasons. However, to minimise a possible bias resulting from technical issues, we used the same laboratory kits for NSE and S100B evaluation in both Finland and Denmark.
Conclusions
Targeting low-normal or high-normal MAP was feasible in comatose patients resuscitated from OHCA and admitted to ICU. However, the blood pressure level did not affect markers of cerebral or myocardial injury, cerebral oxygenation, or EEG findings.
Other information
Registration
ClinicalTrials.gov, NCT02698917. Registered on 26 January 2016.
Protocol
The protocol of the COMACARE study has been previously published.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. Comacare study group: Aarhus University Hospital: Thomas Birkelund, Susanne Ilkjaer, Hans Kirkegaard; Central Finland Central Hospital: Raili Laru-Sompa, Anni Pulkkinen, Mikko Reilama, Sinikka Tolmunen; Helsinki University Hospital: Minna Bäcklund, Jonna Heinonen, Johanna Hästbacka, Pekka Jakkula, Nina Lundblom, Marcus Norrgård, Marjatta Okkonen, Ville Pettilä, Markus B Skrifvars, Tarja Suhonen, Marjaana Tiainen, Tuukka Tikka, Marjut Timonen, Jussi Toppila, Miia Valkonen, Erika Wilkman; Jorvi Hospital: Teemu Hult, Tuomas Oksanen; Kuopio University Hospital: Stepani Bendel, Elina Halonen, Sari Rahikainen, Saija Rissanen, Eija Vaskelainen; North Karelia Central Hospital: Tanja Eiserbeck, Sirkku Heino, Helena Jyrkönen, Matti Reinikainen, Johanna Räsänen, Tero Surakka; Päijät-Häme Central Hospital: Talvikki Koskue, Petteri Kujala, Pekka Loisa, Marika Lähde; Tampere University Hospital: Jari Kalliomäki, Sari Karlsson, Atte Kukkurainen, Simo Varila. We thank Tuomas Selander, MSc, biostatistician, for help with the statistical analyses.
Funding
Independent funding support has been received from Helsinki University; Helsinki University Hospital (State funding, Finland); Stiftelsen Dorothea Olivia, Karl Walter och Jarl Walter Perkléns minne; The Laerdal Foundation for Acute Medicine; Medicinska Understödsföreningen Liv och Hälsa; Finska Läkaresällskapet; The Finnish Society of Anaesthesiologists; Orion Research Foundation and Svenska kulturfonden. The funding bodies had no input regarding the design, management, or reporting of the trial.
References
- 1.Bhate TD, McDonald B, Sekhon MS, Griesdale DEG. Association between blood pressure and outcomes in patients after cardiac arrest: a systematic review. Resuscitation. 2015;97:1–6. doi: 10.1016/j.resuscitation.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Laurent I, Monchi M, Chiche J-D, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/S0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 3.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.CIR.0000023891.80661.AD. [DOI] [PubMed] [Google Scholar]
- 4.Hékimian G, Baugnon T, Thuong M, et al. Cortisol levels and adrenal reserve after successful cardiac arrest resuscitation. Shock. 2004;22:116–119. doi: 10.1097/01.shk.0000132489.79498.c7. [DOI] [PubMed] [Google Scholar]
- 5.Sundgreen C, Larsen FS, Herzog TM, et al. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32:128–132. doi: 10.1161/01.STR.32.1.128. [DOI] [PubMed] [Google Scholar]
- 6.Nolan JP, Soar J, Cariou A, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Hachimi-Idrissi S, Corne L, Huyghens L. The effect of mild hypothermia and induced hypertension on long term survival rate and neurological outcome after asphyxial cardiac arrest in rats. Resuscitation. 2001;49:73–82. doi: 10.1016/S0300-9572(00)00268-9. [DOI] [PubMed] [Google Scholar]
- 8.Laurikkala J, Wilkman E, Pettilä V, et al. Mean arterial pressure and vasopressor load after out-of-hospital cardiac arrest: associations with one-year neurologic outcome. Resuscitation. 2016;105:116–122. doi: 10.1016/j.resuscitation.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Russo JJ, James TE, Hibbert B, et al. Impact of mean arterial pressure on clinical outcomes in comatose survivors of out-of-hospital cardiac arrest: insights from the University of Ottawa Heart Institute Regional Cardiac Arrest Registry (CAPITAL-CARe) Resuscitation. 2017;113:27–32. doi: 10.1016/j.resuscitation.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Roberts BW, Kilgannon JH, Hunter BR, et al. Association between elevated mean arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest. Crit Care Med. 2018 doi: 10.1097/ccm.0000000000003474. [DOI] [PubMed] [Google Scholar]
