Abstract
Aim
This study aimed to determine rates of retention in care, viral suppression, and use of antiretroviral therapy (ART) and identify risk factors for loss to follow-up (FU) in an adult cohort from a tertiary teaching hospital in Florence, Italy.
Methods
We included all newly diagnosed HIV-infected patients aged >18 years who were linked to our clinic from July 2007 to December 2015. On July 31, 2017, we evaluated the proportion of patients retained in care, on ART, and having HIV RNA <50 copies/mL. We assessed predictors of loss to FU through univariate and multivariate analyses.
Results
We included 423 patients. By July 2017, 23 (5.5%) patients died, 25 (5.9%) moved to a different center, and 64 (15.1%) were lost to follow-up. Among the remaining 311 patients (73.5%), 96.5% were on ART and 95% had HIV RNA <50 copies/mL. After adjustment for sex, age at diagnosis, origin, and risk of transmission, our results showed a lower retention rate in those not on ART at the end of the follow-up (adjusted HR [aHR]: 10.33, 95% CI 5.80–18.40, P<0.001), non-Italians (aHR: 1.69, 95% CI: 0.99–2.89, P=0.054) and <35 years old (aHR: 1.85; 95% CI 1.04–3.30, P=0.037).
Conclusion
In our hospital in Florence, we found a gap in retention in care among foreigners, people <35 years old, and those who were not in treatment at the end of the follow-up. The results of this study may help to identify opportunities for appropriate future interventions.
Keywords: HIV-1, continuum of care, retention in care, Italy, 90-90-90 target, predictors associated to loss to follow-up
Introduction
The HIV care continuum is a powerful tool for describing HIV epidemiology and movement of HIV-positive individuals through discrete stages of care. The continuum spans the range from patients not in care and unaware of their HIV infection status to those virologically suppressed and fully engaged in HIV care.1 The HIV care continuum is also a useful framework for assessing progress against The Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 HIV/AIDS regional targets for the year 2020. Under this initiative, 90% of people with HIV should be diagnosed, 90% of those diagnosed should be treated with antiretroviral therapy (ART), and 90% of those on ART should achieve an undetectable viral load (VL).2 Monitoring of care continuum is associated with higher virological success, and it is considered an important indicator of provided assistance quality level.3 On the contrary, failed retention in care is associated with a worse outcome, including virological failure and increased mortality and loss to follow-up (FU).4
According to UNAIDS, percentage of all people living with HIV accessing ART varies widely across different countries, especially between high- and low-income countries, ranging from 40% in Western and Central Africa to 79% in Western/Central Europe and North America.5 In the US, 1.1 million people are living with HIV, 85% are diagnosed, and only 49% have the virus under control through HIV treatment.6
Italian health care service provides ART to all the individuals in need and has recommended universal HIV treatment since 2015 regardless of the initial CD4 count.7 The latest estimates from Italian Istituto Superiore di Sanità indicate that 130,000 (120,000–150,000) people are living with HIV. People diagnosed and cared accounted for 107,000 (82.3%); 87.6% were on ART and, among them, 86.0% reached viral suppression (<50 copies/mL) at the last visit.8,9 However, national data on the number of HIV-diagnosed individuals and their characteristics (clinical, immunological, behavioral, and treatment) are lacking because prevalence surveys are not routinely carried out.8
Moreover, few studies in Italy paid attention to predictors associated with loss to FU.3 Thus, although HIV care continuum is a framework developed to be applied at a population level, we applied the model to a single university teaching hospital in Florence, Italy, to evaluate the rate of retention in care, the alignment with the “90–90–90” target, and the predictors associated with loss to FU over a 10-year period.
Materials and methods
Clinical settings and data sources
Azienda Ospedaliero – Universitaria Careggi (AOUC) is a tertiary teaching hospital in Florence, Italy. The hospital’s infectious diseases outpatient unit provides care to ~1,500 HIV-positive patients, one-third from Florence itself and two-thirds from the Florence area (municipalities surrounding Florence and all Tuscany region). Laboratory data were collected from an electronic database system, which has been implemented in our hospital in July 2007. Clinical data have been obtained from paper medical records at the time of medical consultations. The research database was supplemented with the hospital pharmacy database, which captures antiretroviral drug dispensations. Investigators checked data for internal consistency and missing data. Patients signed a form providing informed consent for inclusion in epidemiologic studies at the time of first clinical visit. The study was approved by our local ethics committee (Comitato Etico Area Vasta Centro – AOU Careggi, rif n.10452_oss). The study was conducted according to the Declaration of Helsinki.
