Abstract
Background
Mounting evidence suggests that nutritional exposures during pregnancy influence the fetal epigenome, and that these epigenetic changes can persist postnatally, with implications for disease risk across the life course.
Methods
We review human intergenerational studies using a three-part search strategy. Search 1 investigates associations between preconceptional or pregnancy nutritional exposures, focusing on one-carbon metabolism, and offspring DNA methylation. Search 2 considers associations between offspring DNA methylation at genes found in the first search and growth-related, cardiometabolic and cognitive outcomes. Search 3 isolates those studies explicitly linking maternal nutritional exposure to offspring phenotype via DNA methylation. Finally, we compile all candidate genes and regions of interest identified in the searches and describe their genomic locations, annotations and coverage on the Illumina Infinium Methylation beadchip arrays.
Results
We summarize findings from the 34 studies found in the first search, the 31 studies found in the second search and the eight studies found in the third search. We provide details of all regions of interest within 45 genes captured by this review.
Conclusions
Many studies have investigated imprinted genes as priority loci, but with the adoption of microarray-based platforms other candidate genes and gene classes are now emerging. Despite a wealth of information, the current literature is characterized by heterogeneous exposures and outcomes, and mostly comprise observational associations that are frequently underpowered. The synthesis of current knowledge provided by this review identifies research needs on the pathway to developing possible early life interventions to optimize lifelong health.
Keywords: Epigenetics, DNA methylation, fetal programming, Developmental Origins of Health and Disease, one-carbon metabolism, candidate genes, metastable epialleles, cognitive development, cardiometabolic outcomes, growth
Key Messages
The body of evidence linking maternal nutritional exposure to offspring phenotype via DNA methylation in humans is rapidly growing yet currently remains complex and inconsistent.
Candidate genes in the field of intergenerational nutritional epigenetics go beyond imprinted genes to include other gene classes such as metastable epialleles.
Going forwards, there is a continued need for adequately powered prospective cohort studies with repeated longitudinal measurements and randomized nutritional interventions to track the full continuum from maternal exposure to offspring epigenotype to later phenotype.
Introduction
Epigenetic modifications influence gene expression without altering the nucleotide sequence, through the action of a diverse array of molecular mechanisms including DNA methylation, histone modifications and RNA-mediated effects.1 Epigenetic processes have been implicated in the aetiology of a variety of diseases,2 most prominently cancer3 and fetal growth disorders.4 Epigenetic marks are mitotically heritable and can be influenced by the environment,5 suggesting a potential mechanism linking early life exposures to later phenotype,6,7 a notion supported by animal studies.8–10 However, the extent to which epigenetics plays a role in fetal programming in humans remains relatively unexplored. In this review we collate evidence from human intergenerational studies, exploring which nutritional exposures during pregnancy may affect DNA methylation in the offspring, and the possible impact of such modifications on health and disease risk across the life course.
DNA methylation and gene expression
Many biological processes rely on DNA methylation, including genomic imprinting, X-chromosome inactivation and tissue-specific gene expression.11 DNA methylation describes the addition of a methyl group to a cytosine base at the 5’ carbon position to form 5-methylcytosine, catalyzed by DNA methyltransferases (DNMTs). This most commonly occurs at cytosine bases adjacent to guanine, termed CpG (‘cytosine-phosphate-guanine’) sites. Regions of high CpG density are known as ‘CpG islands’, and approximately two-thirds of human genes contain these in their promoter regions.12 DNA methylation has been shown to influence transcriptional activity either by blocking transcription factors binding to the DNA, or by the recruitment of histone modifiers which promote a closed chromatin structure and gene silencing.1 CpG methylation within promoters is typically associated with transcriptional silencing,13 although not consistently, and the effect of DNA methylation may vary depending on which region within the gene is methylated.14 There is also increasing evidence that DNA methylation and histone modifications work in concert with non-coding RNAs to regulate gene expression.15 DNA methylation plays a role in chromatin remodelling, as DNMT enzymes at CpG sites can be physically linked to enzymes which bring about histone methylation and de-acetylation.13 MicroRNAs (miRNAs) affect gene expression through binding to messenger RNAs (mRNAs) and repressing translation,16 including mRNAs that control the expression of DNMTs and histone deacetylases.15 The transcription of some miRNA classes can be influenced by CpG methylation and histone modifications.16
Epigenetics, windows of plasticity and the Developmental Origins of Health and Disease
The Developmental Origins of Health and Disease (DOHaD) hypothesis posits that early life exposure to environmental insults can increase the risk of later adverse health outcomes.7 David Barker’s early cohort studies showed that lower birthweight was associated with an increased risk of hypertension, type 2 diabetes (T2D) and cardiovascular disease in later life,17 findings that were widely replicated.18 Risk of disease was further exacerbated by rapid childhood weight gain, adult obesity and other lifestyle factors such as unhealthy diets, smoking and lack of exercise.19,20 The Dutch Hunger Winter studies showed that exposure to famine during pregnancy was associated with a wide range of phenotypes in the adult offspring, including increased blood pressure,21 obesity22 and schizophrenia,23 effects that depended on the timing of the exposure during pregnancy.22
Epigenetic processes are emerging as potential mechanisms to explain these and other associations found in the DOHaD literature. For example the ‘thrifty epigenome’ hypothesis proposes that in utero exposures can shape an epigenetic signature, resulting in a phenotype that is ‘adapted’ to the early life environment but which may prove to be ‘maladapted’ if the environment changes in later life.24 Therefore famine exposure during pregnancy could programme ‘thrifty epigenotypes’ that are adapted to a nutritionally poor environment, but this may subsequently trigger metabolic disease if the adult environment changes to one that is nutritionally abundant.
The periconceptional period is a time of rapid cell differentiation and epigenetic remodelling, and may therefore represent a critical window during which the developing epigenome is sensitive to environmental influences.25 We define the periconceptional window from 14 weeks preceding conception until 10 weeks after conception.26 Within 48 hours of fertilization, there is rapid erasure of methylation marks to render the developing cells pluripotent.11 After implantation, re-methylation occurs in a tissue-specific manner, and continues throughout pregnancy, enabling differentiation of somatic cells. A second wave of demethylation occurs in the primordial germ cells as they migrate to the genital ridge.27 At this stage most parental imprints are erased, so that sex-specific imprints can be laid down. In boys the prospermatogonia then undergo re-methylation throughout gestation, whereas in girls the oocytes continue to be re-methylated over the duration of their maturation, with evidence of high activity as each egg ripens before ovulation.27
Notable classes of loci that may be especially sensitive to early environmental exposure include imprinted genes, metastable epialleles (MEs) and transposable elements (TEs).6 Imprinted genes exhibit monoallelic expression, whereby only the maternally or paternally inherited allele is expressed, with expression controlled by regulatory regions whose methylation state is inherited in a parent of origin-specific manner.28 MEs are genomic loci showing variable methylation between individuals, but showing high correlation in methylation status across tissues within the same individual, indicating establishment of methylation state in the first few days after conception, preceding gastrulation.29 MEs therefore help to pinpoint the timing of an exposure influencing ME methylation to the periconceptional period.30,31 TEs are small, mobile sequences of DNA that are thought to comprise 45% of the human genome.32 They can insert into new genomic locations and become disruptive if transposed into a functional gene or when increasing copy number. Whereas most TEs are silenced epigenetically,33 some have variable methylation patterns that have been shown to be influenced by nutrition in mice.9 Their methylation states can alter neighbouring gene expression, exemplified by the Agouti mouse model detailed later.
Influence of nutrition on DNA methylation
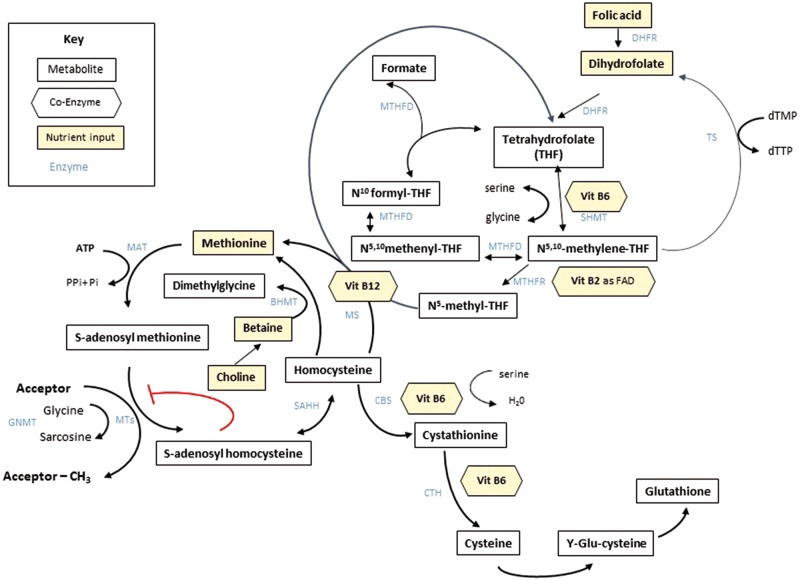
A range of maternal exposures have been associated with DNA methylation including nutrition, stress, infection, pollutants, smoking, radiation, level of exercise and parental body composition.34–36 Animal studies suggest that the epigenome is particularly sensitive to such environmental factors in early life, notably during the prenatal and neonatal periods.9,25,37 Studies of the effects of early life nutrition on DNA methylation have shown that maternal under- or over-nutrition or differences in protein, fat, sugar or micronutrient intake during gestation can induce epigenetic and phenotypic changes in the offspring.8,38 Recent studies have also shown that variations in paternal diet or body composition might also induce long-term epigenetic and phenotypic changes in the offspring.39 One-carbon nutrients and metabolites are thought to be particularly important in the periconceptional period and during embryonic development.26 One-carbon metabolism (OCM) pathways link the folate, methionine, homocysteine, transsulphuration and transmethylation metabolic pathways together (Figure 1). These are crucial for many biochemical processes, including DNA methylation.
Figure 1.
A simplified summary of one-carbon metabolism. BHMT, Betaine Homocysteine MethylTransferase; CBS, Cystathionine-Beta-Synthase; CTH, Cystathionine Gamma-Lyase; DHFR, Dihydrofolate Reductase; dTMP, Deoxythymidine Monophosphate; dTTP, Deoxythymidine Triphosphate; FAD, Flavin Adenine Dinucleotide; GNMT, Glycine N-MethylTransferase; MAT, Methionine AdenosylTransferase; MS, Methionine Synthase; MT, Methyl Transferases; MTHFD, MethyleneTetraHydroFolate Dehydrogenase; MTHF, MethyleneTetraHydroFolate Reductase; SAHH, S-Adenosyl Homocysteine Hydrolase; SHMT, Serine HydroxyMethylTransferase; TS, Thymidylate Synthase. Source: reproduced with permission from James et al. Epigenetics, nutrition and infant health. In: Karakochuk C, Whitfield K, Green T, Kraemer K (eds). The Biology of the First 1000 Days. Boca Raton, FL: CRC Press, 2017.
Nutrition plays a key role in OCM by providing substrates (folate, methionine, choline and betaine) and essential co-factors (vitamins B12, B6 and B2). For example, B12 is required by methionine synthase to methylate homocysteine, B6 is essential in the homocysteine transsulphuration pathway, and both B6 and B2 are needed to reduce dietary folate to methyltetrahydrofolate. A more detailed overview of OCM and the role of nutrients in these pathways is provided in Supplementary Material 1, available as Supplementary data at IJE online.
The potential for maternal nutrition to both alter offspring DNA methylation and influence phenotype is famously illustrated by the Agouti mouse experiments. Two groups of pregnant dams were fed diets that differed only in nutrients essential to OCM (folic acid, choline, betaine and B12). Increased levels of one-carbon nutrients increased methylation in the isogenic pups at a retrotransposon locus [Intracisternal A Particle (IAP), also an ME] upstream of the Agouti gene. The degree of expression of the Agouti gene depended on the level of IAP methylation, and this in turn altered the pups’ fur colour, as well as their appetite, adiposity and glucose tolerance in adulthood.6,9
Review methodology
We performed a narrative review of the literature in three stages to form the thematic analysis in this paper. First we searched for studies describing associations between preconceptional or pregnancy nutritional exposures and DNA methylation in offspring. We limited this search to human studies that used an intergenerational design. We included nutritional exposures in dietary or supplemental form related to OCM, or broader measures that could influence availability of such nutrients (famine, seasonal diets and macronutrients). We excluded paternal exposures and nutrients not directly involved in OCM, and we only considered epigenetic studies focusing on DNA methylation. Second, we searched for human studies linking infant DNA methylation to a subset of phenotypic outcomes (growth-related, cardiometabolic and cognitive), restricting the included studies to those describing methylation at genetic loci identified in the first search (‘nutrition-sensitive’ loci). Third, we isolated those studies explicitly linking maternal nutritional exposure to offspring phenotype via DNA methylation. Three authors (P.J., S.S., A.S.T.) performed the searches in PubMed and Google Scholar, assessing titles and abstracts against the inclusion criteria. Reference sections of included studies and relevant review papers were also used to help confirm that key studies had been included. Searches took place from January to March 2017. Supplementary Material 2, available as Supplementary data at IJE online, details the strategy and gives an example of the search terms used in PubMed.
