Abstract
Background
Cognitive impairment affects many patients with multiple sclerosis (MS). NeuroTrax, a computerized cognitive screen that can be administered during routine clinical care, provides a consistent, validated, objective cognitive profile measure with a global cognitive score (GCS) and seven individual domain scores. Natalizumab is an efficacious therapy for relapsing MS, demonstrating reductions in disability worsening and MS disease activity measured by magnetic resonance imaging.
Objective
The aim of this study was to assess cognitive function as measured by NeuroTrax in MS patients treated with natalizumab for ≥ 2 years.
Methods
This retrospective observational study included adult MS patients in the United States who received 300 mg intravenous natalizumab every 4 weeks for ≥ 2 years. NeuroTrax data were evaluated at baseline and yearly thereafter. Changes in GCS and the seven individual cognitive domain scores from baseline to after 24 infusions of natalizumab were analyzed.
Results
In the study population at baseline (N = 52), 22 patients (42.3%) had disease duration of 0–5 years; 12 patients (23.1%) were treatment naive. GCS score improved significantly from baseline [mean 95.5, standard deviation (SD) 12.9] to year 2 (mean 98.9, SD 13.2; change from baseline 3.4; p = 0.003). After 2 years on natalizumab, 17 patients (32.7%) demonstrated clinically significant improvement (increase from baseline > 1 SD) in GCS. Results were similar regardless of whether patients had previously received MS therapy.
Conclusions
Patients treated with natalizumab demonstrated significant improvement in cognitive function, measured by NeuroTrax GCS, over 2 years of treatment.
Key Points
| Natalizumab significantly improved NeuroTrax global cognitive score over 2 years of treatment, regardless of whether patients were treatment-naive or were previously treated with other disease-modifying therapies. |
| Numerical improvements were observed for all seven individual cognitive domains from baseline to 2 years. |
| These results indicate that some aspects of cognitive impairment can be reduced in patients with multiple sclerosis treated with natalizumab. |
Introduction
Cognitive impairment affects approximately 40‒65% of patients with multiple sclerosis (MS) [1, 2], but may be insufficiently recognized or monitored [3]. Nonetheless, cognitive impairment has a significant adverse impact on the quality of life and functional ability of MS patients; patients with MS and cognitive impairment experience greater difficulties with activities of daily living than patients who remain cognitively intact [3, 4]. Cognitive impairment has adverse effects on outcomes such as mobility and fall risk, driving safety, and employment status [4–8]. Although cognitive function is an important consideration when treating patients with MS, screening for deficits using traditional neuropsychological testing can be time-consuming and therefore difficult to complete in clinical practice [9], limiting its use in the management of MS.
NeuroTrax™ (NeuroTrax Ltd, Modiin, Israel; also referred to as the Mindstreams Computerized Cognitive Battery or the Mindstreams Global Assessment Battery) is a computerized cognitive screening test that can be administered in an office setting in approximately 45 min [10–12]. It provides an objective cognitive function profile with a global cognitive score (GCS) and seven individual domain scores (memory, executive function, visual-spatial processing, verbal function, attention, information processing speed, and motor function). Learning effects associated with NeuroTrax appear to be minimal [10, 13, 14]. The test has been validated for screening patients with MS for cognitive impairment and for monitoring them for cognitive change over time [10, 11]. Additionally, the GCS has demonstrated a strong correlation with cognitive impairment as assessed by the Symbol Digit Modalities Test [15].
A recent study using NeuroTrax showed that patients with MS (N = 1500) exhibited poorer cognitive performance than healthy subjects [10]. Cognitive impairment in these patients was most common in the information processing speed and executive function domains.
In addition to a need for cognitive screening to determine the cognitive effect of MS at diagnosis and to monitor disease worsening, there is also a need for screening to evaluate the effects of treatment on cognitive function in MS. Natalizumab has demonstrated efficacy in the treatment of patients with relapsing MS (RMS) [16]. In the 2-year AFFIRM trial, natalizumab significantly reduced disease activity, as reflected by brain magnetic resonance imaging, clinical relapse rate, and rate of confirmed disability worsening, and significantly improved health-related quality of life relative to placebo [16–18]. Some patients with RMS treated with natalizumab also exhibit sustained improvement in disability [19, 20]. Improvement or stabilization of cognition has also been reported in patients treated with natalizumab for 1–3 years [21–24].