Study design and definitions
We performed a retrospective cohort analysis. We enrolled all newly HIV-diagnosed patients aged ≥18 years who were linked to our clinic from July 1, 2007, to December 31, 2015, and performed at least one medical visit. The end of the follow-up (EFU) was July 31, 2017. Electronic and paper hospital records were consulted to determine their current status at the EFU.
Categories within our HIV care cascade model were defined as follows. Patients were considered “linked to care” if they had within the study period at least one visit after being HIV diagnosed. “Retention in care” was defined as patients still in medical FU at the end of the observational period with at least one clinic visit in the previous 12 months. We defined “On ART” those who were retained in care with a regular ART dispensation from the pharmacy from July 31, 2016 to July 31, 2017 regardless of changes in the first prescribed ART and therapeutic vacations during the study period. “Lost to follow-up” (LTFU) was defined as no visit attendance within 12 months and unavailability for at least three phone attempts with no further information from medical records, local doctor, or his or her next of kin. “Virologic suppression” was defined as HIV-1 VL <50 copies/mL at the last available measurement. Individuals were considered “moved to another center” and “deceased” when it was clearly reported in the medical records.
Baseline CD4 was the CD4 count closest to the first visit, within 30 days before or after that visit, and no more than 7 days after ART initiation. Baseline VL was the VL closest to the first visit, within 30 days before or after that visit, and before of ART start.
Statistical analyses
We used Stata version 14.0 (StataCorp LP, College Station, TX, USA). Individuals contributed data from the first physician visit until death, transfer to another center, or last contact.
The primary outcome was the patient status at the EFU. Patients were categorized as follows: retained in care, on ART, on ART and HIV RNA <50 copies/mL, LTFU, transferred to another center, and deceased. “Age at the diagnosis” was categorized into three levels (<35 years, 35–50 years, and >50 years) using the stratum >50 years as a reference category. Nadir CD4 was also categorized into a three-level variable as follows: <200 cell/mm3, 200–500 cell/mm3, and >500 cell/mm3. Nadir CD4 >500 cell/mm3 was used as a reference category. Other variables were categorized as a dichotomous variable (no/yes). The incidence rate was calculated by the number of LTFU divided by the person-time of the at-risk population.
Deceased individuals and those who moved to another center were excluded from comparative analysis between retained in care and LTFU patients. We used descriptive statistics including mean (and standard deviation) values and medians (and interquartile ranges [IQRs]) to describe continuous data and proportions for categorical data, respectively. Associations between explanatory variables and the primary outcome (retained in care/LTFU) were compared using chi-squared test/Wilcoxon test for dichotomized or categorical variables and Student’s t-test for continuous variables.
We used a Cox proportional hazard model as a multivariate regression model to identify factors associated with LTFU. We included in the final model all the possible confounders when in the univariate analysis, P-values were less than 0.150 and no multicollinearity problems, data sparsity, or missing data had arisen. Likelihood ratio (LR) test was also used for comparing the goodness-of-fit of the model adding a variable at a time.
Results
The study included 423 patients. The majority (81%) was male; median age was 46 years [IQR 55–38]. Sexually transmitted HIV infection had occurred in 376 cases (88.8%): 214 (50.6%) reported to be men who have sex with men (MSM), 162 (38.3%) had a heterosexual transmission. Eighteen out of 423 (4.3%) were intravenous drug users (IVDUs). Twenty-six patients (6.1%) had an unknown transmission route. The CD4 median count nadir was 310 cell/mm3 (IQR: 452–112), and the median VL Zenith was 5.1 Log10 [IQR: 5.5–4.5]. Table 1 summarized the baseline characteristics of the cohort.
Table 1.
