Abstract
Context
Many international clinical guidelines recommend that overweight and obese women lose weight prior to pregnancy to reduce the risk of adverse pregnancy outcomes. Women who have recently given birth and plan future pregnancies are an important target population for preconception weight-loss interventions.
Objective
A systematic review to evaluate postpartum dietary and/or physical activity interventions to promote weight loss and improve health in a subsequent pregnancy was conducted.
Data Sources
Five databases—the Cochrane Central Register of Controlled Trials, MEDLINE (through PubMed), Embase, the Australian New Zealand Clinical Trials Registry, and the International Clinical Trials Registry—were searched using the following terms: preconception, pregnancy, postpartum, pregnancy outcomes, body mass index, weight gain, weight loss, weight change, postpartum weight retention, dietary or lifestyle intervention, and randomiz(s)ed controlled trial. The date of last search was November 2017.
Data Extraction
Data were extracted from each identified study using a standard form. The primary outcomes were weight loss at the completion of the intervention and at follow-up assessments. Secondary endpoints included maternal and infant outcomes in a subsequent pregnancy.
Data Analysis
Mean differences (MDs) were calculated for continuous data and risk ratios for dichotomous data, both with 95%CIs.
Results
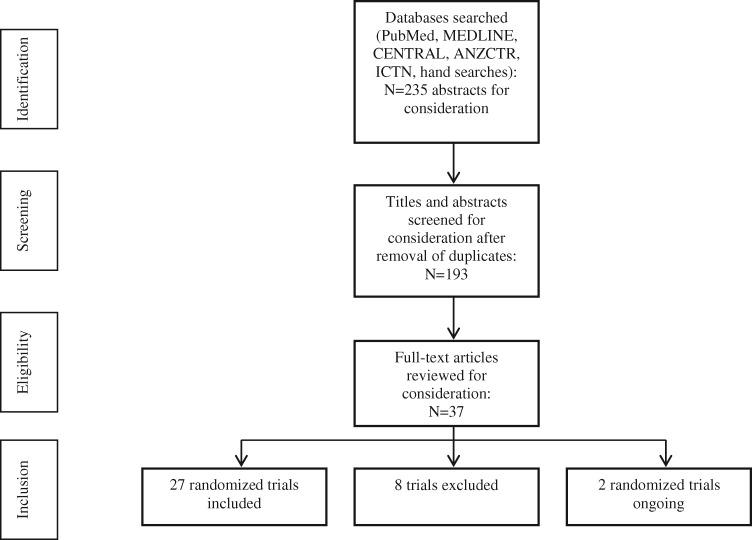
A total of 235 abstracts (193 after duplicates were excluded) were identified, from which 37 manuscripts were selected for full-text review. In total, 27 trials were identified for inclusion. Outcome data were available for approximately 75% of participants (n = 3485). A combined dietary and physical activity intervention provided post partum produced greater postpartum weight loss (MD, −2.49 kg; 95%CI, −3.34 to −1.63 kg [random-effects model]; 12 studies, 1156 women), which was maintained at 12 months post partum (MD, −2.41 kg; 95%CI, −3.89 to −0.93 kg [random-effects model]; 4 studies, 405 women), compared with no intervention. No studies reported maternal or infant health outcomes in a subsequent pregnancy.
Conclusions
Providing a postpartum intervention is associated with weight loss after birth, but effects on maternal and infant health in a subsequent pregnancy are uncertain.
Keywords: dietary and physical activity interventions, interpregnancy weight loss, overweight and obesity, systematic review
INTRODUCTION
Across developed nations, approximately 1 in 2 women enter pregnancy overweight or obese, their body mass index (BMI) exceeding 25 kg/m2.1 Maternal overweight and obesity increases the risk of virtually all pregnancy and birth complications, the magnitude of which increases with the severity of obesity.2 Efforts to improve outcomes have focused largely on dietary and lifestyle interventions during pregnancy to limit gestational weight gain, and while such strategies have generated some improvements in maternal diet and physical activity,3–5 they have been associated with only modest changes in gestational weight gain and little clinical impact.6 Additionally, prepregnancy overweight and obesity represents a stronger, independent risk for many of these adverse outcomes,7 a risk that is not addressed by intervention during pregnancy.
Many international clinical guidelines recommend that overweight and obese women lose weight prior to pregnancy to reduce the risk of adverse pregnancy outcomes.8,9 An important target population for such preconception weight-loss interventions are women who have recently given birth. Pregnancy represents a significant turning point for many women in terms of their cardiovascular and health trajectory, as metabolic changes, including relative insulin resistance, promote weight gain during pregnancy and weight retention after birth10 and therefore increase a woman’s risk of either remaining or becoming overweight or obese.11,12 It has been estimated that women of reproductive age gain approximately 700 g of weight per year,11 and in any given 5-year period, 20% of women will gain sufficient weight to progress into a higher BMI category.13 This, in turn, contributes to an increase in interpregnancy BMI and an increased risk of adverse pregnancy outcomes and perpetuates longer-term risks of obesity and its health consequences.14
The effect of a change in interpregnancy BMI on birth outcomes in a subsequent pregnancy has been investigated in a number of observational studies. Increasing BMI is associated with an increased risk of gestational diabetes, hypertension, cesarean birth, high birth weight, and perinatal mortality.15,16 In contrast, even modest weight loss (≈ 3 − 5 kg) between consecutive pregnancies in overweight or obese women has been associated with a reduction in the risk of an infant being born large for gestational age.16 However, there has been little systematic synthesis of the literature to evaluate the effect of weight-loss strategies after birth on a woman’s subsequent pregnancy outcomes.
The aim of this systematic review of the literature and meta-analysis was to evaluate the effects of dietary and/or lifestyle interventions for women in the postpartum period as a strategy to promote weight loss after birth and to improve maternal and infant health outcomes in a subsequent pregnancy.
METHODS
Sources
The Cochrane Central Register of Controlled Trials, MEDLINE though PubMed, MEDLINE, Embase, the Australian New Zealand Clinical Trials Registry, and International Clinical Trials Registry Platform (see Table S1 in the Supporting Information online) were searched using the following keywords: preconception, pregnancy, postpartum, pregnancy outcomes, BMI, weight gain, weight loss, weight change, postpartum weight retention, dietary or lifestyle intervention, and randomiz(s)ed controlled trial (see Table S2 in the Supporting Information online). The date of the last search was November 2017. The methodology followed the Cochrane Handbook for Systematic Reviews of Interventions17 and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (see Appendix S1 in the Supporting Information online),18 without date or language restrictions.