Review of studies linking maternal nutritional exposure to offspring DNA methylation
We provide a broad overview of the associations found in the literature between maternal nutritional exposure and offspring DNA methylation in Table 1. Below we briefly review the associations by type of exposure, but refer the reader to detailed information on the individual studies (n = 34) in Supplementary Table 1, available as Supplementary data at IJE online, which includes information on the nutritional exposures, timing of exposures, study design, DNA tissue, age of offspring and associated genes. All gene names are defined in Table 4 (see candidate gene data summary, below).
Table 1.
Summary of associations between maternal one-carbon metabolites and broader nutritional exposures with offspring DNA methylation
| Timing of exposure | Maternal exposurea | Offspring DNA methylation association |
|---|---|---|
| (↑/↓: increased/decreased methylation) | ||
| Periconception | ↑B2 | ↑PLAGL1 (ZAC1),40↑VTRNA2-141 |
| ↑Betaine | ↑ DNMT1,42↑POMC,43↑RXRA44 | |
| Famine | ↓IGF2,45↑↓bIGF2,46↓INSIGF,46,47↑IL10,47↑GNASAS,47↑LEP,47↑ABCA1,47↑MEG3,47↑TACC1,48↑ZNF385A,48↓TMEM105,48↑PAX8,49↓ZFP57,4, ↓PRDM949 | |
| ↑Folates | ↓STX11,50↓OTX2,50↓TFAP2A,50↓CYS1,50↓LEP,44↑RXRA44 | |
| ↑Folic acid | ↑LEP,42↓H19,51↑IGF2,52↓IGF244 | |
| ↑Multiple micronutrients | ↓GNASAS,53↓MEG3,53↓IGF2R,53↓MEST53 | |
| Seasonality of one-carbon metabolitesc | ↑POMC,43↑VTRNA2-1,41↑BOLA3,30↑FLJ20433,30↑PAX8,30↑SLITRK1,30↑ZFYVE28,30↑RBM4631 | |
| 1st and 2nd trimester | ↑B6 | ↑MEG354 |
| ↑Betaine | ↓ LEP42 | |
| ↑Carbohydrates | ↓RXRA55 | |
| ↑Choline | ↓DNMT142 | |
| Famine | ↑FAM150B,48↑SLC38A2,48↑PPAP2C,48↓OSBPL5/MRGPRG,48↑TACC1,48 ↑ZNF385A,48↑PAX8,49↓ZFP57,49↓PRDM949 | |
| ↑Folates | ↓PEG3,56↑NR3C1,57↓MEG3,56↓PLAGL1,56↑IGF2,56↓LEP,42↓DNMT142 | |
| ↑Folic acid | ↓PEG3,58↑IGF2,58↓DNMT144 | |
| 3rd trimester | ↑B2 | ↑PLAGL1 (ZAC1)40 |
| ↑B12 | ↓IGF259 | |
| ↑Choline | ↑↓dNR3C1,60↑↓dCRH,60↑DNMT1,42,44 | |
| Famine | ↓GNASAS,47↑TACC1,48↑ZNF385A,48↑PAX8,49↓ZFP57,49↓PRDM949 | |
| ↑Folates | ↑DNMT1,44↓RXRA,42↑LASP1,61↑ACADM,61↑WNT9A,61↑FZD7,61↓ZFP57,61↓LY6E,61↓C21orf5661 | |
| ↑Folic acid | ↑RXRA42 | |
| ↑ Meat and fish intake | ↑HSD262 | |
| ↑ High sugar, high fat diet | ↑IGF263 | |
| ↑Omega-3 PUFA | ↓H19,64↑IGF2,6 mostly ↓associations in EWAS65 | |
| ↑Omega-6 PUFA | ↓MIRLET7BHG66 | |
Like nutrients are shaded in the same colour during each time period.
Different associations at different loci within gene.
Rainy season (higher concentration of most one-carbon metabolites) versus dry season.
Different associations between different tissues.
EWAS, epigenome-wide association study; PUFA, polyunsaturated fatty acids.
Table 4.
Candidate genes exhibiting associations between nutritional exposures during periconception and pregnancy and offspring DNA methylation. Links between methylation at nutrition-sensitive genes and offspring phenotype are also included
| Gene/region of Interest | Genomic featuresc | Exposure (↑/↓: increased/decreased) | Outcome (↑/↓: increased/decreased) | Coordinates of ROI in studiesd,e (number of CpGs on 450ka and EPICb arrays) |
|---|---|---|---|---|
| Blue = ME | ||||
| Brown = imprinted | ||||
| Yellow = ME and imprinted | ||||
| ABCA1 (ATP Binding Cassette Subfamily A Member 1) | Promoter marks; CpG island; binding site for multiple TFs | Famine | ↑Methylation47 | chr9: 107, 690, 502-107, 690, 821 (1)a(5)b |
| ACADM (Acyl-CoA Dehydrogenase, C-4 To C-12 Straight Chain) | Multiple TFs binding sites; Promoter mark; Active Enhancer mark | ↑Folate | ↑Methylation61 | chr1: 76, 189, 707-76, 190, 008 (6)a(7)b |
| BOLA3 (BolA Family Member 3) | Enhancer and Promoter marks; CpG island; binding site for multiple TFs | Rainy season conception | ↑Methylation30 | chr2: chr2: 74, 357, 632-74, 357, 837 (1)a,b |
| CRH (Corticotropin-Releasing Hormone) | Enhancer mark | ↑Choline | ↓Methylation60 | chr8: 67, 090, 692-67, 091, 132 (5)a(8)b |
| CYS1 (Cystin 1) | Multiple TFs binding sites; Promoter mark | ↑Folate | ↓Methylation50 | chr2: 10, 220, 719 |
| DNMT1 (DNA Methyltransferase 1) | Multiple TFs binding sites; Promoter mark; Active Enhancer mark | ↑Folate | ↑Methylation44, ↓Methylation42 | chr19: 10, 305, 774-10, 305, 811 (2)a,b |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark | ↑Folic acid | ↓Methylation44 | chr19: 10, 305, 774-10, 305, 811 (2)a,b | |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark | ↑Choline | ↑Methylation44, ↑↓Methylation42 | chr19: 10, 305, 774-10, 305, 811 (2)a,b | |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark | ↑Betaine | ↑Methylation42 | chr19: 10, 305, 774-10, 305, 811 (2)a,b | |
| EXD3 (FLJ20433) (exonuclease 3'-5' domain containing 3) | Active Enhancer mark; CpG island | Rainy season conception | ↑Methylation30 | chr9: 140, 312, 206-140, 312, 339 |
| FAM150B (Family With Sequence Similarity 150, Member B) | None | Famine | ↑Methylation48 | chr2: 366, 113 (1)a,b |
| FZD7 (Frizzled Class Receptor 7) | Multiple TFs binding sites; Promoter mark | ↑Folate | ↑methylation61 | chr2: 202, 901, 045-202, 901, 470 (5)a(4)b |
| GNASAS (Guanine Nucleotide Binding Protein (G Protein), Alpha Stimulating Activity Antisense RNA 1) | Enhancer marks; Multiple TFs binding sites | Famine (periconceptional)/Famine (late gestation) | ↑Methylation/ ↓Methylation47 | chr20: 57, 425, 815-57, 426, 108 (3) a,b |
| CpG island; MYC binding site | UNIMMAP (supplementation) | ↓Methylation53 | chr20: 57, 429, 802-57, 430, 242 (1)a(2)b | |
| H19 | Multiple TFs binding sites | ↑Methylation | ↑Birthweight56 | chr11: 2, 011, 131-2, 011, 275 (2)a,b |
| MYC and CTCF binding sites; Active promoter mark; weak enhancer mark | ↑Methylation | ↑Small for gestational age92 | chr11: 2, 019, 727-2, 019, 921 (7)a(6)b | |
| Multiple TFs binding sites | ↑ Omega-3 PUFA | ↓Methylation64 | chr11: 2, 024, 197-2, 024, 340 | |
| Multiple TFs binding sites | ↑Folic acid | ↓Methylation51 | chr11: 2, 024, 254-2, 024, 261 | |
| Enhancer Mark; CTCF-binding site | ↑Methylation | ↓Birth length, ↑weight in adulthood, ↑adult BMI, ↑adult blood pressure62 | chr11: 2, 021, 072-2, 021, 291 (2)a,b | |
| HSD11B2 (Hydroxysteroid 11-Beta Dehydrogenase 2) (HSD2) | Multiple TFs binding sites; CpG island | ↑Methylation | ↓Neonatal ponderal index, ↑birthweight, ↑adult adiposity, ↑adult blood pressure62 | chr16: 67464346-67464649 (3)a(4)b |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark; CpG island | ↑Meat and fish intake | ↑Methylation62 | chr16: 67, 464, 981-67, 465, 111 (1)a(2)b | |
| Multiple TFs binding sites, Active Enhancer mark | ↓Methylation | ↑Risk of being in a poorly regulated neurobehavioral profile103,104 | chr16: 67, 464, 387-67, 464, 417 | |
| IGF2 (Insulin-like Growth Factor 2) | POL2A binding site | ↑Folic acid | ↓Methylation44 | chr11: 2, 151, 629-2, 151, 721 (3) a,b |
| POL2A binding site | ↑Folate | ↑Methylation56 | chr11: 2, 151, 629-2, 151, 721 (3) a,b | |
| 1 reported SNP (rs3741210) | ↑Omega-3 PUFA | ↑Methylation64 | chr11: 2, 169, 425-2, 169, 556 | |
| CTCF binding site; Enhancer mark; 2 reported SNPs (rs3741210, rs3741208) | ↑Folic acid | ↑Methylation52 | chr11: 2, 169, 459 -2, 169, 796 | |
| CTCF binding site; Enhancer mark; 2 reported SNPs (rs3741210, rs3741208) | ↑Methylation | ↓Birthweight52 | chr11: 2, 169, 459 -2, 169, 796 | |
| CTCF binding site; Enhancer mark; 2 reported SNPs (rs3741210, rs3741208) | Famine | ↓Methylation45,46 | chr11: 2, 169, 459-2, 169, 796 | |
| POL2A and USF1 binding sites; 1 CpG island; 1 reported SNP (rs1803647) | ↑Folic acid | ↑Methylation58 | chr11: 2, 154, 262-2, 154, 977 (5)a,b | |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark | ↑Methylation | ↑ADHD in early‐onset persistent youth63 | (37)a(35)b,f | |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark | High‐fat and ‐sugar diet | ↑Methylation63 | (37)a(35)b,f | |
| POL2A binding site; Promoter mark; Active Enhancer mark; CpG island | ↑Omega-3 PUFA | ↑Methylation64 | chr11: 2, 159, 107-2, 159, 965 (3)a(4)b | |
| EZH2 and CTCF binding site; Promoter mark; CpG island | ↑Vitamin B12 | ↓Methylation59 | chr11: 2, 161, 115-2, 161, 275 (4)a,b | |
| CTCF binding site; Enhancer mark; 2 reported SNPs (rs3741210, rs3741208) | Famine | ↓Methylation46 | chr11: 2, 169, 385-2, 169, 489 | |
| Enhancer mark | Famine | ↓Methylation46 | chr11: 2, 170, 541-2, 170, 644 | |
| CTCF binding site; Enhancer mark; 2 reported SNPs (rs3741210, rs3741208) | ↓Methylation | ↑Small for gestational age83 | chr11: 2, 169, 458-2, 169, 796 | |
| EZH2, RAD21 and CTCF binding site; Promoter mark; CpG island | Famine | ↑Methylation46 | chr11: 2, 160, 906-2, 161, 372 (14)a(13)b | |
| EZH2, ZBTB7A and CTCF binding site; Promoter mark; CpG island | Famine | ↑Methylation46 | chr11: 2, 161, 550-2, 161, 846 (1)a(2)b | |
| Enhancer mark; 1 reported SNPs (rs3741210) | ↓Methylation | ↑Small for gestational age95 | chr11: 2, 169, 467-2, 169, 640 | |
| POLR2A and ZBTB7A binding site | Famine | ↓Methylation46 | chr11: 2, 155, 447-2, 155, 736 (1)a,b | |
| CpG island; USF1 and POL2A binding sites | ↑Methylation | ↑Birthweight, birth height, head and thorax circumference at birth91 | chr11: 2, 154, 263-2, 154, 457 (2)a,b | |
| None | ↑Methylation | ↑Birthweight90 | chr11: 2, 169, 518-2, 169, 499 | |
| CTCF and REST binding sites; CpG island | ↑Methylation | ↑TG and TG: HDL102 | chr11: 2, 160, 374-2, 160, 610 (4)a,b | |
| IGF2R (Insulin-like Growth Factor 2 Receptor) | CpG island; associated with SNP rs677882 and rs8191722 | ↑UNIMMAP (supplementation) | ↓Methylation53 | chr6: 160, 426, 403-160, 426, 850 |
| IGF2/H19 ICR | None | ↑Methylation | ↓Head circumference between 1–10 years; ↑subcutaneous fat measures at age 17 years97 | chr11: 2, 064, 402-2, 064, 717 |
| IL10 (Interleukin 10) | Enhancer and Promoter marks; binding site for multiple TFs | Famine | ↑Methylation47 | chr1: 206, 946, 011-206, 946, 339 (2)a(3)b |
| INSIGF (Insulin- Insulin-like Growth Factor 2) | None | Famine | ↓Methylation46,47 | chr11: 2, 182, 336-2, 182, 640 (5)a(4)b |