Baseline characteristics and retention in care at the EFU (N=423)
| Characteristics | |
|---|---|
|
| |
| Age (years), median (IQR) | 46 (55–38) |
| Male sex, n (%) | 346 (81.0) |
| FU (months), median (IQR) | 64 (98–41) |
| Origin, n (%) | |
| • Italy | 309 (73.0) |
| • Europe (not Italy) | 8 (1.9) |
| • East Europe | 28 (6.6) |
| • Middle East | 5 (1.2) |
| • Asia | 7 (1.6) |
| • Latin America | 28 (6.6) |
| • Africa | 38 (9.0) |
| Risk factor, n (%) | |
| • Heterosexual | 162 (38.3) |
| • MSM | 214 (50.6) |
| • IVDU | 18 (4.3) |
| • Transfusion | 3 (0.7) |
| • Unknown | 26 (6.1) |
| HCV Ab positive, n (%) | 37 (9.2) |
| HBsAg positive, n (%) | 21 (5.1) |
| AIDS, n (%) | 78 (18.5) |
| Acute infection, n (%) | 36 (8.5) |
| Nadir CD4 (cells/μL), median (IQR) | 310 (452–112) |
| Zenith (log10), median (IQR) | 5.1 (5.5–4.5) |
| Status at the EFU | |
| • Retained in care, n (%) | 311 (73.5) |
| • Moved to another center, n (%) | 25 (5.9) |
| • LTFU, n (%) | 64 (15.1) |
| • Death, n (%) | 23 (5.5) |
Abbreviations: EFU, end of the follow-up; FU, follow-up; HBsAg, hepatitis B surface antigen; HCV Ab, hepatitis C virus antibodies; MSM, men who have sex with men; IVDU, intravenous drug user; LTFU, lost to follow-up.
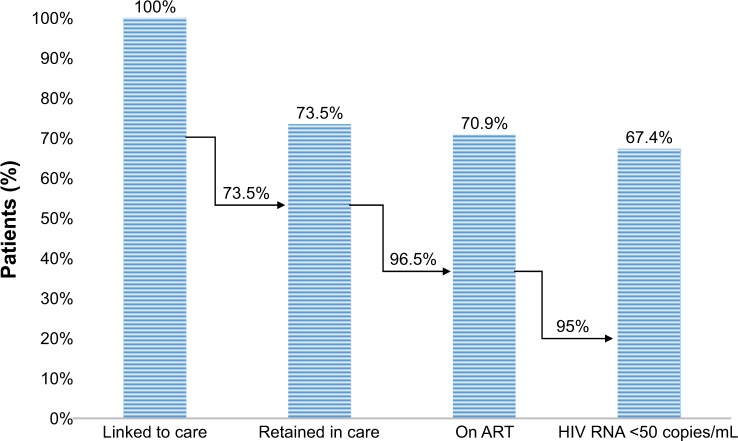
Among the 423 patients, 25 (6.3%) moved to another center, 64 (15.1%) were LTFU, and 23 (5.5%) died (Table 1). Among the 311 (73.5%) patients retained in care, 300 (96.4%) were on ART and 285 (95%) of them had <50 copies/mL (Figure 1). The overall incidence rate of loss to FU excluding deaths and transferred patients was 29.78×1,000 person-years (95% CI 23.26–38.12). The incidence rate at the end of the first year of FU was 44.21×1,000 person-years (95% CI 27.08–72.16) and 34.30×1,000 person-years (95% CI 19.47–60.36) within the second year. After the third year, the incidence rate of loss to FU was 19.55×1,000 person-years (95% CI 12.74–29.98).
Figure 1.
The cascade of continuum of care at AOUC, Florence, Italy.
Abbreviations: AOUC, Azienda Ospedaliero – Universitaria Careggi; ART, antiretroviral treatment.
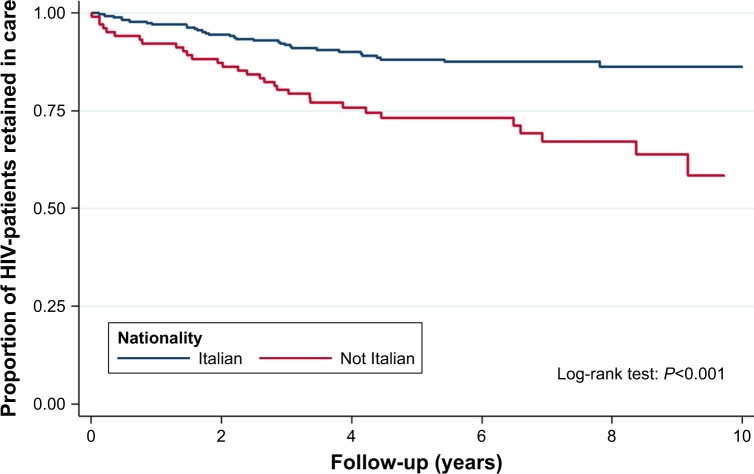
In the univariate analysis, the most associated variables with LTFU was “Being off ART at the EFU” and “Being not Italian” (Figure 2 and Table 2). Patients LTFU had a higher incidence rate in the category <35 years compared to individuals >50 years (Figure 3 and Table 2; crude risk ratio [RR] 3.80, 95% CI 1.90–7.58, P<0.001). No difference was observed in gender, transmission route, AIDS events, acute infection, presence of hepatitis C virus antibodies (HCV Ab) and/or hepatitis B surface antigen (HBsAg) at baseline, and being on a single tablet regimen (STR; Table 2).