Study selection
All published and ongoing randomized trials in which a dietary and/or lifestyle intervention to promote weight loss in women in the postpartum period was compared with standard postnatal care or no intervention to promote weight loss were included. Participants included women who had given birth to a healthy singleton infant and (1) were overweight or obese; (2) had a normal BMI upon commencing pregnancy but whose gestational weight gain exceeded the Institute of Medicine guidelines19; or (3) had retained weight at the time of trial recruitment as defined by the trial authors.
Studies were excluded if a quasi-randomized design was used, if information was available in abstract form only, if underweight women were recruited, or if the intervention began prior to birth.
Outcomes
The following primary outcomes were assessed: (1) mean change in weight (in kilograms) between trial entry and end of the intervention (or at time of conception in a subsequent pregnancy, if recorded); (2) mean change in weight (in kilograms) between trial entry and final assessment (after completion of the intervention, to determine weight maintenance); (3) proportion of women who returned to their prepregnancy weight at final assessment; (4) proportion of women whose BMI was within the normal range (18.5–24.9 kg/m2) following completion of the intervention; and (5) proportion of overweight or obese women who lost 5% or more20 of their prepregnancy body weight following completion of the intervention.
Secondary maternal outcomes related to pregnancy and to birth outcomes in a subsequent pregnancy included gestational diabetes; preeclampsia; gestational hypertension; preterm birth before 37 weeks; gestational weight gain below, within, or above the Institute of Medicine recommendations; cesarean birth; and postpartum hemorrhage. Secondary infant outcomes related to birth outcomes in a subsequent pregnancy included birth weight in kilograms; birth weight ≥ 4.0 kg; birth weight ≤ 2.5 kg; neonatal intensive care admission; and breastfeeding. A range of longer-term maternal and child health outcomes were considered, including maternal type 2 diabetes; maternal essential hypertension; and childhood obesity.
J.M.D., A.R.D. and C.M.O’B. reviewed articles independently for inclusion and risk of bias. Discrepancies were resolved by discussion, with no blinding of authorship. Factors assessed for risk of bias included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential biases.17 Cluster trials were additionally assessed for recruitment bias, baseline characteristic imbalance, and the possibility of an additional herd effect of the intervention.17
Statistical analyses were conducted using the Review Manager software.21 Fixed-effect inverse variance meta-analysis was used to combine data from trials that examined similar interventions and when the population and methods of the trials were considered similar. Statistical heterogeneity was evaluated for each analysis using the τ2, I2, and χ2 statistics, with heterogeneity considered significant if the I2 statistic exceeded 30% and either the τ2 statistic was greater than zero or the chi-squared statistic revealed P < 0.10.17 Where there was evidence of heterogeneity, reasons were investigated. Use of a random-effects model was considered for analysis, and heterogeneity was further explored using sensitivity analyses.
Mean differences (MDs) were calculated for continuous data and risk ratios (RRs) for dichotomous data, both with 95%CIs. Sensitivity analyses were planned to evaluate the effects of the intensity of the intervention (defined as at least 14 intervention sessions, consistent with the available literature supporting weight loss in the general population),22 the nature of the intervention (diet alone, physical activity alone, mixed diet, and physical activity), maternal BMI subgroups at the commencement of the index pregnancy (normal BMI vs overweight vs obese), and trial quality (adequate process of randomization, allocation concealment, postrandomization exclusions, and loss to follow-up) on the primary outcomes.
When using data from cluster randomized trials, sample sizes (and numbers of events for dichotomous outcomes) were reduced to an effective sample size (or event number) by dividing by the design effect to apportion correct weighting, as recommended by the Cochrane Collaboration.17
RESULTS
Figure 1 shows the literature search strategy. A total of 235 abstracts (193 after removal of duplicates) were identified, from which 37 full-text manuscripts were reviewed and 27 were included.23–49Table 1 shows the characteristics of the included trials. One completed trial with a published protocol50 and 1 ongoing randomized trial51 were identified (see Table S3 in the Supporting Information online) and 8 studies were excluded (see Table S4 in the Supporting Information online).52–59
Figure 1.
Flow diagram of the literature search process. Abbreviations: ANZCTR, Australian New Zealand Clinical Trials Registry; ICTN, International Clinical Trials Registry.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Description |
|---|---|
| Population | Women in the postpartum period who had given birth to a healthy singleton infant |
| Intervention | Dietary and/or physical activity interventions to promote weight loss |
| Comparison | Standard postpartum care or no intervention |
| Outcomes | Weight loss at the completion of the intervention and 12 months after completion of the intervention; maternal and infant outcomes in a subsequent pregnancy |
| Study design | Randomized controlled trials |
Study descriptions
Twenty-seven randomized trials were included (Table 2),23–49 involving 4610 women to whom an intervention was delivered in the postpartum period. Outcome data were available for approximately 75% of participants (n = 3485). Twenty-three trials reported weight change at the end of the intervention period,24–27,29–36,38–43,45,46,48,49 with 6 trials reporting weight change at 12 months post partum.24,34,35,37,45,46 Fourteen trials (n = 1511) recruited only women who were overweight or obese at the time of enrollment.23–28,30,32,34,35,43–45,49
Table 2.