| LASP1 (LIM And SH3 Protein 1) | Multiple TFs binding sites; Promoter marks; Enhancer marks; 4 CpG islands; 25 reported SNPs | ↑Folate | ↑Methylation61 | chr17: 37, 123, 638-37, 123, 949 (9)a,b |
| LEP (Leptin) | None | ↑Folate | ↓Methylation42,44 | chr7: 127, 881, 035-127, 881, 054 |
| None | ↑Betaine | ↓Methylation42 | chr7: 127, 881, 035-127, 881, 054 | |
| None | ↑Folic acid | ↑Methylation42 | chr7: 127, 881, 035-127, 881, 054 | |
| CpG island; CEBP binding site; 2 reported SNPs (rs791620, rs2167270) | Famine | ↑Methylation47 | chr7: 127, 881, 054-127, 881, 410 (4)a(6)b | |
| CpG island; CEBP binding site; 2 reported SNPs (rs791620, rs2167270) | ↑Methylation | ↑Small for gestational age94 | chr7: 127, 881, 127-127, 881, 350 (4)a(6)b | |
| CpG island; 1 reported SNP (rs2167270) | ↓Methylation | ↑BMI100 | chr7: 127, 881, 280-127, 881, 300 (2)a(3)b | |
| CpG island; CEBP binding site; 2 reported SNPs (rs791620, rs2167270) | ↓Methylation | ↑BMI; ↑hip circumference98 | chr7: 127, 881, 126-127, 881, 474 (3)a(4)b | |
| CpG island; CEBP binding site; 2 reported SNPs (rs791620, rs2167270) | ↑Methylation | ↑Fasting LDL-C98 | chr7: 127, 881, 126-127, 881, 474 (3)a(4)b | |
| CpG island | ↓Methylation | ↑BMI99 | chr7: 127, 881, 036 -127, 881, 057 | |
| CpG island; CEBP binding site; 2 reported SNPs (rs791620, rs2167270) | ↑Methylation | ↑Lethargy and hypotonicity105 | chr7: 127, 881, 127-127, 881, 350 (4)a(6)b | |
| CpG island; CEBP binding site; 2 reported SNPs (rs791620, rs2167270) | ↓Methylation | ↑HDL101 | chr7: 127, 881, 053-127, 881, 410 (4)a(6)b | |
| LY6E (Lymphocyte Antigen 6 Family Member E) | Multiple TFs binding sites; Promoter mark; Active Enhancer mark | ↑Folate | ↓Methylation61 | chr8: 144, 120, 106-144, 120, 706 (8)a(9)b |
| MEG3 (Maternally Expressed 3) (GTL-2) | CpG island; Promoter mark | ↑Vitamin B6 | ↑Methylation54 | chr14: 101, 294, 220-101, 294, 391 |
| CpG island; Promoter mark | ↑Folate | ↓Methylation56 | chr14: 101, 294, 220-101, 294, 391 | |
| Enhancer and Promoter marks; CpG island; POLR2A binding site | ↑ UNIMMAP (supplementation) | ↓Methylation53 | chr14: 101, 292, 283– 101, 292, 796 (4)a(5)b | |
| CpG island; Promoter mark | ↓Methylation | ↑Birthweight56 | chr14: 101, 294, 220-101, 294, 391 | |
| None | Famine | ↑Methylation47 | chr14: 101, 291, 413-101, 291, 642 (5)a(6)b | |
| MEST (Mesoderm-Specific Transcript) (PEG1) | CpG island | ↑UNIMMAP (supplementation) | ↓Methylation53 | chr7: 130, 131, 325-130, 131, 792 (11)a(9)b |
| Multiple TFs binding sites; Promoter mark; Enhancer mark; CpG island | ↑Methylation | ↑Small for gestational age93 | chr7: 130, 125, 200-130, 126, 400 (16)a(17)b | |
| MIRLET7BHG (MicroRNA Let-7b Host Gene) | Active Enhancer mark | ↑Omega-6 PUFA | ↓Methylation66 | chr22: 46, 473, 721 (1)a,b |
| Active Enhancer mark | ↓Methylation | ↑Birthweight66 | chr22: 46, 473, 721 (1)a,b | |
| NR3C1 (Nuclear Receptor Subfamily 3 Group C Member 1) (GR) | Multiple TFs binding sites; Promoter mark; Enhancer mark; CpG island; 2 reported SNPs (rs10482604, rs10482605) | ↑Methylation | ↑Risk of being in a poorly regulated neurobehavioural profile103,104 | chr5: 142, 783, 501-142, 783, 640 (4)a,b |
| Multiple TFs binding sites; Promoter mark; Enhancer mark; CpG island; 2 reported SNPs (rs10482604, rs10482605) | ↑Choline | ↑Methylation60 | chr5: 142, 783, 501-142, 783, 908 (5)a(7)b | |
| Multiple TFs binding sites; Promoter mark; Enhancer mark; CpG island | ↑Methylation | ↑Adult waist circumference, ↑adult BMI62 | chr5: 142, 782, 759-142, 783, 164 (2)ab | |
| Multiple TFs binding sites; Promoter mark; Enhancer mark; CpG island; 1 reported SNP (rs10482604) | ↑Meat/fish and vegetable intake, ↓bread/potato intake in late pregnancy | ↑Methylation62 | chr5: 142, 783, 579-142, 783, 714 (3)a,b | |
| Multiple TFs binding sites; Promoter mark; Enhancer mark; CpG island; 1 reported SNP (rs10482604) | ↑Methylation | ↓Adult blood pressure62 | chr5: 142, 783, 578 -142, 783, 714 (3)a,b | |
| OSBPL5/MRGPRG (Oxysterol-Binding Protein Like 5/MAS Related GPR Family Member G) | Enhancer mark; CpG island | Famine | ↓Methylation48 | chr11: 3, 225, 076 (1)a,b |
| OTX2 (Orthodenticle Homeobox 2) | CpG island; EZH2 binding site | ↑Folate | ↓Methylation50 | chr14: 57, 278, 729 (1)a,b |
| PAX8 (Paired Box8) | Multiple TFs binding sites; Promoter mark; Active Enhancer mark | Rainy season conception | ↑Methylation30 | chr2: 113, 993, 262-113, 993, 391(2)a,b |
| chr2: 113, 992, 866-113, 993, 036(2)a,b | ||||
| Multiple TFs binding sites; Promoter mark; Active enhancer mark | Famine | ↑Methylation49 | chr2: 113, 992, 762-113, 993, 313 (8)a(7)b | |
| PEG3 (Paternally Expressed 3) | Multiple TFs binding sites; 2 CpG islands; 1 reported SNP (rs2302376) | ↑Folate | ↓Methylation56 | chr19: 57, 351, 945-57, 352, 096 (4)a(3)b |
| Multiple TFs binding sites; 2 CpG islands; 1 reported SNP (rs2302376) | ↑Folic acid | ↓Methylation56 | chr19: 57, 351, 945-57, 352, 096 (4)a(3)b | |
| Multiple TFs binding sites; 2 CpG islands; 1 reported SNP (rs2302376) | ↑Folic acid | ↓Methylation58 | chr19: 57, 351, 944-57, 352, 096 (4)a(3)b | |
| PLAGL1 (PLAG1-Like Zinc Finger 1) (ZAC1) | Multiple TFs binding sites; Promoter mark; Active Enhancer mark; CpG island | ↑Folate | ↓Methylation56 | chr6: 144, 329, 109-144, 329, 231 (1)a,b |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark; CpG island | ↑Methylation | ↑Birthweight56 | chr6: 144, 329, 109-144, 329, 231 (1)a,b | |
| Multiple TFs binding sites; Promoter mark; CpG island | ↑Methylation index | ↑Fetal weight at 32 weeks of gestation, weight and BMI at 1 year40 | chr6: 144, 329, 390-144, 329, 740 (4)a,b | |
| Multiple TFs binding sites; Promoter mark; CpG island | ↑ Vitamin B2 | ↑Methylation index40 | chr6: 144, 329, 390-144, 329, 740 (4)a,b | |
| POMC (Proopiomelanocortin) | Multiple TFs binding sites; Promoter mark; Active Enhancer mark; CpG island | ↑Methylation | ↑BMI43,96 | chr2: 25, 384, 508-25, 384, 832 (3)a,b |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark; CpG island | ↑SAM:SAH ratio; ↑betaine | ↑Methylation43 | chr2: 25, 384, 508-25, 384, 832 (3)a,b | |
| PPAP2C (PLPP2) (Phosphatidic Acid Phosphatase 2c) | CpG island | Famine | ↑Methylation48 | chr19: 292, 167 (1)a,b |
| PRDM9 (PR-Domain Containing Protein 9) | Multiple transcription factor binding sites; Promoter mark, Active enhancer mark; 2 reported SNPs (rs10077095, rs1994929) | Famine | ↓Methylation49 | chr5: 23, 507, 030-23, 507, 752 (12)a(11)b |
| RBM46 (RNA-Binding Motif Protein 46) | CpG island | Rainy season conception | ↑Methylation31 | chr4: 155, 702, 818-155, 703, 110 (1)a,b |
| RXRA (Retinoid X Receptor Alpha) | Multiple TFs binding sites; Enhancer mark | ↑Methylation | ↑Fat mass; % fat mass55 | chr9: 137, 215, 697 -137, 216, 117 (1)a,b |
| Multiple TFs binding sites; Enhancer mark | ↑Methylation | ↑BMI55 | chr9: 137, 215, 697 -137, 216, 117 (1)a,b | |
| Multiple TFs binding sites; Enhancer mark | ↑Carbohydrate intake | ↓Methylation55 | chr9: 137, 215, 697 -137, 216, 117 (1)a,b | |
| Multiple TFs binding sites; Enhancer mark | ↑Methylation | ↓Bone mineral content; % BMC75 | chr9: 137, 215, 697 -137, 216, 117 (1)a,b | |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark; CpG island | ↑Folate | ↓Methylation42 | chr9: 137, 217, 097-137, 217, 132 | |
| Multiple TFs binding sites; Promoter mark; Active Enhancer mark; CpG island | ↑Folate | ↑Methylation44 | chr9: 137, 217, 097-137, 217, 132 | |
| SLC38A2 (Solute Carrier Family 38 Member 2) | Enhancer mark | Famine | ↑Methylation48 | chr12: 46, 737, 123 (1)a,b |
| SLITRK1 (SLIT And NTRK-like Family Member 1) | Promoter mark; Enhancer mark; CpG island | Rainy season conception | ↑Methylation30 | chr13: 84, 453, 741-84, 453, 828 |
| chr13: 84, 454, 210-84, 454, 281 | ||||
| SPATC1L (C21orf56) (Spermatogenesis And Centriole Associated 1 Like) | Multiple TFs binding sites; Promoter mark; Active Enhancer mark | ↑Folate | ↓Methylation61 | chr21: 47, 604, 052-47, 604, 654 (5)a,b |
| STX11 (Syntaxin 11) | Multiple TFs binding sites; Promoter mark; CpG island | ↑Folate | ↓Methylation50 | chr6: 144, 471, 564 (1)a,b |
| TACC1 (Transforming Acidic Coiled-Coil Containing Protein 1) | Promoter mark; Enhancer mark | Famine | ↑Methylation48 | chr8: 38, 586, 183 (1)a,b |
| TFAP2A (Transcription Factor AP-2 Alpha) | E2F1 and EZH2 binding site; Promoter mark; Active Enhancer mark; CpG island | ↑Folate | ↓Methylation50 | chr6: 10, 411, 911 (1)a,b |
| TMEM105 (Transmembrane Protein 105) | Enhancer mark; Active Enhancer mark; CpG island | Famine | ↓Methylation48 | chr17: 79, 283, 915 (1)a,b |
| VTRNA2-1 (Vault RNA 2-1) | Multiple TFs binding sites; Promoter mark; Active Enhancer mark; CpG island | Rainy Season; ↑vitamin B2; ↑methionine; ↓dimethylglycine | ↑Methylation41 | chr5: 135, 415, 762-135, 416, 613 (15)a(13)b |
| WNT9A (Wnt Family Member 9A) | NRF1 binding site; Promoter mark; Active Enhancer mark; CpG island | ↑Folate | ↑Methylation61 | chr1: 228, 075, 423-228, 075, 749 (5)a(3)b |
| ZFP57 (Zinc Finger Protein 57) | YY1 binding site; Promoter mark; Active Enhancer mark; multiple reported SNPs | ↑Folate | ↓Methylation61 | chr6: 29, 648, 161-29, 649, 084 (24)a(25)b |
| Promoter mark; Active Enhancer mark; multiple reported SNPs | Famine | ↓Methylation49 | chr6: 29, 648, 345-29, 649, 024 (19)a(18)b | |
| ZFYVE28 (Zinc Finger FYVE-Type Containing 28) | Multiple TFs binding sites; Promoter mark; CpG island | Rainy season conception | ↑Methylation30 | chr4: 2, 366, 658-2, 366, 739 (1)a,b |
| chr4: 2, 366, 909-2, 367, 003 | ||||
| ZNF385A (Zinc Finger Protein 385A) | Multiple TFs binding sites; Promoter mark; CpG island | Famine | ↑Methylation48 | chr12: 54, 764, 265 (1)a,b |
LBW, low birthweight; LDL-C, low-density lipoprotein cholesterol; ME, metastable epiallele; ROI, region of interest; SAH, s-adenosyl homocysteine; SAM, s-adenosyl methionine; UNIMMAP, United Nations International Multiple Micronutrient Preparation.
Number of CpGs covered on Infinium HumanMethylation450K BeadChip array.