- 11.Ameloot K, Meex I, Genbrugge C, et al. Hemodynamic targets during therapeutic hypothermia after cardiac arrest: a prospective observational study. Resuscitation. 2015;91:56–62. doi: 10.1016/j.resuscitation.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Bro-Jeppesen J, Annborn M, Hassager C, et al. Hemodynamics and vasopressor support during targeted temperature management at 33°C Versus 36°C after out-of-hospital cardiac arrest. Crit Care Med. 2015;43:318–327. doi: 10.1097/CCM.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 13.Jakkula P, Reinikainen M, Hästbacka J, et al. Targeting low- or high-normal Carbon dioxide, Oxygen, and Mean arterial pressure After Cardiac Arrest and REsuscitation: study protocol for a randomized pilot trial. Trials. 2017;18:1–9. doi: 10.1186/s13063-017-2257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crepeau AZ, Rabinstein AA, Fugate JE, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest. Neurology. 2013;80:339–344. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- 15.Vaahersalo J, Hiltunen P, Tiainen M, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in Finnish intensive care units: the FINNRESUSCI study. Intensive Care Med. 2013;39:826–837. doi: 10.1007/s00134-013-2868-1. [DOI] [PubMed] [Google Scholar]
- 16.Kilgannon JH, Roberts BW, Jones AE, et al. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest. Crit Care Med. 2014;42:2083–2091. doi: 10.1097/CCM.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 17.Russo JJ, Di Santo Pietro, Simard T, et al. Optimal mean arterial pressure in comatose survivors of out-of-hospital cardiac arrest: an analysis of area below blood pressure thresholds. Resuscitation. 2018;128:175–180. doi: 10.1016/j.resuscitation.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Beylin ME, Perman SM, Abella BS, et al. Higher mean arterial pressure with or without vasoactive agents is associated with increased survival and better neurological outcomes in comatose survivors of cardiac arrest. Intensive Care Med. 2013;39:1981–1988. doi: 10.1007/s00134-013-3075-9. [DOI] [PubMed] [Google Scholar]
- 19.Bouzat P, Suys T, Sala N, Oddo M. Effect of moderate hyperventilation and induced hypertension on cerebral tissue oxygenation after cardiac arrest and therapeutic hypothermia. Resuscitation. 2013;84:1540–1545. doi: 10.1016/j.resuscitation.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Ameloot K, Genbrugge C, Meex I, et al. An observational near-infrared spectroscopy study on cerebral autoregulation in post-cardiac arrest patients: time to drop “one-size-fits-all” hemodynamic targets? Resuscitation. 2015;90:121–126. doi: 10.1016/j.resuscitation.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Moonen C, Lemmens R, Van Paesschen W, et al. The impact of global hemodynamics, oxygen and carbon dioxide on epileptiform EEG activity in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2018;123:92–97. doi: 10.1016/j.resuscitation.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Roth D, Van Tulder R, Heidinger B, et al. Admission blood pressure and 1-year mortality in acute myocardial infarction. Int J Clin Pract. 2015;69:812–819. doi: 10.1111/ijcp.12588. [DOI] [PubMed] [Google Scholar]
- 23.Brunauer A, Koköfer A, Bataar O, et al. The arterial blood pressure associated with terminal cardiovascular collapse in critically ill patients: a retrospective cohort study. Crit Care. 2014;18:1726–1728. doi: 10.1186/s13054-014-0719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.