In this retrospective study, we evaluated longitudinal changes in cognitive function, as measured by NeuroTrax, in patients with MS who initiated and received continuous treatment with natalizumab for ≥ 2 years.
Methods
Study Design
The study sample consisted of patients with MS who initiated natalizumab between May 2007 and August 2012 at one study center (Patchogue, NY, USA). Electronic health records from the study center were retrospectively reviewed for this observational study. Patients had two or more cognitive test dates > 10 and ≤ 30 months apart in the course of routine clinical care while receiving 300 mg intravenous natalizumab every 4 weeks for ≥ 2 years.
To be included in the study, patients had to have available NeuroTrax tests at baseline (prior to initiating natalizumab) and after 1 and 2 years of treatment, and were required to have met the McDonald criteria for relapsing–remitting MS diagnosis [25] and be 18–70 years of age at each cognitive testing date. Patients were excluded if they had an interruption in natalizumab treatment (missed more than one dose during the course of the 28-day dosing cycle), were not compliant with study timelines, or had comorbid neurological or psychiatric disease or other documented cognitive impairment. Included patients did not have any relapses or exacerbations recognized or treated with steroids during the study period.
A waiver of informed consent was obtained from the Institutional Review Board on the basis of non–personally-identifiable retrospective data collection and minimal risk.
Assessments
All cognitive assessments were performed using NeuroTrax; the specific methodology for conducting the NeuroTrax test has been previously reported [10]. Three different forms of the test were used to minimize learning effects. Chart information was retrospectively reviewed to assess eligibility criteria and to extract NeuroTrax data at baseline (prior to natalizumab initiation) and annually thereafter. Index scores were normalized and fit to an IQ-like scale [mean 100, standard deviation (SD) 15] stratified by age and education [10, 26], with lower scores indicating greater cognitive impairment. The study measured change from baseline in GCS and the seven individual domain scores after 24 infusions of natalizumab. The proportion of patients achieving a clinically significant change in cognition from baseline and the proportion of patients exhibiting cognitive impairment at each time point were also evaluated. Previously used definitions of clinically significant change (an increase in score > 1 SD from baseline) and cognitive impairment [a score > 1 SD below the mean for cognitively healthy individuals (i.e. score < 85)] [10] were employed for this analysis.
This was a non-interventional, post-authorization study based on secondary use of data. Therefore, adverse event reporting in the form of individual case safety reports was not required.
Statistical Analyses
Demographics and baseline characteristics were analyzed using summary statistics [i.e. mean (SD), median, minimum, and maximum values for continuous variables, and frequency tables for categorical variables]. Change from baseline in global and individual domain scores at 1 and 2 years was analyzed using a generalized estimating equation model adjusted for baseline Expanded Disability Status Scale score and age. Data within 30 days of a relapse were censored.
Results
Patients
The study population included 52 patients. At baseline, 22 patients (42.3%) had a disease duration of 0–5 years, and 30 patients (57.7%) had a disease duration ≥ 6 years (Table 1). Before initiating natalizumab, most patients (n = 40; 76.9%) had prior treatment with another disease-modifying therapy (DMT), whereas 12 patients (23.1%) were treatment-naive.
Table 1.