Number of CpGs covered on Infinium MethylationEPIC array.
The following regulatory features were checked: enhancer/promoter marks (histone), overlapping binding sites for various transcription factors (e.g. CTCF, POL2A etc.) within region of interest (ROI) and presence of nearby reported GWAS single nucleotide polymorphisms (SNPs).
Coordinates based on genome build hg19. The BiSearch Web server122 was used to find genomic coordinates for ROIs where only primers were available.
HumanMethylation450 v1.2 and Infinium MethylationEPIC v1.0 B4 Manifest Files were referred to report ROI coverage on Illumina Infinium Methylation BeadChip arrays.
A total of 37 probes from 450k array were found within the gene and considered for analysis.
Folate
Associations between maternal folate exposure and the offspring methylome are inconsistent, with varying effects according to the form of folate (dietary folates or folic acid supplements)58 the timing of exposure,42,58 baseline maternal folate status,50,61 underlying genotype,67 the genomic region affected68 and individual CpG site.42
Periconceptional folic acid has been positively associated with offspring methylation at LEP,42 inversely associated with methylation at H19,51 and has demonstrated both positive52 and inverse44 associations at IGF2. Not all studies have found an effect of periconceptional folic acid exposure.58 Supplementation started after 12 weeks of gestation has been associated with increased offspring methylation at IGF2 and decreased methylation at PEG3.58 Folic acid taken up to the end of the second trimester has been inversely associated with DNMT1 methylation, but positively correlated at the same locus when the folic acid consumption was extended into the third trimester.44
Data for dietary folate intakes (assessed using questionnaires or plasma samples) are equally variable. Periconceptional folate intake and offspring DNA methylation were inversely associated with the majority of differentially methylated CpGs in an epigenome-wide screen, although this trend reversed in stratified analysis among women with low intakes (<200 µg/day).50 Periconceptional intakes have also been inversely associated with methylation at LEP and positively associated at RXRA.44 First trimester folate exposure has shown positive associations with DNA methylation at IGF256 and NR3C1,57 and inverse associations at MEG3, PLAGL1 and PEG3.56 For second trimester folate exposure, studies have reported inverse associations at multiple differentially methylated CpG sites,68 and at LEP and DNMT1.42 Third trimester folate exposure has shown positive associations with methylation at DNMT1,44 and at LASP1, ACADM, WNT9A, C21orf56 and FZD7,61 but inverse associations at ZFP57, LY6E and RXRA.42,61
B vitamins
Maternal serum B12 at first antenatal visit has been inversely associated with cord blood global methylation levels,67 and inversely associated with offspring IGF2 methylation when exposure timing was at delivery.59 Some studies have assessed joint effects of B vitamins. One study assessed pre-pregnancy and third trimester maternal B2, B3, B6, folate and B12 intake, and found a positive correlation between maternal B2 and offspring methylation at PLAGL1 (ZAC1) at both time points.40 Another study found no associations between first trimester maternal plasma B12 and B6 concentrations with offspring methylation at H19, PEG10/SGCE and PLAGL1, but there was a positive trend in methylation at MEG3 across maternal B6 quartiles.54
Choline and betaine
To date there is one human intervention study investigating the effect of supplementing mothers’ diets with choline (480 mg vs 930 mg) in the third trimester on offspring DNA methylation. The intervention increased methylation at NR3C1 and CRH in fetal placental tissue but reduced methylation in cord blood. No effect was seen at GNAS-AS, IGF2, IL10 or LEP.60 In observational studies, second trimester choline intake has been inversely associated with DNMT1 methylation in cord blood.42 Third trimester choline intake has been positively associated with DNMT1 methylation in cord blood and in infant buccal cells.42,44 Maternal periconceptional betaine intake has been positively associated with cord blood methylation at DNMT1, RXRA and POMC,42–44 and second trimester intake inversely associated with LEP methylation.42
Polyunsaturated fatty acids
Polyunsaturated fatty acids (PUFAs) are thought to influence OCM by upregulating enzymes responsible for the methylation of homocysteine to methionine and by directly influencing demand for methyl groups via phosphatidylcholine (described in Supplementary Material 1, available as Supplementary data at IJE online). There have been several studies of PUFA supplementation in mothers. In one trial, omega-3 PUFA supplementation in the second and third trimesters showed no difference in the cord blood methylation of various gene promoter sites, but the intervention increased global methylation (LINE-1) in offspring of mothers who smoked.69 It also decreased H19 methylation, and increased IGF2 methylation in offspring of overweight mothers.64 A more recent trial, also implemented in the second and third trimesters, found omega-3 PUFA supplementation was associated with 21 differentially methylated regions (DMRs) at birth.65 These were predominantly hypomethylated in the intervention group. However, not all omega-3 PUFA supplementations trials have demonstrated an effect on methylation.70 Maternal plasma omega-6 PUFA concentrations in the third trimester have been inversely associated with offspring MIRLET7BHG methylation.66
Broader nutrition measures: famine studies, seasonal exposures, macronutrients
Several studies have used broader measures of maternal nutritional exposure, such as famine, season of conception and macronutrient intake. During the Dutch Famine of 1944, there was a large drop in all food intakes, with average energy intake reduced to 500–1000 kcal per day.71 In follow-up studies of adults who were exposed to famine in utero, exposure in early pregnancy (periconception and up to 10 weeks of gestation) was associated with lower methylation of INSIF and TMEM105, increased methylation at IL10, GNASAS, LEP, ABCA1, MEG3, TACC1 and ZNF385A, and both increased and decreased methylation at IGF2 depending on the loci within the gene.45–48 Not all these effects were seen in those exposed during late gestation.45,48 In a candidate gene analysis of putative metastable epialleles, offspring exposed to famine for at least 7 months during gestation in Bangladesh had higher methylation at PAX8 and lower methylation at PRDM9 and ZFP57, compared with unexposed controls.49
One study found an inverse association between maternal second trimester carbohydrate intake and infant RXRA methylation.55 Another study looked at the effect of a prenatal diet high in fat and sugar and found a positive association with offspring IGF2 methylation.63 Higher methylation at GR has been observed in infants of mothers having higher meat/fish/vegetables and lower bread/potato intake in late pregnancy (>20 weeks of gestation compared with earlier in pregnancy) and increased infant methylation at HSD2 has been associated with increased maternal meat and fish intake in late pregnancy.62 In a pilot trial of periconceptional multiple micronutrient supplementation (UNIMMAP) for mothers, there were sex-specific effects on infant methylation at IGF2R, GNASAS, MEG3 and MEST.53 The difficulty of such studies, however, is that it is not possible to know which nutrient deficits or imbalances caused the epigenetic effects. In The Gambia, where season has marked effects on maternal diet and body weight,72 children conceived in the rainy season had higher methylation in peripheral blood lymphocytes at six MEs, at VTRNA2–1 and at POMC compared with those conceived in the dry season.31,41,43 This may reflect a role of one-carbon-related nutrients; in the rainy season, maternal periconceptional plasma showed higher concentrations of folate, B2, methionine, betaine, S-adenosyl methionine (SAM):S-adenosyl homocysteine (SAH) ratio and betaine:dimethylglycine (DMG) ratio, and lower B12 and homocysteine, indicating higher methylation potential.
Aside from those considered above, the list of maternal exposures associated with changes in infant DNA methylation continues to grow. These include further nutrition-related exposures (e.g. dietary polyphenols,73 vitamin D74,75 and vitamin A76) non-nutrition-related exposures (e.g. maternal stress77 and toxin exposure78) and factors that span the spectrum of nutrition and health-related considerations (e.g. maternal hyperglycaemia,79 maternal body mass index (BMI),80–82 intrauterine growth restriction (IUGR),83–85 the microbiome86 and infection87). The ongoing challenge is not only to identify relevant exposures, but also to delineate the consequences for human health across the life course. It is to this latter point that we now turn.
Review of studies linking nutrition-associated DNA methylation loci to health outcomes
In animal studies, nutritional exposures in pregnancy bring about distinct phenotypic effects in offspring via epigenetic mechanisms. Differential methylation of genes may induce phenotypic variation by the modulation of gene expression which may alter tissue structure, homeostatic control processes and the activity of metabolic pathways.88 Often cited examples include the effects of maternal methyl donor supplementation on offspring coat colour and adiposity in the Agouti mouse, and the development of the fertile queen bee from genetically identical larvae by epigenetic silencing of DNMT3, caused by preferential feeding of royal jelly.9,89
In this section we focus on evidence provided by two types of studies:
Those reporting associations between methylation at the nutrition-sensitive epigenetic loci described above and offspring phenotypes; these are summarized in Table 2, with detailed information on all included studies (n = 31) in Supplementary Table 2, available as Supplementary data at IJE online;
Those linking maternal nutrition exposure, infant DNA methylation and offspring phenotypic effects in a single study (n = 8); these are summarized in Table 3.
Table 2.
Summary of associations between methylation at nutrition-sensitive genetic loci and phenotypes
| Direction of DNA methylation/locus | Associated phenotype/direction | Tissue analysed | Age at methylation measurement |
|---|---|---|---|
| (↑/↓: increased/decreased) | |||
| Birth size | |||
| ↑H19,56↑PLAGL1,56↓MEG3,56↓MIRLET7BHG,66↑IGF290 | ↑Birthweight | Cord blood | Birth |
| ↑IGF2 DMR291 | ↑Birthweight | Placenta | Birth |
| ↓IGF2,52↑HSD262 | ↑Birthweight | Peripheral blood | 17 months,52 40 years62 |
| ↑H19 ICR62 | ↓Birth length | Peripheral blood | 40 years |
| ↑PLAGL140 | ↑Estimated fetal weight at 32 weeks of gestation | Cord blood | Birth |
| ↑HSD262 | ↓Neonatal ponderal index | Peripheral blood | 40 years |
| ↓IGF2 DMR0,83↑H1992 | ↑Small for gestational age | Cord blood | Birth |
| ↑MEST,93↑LEP94 | ↑Small for gestational age | Placenta,93 cord blood94 | Birth |
| ↓IGF2 DMR095 | ↑Small for gestational age | Peripheral blood | 11 years |
| Anthropometric measures/adiposity | |||
| ↑PLAGL140 | ↑Weight at age 1 year | Cord blood | Birth |
| ↑PLAGL140 | ↑Body mass index (BMI) z-score at age 1 year | Cord blood | Birth |
| ↑IGF2 DMR291 | ↑Height, head and thorax circumference at birth | Placenta | Birth |
| ↑POMC96 | ↑Obesity at age 11 years | Peripheral blood | 11 years |
| ↑IGF2/H19 ICR97 | ↓Early childhood head circumference | Peripheral blood | 1–10 years |
| ↑H19 ICR,62↑HSD262 | ↑Weight in adulthood | Peripheral blood | 40 years |
| ↑H19 ICR,62↑HSD2,62↑NR3C1 exon 1C62 | ↑Waist circumference in adulthood | Peripheral blood | 40 years |
| ↑POMC,43↑H19 ICR,62↑HSD2,62↑ NR3C1 exon 1C,62↓LEP98 | ↑BMI in adulthood | Peripheral blood | 48,43 40,62 34.798 years |
| ↑RXRA55 | ↑Adiposity at age 9 years | Cord blood | Birth |
| ↓LEP99 | ↑Obesity at age 10–15 years | Saliva | 10–15 years |
| ↓LEP100 | ↑Obese subjects with insulin resistance at age 10-16 years | Peripheral blood | 10–16 years |
| ↑IGF2/H19 ICR97 | ↑Skinfold thickness and subcutaneous adiposity at age 17 years | Peripheral blood | 17 years |
| Skeletal growth and bone quality | |||
| ↓RXRA75 | ↑Bone mineral content at age 4 years | Cord blood | Birth |
| Cardiometabolic outcomes | |||
| ↑LEP98 | ↑Fasting low-density lipoproteincholesterol levels in adulthood | Peripheral blood, Subcutaneous adipose tissue | 34.7 years |
| ↑H19 ICR,62↓ NR3C1 exon 1F,62↑HSD262 | ↑Blood pressure in adulthood | Peripheral blood | 40 years |
| ↓LEP101 | ↑High-density lipoprotein (HDL) profile | Peripheral blood | 17 months |
| ↑IGF2102 | ↑Triglycerides (TG), ↑TG:HDL | Peripheral blood | 11.6 years |
| Cognitive outcomes | |||
| ↑IGF263 | ↑Early onset conduct problem, attention-deficit/hyperactivity disorder | Cord blood | Birth |
| ↑NR3C1,103,104↓HSD2103,104 | ↑Risk of being in a poorly regulated neurobehavioural profile | Placenta, Buccal cells | Birth |
| ↑LEP105 | ↑Lethargy and hypotonicity | Placenta | Birth |
Table 3.