Characteristics of the studies included in the systematic review and meta-analysis
| Reference | Methods | Participants | Intervention | Outcomes |
|---|---|---|---|---|
| Berry et al. (2015)23 | Setting: Raleigh, NC, USA. Design: RCT. Random sequence generation: Computer generated. Allocation concealment: NS. Completeness of outcome data: 60 women in total were randomized; 16 women (27%) lost to follow-up | Inclusion criteria: Women aged > 18 y who were at least 6 wk post partum and had a BMI > 25 kg/m2 were eligible. Exclusion criteria: Participation in another clinical trial or weight-management program | Intervention: Combined diet and physical activity intervention for weekly 60-min sessions over a 12-wk period, followed by thrice-monthly follow-up sessions. Resources included written information and lessons about social cognitive skills to facilitate healthier choices related to diet and physical activity, including a home exercise program to increase activity to at least 30 min on most days of the week. Control: Standard postnatal care | Acceptability and feasibility of larger RCT; anthropometric measures; completed questionnaire that assessed changes in health behaviors and self-efficacy |
| Bertz et al. (2012)24 | Setting: Gothenburg, Sweden. Design: RCT, 2 × 2 factorial design. Random sequence generation: A blocked randomization (block size of 4) was used within each stratum. In all, 16 blocks of 4 (n = 64) were completed and 2 blocks of 4 were partially used, until a total of 68 women were randomly assigned. Allocation concealment: Group allocation was concealed to all until completion of baseline measurements Completeness of outcome data: 68 women in total were randomized; 6 women (8.9%) lost to follow-up | Inclusion criteria: Self-reported prepregnancy BMI of 25–35 kg/m2; nonsmoker; singleton term birth; intention to breastfeed for 6 mo; providing 20% of infant energy intake as complementary foods; birth weight of infant 2500 g or more; and no illness in the mother or infant. Exclusion criteria: BMI > 35 kg/m2 | Intervention, arm 1 (group D): 3 appointments over a 12-wk intervention, which included meeting with a dietitian and provision of a diet plan and written information about the dietary aspect of weight loss. Intervention, arm 2 (group E): 12-wk intervention that included an exercise plan provided by a physical therapist. Exercise escalated in intensity over the intervention period; the aim was to achieve 45-min brisk walks on 4 d/wk. Intervention, arm 3 (group DE): Participated in the dietary intervention (arm 1 [group D]) and the exercise intervention (arm 2 [group E]) simultaneously. Control, arm 4 (group C): Usual care | Primary outcomes were changes from baseline in body weight and body composition. Measures of treatment included energy intake and expenditure and daily steps. Involuntary cessation of breastfeeding, reduced duration of breastfeeding, and inadequate child growth were indicators of adverse effects |
| Chang et al. (2017)25 | Setting: Michigan, USA. Design: RCT. Random sequence generation: not stated. Allocation concealment: NS. Completeness of outcome data: 602 women randomized (410 in intervention group and 202 in control group); 191 in intervention group and 78 in control group were lost to follow-up (43% losses to follow-up) | Inclusion criteria: Low-income women participating in WIC, between 6 wk and 4.5 y post partum, with BMI between 25 and 39.9 kg/m2 | Intervention: 16-wk intervention comprising 11 theory-based, culturally sensitive videos featuring peers from the target audience to teach skills about stress management, healthier eating, and being more physically active. Supplemented with 11 peer support group teleconferences with 9 other women, either weekly or fortnightly. Control: Received printed materials on stress management, healthy eating and physical activity from standard reliable sources | Weight change at completion of intervention and at 3 mo after completion of intervention |
| Colleran et al. (2012)26 | Setting: Greensboro, NC, USA. Design: RCT. Random sequence generation: A computer-generated random numbers table was used to stratify primiparous and multiparous women into 1 of 2 groups. Allocation concealment: Opaque envelopes. Completeness of outcome data: 31 women randomized; 4 postrandomization withdrawals (12.9%) | Inclusion criteria: Women < 3 wk post partum who delivered vaginally, had a self-reported BMI between 25 and 30 kg/m2, and were fully breastfeeding (ie, not supplementing with formula), aged 23–37 y, and participating in physical activity < 3 d/wk for the past 3 mo. Exclusion criteria: Cesarean birth, history of smoking during pregnancy, preexisting medical conditions that affected hormone levels, or known contraindications to exercise | Intervention: Participation in a 16-wk diet and exercise program, including home visits, from wk 4 to wk 20 post partum. Exercise protocol consisted of walking 10 000 steps or 3000 aerobic steps per day and strength training 5 times and 3 times weekly, respectively. A registered dietitian worked with participants using an internet-based program (MyPyramid Menu Planner for Moms) and met with them weekly for a 30-min session. Control: Received standard public health information at 9 wk and 16 wk post partum. The minimal care group was asked not to participate in structured exercise or change their dietary habits for 16 wk | At completion of intervention (16 wk): weight change; bone mineral density; dietary intake; cardiovascular fitness; muscle strength and endurance; and hormone levels (prolactin, estradiol, and growth hormone) |
| Craigie et al. (2011)27 | Setting: Areas of low socioeconomic status across Scotland. Design: Pilot randomized trial. Random sequence generation: Computer generated. Allocation concealment: NS. Completeness of outcome data: 52 women in total were randomized; 3 postrandomization withdrawals; 13 women (31%) lost to follow-up | Inclusion criteria: Women 6–18 mo post partum, of low socioeconomic status, with BMI > 25 kg/m2, recruited from areas of moderate to high social deprivation in Scotland. Exclusion criteria: Pregnancy | Intervention: 12-wk combined diet and physical activity intervention. Thrice-monthly face-to-face meetings with counselor, interspersed with 3 structured phone calls. Resources included weight-loss booklet. Intervention focused on daily energy deficit of 500 kcal as well as 150 min of moderate to vigorous activity per week. Control: Received weight-loss booklet only | Acceptability and feasibility of larger RCT; weight change at end of intervention; anthropometric measures; and completed questionnaire that assessed changes in diet; physical activity |
| deRosset et al. (2013)28 | Setting: North Carolina, USA. Design: cluster RCT; unit of randomization is center. Random sequence generation: Unclear but was performed by a statistician not involved in enrollment. Allocation concealment: Treatment allocation of each center revealed by statistician after all women had enrolled. Neither women nor clinicians could be blinded. Completeness of outcome data: 24 women randomized; no women lost to follow-up | Inclusion criteria: Postpartum women with limited English proficiency, aged > 21 y (or married), able to understand spoken Spanish, cleared for participation at 6-wk postpartum visit, self-identifying as Hispanic and overweight or obese. Exclusion criteria: Women with heart murmur or heart disease, family history of sudden death, or participation in another weight-management program | Intervention: Women (n = 13) received 12 weekly group classes for 60 min of nutrition and exercise education and coping skills training delivered in Spanish. Women were encouraged to walk with their infants daily, increasing to 6 min/d, and to form walking groups outside of classes. Women received a weekly reminder phone call prior to each class. Control: Received no intervention but were offered participation at the completion of the study | Demographic information; self-reported health history; height, weight, BMI, and waist circumference; triceps and subscapular skinfold thicknesses; and health behavior assessment questionnaire |