Patient characteristics at baseline
| Baseline characteristic | Natalizumab-treated patients (N = 52) |
|---|---|
| Age, years (mean [SD]) | 45.9 (8.80) |
| Female | 39 (75.0) |
| Time from diagnosis of MS, years | |
| 0–5 | 22 (42.3) |
| 6–10 | 14 (26.9) |
| 11–15 | 13 (25.0) |
| ≥ 16 | 3 (5.8) |
| EDSS score | |
| Mean (SD) | 2.2 (1.74) |
| Median (range) | 2.0 (0.0–8.0) |
| Prior DMT treatment | 40 (76.9) |
Data are expressed as n (%) unless otherwise specified
DMT disease-modifying therapy, EDSS Expanded Disability Status Scale, MS multiple sclerosis, SD standard deviation
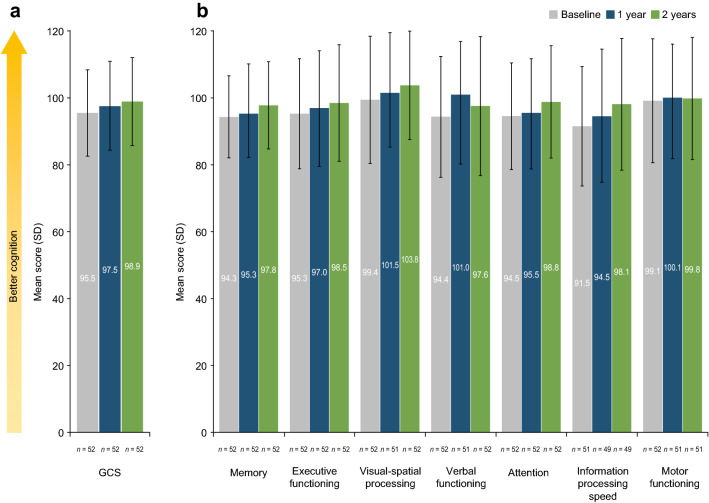
Prior to natalizumab initiation, mean scores in this population for visual-spatial processing (99.4) and motor function (99.1) were near the population-normalized mean for a healthy sample (100) (Fig. 1). Mean scores for GCS and all other domains were lower than the population-normalized mean, with the lowest mean scores observed in information processing speed (91.5).
Fig. 1.
Mean a GCS and b cognitive domain scores at baseline and after 1 and 2 years of natalizumab treatment (N = 52). SD standard deviation, GCS global cognitive score
Overall Improvement in Global Cognitive Score and Cognitive Domains
In the overall study population, GCS improved numerically from baseline to 1 year (mean change from baseline 2.06; p = 0.064), and improved significantly from baseline to 2 years (mean change from baseline 3.43; p = 0.003) (Fig. 1a; Table 2). From baseline to 1 year, verbal functioning score improved significantly (mean change from baseline 6.36; p = 0.007), while other cognitive domains exhibited numerical improvements, with mean changes from baseline ranging from 0.94 (memory) to 2.54 (information processing speed) (Table 2). All seven cognitive domains demonstrated improvement from baseline to 2 years, and significant changes from baseline were identified for memory, visual-spatial processing, attention, and information processing speed (Fig. 1b; Table 2).
Table 2.
Change from baseline for GCS and the seven cognitive domains at 1 and 2 years of natalizumab treatment (N = 52)
| 1 year | 2 years | |||||