Studies linking maternal one-carbon metabolites or broader nutritional exposures to offspring DNA methylation and phenotype
| Study | Exposure (exposure timing) | Offspring tissue analysed | Genes analysed | Phenotype investigated | Key findings (↑/↓: increased/decreased, ˜ associated with) |
|---|---|---|---|---|---|
| Azzi S et al.40 | Pre-pregnancy BMI, vitamins B2, B3, B6, folate, B12 (3 months before conception and last trimester) | Cord blood | PLAGL1 (ZAC1) | Pre- and post-natal growth |
|
| Drake AJ et al.62 | Maternal diet: food group analysis (‘Early’ <20 weeks and ‘late’ >20 weeks of gestation) | Peripheral blood | IGF2, H19 ICR, HSD2, NR3C1 | Birthweight, current height, weight, waist circumference, blood pressure |
|
| Godfrey KM et al.55 | Maternal carbohydrate intake (2nd trimester) | Cord blood | RXRA, NOS3, SOD1, IL8, PIK3CD | Adiposity |
|
| Hoyo C et al.56 | Maternal erythrocyte folate (1st trimester, median 12 weeks of gestation)) | Cord blood | IGF2, H19, PEG1/MEST, PEG3, PLAGL1, MEG3-IG, PEG10/SGCE, NNAT, DLK1/MEG3 | Birthweight |
|
| Kühnen P et al.43 | Maternal 1-carbon metabolites/season of conception (periconception) | Peripheral blood/MSH-positive neurons | POMC | Obesity/BMI |
|
| Lin X et al.66 | Maternal BMI, glucose, plasma fatty acids, plasma vitamin D, serum B12, B6, folate, iron, zinc, magnesium (3rd trimester; 26-28 weeks of gestation) | Cord blood | Epigenome-wide association study | Birthweight, size and adiposity at 4 years |
|
| Rijlaarsdam J et al.63 | High-fat and -sugar diet (3rd trimester, 32 weeks of gestation) | Cord blood, peripheral blood at age 7 years | IGF2 | ADHD |
|
| Steegers-Theunissen RP et al.52 | Maternal folic acid supplementation (periconception) | Peripheral blood | IGF2 | Birthweight |
|
ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index; ICR, imprinting control region; PUFA, polyunsaturated fatty acids.
We consider three broad categories of offspring phenotypic outcomes: growth and body composition, cardiometabolic risk markers and cognitive function.
Growth and body composition
DNA methylation signatures in different tissues such as cord and peripheral blood, placenta, subcutaneous and visceral adipose tissue and buccal cells have been associated with growth outcomes such as size at birth (usually birthweight, with or without adjustment for gestational age), child/adult adiposity and skeletal growth or bone size/quality (see Supplementary Table 2, available as Supplementary data at IJE online).
Birth size: most studies investigating growth-related phenotypes have analysed imprinted genes due to their known role in fetal growth regulation.106 Chromosomal region 11p15.5 contains two imprinting control regions (ICRs): the H19/IGF2 (ICR1) and KCNQ1/CDKN1C (ICR2) domains.107 Russell–Silver Syndrome (RSS, a disorder of impaired growth) is associated with hypomethylation of ICR1 and hypermethylation of ICR2. Beckwith-Wiedemann Syndrome (BWS, an over-growth disorder) is associated with hypermethylation of ICR1 and hypomethylation of ICR2.108 Some studies indicate that patients with RSS and BWS exhibit abnormal methylation at multiple gene loci.109 Differences in methylation at these loci have also been associated with less extreme growth-related phenotypes. In a study of 50 French-Canadian mothers and infants, 31% of variance in birthweight was attributed jointly to differential IGF2/H19 methylation and genotype of a particular IGF2/H19 polymorphism (rs2107425).91 The direction of association between methylation and birthweight, however, varies by study and tissue analysed.90,91 For example, hypomethylation at IGF2 DMRs have been associated with both increased and decreased birthweight.52,83,90,95,110 Some studies have found no association with birthweight.111 Further examples of the complex relationship between DNA methylation at various IGF2/H19 DMRs and infant growth phenotypes are detailed in Supplementary Table 2, available as Supplementary data at IJE online.
The paternally expressed imprinted gene MEST acts as an inhibitor of human adipogenesis and is involved in skeletal muscle growth and development.112 In placenta, increased methylation at the MEST transcription start site is correlated with reduced gene expression and IUGR.93,113 Increased methylation at the paternally expressed PLAGL1, which codes for a cell growth suppressor protein, is associated with higher birthweight and weight at 1 year of age.40
Some studies have associated other (non-imprinted) genes with birth size. For example, small-for-gestational age newborns had higher methylation at LEP in cord blood than appropriate-for-gestational age infants.94 Methylation at CpGs within HSD11B2, which codes for the enzyme responsible for catalyzing the conversion of cortisol to inactive cortisone, has been inversely related to newborn ponderal index in a cohort study.62
A small number of studies have investigated links between maternal nutrition, DNA methylation and newborn size. One study found that higher maternal erythrocyte folate levels in the first trimester were associated with decreased methylation in cord blood at MEG3, PLAGL1 and PEG3, and increased methylation at IGF2.56 Folate concentration and methylation at five DMRs were positively associated with birthweight. The authors hypothesiszed that the association of folate with birthweight could be mediated by differential methylation at MEG3, H19 and PLAGL1, with MEG3 contributing the strongest effect. Another cohort study found that higher maternal plasma glucose and omega-6 PUFA concentrations in the third trimester were associated with increased infant methylation at IGDCC4 and CACNA1G, and decreased methylation at MIRLET7BHG. These methylation patterns were all associated with higher birthweight.66
Adiposity: a case-control study in Germany found that obese adults (BMI >35 kg/m2) demonstrated lower methylation at MEST than in controls (BMI <25 kg/m2), and used a separate dataset to suggest that such outcomes may be partially caused by intrauterine exposure to gestational diabetes mellitus.114 In obese boys from the USA, an inverse association was reported between LEP methylation in buccal DNA and BMI, waist circumference (as z-scores) and percentage body fat.99NR3C1 Exon 1 C methylation has been positively associated with waist circumference and BMI at age 40 years,62 and increased IGF2/H19 methylation has been associated with increased skinfold thickness and subcutaneous adiposity at age 17 years.97
A number of studies have investigated maternal nutritional exposure, DNA methylation and child adiposity. POMC codes for melanocyte-stimulating hormone (MSH) and is involved with leptin in the regulation of body weight. POMC is an ME, and children conceived in the dry season in The Gambia had lower DNA methylation at a POMC variably methylated region (VMR) compared with those conceived in the rainy season.43POMC VMR methylation influences POMC expression,96 and methylation at this locus in blood and MSH-positive neurons is associated with BMI and obesity in children and adults.43 Godfrey et al. (2011) found that lower carbohydrate intake during early pregnancy was associated with increased umbilical cord tissue methylation at RXRA, which in turn was associated with greater adiposity in the offspring at 9 years of age.55
Skeletal growth and bone quality: RXRA forms heterodimers with vitamin D (and other nuclear) receptors, facilitating their role in the regulation of bone metabolism.115,116 Differential methylation of specific CpGs in RXRA in cord blood DNA has been inversely associated with percentage bone mineral content and bone mineral content adjusted for body size, measured at age 4 years, and also with maternal free 25(OH)-vitamin D index.75
Cardiometabolic outcomes
Maternal nutritional status during pregnancy and factors influencing fetal growth have been implicated in the aetiology of cardiometabolic outcomes such as dyslipidaemia, hypertension, type 2 diabetes (T2D) and cardiovascular disease later in life.117,118
Leptin has been studied extensively in the domain of cardiometabolic outcomes, owing to its role in metabolism and regulation of body weight.119LEP methylation at a specific CpG in blood and subcutaneous adipose tissue has been positively associated with low-density lipoprotein cholesterol levels in very obese (BMI >40 kg/m2) adults.98 In the same study, methylation at the LEP promoter was inversely correlated with BMI.98 A different study found an inverse relationship between LEP methylation in whole blood and high-density lipoprotein cholesterol levels in 17-month-old infants.101 Furthermore, lower methylation in CpGs near the LEP transcription start site has been observed in adolescents with obesity and insulin resistance, although not with obesity alone.100IGF2 methylation has also been related to lipid profile in obese children aged 11 years; those with intermediate methylation at the IGF2 P3 promoter had higher triglycerides (TG) and a higher TG:high-density lipoprotein cholesterol ratio than those with hypomethylation.102HSD2 methylation has been positively associated with systolic blood pressure,62 and NR3C1 exon1F and H19 ICR methylation also show positive associations with both systolic and diastolic blood pressures in adults.62 Note that adiposity and obesity (reviewed above) are also important risk factors that, alongside other markers, can signal increased risk of adverse cardiometabolic outcomes.120
Cognitive outcomes
The glucocorticoid receptors modulate the action of glucocorticoids and are involved in brain development and function.121NR3C1 and HSD11B2 genes regulate the action of cortisol and have been well studied in relation to neurobehaviour. Increased methylation at the NR3C1 promoter and decreased methylation in HSD11B2 in placental and infant buccal cell DNA have been associated with a high-risk neurobehavioural profile characterized by poor attention, high excitability, low quality of movement and signs of stress.103,104 An increase in LEP methylation in placental DNA has been associated with an increased risk of lethargy and hypotonia among male infants.105 Increased methylation at IGF2 in cord blood has been associated with early onset persistent attention-deficit/ hyperactivity disorder (ADHD) in children between 7 and 13 years of age.63
Candidate gene data summary
In Table 4 we provide further details of the 45 ‘candidate genes’ highlighted so far in this review. This includes information on their genomic location, the studies that considered them, regions of interest (ROIs) analysed and the coverage of ROIs on Illumina Infinium Methylation beadchip arrays.
Discussion
In this review we have described evidence in humans linking maternal nutrition during pregnancy with DNA methylation in the offspring, and linking DNA methylation at nutrition-sensitive loci to phenotypes at birth and outcomes in later life. As with all reviews, publication bias can mean that null findings may have been under-reported, and studies that do report associations may sometimes rely on post hoc subgroup analyses for significant findings. There are also numerous challenges specific to both the design and interpretation of intergenerational nutritional epigenetics studies which we discuss in the following sections.
Measuring nutritional exposures
Methods for measuring maternal nutritional exposure have limitations. For example, one of the most commonly used methods for this purpose are food frequency questionnaires, which suffer from recall bias and have differing validity by micronutrient.123 Weighed records require accurate, context-specific dietary databases and well-trained data collectors, and may not accurately reflect normal eating habits.124 However, these two approaches have the advantage of capturing food groups and combinations of nutrients that more direct tissue nutritional biomarkers can overlook.125 Plasma biomarkers are challenging to interpret, given that they represent nutrient levels after absorption and through interaction with genotype, and are not simple reflections of dietary intake. Concentrations do not capture metabolite flux, and can be misleadingly low if tissue uptake is rapid. Of particular relevance to maternal gestational samples is the effect of haemodilution, which can lower several biomarker concentrations.126 Maternal plasma nutrient concentrations are assumed to reflect dietary intake, and to correlate with cord blood concentrations and nutrient levels in fetal tissue, which may not be the case. Whereas positive correlations between maternal serum and cord blood serum are found for homocysteine, betaine, folate and B12, cord blood levels are multiple times higher, suggesting that these nutrients are homeostatically controlled to ensure fetal supply.127 In the context of periconceptional studies, more research is needed on which accessible tissues best represent the nutritional milieu surrounding the developing embryo in the initial days after fertilization. In the meantime, serum or plasma levels, though imperfect, are likely to offer a more accurate representation of fetal nutrient exposure than dietary intake methods.
Most of the attention on nutritional exposures has focused on the provision of methyl groups and the necessary co-factors for DNA methylation. However, the periconceptional period is marked by an initial wave of demethylation to erase parental epigenetic marks, before the process of remethylation.27 It is therefore important to consider the role nutrition could play in influencing demethylation. In demethylation, 5-methylcytosine is sequentially oxidized to 5-hydroxymethylcytosine and 5-formylcytosine (5fC) by 10-11 translocation (TET) dioxygenases that use vitamin C (ascorbate) as a co-factor.128 5fC can then either be further oxidized to 5-carboxylcytosine or converted to an unmethylated cytosine by base excision repair. Adding vitamin C to mouse or human embryonic stem cells in vitro increases the activity of TET enzymes, resulting in active demethylation in the germline.129 However, to our knowledge there have been no human in vivo studies exploring effects of periconceptional vitamin C deficiency on offspring DNA methylation.
Nutritional compounds do not act in isolation, and ideally analyses should recognize this by considering their interactions in metabolic pathways. For example, one-carbon metabolism is governed by intricately controlled feedback loops which help protect the flux of metabolites, through key reactions over a range of nutrient and co-factor concentrations.130,131 This means that associations between individual micronutrients and methylation (e.g. the commonly analysed methyl donors folate and betaine) can disappear after adjustment for other metabolites (e.g. SAM and DMG, which can inhibit transmethylation reaction rates). Advances in measurement technology that allow the measurement of a greater range of nutritional biomarkers (e.g. metabolomics), combined with more sophisticated analytical techniques,132,133 should enable a more nuanced understanding of the ways in which nutritional biomarkers combine to jointly influence methylation.
Measuring DNA methylation
A single CpG site in a single cell is either methylated or unmethylated, but measurements are typically made at the tissue level where methylation is a quantitative measure corresponding to the proportion of methylated cells.134 Accurate assessment of tissue-level DNA methylation patterns presents a challenge, given the sensitivity of the measurements to both technical and biological variation. The advent of high-throughput, genome-wide microarray platforms, such as the Illumina HumanMethylation 450 K and EPIC arrays,135–137 has helped in this regard, first by helping to standardize aspects of epigenome-wide association study (EWAS) design, and second by reducing the cost of genome-wide methylation assays required for adequately powered large studies.