| Dewey et al. (1994)29 | Setting: California, USA Design: RCT. Random sequence generation: NS. Allocation concealment: NS. Completeness of outcome data: 38 women in total were randomized; 3 postrandomization withdrawals and 2 women lost to follow up (13%) | Inclusion criteria: Women 6–8 wk post partum who were sedentary and breastfeeding their infant (healthy infant born at term) were eligible. Exclusion criteria: History of chronic illness, smoking, or medication use | Intervention: Physical activity intervention using stationary bike. Consisted of 45-min supervised exercise sessions, 5 times/wk (12-wk duration). Control: Exercise no more than once weekly for 12 wk | Energy expenditure; weight change at end of intervention; anthropometric measures; breast milk composition; heart rate monitoring |
| Gilmore et al. (2017)30 | Setting: Baton Rouge, LA, USA. Design: RCT. Random sequence generation: NS. Allocation concealment: NS. Completeness of outcome data: 40 women randomized; 5 women (13%) lost to follow-up | Inclusion criteria: Women aged > 18 y, < 8 wk post partum, English speaking, with a BMI ≥ 25 kg/m2 and < 40 kg/m2, who were participating in WIC. Exclusion criteria: Enrollment in the Nurse Family Partnership Program, multiple gestational pregnancy, history of psychiatric illness or chronic conditions that affect BMI or appetite, or use of any medications or supplements that aid weight loss | Intervention: Women received the SmartLoss smartphone application, which delivered a personalized 16-wk weight-loss intervention aimed at increasing physical activity, monitoring body weight, and adjusting energy intake to facilitate a 10% weight-loss goal over the 16-wk period. Control: Received standardized advice and services for postpartum nutrition and weight-management but no personalized dietary prescription | Weight change at end of intervention; blood pressure; heart rate; waist and hip circumferences; and body composition |
| Haire-Joshu et al. (2015)31 | Setting: St Louis, MO, USA. Design: Randomized trial; nested cohort design. Random sequence generation: NS; described as a “group randomized nested cohort design.” Allocation concealment: NS. Completeness of outcome data: 1325 adolescent girls in total were randomized; 420 girls (32%) lost to follow up | Inclusion criteria: Adolescent girls < 12 mo post partum who were participating in the Parents as Teachers teen program | Intervention: Girls received access to community parent educators, with five 60-min home visits and school-based classroom group meetings focusing on dietary and physical activity behaviors. Control: Received standard child development information as part of the Parents as Teachers program | Weight change at end of intervention; dietary and physical activity changes; and BMI success (described as maintenance of normal BMI, decrease in overweight BMI, or decrease in obese BMI) |
| Herring et al. (2014)32 | Setting: Philadelphia, PA, USA. Design: Pilot randomized trial. Random sequence generation: Computer generated. Allocation concealment: Sealed opaque envelopes. Completeness of outcome data: 18 women in total were randomized; 1 woman (5.6%) lost to follow up | Inclusion criteria: Women recruited at 2 wk to 12 mo post partum, aged >18 y, with BMI ≥25 kg/m2 or weight >5 kg above early pregnancy weight, who had cell phone access and were members of Facebook. Exclusion criteria: Smoking or report of a cardiac, gastrointestinal, cognitive, or psychiatric disorder | Intervention: Technology-based intervention focusing on diet and physical activity, weight-loss behavior strategies, energy deficit, and goal setting; supplemented with biweekly phone calls over 14-wk intervention period. Control: Standard postpartum care | Weight change at end of 14-wk intervention and BMI success (described as maintenance of normal BMI, decrease in overweight BMI, or decrease in obese BMI) |
| Huang et al. (2011)33 | Setting: Taiwan Design: Randomized trial. Random sequence generation: Random number table. Allocation concealment: NS. Completeness of outcome data: 240 women in total were randomized; 51 women (21.3%) lost to follow-up | Inclusion criteria: Women < 16 wk gestation, aged > 18 y, and able to speak and read Chinese. Exclusion criteria: History of a cognitive or psychiatric impairment | Intervention arm 1: 6 one-to-one counseling sessions (1 primary and 5 boosters) related to combined diet and physical activity commenced during pregnancy and continued through 3 mo post partum. This group was not included in the analyses because the intervention was delivered during pregnancy. Intervention arm 2: 3 counseling sessions (1 primary and 2 boosters) related to combined diet and physical activity at birth and at 6 wk and 3 mo post partum. Women were also provided with a brochure. Control: Standard hospital care | Weight change after the intervention; weight retention of < 5 kg; and changes in diet and physical activity |
| Huseinovic et al. (2016)34 | Setting: Gothenburg, Sweden. Design: Randomized trial. Random sequence generation: Random number table. Allocation concealment: Sealed numbered envelopes. Completeness of outcome data: 110 women in total were randomized; 17 women (15.5%) lost to follow-up | Inclusion criteria: Women 6–15 wk post partum, with BMI > 27 kg/m2. Exclusion criteria: History of serious maternal or infant disease; inability to understand and read Swedish | Intervention: Combined diet and physical activity intervention (12 wk duration). Face-to-face meeting with dietitian, supplemented by biweekly text messages and phone calls. Intervention focused on daily energy deficit of 500 kcal as well as brisk walking at least 4 times/wk. Control: Standard postnatal care | Weight change after intervention and at 12 mo and 2 y after intervention; changes in diet and physical activity; anthropometric measures |
| Keller et al. (2014)35 | Setting: Phoenix, AZ, USA. Design: Randomized trial. Random sequence generation: Random number table. Allocation concealment: Sealed envelopes. Completeness of outcome data: 139 women in total were randomized; 46 women (33.0%) lost to follow-up; however, loss to follow-up was greater in control group than in intervention, 45% vs 21% | Inclusion criteria: Women 6 wk to 6 mo post partum, habitually sedentary, Latina origin, aged 18–40 y, with a BMI ranging 25 − 35 kg/m2. Exclusion criteria: Current pregnancy, severe musculoskeletal or cardiorespiratory disease, infectious illness, osteoporosis, use of oral steroids or medications affecting coagulation | Intervention: 12-wk intervention involving weekly sessions promoting social support and group walking classes to increase physical activity. Control: Standard postnatal care; monthly newsletters; phone calls to address common postpartum or newborn concerns | Weight change after intervention and at 12 mo after intervention; changes in diet and physical activity; emotional well-being |