|---|---|---|---|---|---|---|
| Mean | 95% CI | p valuea | Mean | 95% CI | p valuea | |
| GCS | 2.06 | − 0.12, 4.24 | 0.064 | 3.43 | 1.18, 5.69 | 0.003 |
| Memory | 0.94 | − 2.30, 4.18 | 0.570 | 3.44 | 0.97, 5.92 | 0.006 |
| Executive function | 1.68 | − 1.75, 5.11 | 0.337 | 3.21 | − 0.24, 6.66 | 0.068 |
| Visual-spatial processing | 1.39a | − 2.39, 5.16 | 0.471 | 4.31 | 0.54, 8.08 | 0.025 |
| Verbal functioning | 6.36 | 1.75, 10.97 | 0.007 | 3.19 | − 2.28, 8.66 | 0.253 |
| Attention | 1.00 | − 2.11, 4.11 | 0.529 | 4.27 | 0.76, 7.79 | 0.017 |
| Information processing speed | 2.54b | − 0.59, 5.68 | 0.112 | 5.15b | 1.96, 8.34 | 0.002 |
| Motor function | 1.02a | − 1.87, 3.92 | 0.487 | 0.05a | − 3.38, 3.48 | 0.976 |
p Value for change based on generalized estimating equation model of change with treatment group, visit, and their interaction as effects, adjusted for baseline Expanded Disability Status Scale score and baseline age
GCS global cognitive score, CI confidence interval
an = 51
bn = 49
Clinically Significant Cognitive Improvement
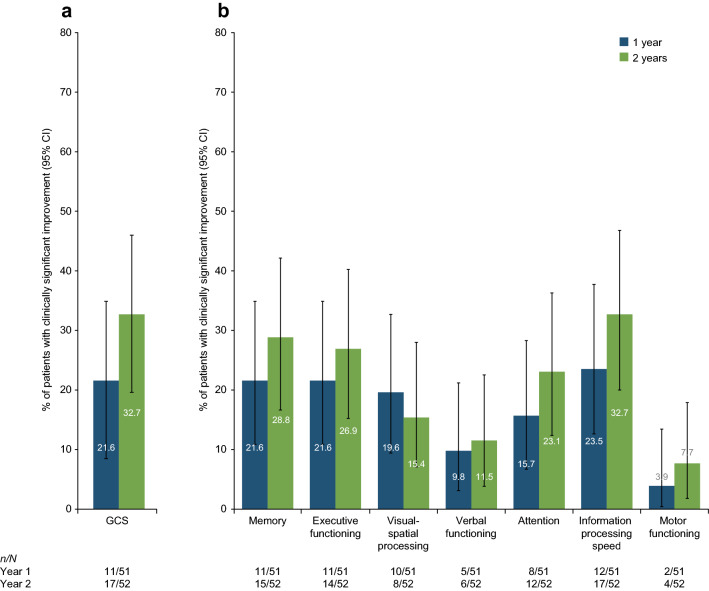
The proportions of patients achieving a clinically significant improvement in cognition scores (i.e. > 1 SD [10]) from baseline to 1 and 2 years are shown in Fig. 2. The percentage of patients experiencing a significant change in GCS increased from 21.6% at 1 year to 32.7% at 2 years. When looking at individual domain scores, the percentage of patients experiencing a clinically significant improvement increased in all domains from baseline to year 1, and in all but one domain (visual-spatial processing) from year 1 to year 2.
Fig. 2.
Percentage of patients exhibiting a clinically significant change from baseline to 1 and 2 years of natalizumab treatment in a GCS and b cognitive domains. 95% CI is based on exact binomial. CI confidence interval, GCS global cognitive score
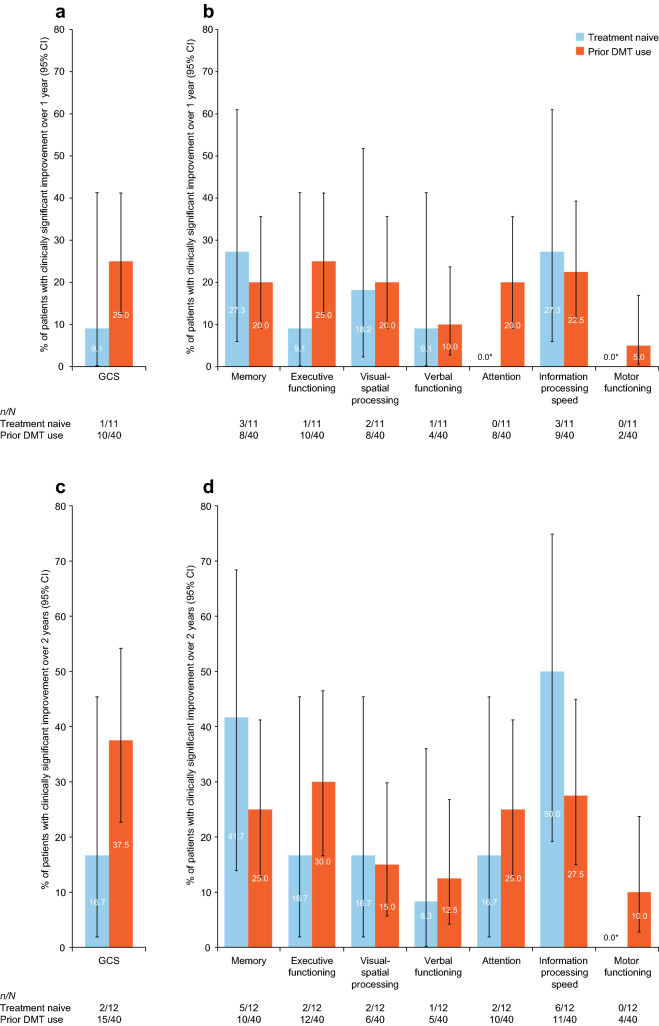
Both treatment-naive patients and those with prior DMT use experienced clinically significant improvement (Fig. 3), and no statistically significant differences were observed between patients with or without prior DMT use.
Fig. 3.