Microarray-based EWAS have a number of limitations. First, by design, only a small proportion of the methylome is interrogated. These platforms attempt to include CpGs sites from all annotated genes, but the number of CpG sites per gene is low and equal coverage is typically not given to all genomic features and/or CpG contexts, with the focus having traditionally been on sites in promoters and CpG islands. Second, arrays provide no information on sequence-level variation, which is known to influence methylation status.138,139 Finally, bioinformatics and analytical expertise are required (as well as the necessary computational resources) to process and model the data, and to correct for batch and other technical effects, in order to obtain reliable, high-quality methylation profiles.140 As an alternative, true genome-wide approaches such as whole-genome bisulphite sequencing (WGBS) are available which interrogate all ∼28 million CpG sites in the methylome, although this is currently prohibitively expensive for larger samples. Targeted high-resolution platforms141,142 offer a potential compromise between coverage and cost, but their utility, convenience and cost-effectiveness for performing EWAS remain to be established. Given the importance of demethylation during periconceptional epigenetic remodelling, it may also be important to consider the oxidized forms of 5-methyl cytosine (e.g. 5-hydroxymethylcytosine) which occur as intermediate products in the demethylation pathway.143
Tissue specificity, confounding and stability of methylation across the life course
The tissue-specific nature of DNA methylation presents a major challenge for epigenetic association studies.134,144 The majority of studies reported in this review are constrained to accessible tissues such as cord blood that may be unrelated to the phenotype of interest, and different tissues may be sensitive to different environmental exposures. In this case reference epigenomes from different tissues and cell types in both healthy and diseased individuals145 may inform the choice of tissue as well as providing data for investigating the tissue specificity of identified signals. Where exposure-related effects occur during early embryonic development, before gastrulation, methylation changes may be concordant across multiple tissues,146 so that methylation states in accessible tissues such as blood and buccal cells may serve as a proxy for methylation in the target tissue.
Furthermore, numerous biological factors may act as potential confounders, for example age, sex, smoking status and BMI. Tissue-specific methylation differences arising from cell type heterogeneity, notably in blood, can also act as confounders,147 although there are well-established methods that can be used to correct for this.147,148
DNA sequence polymorphisms are also known to influence DNA methylation status and may confound observed associations.149 Heritability of DNA methylation is estimated to be in the range of 18% to 37%.150,151 Consistent with this, many studies have shown that methylation quantitative trait loci (mQTL)—genetic variants associated with methylation differences at the population level—are widespread. To account for this, ideally high-throughput genotype data on the sample being studied should be used149 but, if such data are unavailable, population-level reference mQTL data can be informative.139
Finally, methylation changes associated with an early-life exposure may change throughout the life course, with implications for their utility as biomarkers of exposure or predictors of later phenotype.152–154 Depending on the research question, this may suggest the need to assess long-term stability of methylation at specific loci, through the collection of longitudinal samples.
Linking methylation changes to gene function
Many of the DNA methylation changes reported in studies covered in this review are small, often within the margins of error of the measuring technology, making it difficult to draw conclusions on their functional relevance.155 Indeed, relatively few methylation studies measure gene expression. The link between DNA methylation and expression is complex, depending on genomic context (e.g. location with gene bodies, promoters and enhancers).156 This could in part explain seemingly contradictory findings from different studies measuring associations at the same gene. Moreover, a change in methylation may influence transcription factor binding and the induction of a specific signalling pathway in order to observe a change in gene expression. To aid further understanding, future studies should therefore consider measuring transcription factor binding, markers of gene transcription (mRNA levels), and/or translation (protein levels), to better map the potential effects of DNA methylation differences on gene function.157
Capturing phenotypes
In this review we have focused on phenotypic outcomes most commonly considered in the DOHaD context. However, we do not wish to exclude the possibility that there may be a broader range of phenotypes that are implicated. For example, exposure to the Dutch Hunger Winter famine during pregnancy has been associated with a wide variety of offspring phenotypes, varying according to the timing of famine exposure during gestation.45,47 Consideration of the ‘thrifty epigenotype’ hypothesis24 would suggest that famine-imposed epigenetic modifications in early life are adaptive where similar environment conditions persist, but maladaptive otherwise. There could therefore be a spectrum of phenotypes according to how great the mismatch is between in utero and later life environments. In the case of complex traits such as obesity, the resultant phenotype may also be influenced by factors such as diet and lifestyle in conjunction with methylation differences and genotype of the individual.158
Causal inference
A major goal of nutritional epigenetic studies, also covered in this review, is to assess the potential for epigenetic marks to mediate links between nutritional exposures and health outcomes. In this context, the use of prospective study designs with randomization including negative controls, and techniques such as mediation analysis based on regression systems,159 structural equation modelling160 or network-based techniques,161 parametric/semi-parametric methods,162 or instrumental variable approaches such as Mendelian randomization,80,163,164 can help to strengthen causal inference. More broadly, triangulating findings from diverse studies, each with their own strengths, limitations, assumptions and opposing biases, will maximize the potential for robust findings.165,166
Study design considerations
The literature in this area is dominated by observational studies. This increases the risk of spurious associations due to confounding or reverse causation,149 the latter being a particular problem with methylation association studies where the direction of causality can be hard to establish. Added to this, effect sizes are generally modest, with group-level differences in mean methylation typically less than 10% and often in the region of 1–5% for many of the exposures and phenotypes studied.155,167,168 This has implications for the design of studies characterizing genome-wide, population-level methylation differences, as they need to be adequately powered to detect potentially small effects after adjusting for multiple testing.169
Current interest in periconceptional nutrition has stimulated a number of preconceptional nutrition trials.170–174 In these studies, supplementation before conception is necessary to ensure that the conception period is covered and that a maximal effect on maternal nutritional status at conception is achieved. Nonetheless, accurately pinpointing the timing of nutritional exposures to conception is challenging.
Conclusions
The body of evidence linking maternal nutritional exposure to offspring phenotype via DNA methylation in humans is rapidly growing yet currently remains complex and inconsistent. It is characterized by heterogeneous exposures and outcomes, and mainly observational associations that are frequently under-powered. Existing evidence suggests that the effect of nutritional exposures on DNA methylation depends on the form of the nutritional component, the timing of exposure during periconception and pregnancy, the underlying nutritional status of the mother, maternal and offspring genotype and the specific loci under investigation. The picture is more complex than methylation being determined simply by availability of methyl donors. Many studies have investigated imprinted genes as priority loci for their vulnerability to nutritional exposures, but with the adoption of microarray-based platforms, other candidate genes and gene classes are emerging, for example metastable epialleles.
The utility of this emerging evidence in terms of its translation into effective interventions and therapies remains an open question. Epigenetic marks like DNA methylation may act as integrators of multiple exposures and genetic risk factors, as well as molecular mediators of the effect of exposures on phenotype. Where robust associations are established, DNA methylation can serve as a proxy measure or biomarker of earlier nutritional exposures.175 As mediators of the effect on later phenotype, nutritionally sensitive DNA methylation changes can provide a means to identify genes and pathways for targeted interventions. Whereas there is still much work to do in this area, there are grounds for optimism that epigenomic approaches will provide insights into the molecular basis of the developmental origins of health and disease, which could in turn lead to the development of next-generation interventions.
Funding
This work was supported by the Newton Fund initiative, jointly funded by the Medical Research Council [MR/N006208/1], the Department for International Development, UK, and the Department of Biotechnology, Ministry of Science and Technology, Government of India [BT/IN/DBT-MRC/ DFID/24/GRC/2015–16]. The funding bodies played no role in the design of the review, data collection, analysis, interpretation of data or writing the manuscript.
The EMPHASIS study group includes
Lena Acolatse, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, The Gambia, Meraj Ahmed, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Modupeh Betts, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, The Gambia, Giriraj R Chandak, CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Harsha Chopra, Centre for the Study of Social Change, Mumbai, India, Cyrus Cooper, MRC Life Course Epidemiology Unit, University of Southampton, UK, Momodou K Darboe, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, The Gambia, Chiara Di Gravio, MRC Life Course Epidemiology Unit, University of Southampton, UK, Caroline HD Fall, MRC Life Course Epidemiology Unit, University of Southampton, UK, Meera Gandhi, Centre for the Study of Social Change, Mumbai, India, Gail R Goldberg, MRC Elsie Widdowson Laboratory, Cambridge, UK, Prachand Issarapu, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Philip James, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, UK, Ramatoulie Janha, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, The Gambia, Landing M A Jarjou, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, The Gambia, Lovejeet Kaur, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Sarah H Kehoe, MRC Life Course Epidemiology Unit, University of Southampton, UK, Kalyanaraman Kumaran, MRC Life Course Epidemiology Unit, University of Southampton, UK and CSI Holdsworth Memorial Hospital, Mysore, India, Karen A Lillycrop, University of Southampton, UK, Mohammed Ngum, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, The Gambia, Suraj S Nongmaithem, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Stephen Owens, Institute of Health and Society, Newcastle University, UK, Ramesh D Potdar, Centre for the Study of Social Change, Mumbai, India, Andrew M Prentice, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, The Gambia, Ann Prentice, MRC Unit The Gambia, Elsie Widdowson Laboratory, Cambridge, UK and MRC Life Course Epidemiology Unit, University of Southampton, UK, Tallapragada Divya Sri Priyanka, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Ayden Saffari, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, UK, Sirazul Ameen Sahariah, Centre for the Study of Social Change, Mumbai, India, Sara Sajjadi, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Harshad Sane, Centre for the Study of Social Change, Mumbai, India, Smeeta Shrestha, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Matt J Silver, MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, UK, Ashutosh Singh Tomar, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Kate A Ward, MRC Elsie Widdowson Laboratory, Cambridge and MRC Life course Epidemiology Unit, University of Southampton, UK, Dilip Kumar Yadav, Genomic Research on Complex diseases (GRC Group), CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India, Chittaranjan S Yajnik, Diabetes Unit, KEM Hospital and Research Centre, Pune, India.
Conflict of interest: None declared.