| Krummel et al. (2010)36 | Setting: West Virginia, USA. Design: Randomized trial. Random sequence generation: NS. Allocation concealment: NS. Completeness of outcome data: 151 women in total were randomized; 87 women (57.6%) lost to follow-up | Inclusion criteria: Women < 2 y post partum, aged > 18 y, BMI > 18 kg/, and enrolled in WIC. Exclusion criteria: Type 1 or 2 diabetes; multiple pregnancy; presence of physical disability, substance abuse, or psychiatric illness | Intervention: Combined diet and physical activity intervention. Initial face-to-face counseling session followed by 10 sessions of facilitated group discussion; focus on dietary changes and increased physical activity; monthly newsletters provided. Control: Received initial face-to-face counseling session followed by monthly newsletters | Weight change after intervention; changes in diet and physical activity |
| Leermakers et al. (1998)37 | Setting: Pittsburgh, PA, USA. Design: Randomized trial. Random sequence generation: Stated as “randomly assigned” but was not clarified. Allocation concealment: NS. Completeness of outcome data: 90 women in total were randomized; 28 women (31.1%) lost to follow-up | Inclusion criteria: Women aged > 18 y, 3–12 mo post partum, who exceeded prepregnancy weight by > 6.8 kg were eligible. Exclusion criteria: Women with BMI < 22 kg/m2 who were currently breastfeeding | Intervention: 6-mo intervention with a focus on low-fat/low-calorie diet and increased physical activity, delivered via 2 group sessions with correspondence materials (16 written lessons related to nutrition, physical activity and behavior change) and phone calls. Correspondence consisted of weekly lessons for wk 1 to wk 12; every 2 wk for 4 wk thereafter, and monthly for the remaining 8 wk. Control: Received brochure about healthy eating and physical activity | Weight change at 12 mo post partum; proportion of women returning to prepregnancy weight; changes in diet and physical activity |
| Lovelady et al. (2000)38 | Setting: Greensboro, NC, USA. Design: Randomized trial. Random sequence generation: Random number table. Allocation concealment: NS. Completeness of outcome data: 48 women in total were randomized; 8 women (16.7%) lost to follow-up | Inclusion criteria: Women 4 wk post partum who were healthy, sedentary, nonsmokers, and exclusively breastfeeding their term infant following vaginal birth | Intervention: Combined diet and physical activity intervention consisting of 10 weekly group sessions over a 10-wk period. Intervention focused on a daily energy deficit of 500 kcal as well as 45-min exercise sessions 4 times/wk. Control: Standard postnatal care | Weight change after intervention; changes in diet and physical activity |
| Maturi et al. (2011)39 | Setting: Abadan, Iran. Design: Randomized trial. Random sequence generation: Random number table. Allocation concealment: NS. Completeness of outcome data: 70 women in total were randomized; 4 women (5.7%) lost to follow-up | Inclusion criteria: Women 6 wk to 6 mo post partum who were 18–40 y of age, sedentary, literate, lactating, and had birthed a singleton infant. Exclusion criteria: History of preeclampsia, musculoskeletal disorders, or BMI > 29 kg/m2 | Intervention: 12-wk pedometer exercise program with goal to reach 10 000 steps/d, with a baseline face-to-face counseling session and subsequent weekly text messages, fortnightly phone calls, and brochure sent at 8-wk mark. Control: Standard postnatal care | Weight change after intervention; changes in physical activity |
| McCrory et al. (1999)40 | Setting: Davis, CA, USA. Design: Randomized trial. Random sequence generation: Computer generated. Allocation concealment: NS. Completeness of outcome data: 68 women in total were randomized; 1 woman (1.5%) lost to follow-up | Inclusion criteria: Women 8–16 wk post partum, using no medication, who were nonsmokers, had no history of chronic illness, had a term singleton birth, and were willing to exercise > 1 mo prior to delivery of the intervention and randomization | Intervention arm 1: 11-d dietary intervention through personalized diet plans with provision of pre-prepared and portioned foods. Intervention arm 2: 11-d dietary and physical activity intervention via same method as intervention arm 1, with addition of self-supervised exercise sessions that occurred at least once daily. Participants checked every 1–3 d through heart rate monitoring. Control: Standard hospital care | Weight change after intervention; changes in diet and physical activity; cardiorespiratory fitness; breast milk composition |
| McIntyre et al. (2012)41 | Setting: Brisbane, Australia. Design: RCT. Random sequence generation: NS. Allocation concealment: NS. Completeness of outcome data: 28 women in total were randomized; 2 women (7.1%) lost to follow-up | Inclusion criteria: Women < 6 wk post partum with recent gestational diabetes. Exclusion criteria: Postnatal glucose tolerance test indicating overt diabetes | Intervention: 12-wk intervention consisting of a baseline face-to-face consultation with an exercise physiologist. Participants were also contacted weekly by phone for the next 4 wk and every 2 wk thereafter. Control: Received brief printed materials outlining the importance of diet and exercise for prevention of diabetes | Primary outcome measure was change in self-reported physical activity. Secondary outcomes were change in insulin resistance (HOMA-IR), change in weight, and changes in body composition assessed at completion of the intervention (18 wk post partum) |
| Nicklas et al. (2014)42 | Setting: Boston, MA, USA. Design: Randomized trial. Random sequence generation: Computer generated. Allocation concealment: Sealed sequentially numbered envelopes. Completeness of outcome data: 75 women in total were randomized; 7 women (9.3%) lost to follow-up | Inclusion criteria: Women ≥ 6 wk post partum with a diagnosis of gestational diabetes in their most recent pregnancy. Exclusion criteria: Diagnosis of type 2 diabetes, previous bariatric surgery, use of medications affecting body weight, or birth before 32 wk of gestation | Intervention: Combined dietary and physical activity intervention based on established Diabetes Prevention Program. Intervention delivered weekly for 12 wk, then every 2 wk for 2 mo, then monthly to 1 y post partum. Control: Standard postnatal care | Weight change after intervention and at 12 mo post partum; changes in diet and physical activity; changes in cardiometabolic markers |
| Ostbye et al. (2009)43 | Setting: Durham, NC, USA. Design: Randomized trial. Random sequence generation: Stated as “Labels blindly drawn.” Allocation concealment: NS. Completeness of outcome data: 450 women in total were randomized; 29 women (6.4%) lost to follow-up | Inclusion criteria: Women 6 wk post partum who were overweight or obese prior to pregnancy | Intervention: Combined diet and physical activity intervention, delivered over 9 mo at 6-wk intervals. Consisted of 8 healthy eating classes, 10 physical activity classes, and 6 counseling sessions by phone. Control: Standard postnatal care | Weight change after intervention; changes in diet and physical activity |
| Ostbye et al. (2012)44 | Setting: North Carolina, USA. Design: RCT. Random sequence generation: Permutated 8-block randomization, computer generated. Allocation concealment: Computer generated. Unclear if outcome assessors were blinded. Completeness of outcome data: 400 women in total were randomized; 92 women (23.0%) lost to follow-up | Inclusion criteria: Women who were 1–6 mo post partum, overweight or obese prior to pregnancy, and had a preschool child aged 2–5 y. Exclusion criteria: BMI ≤25 kg/m2 | Intervention: 8-mo KAN-DO intervention. Participants received 8 interactive family kits (1 kit/mo) and a 20–30 min supportive phone counseling session. Focus was on parenting skills, stress management, and healthy behaviors, including diet and physical activity. Control: Minimal care. Women received pamphlets about prereading skills in preschoolers (Reading is Fundamental program) | Primary analysis was weight change in preschool children. Secondary outcomes were weight change in women and changes in parenting skills. Weight change reported as change in BMI in primary paper only |