Proportions of patients with and without prior DMT use achieving a clinically significant change in cognition score from baseline to 1 year (a, b) and to 2 years (c, d) of natalizumab treatment. All p values for between-group comparisons were ≥ 0.05. *CI is not shown. GCS global cognitive score, DMT disease-modifying therapy, CI confidence interval
Reduction in Cognitive Impairment
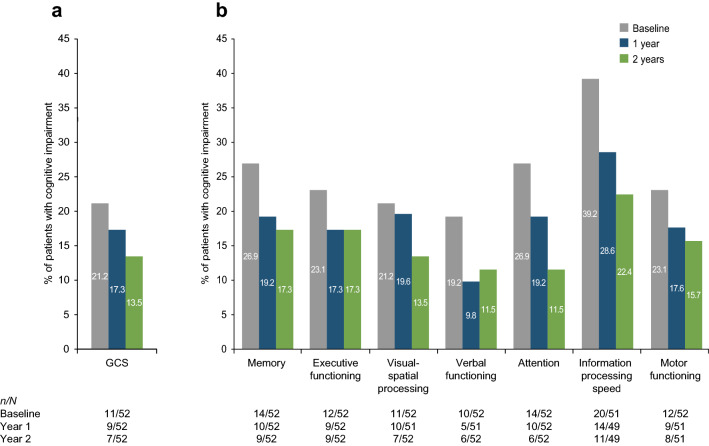
Prior to natalizumab initiation, 21.2% of patients (11/52) exhibited cognitive impairment (score < 85) as measured by GCS (Fig. 4). For the individual domains, percentages of patients with impairment ranged from 19.2% (verbal function) to 39.2% (information processing speed). Compared with baseline, the percentages of patients who had cognitive impairment assessed by GCS or any of the seven domains were lower at years 1 and 2 (Fig. 4). At 2 years, 13.5% of patients (7/52) exhibited cognitive impairment as shown by the GCS. The greatest relative reductions in the percentage of patients with impairment were observed for the attention [26.9% (14/52) to 11.5% (6/52)] and information processing speed [39.2% (20/51) to 22.4% (11/49)] domains.
Fig. 4.
Proportion of patients with cognitive impairment (score < 85) at baseline and after 1 and 2 years of natalizumab treatment in a GCS and b cognitive domains. GCS global cognitive score
Discussion
Patients with MS who were treated with natalizumab exhibited significant improvements in cognitive function as measured by NeuroTrax. After 2 years of treatment, global scores were significantly improved from baseline, and approximately one-third of patients exhibited global score improvement that was clinically significant. Numerical score improvements and clinically significant improvements from baseline to 1 and 2 years were observed for all seven individual cognitive domains. Scores did not differ significantly at 2 years, based on whether or not patients had received prior DMT, although the patient numbers in each group were small. Both treatment-naive patients and those previously treated with other DMTs experienced clinically significant improvements over 2 years of treatment. In addition, the percentages of patients with cognitive impairment decreased over 1 and 2 years of natalizumab treatment.
The findings of the current analysis are consistent with previous studies demonstrating that natalizumab therapy is associated with stable or improved cognitive performance after 1‒3 years of treatment [21–23, 27]. While the current study was not designed to assess the mechanism underlying this improvement in cognitive function, previous work has suggested that these effects may be due to natalizumab’s strong anti-inflammatory effect [28, 29]. Research in animal models has further suggested that inflammation may cause synaptic degeneration and, subsequently, the cognitive dysfunction observed in patients with MS [30, 31]. Natalizumab reduces levels of proinflammatory cytokines in the cerebrospinal fluid [32] and plasma [23, 33], and these reductions have been associated with improvement in cognitive function [23]. Natalizumab’s neuroprotection of cortical brain regions, potentially as a result of reduced inflammation, has also been suggested as an explanation for its effect on preserving cognitive function [21].
The prevalence of cognitive impairment in MS may be high, but varies depending on the study setting [3], and increases with disease duration [10]. One study using NeuroTrax to screen for cognitive function showed that 20.9% of patients with a disease duration of 5 years exhibited cognitive impairment (using the > 1 SD cut-off also applied in this analysis); that percentage of patients increased to 29.3% at a disease duration of 10 years. More severe cognitive impairment was found in 6.0% of patients at 5 years and 9.0% at 10 years [10].