Supplementary Material
Contributor Information
EMPHASIS study group:
Lena Acolatse, Meraj Ahmed, Modupeh Betts, Giriraj R Chandak, Harsha Chopra, Cyrus Cooper, Momodou K Darboe, Chiara Di Gravio, Caroline H D Fall, Meera Gandhi, Gail R Goldberg, Prachand Issarapu, Philip James, Ramatoulie Janha, Landing M A Jarjou, Lovejeet Kaur, Sarah H Kehoe, Kalyanaraman Kumaran, Karen A Lillycrop, Mohammed Ngum, Suraj S Nongmaithem, Stephen Owens, Ramesh D Potdar, Andrew M Prentice, Ann Prentice, Tallapragada Divya Sri Priyanka, Ayden Saffari, Sirazul Ameen Sahariah, Sara Sajjadi, Harshad Sane, Smeeta Shrestha, Matt J Silver, Ashutosh Singh Tomar, Kate A Ward, Dilip Kumar Yadav, and Chittaranjan S Yajnik
References
- 1. Jaenisch R, Bird A.. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 2003;33(Suppl):245–54. [DOI] [PubMed] [Google Scholar]
- 2. Egger G, Liang G, Aparicio A, Jones PA.. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004;429:457–63. [DOI] [PubMed] [Google Scholar]
- 3. Esteller M. Epigenetics in cancer. N Engl J Med 2008;358:1148–59. [DOI] [PubMed] [Google Scholar]
- 4. Moore GE, Ishida M, Demetriou C. et al. The role and interaction of imprinted genes in human fetal growth. Philos Trans R Soc B Biol Sci 2015;370:20140074.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernstein BE, Meissner A, Lander ES.. The mammalian epigenome. Cell 2007;128:669–81. [DOI] [PubMed] [Google Scholar]
- 6. Waterland RA, Michels KB.. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr 2007;27:363–88. [DOI] [PubMed] [Google Scholar]
- 7. Burdge GC, Lillycrop KA.. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr 2010;30:315–39. [DOI] [PubMed] [Google Scholar]
- 8. Lillycrop KA, Burdge GC.. Epigenetic mechanisms linking early nutrition to long term health. Best Pract Res Clin Endocrinol Metab 2012;26:667–76. [DOI] [PubMed] [Google Scholar]
- 9. Waterland RA, Jirtle RL.. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 2003;23:5293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients 2014;6:2165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Messerschmidt DM, Knowles BB, Solter D.. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev 2014;28:812–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Leung FCC.. An evaluation of new criteria for CpG islands in the human genome as gene markers. Bioinformatics 2004;20:1170–77. [DOI] [PubMed] [Google Scholar]
- 13. Klose RJ, Bird AP.. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 2006;31:89–97. [DOI] [PubMed] [Google Scholar]
- 14. Illingworth RS, Bird AP.. CpG islands—‘a rough guide’. FEBS Lett 2009;583:1713–20. [DOI] [PubMed] [Google Scholar]
- 15. Guil S, Esteller M.. DNA methylomes, histone codes and miRNAs: tying it all together. Int J Biochem Cell Biol 2009;41:87–95. [DOI] [PubMed] [Google Scholar]
- 16. Sato F, Tsuchiya S, Meltzer SJ, Shimizu K.. MicroRNAs and epigenetics. FEBS J 2011;278:1598–609. [DOI] [PubMed] [Google Scholar]
- 17. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME.. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989;298:564–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJP.. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 1999;318:427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barker DJP, Osmond C, Kajantie E, Eriksson JG.. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol 2009;36:445–58. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Ley SH, Tobias DK. et al. Birth weight and later life adherence to unhealthy lifestyles in predicting type 2 diabetes: prospective cohort study. BMJ 2015;351:h3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roseboom TJ, van der Meulen JH, van Montfrans GA. et al. Maternal nutrition during gestation and blood pressure in later life. J Hypertens 2001;19:29–34. [DOI] [PubMed] [Google Scholar]
- 22. Ravelli GP, Stein ZA, Susser MW.. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976;295:349–53. [DOI] [PubMed] [Google Scholar]
- 23. Susser E, Neugebauer R, Hoek HW. et al. Schizophrenia after prenatal famine. Arch Gen Psychiatry 1996;53:25–31. [DOI] [PubMed] [Google Scholar]
- 24. Stöger R. The thrifty epigenotype: an acquired and heritable predisposition for obesity and diabetes? BioEssays 2008;30:156–66. [DOI] [PubMed] [Google Scholar]
- 25. Langley-Evans SC. Nutrition in early life and the programming of adult disease: a review. J Hum Nutr Diet 2015;28:1–14. [DOI] [PubMed] [Google Scholar]
- 26. Steegers-Theunissen RPM, Twigt J, Pestinger V, Sinclair KD.. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update 2013;19:640–55. [DOI] [PubMed] [Google Scholar]
- 27. Smallwood SA, Kelsey G.. De novo DNA methylation: a germ cell perspective. Trends Genet 2012;28:33–42. [DOI] [PubMed] [Google Scholar]
- 28. Ishida M, Moore GE.. The role of imprinted genes in humans. Mol Aspects Med 2013;34:826–40. [DOI] [PubMed] [Google Scholar]
- 29. Rakyan VK, Blewitt ME, Druker R, Preis JI, Whitelaw E.. Metastable epialleles in mammals. Trends Genet 2002;18:348–51. [DOI] [PubMed] [Google Scholar]
- 30. Waterland RA, Kellermayer R, Laritsky E. et al. Season of conception in rural Gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet 2010;6:e1001252.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dominguez-Salas P, Moore SE, Baker MS. et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun 2014;5:3746.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hancks DC, Kazazian HH.. Active human retrotransposons: variation and disease. Curr Opin Genet Dev 2012;22:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slotkin RK, Martienssen R.. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet 2007;8:272–85. [DOI] [PubMed] [Google Scholar]
- 34. Perera F, Herbstman J.. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol 2011;31:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reul JMHM, Collins A, Saliba RS. et al. Glucocorticoids, epigenetic control and stress resilience. Neurobiol Stress 2015;1:44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fowden AL, Forhead AJ.. Hormones as epigenetic signals in developmental programming. Exp Physiol 2009;94:607–25. [DOI] [PubMed] [Google Scholar]
- 37. Wolff GL, Kodell RL, Moore SR, Cooney CA.. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 1998;12:949–57. [PubMed] [Google Scholar]
- 38. Jiménez-Chillarón JC, Díaz R, Martínez D. et al. The role of nutrition on epigenetic modifications and their implications on health. Biochimie 2012;94:2242–63. [DOI] [PubMed] [Google Scholar]
- 39. Soubry A. Epigenetic inheritance and evolution: a paternal perspective on dietary influences. Prog Biophys Mol Biol 2015;118:79–85. [DOI] [PubMed] [Google Scholar]
- 40. Azzi S, Sas TCJ, Koudou Y. et al. Degree of methylation of ZAC1 (PLAGL1) is associated with prenatal and post-natal growth in healthy infants of the EDEN mother child cohort. Epigenetics 2014;9:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silver MJ, Kessler NJ, Hennig BJ. et al. Independent genomewide screens identify the tumor suppressor VTRNA2-1 as a human epiallele responsive to periconceptional environment. Genome Biol 2015;16:118.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pauwels S, Ghosh M, Duca RC. et al. Dietary and supplemental maternal methyl-group donor intake and cord blood DNA methylation. Epigenetics 2017;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kühnen P, Handke D, Waterland RA. et al. Interindividual variation in DNA methylation at a putative POMC metastable epiallele is associated with obesity. Cell Metab 2016;24:502–09. [DOI] [PubMed] [Google Scholar]
- 44. Pauwels S, Ghosh M, Duca RC. et al. Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants. Clin Epigenetics 2017;9:16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heijmans BT, Tobi EW, Stein AD. et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 2008;105:17046–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tobi EW, Slagboom PE, van Dongen J. et al. Prenatal famine and genetic variation are independently and additively associated with DNA methylation at regulatory loci within IGF2/H19. PLoS One 2012;7:e37933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tobi EW, Lumey LH, Talens RP. et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet 2009;18:4046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tobi EW, Slieker RC, Stein AD. et al. Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. Int J Epidemiol 2015;44:1211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Finer S, Iqbal MS, Lowe R. et al. Is famine exposure during developmental life in rural Bangladesh associated with a metabolic and epigenetic signature in young adulthood? A historical cohort study. BMJ Open 2016;6:e011768.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gonseth S, Roy R, Houseman EA. et al. Periconceptional folate consumption is associated with neonatal DNA methylation modifications in neural crest regulatory and cancer development genes. Epigenetics 2015;10:1166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoyo C, Murtha AP, Schildkraut JM. et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 2011;6:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Steegers-Theunissen RP, Obermann-Borst SA, Kremer D. et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One 2009;4:e7845.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cooper WN, Khulan B, Owens S. et al. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J 2012;26:1782–90. [DOI] [PubMed] [Google Scholar]
- 54. McCullough LE, Miller EE, Mendez MA, Murtha AP, Murphy SK, Hoyo C.. Maternal B vitamins: effects on offspring weight and DNA methylation at genomically imprinted domains. Clin Epigenetics 2016;8:8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Godfrey KM, Sheppard A, Gluckman PD. et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes 2011;60:1528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoyo C, Daltveit AK, Iversen E. et al. Erythrocyte folate concentrations, CpG methylation at genomically imprinted domains, and birth weight in a multiethnic newborn cohort. Epigenetics 2014;9:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Mil NH, Bouwland-Both MI, Stolk L. et al. Determinants of maternal pregnancy one-carbon metabolism and newborn human DNA methylation profiles. Reproduction 2014;148:581–92. [DOI] [PubMed] [Google Scholar]
- 58. Haggarty P, Hoad G, Campbell DM, Horgan GW, Piyathilake C, McNeill G.. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am J Clin Nutr 2013;97:94–99. [DOI] [PubMed] [Google Scholar]
- 59. Ba Y, Yu H, Liu F. et al. Relationship of folate, vitamin B12 and methylation of insulin-like growth factor-II in maternal and cord blood. Eur J Clin Nutr 2011;65:480–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jiang X, Yan J, West AA. et al. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J 2012;26:3563–74. [DOI] [PubMed] [Google Scholar]
- 61. Amarasekera M, Martino D, Ashley S. et al. Genome-wide DNA methylation profiling identifies a folate-sensitive region of differential methylation upstream of ZFP57-imprinting regulator in humans. FASEB J 2014;28:4068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Drake AJ, McPherson RC, Godfrey KM. et al. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin Endocrinol 2012;77:808–15. [DOI] [PubMed] [Google Scholar]
- 63. Rijlaarsdam J, Cecil CAM, Walton E. et al. Prenatal unhealthy diet, insulin-like growth factor 2 gene (IGF2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. J Child Psychol Psychiatr 2017;58:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee H-S, Barraza-Villarreal A, Biessy C. et al. Dietary supplementation with polyunsaturated fatty acid during pregnancy modulates DNA methylation at IGF2/H19 imprinted genes and growth of infants. Physiol Genomics 2014;46:851–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dijk SJ. V, Zhou J, Peters TJ. et al. Effect of prenatal DHA supplementation on the infant epigenome: results from a randomized controlled trial. Clin Epigenetics 2016;8:114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lin X, Lim IY, Wu Y. et al. Developmental pathways to adiposity begin before birth and are influenced by genotype, prenatal environment and epigenome. BMC Med 2017;15:50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McKay JA, Groom A, Potter C. et al. Genetic and non-genetic influences during pregnancy on infant global and site specific DNA methylation: role for folate gene variants and vitamin B12. PLoS One 2012;7:e33290.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Joubert BR, Dekker HT. D, Felix JF. et al. Maternal plasma folate impacts differential DNA methylation in an epigenome-wide meta-analysis of newborns. Nat Commun 2016;7:10577.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee H-S, Barraza-Villarreal A, Hernandez-Vargas H. et al. Modulation of DNA methylation states and infant immune system by dietary supplementation with ω-3 PUFA during pregnancy in an intervention study. Am J Clin Nutr 2013;98:480–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Amarasekera M, Noakes P, Strickland D, Saffery R, Martino DJ, Prescott SL.. Epigenome-wide analysis of neonatal CD4 + T-cell DNA methylation sites potentially affected by maternal fish oil supplementation. Epigenetics 2014;9:1570–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lumey LH, Stein AD, Kahn HS. et al. Cohort Profile: The Dutch Hunger Winter families study. Int J Epidemiol 2007;36:1196–204. [DOI] [PubMed] [Google Scholar]
- 72. Prentice A, Whitehead R, Roberts S, Paul A.. Long-term energy balance in child-bearing Gambian women. Am J Clin Nutr 1981;34:2790–99. [DOI] [PubMed] [Google Scholar]
- 73. Fang M, Chen D, Yang CS.. Dietary polyphenols may affect DNA methylation. J Nutr 2007;137(Suppl 1):223–28S. [DOI] [PubMed] [Google Scholar]
- 74. Pereira F, Barbáchano A, Singh PK, Campbell MJ, Muñoz A, Larriba MJ.. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle 2012;11:1081–89. [DOI] [PubMed] [Google Scholar]
- 75. Harvey NC, Sheppard A, Godfrey KM. et al. Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth. J Bone Miner Res 2014;29:600–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Feng Y, Zhao L-Z, Hong L, Shan C, Shi W, Cai W.. Alteration in methylation pattern of GATA-4 promoter region in vitamin A-deficient offspring’s heart. J Nutr Biochem 2013;24:1373–80. [DOI] [PubMed] [Google Scholar]
- 77. Babenko O, Kovalchuk I, Metz GAS.. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev 2015;48:70–91. [DOI] [PubMed] [Google Scholar]
- 78. Anway MD, Skinner MK.. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 2006;147(Suppl 6):S43–49. [DOI] [PubMed] [Google Scholar]
- 79. Hajj NE, Schneider E, Lehnen H, Haaf T.. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 2014;148:R111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sharp GC, Lawlor DA, Richmond RC. et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2015;44:1288–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Azzi S, Brioude F, Bouc YL, Netchine I.. Human imprinting anomalies in fetal and childhood growth disorders: clinical implications and molecular mechanisms. Curr Pharm Des 2014;20:1751–63. [DOI] [PubMed] [Google Scholar]
- 82. Burris HH, Baccarelli AA, Byun H-M. et al. Offspring DNA methylation of the aryl-hydrocarbon receptor repressor gene is associated with maternal BMI, gestational age, and birth weight. Epigenetics 2015;10:913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bouwland-Both MI, van Mil NH, Stolk L. et al. DNA methylation of IGF2DMR and H19 is associated with fetal and infant growth: the generation R study. PLoS One 2013;8:e81731.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Einstein F, Thompson RF, Bhagat TD. et al. Cytosine methylation dysregulation in neonates following intrauterine growth restriction. PLoS One 2010;5:e8887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Toure DM, Baccaglini L, Opoku ST. et al. Epigenetic dysregulation of insulin-like growth factor (IGF)-related genes and adverse pregnancy outcomes: a systematic review. J Matern Fetal Neonatal Med 2016;18:1–11. [DOI] [PubMed] [Google Scholar]
- 86. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 2003;133(Suppl 7):2485–93S. [DOI] [PubMed] [Google Scholar]
- 87. Claycombe KJ, Brissette CA, Ghribi O.. Epigenetics of inflammation, maternal infection, and nutrition. J Nutr 2015;145:1109–15S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA.. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr 2007;97:1036.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kucharski R, Maleszka J, Foret S, Maleszka R.. Nutritional control of reproductive status in honeybees via DNA methylation. Science 2008;319:1827.. [DOI] [PubMed] [Google Scholar]