Figure 2.
Kaplan–Meier plot: retention in care by nationality during a 10-year FU at AOUC, Florence, Italy.
Abbreviations: FU, follow-up; AOUC, Azienda Ospedaliero – Universitaria Careggi.
Table 2.
Factors associated with loss to FU
| Characteristics and categorya | LTFU incidence rateb | Crude RR | 95% CI | P-value | Multivariatec (HR) | 95% CI | P-value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age at entry | |||||||
| • >50 | 17.6 | 1 | ref | – | |||
| • 36–50 | 29.0 | 1.65 | 0.88–3.11 | 0.1183 | 0.98 | 0.48–2.03 | 0.97 |
| • <35 | 66.7 | 3.80 | 1.90–7.58 | <0.0001 | 1.85 | 1.04–3.30 | 0.037 |
| Gender | |||||||
| • Female | 38.9 | 1 | ref | – | |||
| • Male | 27.7 | 0.71 | 0.40–1.27 | 0.2494 | – | – | – |
| Origin | |||||||
| • Italian | 20.2 | 1 | ref | ||||
| • Not Italian | 58.0 | 2.86 | 1.75–4.70 | <0.0001 | 1.69 | 0.99–2.89 | 0.054 |
| Risk | |||||||
| • Not MSM | 34.7 | 1 | ref | ||||
| • MSM | 24.5 | 0.68 | 0.41–1.13 | 0.237 | – | – | – |
| Therapy at the EFU | |||||||
| • On ART | 17.5 | 1 | ref | ||||
| • Off ART | 249.1 | 14.25 | 8.67–23.40 | <0.001 | 10.33 | 5.80–18.40 | <0.001 |
| HCV Ab at entry | |||||||
| • Negative | 31.8 | ||||||
| • Positive | 21.4 | 0.67 | 0.24–1.86 | 0.4382 | – | – | – |
| HBsAg at entry | |||||||
| • Negative | 29.4 | 1 | ref | ||||
| • Postive | 24.5 | 0.83 | 0.26–267 | 0.7579 | – | – | – |
| STR at the EFU | |||||||
| • No | 21.8 | 1 | |||||
| • Yes | 13.1 | 0.60 | 0.30–1.19 | 0.2424 | – | – | – |
| Previous AIDS event | |||||||
| • No | 31.3 | 1 | ref | ||||
| • Yes | 18.4 | 0.59 | 0.25–1.36 | 0.2096 | – | – | – |
| Acute infection | |||||||
| • No | 31.3 | 1 | ref | ||||
| • Yes | 11.8 | 0.38 | 0.09–1.55 | 0.1596 | – | – | – |
| Nadir CD4 (cells/μL) | |||||||
| • >500 | 49.2 | 1 | ref | – | |||
| • 200–500 | 27.3 | 0.55 | 0.30–1.03 | 0.0579 | 1.61 | 0.80–3.24 | 0.177 |
| • <200 | 25.1 | 0.51 | 0.25–1.00 | 0.0480 | 1.73 | 0.72–4.12 | 0.214 |
| Zenith | |||||||
| HIV-RNA (copies/mL) | |||||||
| • Low <100,000 | 41.5 | 1 | ref | ||||
| • High >100,000 | 18.6 | 0.49 | 0.26–0.77 | 0.0027 | 0.74 | 0.40–1.36 | 0.338 |
Notes:
n=375.
Per 1,000 person-years.
Adjusted for age at entry, origin, nadir, zenith viremia, and therapy at the EFU.
Abbreviations: FU, follow-up; LTFU, lost to follow-up; RR, risk ratio; ref, reference; MSM, men who have sex with men; EFU, end of the follow-up; ART, antiretroviral treatment; VL, viral load; STR, single tablet regimen.
Figure 3.