Cognitive impairment is one of the most disabling symptoms of MS [11]. It negatively impacts a number of aspects of everyday life, including social and familial interactions, competence in legal and financial matters, adjustment to disability, driving skills, behavior, treatment adherence, and the ability to benefit from rehabilitation [3–8]. For a substantial number of patients, the cognitive effects of MS lead to the loss of employment and financial hardship [3, 4].
Given the considerable toll that cognitive impairment exacts from patients, and the likelihood that patients with such impairment will be inadequately identified, therapy that could mitigate this adverse effect of the disease would be highly beneficial. It has been suggested that prolonged response times on certain cognitive tasks in MS indicates abnormal conduction within demyelinated tracts [11]. Supporting this contention, MS patients have prolonged response time but intact accuracy relative to healthy individuals [11]. This indicates that early therapeutic intervention with a high-efficacy therapy to reduce the nerve damage caused by the inflammation associated with MS may help prevent or delay these adverse cognitive effects. In the current study population, information processing speed showed the greatest deficit of any of the cognitive domains at baseline. The greatest on-treatment improvements were also observed in this domain as the proportion of patients with clinically significant improvement in information processing speed was higher than that for any other domain at both years 1 and 2.
The limitations of the study include the lack of a control group for comparison; however, this was dictated by the retrospective study design and was partially compensated for by the repeated-measures study design. The influence of learning effects is a common concern for longitudinal studies of cognitive function; however, NeuroTrax has good test–retest reliability and has been found to be reliable for longitudinal studies [10, 13, 14]. Moreover, in this study, three different forms of the test were used and patients completed the test only once a year, both of which should have minimized learning effects. As an additional limitation, this was a single-center study conducted in the US, and the sample size was small. Further studies in additional locations and larger and more diverse patient populations are needed to establish more generalizable results. In particular, as many patients in this cohort were relatively early in their disease course (42.3% with ≤ 5 years since MS diagnosis) and displayed minimal disability at baseline, further investigation is needed for patients with higher levels of disability. Longer-term analysis is also needed to confirm that the cognitive changes measured by NeuroTrax will be sustained over longer durations of natalizumab treatment. Continued follow-up is also needed to evaluate cognition in patients who discontinue natalizumab, as previous work has suggested that natalizumab’s cognitive benefits may not be maintained post-discontinuation [34].
Conclusions
Overall, the results of this study support previous findings [21, 22, 27] indicating that cognitive function can be improved in patients with RMS treated with natalizumab. Prospective studies may consider incorporation of cognitive measures as important outcomes for assessing treatment efficacy.
Acknowledgments
The authors gratefully acknowledge Qunming Dong, PhD, formerly of Biogen, for his contributions to the statistical analyses included in this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript and take responsibility for the integrity of the work as a whole. Laurel Ranger and Alison Adams, PhD (Ashfield Healthcare Communications, Middletown, CT, USA), wrote the first draft of this manuscript based on input from the authors and revised subsequent drafts. Joshua Safran (Ashfield Healthcare Communications) copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the manuscript to the authors. The authors had full editorial control of the manuscript and provided their final approval of all content.
Compliance with Ethical Standards
Funding
This study was funded by Biogen, who provided funding for medical writing support in the development of this manuscript. The open access fee for this article was funded by Biogen.
Conflicts of interest
Mark Gudesblatt has received research support from Biogen, EMD Serono, Novartis, Sanofi-Genzyme, and Teva, and speaker/consultant fees from Acorda, Amgen, Biogen, EMD-Serono, Medtronic, Novartis, Sanofi-Genzyme, Saol Therapeutics, and Teva. Myassar Zarif has received speaker fees from Acorda, Biogen, Genzyme, and Teva. Barbara Bumstead has received speaker fees from Biogen, Genentech, Genzyme, and Teva. Jeffrey Wilken has received grants from Biogen and grants and personal fees from Sanofi-Genzyme and George Washington University. Karen Blitz has received speaker fees from Biogen, Genentech, and Teva. Marijean Buhse has received speaker/consultant fees from Biogen, Genentech, and Sanofi-Genzyme. Sourav Santra, Christophe Hotermans, and Lily Lee are employed by and may hold stock and/or stock options in Biogen. Karl Wissemann and Lori Fafard have no conflicts of interest to disclose.