- 90. Hoyo C, Fortner K, Murtha AP. et al. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control 2012;23:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. St-Pierre J, Hivert M-F, Perron P. et al. IGF2 DNA methylation is a modulator of newborn’s fetal growth and development. Epigenetics 2012;7:1125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Qian Y-Y, Huang X-L, Liang H. et al. Effects of maternal folic acid supplementation on gene methylation and being small for gestational age. J Hum Nutr Diet 2016;29:643–51. [DOI] [PubMed] [Google Scholar]
- 93. Kappil MA, Green BB, Armstrong DA. et al. Placental expression profile of imprinted genes impacts birth weight. Epigenetics 2015;10:842–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lesseur C, Armstrong DA, Paquette AG, Koestler DC, Padbury JF, Marsit CJ.. Tissue-specific leptin promoter DNA methylation is associated with maternal and infant perinatal factors. Mol Cell Endocrinol 2013;381:160–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Murphy R, Thompson JM, Tost J, Mitchell EA; Auckland Birthweight Collaborative Study Group. No evidence for copy number and methylation variation in H19 and KCNQ10T1 imprinting control regions in children born small for gestational age. BMC Med Genet 2014;15:67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kuehnen P, Mischke M, Wiegand S. et al. An Alu element-associated hypermethylation variant of the POMC gene is associated with childhood obesity. PLoS Genet 2012;8:e1002543.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Huang R-C, Galati JC, Burrows S. et al. DNA methylation of the IGF2/H19 imprinting control region and adiposity distribution in young adults. Clin Epigenetics 2012;4:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Houde A-A, Légaré C, Biron S. et al. Leptin and adiponectin DNA methylation levels in adipose tissues and blood cells are associated with BMI, waist girth and LDL-cholesterol levels in severely obese men and women. BMC Med Genet 2015;16:29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dunstan J, Bressler JP, Moran TH. et al. Associations of LEP, CRH, ICAM-1, and LINE-1 methylation, measured in saliva, with waist circumference, body mass index, and percent body fat in mid-childhood. Clin Epigenet 2017;9:29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. García-Cardona MC, Huang F, García-Vivas JM. et al. DNA methylation of leptin and adiponectin promoters in children is reduced by the combined presence of obesity and insulin resistance. Int J Obes 2014;38:1457–65. [DOI] [PubMed] [Google Scholar]
- 101. Wijnands KPJ, Obermann-Borst SA, Steegers-Theunissen RPM.. Early life lipid profile and metabolic programming in very young children. Nutr Metab Cardiovasc Dis 2015;25:608–14. [DOI] [PubMed] [Google Scholar]
- 102. Deodati A, Inzaghi E, Liguori A. et al. IGF2 methylation is associated with lipid profile in obese children. Horm Res Paediatr 2013;79:361–67. [DOI] [PubMed] [Google Scholar]
- 103. Paquette AG, Lester BM, Lesseur C. et al. Placental epigenetic patterning of glucocorticoid response genes is associated with infant neurodevelopment. Epigenomics 2015;7:767–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lester BM, Marsit CJ, Giarraputo J, Hawes K, LaGasse LL, Padbury JF.. Neurobehavior related to epigenetic differences in preterm infants. Epigenomics 2015;7:1123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lesseur C, Armstrong DA, Murphy MA. et al. Sex-specific associations between placental leptin promoter DNA methylation and infant neurobehavior. Psychoneuroendocrinology 2014;40:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Reik W, Walter J.. Genomic imprinting: parental influence on the genome. Nat Rev Genet 2001;2:21–32. [DOI] [PubMed] [Google Scholar]
- 107. Nordin M, Bergman D, Halje M, Engström W, Ward A.. Epigenetic regulation of the Igf2/H19 gene cluster. Cell Prolif 2014;47:189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Piedrahita JA. The role of imprinted genes in fetal growth abnormalities. Birth Defects Res Part A Clin Mol Teratol 2011;91:682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Azzi S, Rossignol S, Steunou V. et al. Multilocus methylation analysis in a large cohort of 11p15-related foetal growth disorders (Russell Silver and Beckwith Wiedemann syndromes) reveals simultaneous loss of methylation at paternal and maternal imprinted loci. Hum Mol Genet 2009;18:4724–33. [DOI] [PubMed] [Google Scholar]
- 110. Liu Y, Murphy SK, Murtha AP. et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics 2012;7:735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tobi EW, Heijmans BT, Kremer D. et al. DNA methylation of IGF2, GNASAS, INSIGF and LEP and being born small for gestational age. Epigenetics 2011;6:171–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Karbiener M, Glantschnig C, Pisani DF. et al. Mesoderm-specific transcript (MEST) is a negative regulator of human adipocyte differentiation. Int J Obes 2015;39:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. McMinn J, Wei M, Schupf N. et al. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta 2006;27:540–49. [DOI] [PubMed] [Google Scholar]
- 114. Hajj NE, Pliushch G, Schneider E. et al. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes 2013;62:1320–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ahuja HS, Szanto A, Nagy L, Davies PJA, The retinoid X receptor and its ligands: versatile regulators of metabolic function, cell differentiation and cell death. J Biol Regul Homeost Agents 2003;17:29–45. [PubMed] [Google Scholar]
- 116. Yee YK, Chintalacharuvu SR, Lu J, Nagpal S.. Vitamin D receptor modulators for inflammation and cancer. Mini Rev Med Chem 2005;5:761–78. [DOI] [PubMed] [Google Scholar]
- 117. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS.. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993;341:938–41. [DOI] [PubMed] [Google Scholar]
- 118. Barker DJP. Fetal growth and adult disease. Br J Obstet Gynaecol 1992;99:275–76. [DOI] [PubMed] [Google Scholar]
- 119. Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes 2007;14:17–22. [DOI] [PubMed] [Google Scholar]
- 120. Després J-P, Lemieux I, Bergeron J. et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008;28:1039. [DOI] [PubMed] [Google Scholar]
- 121. Harris A, Seckl J.. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 2011;59:279–89. [DOI] [PubMed] [Google Scholar]
- 122. Arányi T, Tusnády GE.. BiSearch: ePCR tool for native or bisulfite-treated genomic template. Methods Mol Biol 2007;402:385–402. [DOI] [PubMed] [Google Scholar]
- 123. Kroke A, Klipstein-Grobusch K, Voss S. et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr 1999;70:439–47. [DOI] [PubMed] [Google Scholar]
- 124. Black AE, Prentice AM, Goldberg GR. et al. Measurements of total energy expenditure provide insights into the validity of dietary measurements of energy intake. J Am Diet Assoc 1993;93:572–79. [DOI] [PubMed] [Google Scholar]
- 125. Biró G, Hulshof KFAM, Ovesen L, Amorim Cruz JA; EFCOSUM Group. Selection of methodology to assess food intake. Eur J Clin Nutr 2002;56(Suppl 2):S25–32. [DOI] [PubMed] [Google Scholar]
- 126. Faupel-Badger JM, Hsieh C-C, Troisi R, Lagiou P, Potischman N.. Plasma volume expansion in pregnancy: implications for biomarkers in population studies. Cancer Epidemiol Biomarkers Prev 2007;16:1720–23. [DOI] [PubMed] [Google Scholar]
- 127. Wallace JM, Bonham MP, Strain J. et al. Homocysteine concentration, related B vitamins, and betaine in pregnant women recruited to the Seychelles Child Development Study. Am J Clin Nutr 2008;87:391–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Young JI, Züchner S, Wang G.. Regulation of the epigenome by vitamin C. Annu Rev Nutr 2015;35:545–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Blaschke K, Ebata KT, Karimi MM. et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013;500:222–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Reed MC, Gamble MV, Hall MN, Nijhout HF.. Mathematical analysis of the regulation of competing methyltransferases. BMC Syst Biol 2015;9:69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nijhout HF, Best J, Reed MC.. Escape from homeostasis. Math Biosci 2014;257:104–10. [DOI] [PubMed] [Google Scholar]
- 132. Aurich MK, Thiele I.. Computational modeling of human metabolism and its application to systems biomedicine. Methods Mol Biol 2016;1386:253–81. [DOI] [PubMed] [Google Scholar]
- 133. Thiele I, Swainston N, Fleming RMT. et al. A community-driven global reconstruction of human metabolism. Nat Biotechnol 2013;31:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Michels KB, Binder AM, Dedeurwaerder S. et al. Recommendations for the design and analysis of epigenome-wide association studies. Nat Methods 2013;10:949–55. [DOI] [PubMed] [Google Scholar]
- 135. Bibikova M, Le J, Barnes B. et al. Genome-wide DNA methylation profiling using Infinium® assay. Epigenomics 2009;1:177–200. [DOI] [PubMed] [Google Scholar]
- 136. Bibikova M, Barnes B, Tsan C. et al. High density DNA methylation array with single CpG site resolution. Genomics 2011;98:288–95. [DOI] [PubMed] [Google Scholar]
- 137. Pidsley R, Zotenko E, Peters TJ. et al. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol 2016;17:208.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wagner JR, Busche S, Ge B, Kwan T, Pastinen T, Blanchette M.. The relationship between DNA methylation, genetic and expression inter-individual variation in untransformed human fibroblasts. Genome Biol 2014;15:R37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gaunt TR, Shihab HA, Hemani G. et al. Systematic identification of genetic influences on methylation across the human life course. Genome Biol 2016;17:61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Morris TJ, Beck S.. Analysis pipelines and packages for Infinium HumanMethylation450 BeadChip (450k) data. Methods 2015;72:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li Q, Suzuki M, Wendt J. et al. Post-conversion targeted capture of modified cytosines in mammalian and plant genomes. Nucleic Acids Res 2015;43:e81.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Teh AL, Pan H, Lin X. et al. Comparison of methyl-capture sequencing vs. infinium 450K methylation array for methylome analysis in clinical samples. Epigenetics 2016;11:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ulahannan N, Greally JM.. Genome-wide assays that identify and quantify modified cytosines in human disease studies. Epigenetics Chromatin 2015;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Roadmap Epigenomics Consortium: Kundaje A, Meuleman W, Ernst J. et al. Integrative analysis of 111 reference human epigenomes. Nature 2015;518:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bujold D, Morais DAL, Gauthier C. et al. The international human epigenome consortium data portal. Cell Syst 2016;3:496–99.e2. [DOI] [PubMed] [Google Scholar]
- 146. van Baak TE, Coarfa C, Dugué PA. et al. Epigenetic supersimilarity of monozygotic twin pairs. Genome Biol 2018;19:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jaffe AE, Irizarry RA.. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol 2014;15:R31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Houseman EA, Accomando WP, Koestler DC. et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 2012;13:86.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Birney E, Davey Smith G, Greally JM.. Epigenome-wide association studies and the interpretation of disease -omics. PLoS Genet 2016;12:e1006105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bell JT, Tsai PC, Yang TP. et al. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet 2012;8:e1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Grundberg E, Meduri E, Sandling JK. et al. Global analysis of DNA methylation variation in adipose tissue from twins reveals links to disease-associated variants in distal regulatory elements. Am J Hum Genet 2013;93:876–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Richmond RC, Simpkin AJ, Woodward G. et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the life course: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet 2015;24:2201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tsaprouni LG, Yang T-P, Bell J. et al. Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics 2014;9:1382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Richmond RC, Sharp GC, Ward ME. et al. DNA methylation and BMI: investigating identified methylation sites at HIF3A in a causal framework. Diabetes 2016;65:1231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Leenen FAD, Muller CP, Turner JD.. DNA methylation: conducting the orchestra from exposure to phenotype? Clin Epigenetics 2016;8:92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Schultz MD, He Y, Whitaker JW. et al. Human body epigenome maps reveal noncanonical DNA methylation variation. Nature 2015;523:212–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cheung WA, Shao X, Morin A. et al. Functional variation in allelic methylomes underscores a strong genetic contribution and reveals novel epigenetic alterations in the human epigenome. Genome Biol 2017;18:50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Campión J, Milagro FI, Martínez JA.. Individuality and epigenetics in obesity. Obes Rev 2009;10:383–92. [DOI] [PubMed] [Google Scholar]
- 159. Preacher KJ. Advances in mediation analysis: a survey and synthesis of new developments. Annu Rev Psychol 2015;66:825–52. [DOI] [PubMed] [Google Scholar]
- 160. Li R, Tsaih S-W, Shockley K. et al. Structural model analysis of multiple quantitative traits. PLoS Genet 2006;2:e114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Schadt EE, Lamb J, Yang X. et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet 2005;37:710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Millstein J, Zhang B, Zhu J, Schadt EE.. Disentangling molecular relationships with a causal inference test. BMC Genet 2009;10:23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Relton CL, Davey Smith G.. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol 2012;41:161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Yamada L, Chong S.. Epigenetic studies in Developmental Origins of Health and Disease: pitfalls and key considerations for study design and interpretation. J Dev Orig Health Dis 2017;8:30–43. [DOI] [PubMed] [Google Scholar]
- 165. Lin X, Barton S, Holbrook JD.. How to make DNA methylome wide association studies more powerful. Epigenomics 2016;8:1117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Munafò MR, Davey Smith G.. Repeating experiments is not enough. Nature 2018;553:399–401.29368721 [Google Scholar]
- 167. Heijmans BT, Mill J.. Commentary: the seven plagues of epigenetic epidemiology. Int J Epidemiol 2012;41:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Mill J, Heijmans BT.. From promises to practical strategies in epigenetic epidemiology. Nat Rev Genet 2013;14:585–94. [DOI] [PubMed] [Google Scholar]
- 169. Saffari A, Silver MJ, Zavattari P. et al. Estimation of a significance threshold for epigenome-wide association studies. Genet Epidemiol 2018;42:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kumaran K, Yajnik P, Lubree H. et al. The Pune Rural Intervention in Young Adolescents (PRIYA) study: design and methods of a randomised controlled trial. BMC Nutr 2017;3:41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Potdar RD, Sahariah SA, Gandhi M. et al. Improving women’s diet quality preconceptionally and during gestation: effects on birth weight and prevalence of low birth weight—a randomized controlled efficacy trial in India (Mumbai Maternal Nutrition Project). Am J Clin Nutr 2014;100:1257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Owens S, Gulati R, Fulford AJ. et al. Periconceptional multiple-micronutrient supplementation and placental function in rural Gambian women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2015;102:1450–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Nguyen PH, Young M, Gonzalez-Casanova I. et al. Impact of preconception micronutrient supplementation on anemia and iron status during pregnancy and postpartum: a randomized controlled trial in rural Vietnam.. PLoS One 2016;11:e0167416.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hambidge KM, Krebs NF, Westcott JE. et al. Preconception maternal nutrition: a multi-site randomized controlled trial. BMC Pregnancy Childbirth 2014;14:111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ladd-Acosta C, Fallin MD.. The role of epigenetics in genetic and environmental epidemiology. Epigenomics 2016;8:271–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.