| O’Toole et al. (2003)45 | Setting: St Louis, MO, USA. Design: RCT. Random sequence generation: Stated as “Labels blindly drawn.” Allocation concealment: NS. Completeness of outcome data: 40 women in total were randomized; no reported losses to follow-up | Inclusion criteria: Women 6 wk to 6 mo post partum, overweight or obese, with weight gain of > 15 kg during pregnancy and weight of at least 5 kg more than prepregnancy weight at time of enrollment. Exclusion criteria: Current participation or enrollment in an exercise program or formal weight-loss program; contraindications to participation in diet and exercise programs | Intervention: Combined diet and physical activity intervention consisting of group educational sessions delivered weekly for 12 wk, then every 2 wk for 2 mo, then monthly to 1 y post partum. Control: Received single individual session with dietitian | Weight change after intervention and at 12 mo post partum; changes in diet and physical activity |
| Phelan et al. (2017)46 | Setting: California, USA. Design: Cluster RCT. Random sequence generation: Computer generated. Allocation concealment: NS. Blinding of outcome assessment: Yes. Completeness of outcome data: 371 women randomized; 1 woman (0.002%) lost to follow-up | Inclusion criteria: Women 6 wk to 12 mo post partum, with either a BMI > 25 kg/m2 or a BMI 22–24.9 kg/m2 in addition to weight exceeding prepregnancy weight by > 4.5 kg; English or Spanish speaking; and aged 18 − 40 y. Exclusion criteria: Pregnancy, planning pregnancy, planned relocation, current serious medical condition, physical limitations to exercise, history of eating disorders, current drug abuse, or current treatment for psychological condition | Intervention: WIC program delivered within the first postpartum year, with focus on healthy diet and physical activity. Additional web-based intervention that included weekly lessons, 4 weekly text messages, web-based diary, and weight and activity tracker as well as monthly face-to-face group sessions. Control: Received WIC standard program without web-based weight-loss intervention | Weight change at end of intervention (12 mo). Anthropometric measures; changes in dietary intake; accelerometer data related to physical activity; emotional and psychological well-being; and self-efficacy |
| Reinhardt et al. (2012)47 | Setting: Rural New South Wales, Australia. Design: RCT. Random sequence generation: NS. Allocation concealment: NS. Completeness of outcome data: 40 women randomized; 2 women (5.0%) lost to follow-up | Inclusion criteria: Diagnosed gestational diabetes in most recent pregnancy. Exclusion criteria: Women without phone access or with medical disorder/procedures likely to interfere with results | Intervention: Booklet with information on Australian healthy eating and physical activity recommendations, stage of readiness to make behavioral change, strategies such as goal-setting to enable change, and food and physical activity diaries for self-monitoring. Followed by 10 phone-based sessions (10 − 30 min each) with an accredited diabetes educator skilled in motivational interviewing, occurring weekly for 5 wk and then monthly for 5 mo. Control: NS; assumed to be no intervention | Changes in dietary intake, physical activity, weight, and waist circumference |
| Tripette et al. (2014)48 | Setting: Tokyo, Japan. Design: RCT. Random sequence generation: NS. Allocation concealment: NS. Completeness of outcome data: 34 women in total were randomized; no reported postrandomization withdrawals | Inclusion criteria: Women 3–12 mo post partum; nonsmokers. Exclusion criteria: History of cardiovascular disease, or no access to audiovisual equipment for active video games | Intervention: Physical activity intervention delivered over 40 d and involving participation in active video games at least once daily. Control: Standard postnatal care; participants were asked not to change their lifestyle over the following 40 d | Weight change after intervention |
| Walker et al. (2012)49 | Setting: Texas, USA. Design: RCT. Random sequence generation: NS. Allocation concealment: NS. Completeness of outcome data: 71 women randomized; 21 postrandomization withdrawals (29.6%) | Inclusion criteria: Women aged > 18 y who self-identified as Hispanic, African American, or Anglo/white; between 6 wk and 12 mo post partum; with BMI of > 25 kg/m2, retained postpartum weight of at least 5 kg, and parity of 1–3; whose prenatal care was funded by Medicaid; and who delivered a full-term healthy singleton, was conversant in English, and had a phone or pager. Exclusion criteria: Chronic health conditions, such as HIV/AIDS, heart disease, renal disease, or diabetes, or use of medication for weight loss, hypertension, diabetes, thyroid condition, or depression | Intervention: Groups met weekly for 2 h in 13-wk ethnic-specific diet and physical activity weight-loss programs that included weigh-in at the start of each meeting. Duration of interventions was based on estimates of retained weight among low-income women and the corresponding time needed to lose that weight at the rate of about 1 lb/wk. Intervention sessions were delivered by trained registered nurses or health educators. Control: Received intervention materials by mail over 13 wk, along with weekly phone counseling support | Weight change after intervention; scores on psychometric scales to measure health behaviors, perceived stress, self-efficacy, body dissatisfaction, weight distress, and dietary intake |
Abbreviations: BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; KAN-DO, Kids & Adults Now—Defeat Obesity; NS, not stated; RCT, randomized controlled trial; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
Nineteen trials were conducted in the United States,23,25,26,28–32,35–38,40,42–46,49 with 2 studies each conducted in Australia41,47 and Sweden24,34 and single trials conducted in each of Scotland,27 Taiwan,33 Iran,39 and Japan.48 Twenty trials evaluated a combined dietary and physical activity intervention.23,25–28,30–34,36–38,42–47,49 Five assessed physical activity only.29,35,39,41,48 Three trials delivered a comprehensive lifestyle intervention via digital media, 1 utilizing a smartphone application30 and 3 others using a web-based platform.26,46 A further 2 trials used text messaging in addition to face-to-face meetings and phone calls34 or text messaging in addition to phone calls and a closed Facebook support group.32 A third trial in rural Australia delivered the intervention by phone, following an initial study appointment.47
The trial by McCrory et al.40 conducted a 3-arm comparison involving a dietary intervention alone, a combined dietary and physical activity intervention, and no intervention, contributing data to the comparisons assessing dietary interventions alone and a combined intervention. Bertz et al.24 adopted a 2 × 2 factorial design in which 4 arms were compared: diet alone, exercise alone, diet and exercise, and a control group receiving no intervention. The trial by Huang et al.,33 which was included in the comparison assessing combined interventions, compared a combined intervention commencing in the antenatal period (which was not included in the analyses), a combined intervention provided in the postpartum period, and a control group provided with standard postnatal care.
The trials by Phelan et al.46 and deRosset et al.28 were cluster randomized controlled trials.
At least 14 intervention sessions were provided over the course of the trial in 12 trials,23,25,26,29,30,35,36,38,42,43,45,46 with the frequency of study visits considerably less in the remainder.
One completed study with a published protocol50 and 1 ongoing study51 were identified.