Informed consent
A waiver of informed consent was obtained from the Institutional Review Board on the basis of non–personally-identifiable retrospective data collection and minimal risk.
Footnotes
The original version of this article was revised. The name of the second author, which previously read `Karl Wisseman' should read: `Karl Wissemann'.
Change history
9/22/2018
An Online First version of this article was made available online at http://link.springer.com/journal/40263/onlineFirst/page/1 on 24 August 2018. An error was subsequently identified in the article, and the following correction should be noted:
References
- 1.Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci. 2006;245(1–2):41–46. doi: 10.1016/j.jns.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41(5):685–691. doi: 10.1212/WNL.41.5.685. [DOI] [PubMed] [Google Scholar]
- 3.Gold R, Wolinsky JS, Amato MP, Comi G. Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord. 2010;3(6):351–367. doi: 10.1177/1756285610385608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692–696. doi: 10.1212/WNL.41.5.692. [DOI] [PubMed] [Google Scholar]
- 5.D’Orio VL, Foley FW, Armentano F, Picone MA, Kim S, Holtzer R. Cognitive and motor functioning in patients with multiple sclerosis: neuropsychological predictors of walking speed and falls. J Neurol Sci. 2012;316(1–2):42–46. doi: 10.1016/j.jns.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Kalron A. The relationship between specific cognitive domains, fear of falling, and falls in people with multiple sclerosis. Biomed Res Int. 2014;2014:281760. doi: 10.1155/2014/281760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause I, Kern S, Horntrich A, Ziemssen T. Employment status in multiple sclerosis: impact of disease-specific and non-disease-specific factors. Mult Scler. 2013;19(13):1792–1799. doi: 10.1177/1352458513485655. [DOI] [PubMed] [Google Scholar]
- 8.Lincoln NB, Radford KA. Cognitive abilities as predictors of safety to drive in people with multiple sclerosis. Mult Scler. 2008;14(1):123–128. doi: 10.1177/1352458507080467. [DOI] [PubMed] [Google Scholar]
- 9.Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS) Mult Scler. 2012;18(6):891–898. doi: 10.1177/1352458511431076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achiron A, Chapman J, Magalashvili D, Dolev M, Lavie M, Bercovich E, et al. Modeling of cognitive impairment by disease duration in multiple sclerosis: a cross-sectional study. PLoS One. 2013;8(8):e71058. doi: 10.1371/journal.pone.0071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achiron A, Doniger GM, Harel Y, Appleboim-Gavish N, Lavie M, Simon ES. Prolonged response times characterize cognitive performance in multiple sclerosis. Eur J Neurol. 2007;14(10):1102–1108. doi: 10.1111/j.1468-1331.2007.01909.x. [DOI] [PubMed] [Google Scholar]
- 12.Doniger GM, Dwolatzky T, Zucker DM, Chertkow H, Crystal H, Schweiger A, et al. Computerized cognitive testing battery identifies mild cognitive impairment and mild dementia even in the presence of depressive symptoms. Am J Alzheimers Dis Other Demen. 2006;21(1):28–36. doi: 10.1177/153331750602100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweiger A, Doniger G, Dwolatzky T, Jaffe D, Simon E. Reliability of a novel computerized neuropsychological battery for mild cognitive impairment. Acta Neuropsychologica. 2003;1(4):407–413. doi: 10.1016/S0028-3932(02)00173-2. [DOI] [Google Scholar]
- 14.Melton JL. Psychometric evaluation of the mindstreams neuropsychological screening tool. Navy Experimental Diving Unit Technical Report No 06-10. 2005. http://www.dtic.mil/dtic/tr/fulltext/u2/a451462.pdf. Accessed 5 July 2018.