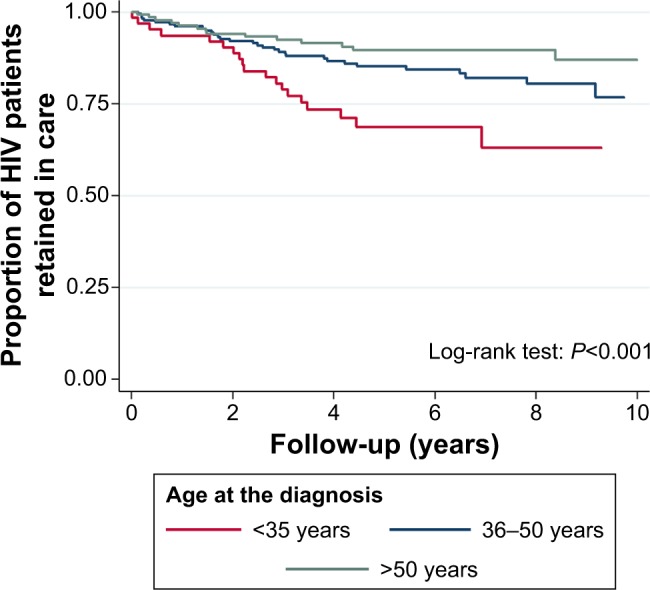
Kaplan–Meier plot: retention in care by age at HIV diagnosis during a 10-year FU at AOUC, Florence, Italy.
Abbreviations: FU, follow-up; AOUC, Azienda Ospedaliero – Universitaria Careggi.
In multivariate analysis, “Being off ART at the EFU” was strongly associated (adjusted HR [aHR]: 10.33, 95% CI 5.80–18.40, P≤0.001) with LTFU. “Being not Italian” and “Age <35 years at baseline” were also associated (aHR: 1.69, 95% CI 0.99–2.89, P=0.054 and aHR: 1.85, 95% CI 1.04–3.30, P=0.037, respectively) with LTFU. Having CD4 Nadir count <200 cell/mm3 at baseline was no more significant after the adjustment.
Discussion
Retention in care rate of HIV-infected patients at the AOUC in Florence is slightly lower than national estimates (73.5% vs 82.3%); an explanation could be that patients transferred to other clinical centers were not included in the analysis but could be in care within or outside the region.
However, our rate of virological success was superior compared to that of the USA where ~50% of known HIV-infected individuals have <50 copies/mL, with a possible explanation of financial barriers that hamper access to HIV care,1,6 and it resulted also higher (95.0% vs 86.0%) with respect to national data.10 Although there are limitations due to the incorrect comparison of local to national realities in different countries, we showed that in our hospital, UNAIDS target was not reached (Figure 1). However, our results are in agreement with those of Prinapori et al (2018) who conducted a similar study in Italy in their hospital.
In the multivariate analysis, “Not being Italian” associated with LTFU (aHR: 1.69, 95% CI 0.99–2.89). As seen throughout Europe, a significant proportion of those searching for HIV care in our center are foreign patients including young asylum seekers and migrants.3,11 Although, in Italy, the treatment is offered free to everyone, the high rate of LTFU among foreign patients could be explained by sociocultural and structural barriers such as language gaps, stigma, fear of disclosure in their community, and difficulty to obtain required documents that are necessary to access to the cures.12 Fear and stigma are common feelings for all individuals recently diagnosed with HIV infection, but foreigners and migrants with HIV infection may be more vulnerable to racial, social, and economic disparities and therefore have difficulty remaining in care.13 Moreover, that key population could be temporarily present and move quickly through and out of Italy. In this perspective, systematic tracing activities in LTFU patients could increase the treatment success rate and should be encouraged. “Being aged <35 years” was also strongly associated with LTFU, which is in accordance with most literature (Table 2).12,14–16 Medical visits or refill from the pharmacy was skipped more often by the younger patients who may experience more HIV-related stigma and apprehension about the risk of losing their jobs than older adults. 13,14
Finally, “Being off ART at the EFU” was the strongest factor to be associated with LTFU. We observed the higher rate of LTFU during the first and second years of FU (44.21×1,000 person-years [95% CI 27.08–72.16] and 34.30×1,000 person-years [95% CI 19.47–60.36], respectively). Since patients on ART at EFU were less likely to disengage from care,4 the recent concept of immediately treating all regardless of CD4 value should help to increase retention in treatment in the next future. Finally, concerning therapies, we found that most of our retained patients were virosuppressed, and this could be related to the excellent tolerability of current ART regimes. Being on STR with respect to LTFU did not result in a significant advantage (Table 2). In fact, data on STR and retention in care are controversial in literature. STR with efavirenz appears to be more favorable in one study although it is compared with outdated therapies.17
Our research has some limitations. First of all, we could not collect data such as education, family, and socialeconomic status. Moreover, there is a lack of a systematic approach to determine health outcomes in LTFU patients, since there was no centralized death registry and some transfers could not have been notified. Finally, this analysis was limited to one site and could not be representative of all HIV patients living with diagnosed HIV infection in Italy.