Excluded studies
Four studies were excluded because the delivery of the intervention commenced during pregnancy,52,55,56,58 and 3 were excluded because they used a nonrandomized design.54,57,59 One study was excluded because it compared a historical control group with 2 intervention arms that were randomized.53
Methodological quality
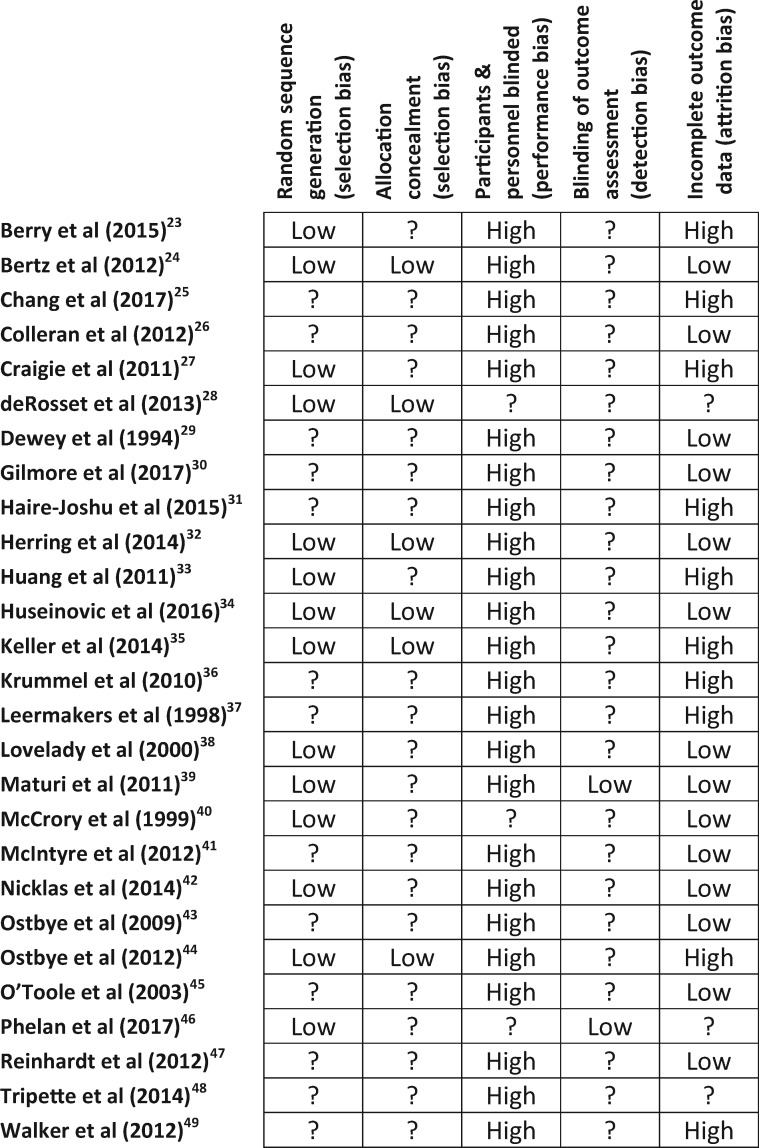
Figure 2 shows details of the risk of bias for the 27 included studies,22–48 which were considered to be at moderate risk of bias and of low to medium quality. Fourteen trials reported use of a computer-generated random number sequence or random number table,23,24,26,27,32–35,38–40,42,44,46 and 4 studies used sealed opaque envelopes for allocation concealment.26,32,34,35 None of the included trials reported blinding of participants, owing to the nature of the interventions, although 2 trials reported blinding of outcome assessors.39,46
Figure 2.
Risk of bias of included studies. Symbol: ?, unclear risk.
Ten trials reported postrandomization withdrawals and loss to follow-up greater than 20% and were considered to be at high risk of attrition bias.23,25,27,31,33,35–37,44,49
No additional risks of biases were identified for the 2 included cluster randomized trials,28,46 including recruitment bias or imbalance of baseline characteristics. It was not possible to assess an additional effect of the intervention within clusters.
Dietary intervention alone vs standard postnatal care or no intervention
Two randomized trials involving a total of 75 women in the postpartum period were included in this comparison.24,40 Women who were provided with a dietary intervention in the postpartum period were more likely to have significant weight loss at the completion of the intervention compared with women who received no intervention (MD, −1.82 kg; 95%CI, −2.19 to −1.44 kg). One of these trials24 reported weight change at 12 months and found that women who received a dietary intervention alone maintained significant weight loss compared with women who received no dietary intervention (MD, −9.30 kg; 95%CI, −13.71 to −4.89; 30 women). No longer-term health outcomes or maternal or infant health outcomes in a subsequent pregnancy were reported.
Physical activity intervention alone vs standard postnatal care or no intervention
Six randomized trials involving 288 women in the postpartum period were included in this comparison.24,29,35,39,41,48 Data from 3 trials could be included in the meta-analysis,24,41,48 with the other 3 studies reporting baseline and postintervention weight outcomes but not change in weight.29,35,39
Women who were provided with a physical activity intervention in the postpartum period had greater weight loss at the completion of the intervention when compared with women who received no intervention (MD, −1.45 kg; 95%CI, −2.41 to −0.50 kg [random-effects model]; 3 studies, 93 women).
Combined dietary and physical activity intervention vs standard postnatal care or no intervention
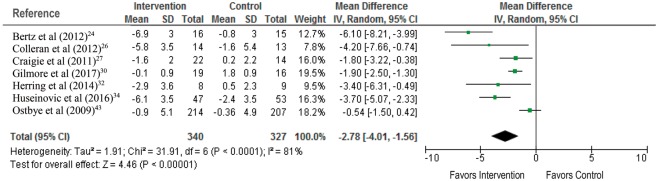
Twenty-two randomized trials involving 3294 women were included in this comparison,23–28,30–34,36–38,40,42–47,49 with 12 studies involving 1156 women contributing data to the primary weight-related outcomes.24,26,27,30,32–34,36,38,40,43,46
Women provided with a combined dietary and physical activity intervention had greater weight loss at the completion of the intervention compared with women who received no intervention (MD, −2.49 kg; 95%CI, −3.34 to −1.63 kg [random-effects model]; 12 studies, 1156 women) (Figure 3).24,26,27,30,32–34,36,38,40,43,46 There was a high degree of heterogeneity for this outcome (I2 statistic = 86%), which persisted after considering only those trials with adequate randomization processes (MD, −3.14 kg; 95%CI, −4.40 to −1.88 kg [random-effects model]; 9 studies, 643 women; I2 statistic = 88%) (see Figure S1 in the Supporting Information online)24,26,27,32–34,38,40,46; only those trials with low rates of postrandomization withdrawals and loss to follow-up (MD, −2.92 kg; 95%CI, −4.00 to −1.83 kg [random-effects model]; 8 studies, 908 women; I2 statistic = 90%) (see Figure S2 in the Supporting Information online)24,30,32,34,38,40,43,46; and only those trials that recruited women who were overweight or obese at the start of their index pregnancy (MD, −2.78 kg; 95%CI, −4.01 to −1.56 kg [random-effects model]; 7 studies, 667 women; I2 statistic = 81%) (Figure 4).24,26,27,30,32,34,43
Figure 3.