- 15.Gudesblatt M, Zarif M, Wissemann K, Bumstead B, Fafard L, Buhse M, et al. Multiple sclerosis and cognitive testing: the relationship between traditional measures and novel computerized analytics: a preliminary analysis. Mult Scler. 2016;22(Suppl 3):449. [Google Scholar]
- 16.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 17.Miller DH, Soon D, Fernando KT, MacManus DG, Barker GJ, Yousry TA, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68(17):1390–1401. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- 18.Rudick RA, Miller D, Hass S, Hutchinson M, Calabresi PA, Confavreux C, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62(4):335–346. doi: 10.1002/ana.21163. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JT, Giovannoni G, Lublin FD, O’Connor PW, Polman CH, Willoughby E, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler. 2011;17(8):970–979. doi: 10.1177/1352458511399611. [DOI] [PubMed] [Google Scholar]
- 20.Butzkueven H, Kappos L, Pellegrini F, Trojano M, Wiendl H, Patel RN, et al. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry. 2014;85(11):1190–1197. doi: 10.1136/jnnp-2013-306936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattioli F, Stampatori C, Bellomi F, Scarpazza C, Capra R. Natalizumab significantly improves cognitive impairment over three years in MS: pattern of disability progression and preliminary MRI findings. PLoS One. 2015;10(7):e0131803. doi: 10.1371/journal.pone.0131803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilken J, Kane RL, Sullivan CL, Gudesblatt M, Lucas S, Fallis R, et al. Changes in fatigue and cognition in patients with relapsing forms of multiple sclerosis treated with natalizumab: the ENER-G study. Int J MS Care. 2013;15(3):120–128. doi: 10.7224/1537-2073.2012-043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iaffaldano P, Ruggieri M, Viterbo RG, Mastrapasqua M, Trojano M. The improvement of cognitive functions is associated with a decrease of plasma osteopontin levels in natalizumab treated relapsing multiple sclerosis. Brain Behav Immun. 2014;35:176–181. doi: 10.1016/j.bbi.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Iaffaldano P, Viterbo RG, Paolicelli D, Lucchese G, Portaccio E, Goretti B, et al. Impact of natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis: a prospective, open-label, two years observational study. PLoS One. 2012;7(4):e35843. doi: 10.1371/journal.pone.0035843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golan D, Doniger GM, Wissemann K, Zarif M, Bumstead B, Buhse M, et al. The impact of subjective cognitive fatigue and depression on cognitive function in patients with multiple sclerosis. Mult Scler. 2018;24(2):196–204. doi: 10.1177/1352458517695470. [DOI] [PubMed] [Google Scholar]
- 27.Jacques FH, Harel BT, Schembri AJ, Paquette C, Bilodeau B, Kalinowski P, et al. Cognitive evolution in natalizumab-treated multiple sclerosis patients. Mult Scler J Exp Transl Clin. 2016;2:2055217316657116. doi: 10.1177/2055217316657116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rorsman I, Petersen C, Nilsson PC. Cognitive functioning following one-year natalizumab treatment: a non-randomized clinical trial. Acta Neurol Scand. 2018;137(1):117–124. doi: 10.1111/ane.12833. [DOI] [PubMed] [Google Scholar]
- 29.Talmage GD, Coppes OJM, Javed A, Bernard J. Natalizumab stabilizes physical, cognitive, MRI, and OCT markers of disease activity: a prospective, non-randomized pilot study. PLoS One. 2017;12(4):e0173299. doi: 10.1371/journal.pone.0173299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandolesi G, Grasselli G, Musumeci G, Centonze D. Cognitive deficits in experimental autoimmune encephalomyelitis: neuroinflammation and synaptic degeneration. Neurol Sci. 2010;31(Suppl 2):S255–S259. doi: 10.1007/s10072-010-0369-3. [DOI] [PubMed] [Google Scholar]
- 31.Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29(11):3442–3452. doi: 10.1523/JNEUROSCI.5804-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellergard J, Edstrom M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler. 2010;16(2):208–217. doi: 10.1177/1352458509355068. [DOI] [PubMed] [Google Scholar]
- 33.Iaffaldano P, Ribatti D, Trojano M. Natalizumab reduces serum pro-angiogenic activity in MS patients. Neurol Sci. 2018;39(4):725–731. doi: 10.1007/s10072-018-3266-9. [DOI] [PubMed] [Google Scholar]
- 34.Iaffaldano P, Viterbo RG, Trojano M. Natalizumab discontinuation is associated with a rebound of cognitive impairment in multiple sclerosis patients. J Neurol. 2016;263(8):1620–1625. doi: 10.1007/s00415-016-8177-1. [DOI] [PubMed] [Google Scholar]