Conclusion
Our study shows that the UNAIDS result 90-90-90-90 was not achieved in our setting. Hence, the results of this study may help identify opportunities for appropriate future interventions, underlining the importance of intercepting fragile populations as young people and foreigners for whom it may be necessary to set up a dedicated path in our outpatient clinics that favors retention in care.
Acknowledgments
Filippo Lagi received the Società Italiana di Terapia Antinfettiva (SITA [Italian Society of Anti-infective Therapy]) scholarship from October 1, 2016, to October 1, 2018.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gardner EM, McLees MP, Steiner JF, Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS 90–90–90 – An ambitious treatment target to help end the AIDS epidemic [2014] [Accessed November 22, 2018]. Available from: http://www.unaids.org/en/resources/documents/2017/90-90-90.
- 3.Prinapori R, Giannini B, Riccardi N, et al. Predictors of retention in care in HIV-infected patients in a large hospital cohort in Italy. Epidemiol Infect. 2018;146(5):606–611. doi: 10.1017/S0950268817003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connell SO, Rourke AO, Sweeney E, Lynam A, Sadlier C, Bergin C. Factors associated with non-retention in HIV care in an era of widespread antiretroviral therapy. Int J STD AIDS. 2017;28(7):679–684. doi: 10.1177/0956462416663868. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS Global HIV & AIDS statistics – 2018 fact sheet [2018] [Accessed November 22, 2018]. Available from: http://www.unaids.org/en/resources/fact-sheet.
- 6.Centers for Disease Control and Prevention (CDC) HIV incidence: estimated annual infections in the U.S., 2008–2014. CDC Fact Sheet. 2017:1–2. [Google Scholar]
- 7.Antinori A, Marcotullio S, Andreoni M, et al. Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. Update 2015. New Microbiol. 2016;39(2):93–109. [PubMed] [Google Scholar]
- 8.Mammone A, Pezzotti P, Regine V, et al. How many people are living with undiagnosed HIV infection? An estimate for Italy, based on surveillance data. AIDS. 2015;2016:1131–1136. doi: 10.1097/QAD.0000000000001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camoni L, Raimondo M, Dorrucci M, et al. Estimating minimum adult HIV prevalence: a cross-sectional study to assess the characteristics of people living with HIV in Italy. AIDS Res Hum Retroviruses. 2015;31(3):282–287. doi: 10.1089/aid.2014.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ECDC Continuum of HIV care. Monitoring implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 progress report [2017] [Accessed November 22, 2018]. Available from: https://ecdc.europa.eu/sites/portal/files/documents/Continuum-of-HIV-care-2017.pdf.
- 11.UNAIDS The gap report 2014: Migrants [2014] [Accessed November 22, 2018]. Available from: http://www.unaids.org/en/resources/documents/2014/Migrants.
- 12.Tripathi A, Youmans E, Gibson JJ, Duffus WA. The impact of retention in early HIV medical care on viro-immunological parameters and survival: a statewide study. AIDS Res Hum Retroviruses. 2011;27(7):751–758. doi: 10.1089/AID.2010.0268. [DOI] [PubMed] [Google Scholar]
- 13.Giordano TP, Gifford AL, White AC, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 14.Yehia BR, Rebeiro P, Althoff KN. The impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr. 2016;68(4):413–419. doi: 10.1097/QAI.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebeiro P, Althoff KN, Buchacz K. Retention among North American HIV–infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr. 2012;2014(62):356–362. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franciotta D, Avolio C, Capello E, Lolli F; AINI. Consensus recommendations of the Italian association for neuroimmunology for immunochemical cerebrospinal fluid examination. J Neurol Sci. 2005;237(1–2):5–11. doi: 10.1016/j.jns.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Hemmige V, Flash CA, Carter J. Single tablet HIV regimens facilitate virologic suppression and retention in care among treatment naïve patients. AIDS Care. 2018;30(8):1017–1024. doi: 10.1080/09540121.2018.1442554. [DOI] [PMC free article] [PubMed] [Google Scholar]