Combined dietary and physical activity intervention for the outcome of weight loss at completion of the intervention.
Figure 4.
Combined dietary and physical activity intervention for the outcome of weight loss at completion of the intervention in women categorized as overweight or obese.
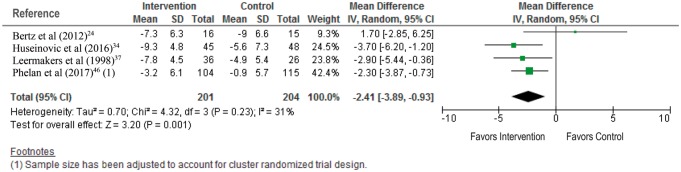
When considering the effects of the intervention at 12 months post partum, a combined dietary and physical activity intervention was associated with greater weight loss when compared with no intervention (MD, −2.41 kg; 95%CI, −3.89 to −0.93 kg [random-effects model]; 4 studies, 405 women; I2 statistic = 31%) (Figure 5).24,34,37,46
Figure 5.
Combined dietary and physical activity intervention for the outcome of weight loss at 12 months post partum.
The proportion of women returning to their prepregnancy weight was greater after a combined dietary and physical activity intervention than after no intervention (RR = 3.13; 95%CI, 0.99–9.88 [fixed-effects model]; 1 study, 62 women).
While the included trials reported improvements in dietary patterns and physical activity, no longer-term health outcomes or maternal or infant health outcomes in a subsequent pregnancy were reported.
Heterogeneity
The results showed evidence of statistical heterogeneity for the reported weight-related outcomes. The random-effects model was used, without any change in the estimated effect size. Similarly, consideration of only those studies with appropriate methods of randomization, low risk of bias owing to adequate follow-up, high (≥ 14) vs low (< 14) frequency of study visits, and maternal BMI category did not influence the magnitude or direction of effect. Because significant heterogeneity persisted and could not be explained, the effect size estimated should be considered as an average effect size, and interpreted with caution.17
DISCUSSION
The findings of this systematic review and meta-analysis indicate that a dietary or physical activity intervention, alone or in combination, provided to women in the postpartum period is associated with greater weight loss at the completion of the intervention and at 12 months post partum, with more women returning to their prepregnancy weight after a combined intervention, compared with no intervention. Providing a combined dietary and physical activity intervention was associated with an average weight loss of 2.49 kg at the completion of the intervention and an average weight loss of 2.4 kg at 12 months post partum. A limited number of trials evaluated dietary or physical activity interventions alone, and while they suggest beneficial weight loss at the completion of the intervention, their findings should be interpreted with caution.
This review is not without limitations, reflecting the methodological quality of the included studies. The description of trial methodology varied considerably across studies, with just over half describing the methods used to generate the randomization sequence23,24,27,28,33–35,38–40,42,44 and only 7 describing adequate allocation concealment,24,26,28,32,34,35,44 thereby introducing potential for selection bias.
While 27 randomized trials involving over 4600 women were identified for inclusion, fewer trials (n = 15) involving 1165 participants (≈ 25%)24,26,27,30,32–34,36–38,40,41,43,46,48 contributed data to the primary outcome of weight change at the end of the intervention period. At 12 months post partum, data from only 4 trials involving 420 women were available.24,34,37,46 Both the high rate of postrandomization loss to follow-up and the participant withdrawal rate of approximately 25% identified in this review introduce significant potential for attrition bias, as does the lack of reporting of outcomes beyond completion of the intervention. These findings also highlight the difficulty in both engaging and maintaining contact with women in the postpartum period, reflecting changes in infant care responsibilities following birth as well as the mobility of this population of women.
Future studies should develop appropriate but flexible strategies to engage and maintain contact with women at this time of significant change in life circumstances. However, such strategies also need to consider the indications that at least 14 sessions with a trained professional over a period of 6 months should to be provided for an intervention to be of sufficient intensity to achieve weight-loss goals.22 While 9 of the included studies provided participants with 10 or more intervention sessions (either face to face [including groups] or by phone),23,25,28,35,36,38,42,45,46 the remainder of the included studies provided considerably fewer sessions.
A limitation of this systematic review is that it does not permit robust recommendations to be made about either the content or the delivery of a postpartum intervention that could be incorporated into clinical care, owing to a lack of reporting of key details about (1) the specific dietary and physical activity content of the intervention; (2) the assessment tools used; and (3) the format of delivering the intervention. Similar limitations attributed to the incomplete reporting of the content of dietary and lifestyle interventions during pregnancy have been identified previously,60 highlighting the need for adequate reporting and complete process evaluation of complex interventions.61
Most included studies reported positive effects, demonstrated by improvements in maternal dietary and physical activity behaviors. While these health behaviors and initial weight changes are important, it remains uncertain whether they are associated with sustained behavioral change. Furthermore, it is unclear if such changes initiated in the postpartum period result in improved maternal and infant health outcomes in a subsequent pregnancy, since none of the studies have reported longer-term follow-up of participants or subsequent pregnancy outcomes.
Weight gain between successive pregnancies is associated with increased risk of gestational diabetes, hypertension, cesarean birth, high birth weight, and perinatal mortality.15,16 However, among women who were overweight or obese at the time of their first pregnancy, weight loss of a single BMI unit (corresponding to weight loss of ≈ 3–5 kg) was associated with a reduction in the risk of giving birth to an infant large for gestational age (odds ratio [OR] = 0.82; 95%CI, 0.72–0.95).16 The effect of interpregnancy weight loss on other pregnancy outcomes, including hypertension (OR = 0.98; 95%CI, 0.70–1.37) and gestational diabetes (OR = 0.96; 95%CI, 0.66–1.37), was less clear.16
Further indirect evidence of a beneficial effect of preconception weight loss on pregnancy and birth outcomes is available from the bariatric surgery literature, although the findings are inconsistent across studies.62 While some reports suggest a reduction in the risk of an infant born large for gestational age and of maternal complications such as hypertension, gestational diabetes, and cesarean birth, others have identified no differences in the risk of pregnancy and birth complications.62
CONCLUSION
Providing women with a dietary and/or physical activity intervention in the postpartum period is associated with modest weight loss after birth, which, on the basis of limited available data, appears to be maintained at 12 months post partum and to be of greater magnitude following a combined dietary and lifestyle intervention. The longer-term effects on sustained behavioral change and on subsequent pregnancy and birth outcomes are still unknown. If improvements in maternal and child health are to be realized, preconception strategies to promote weight loss, including weight loss during the interpregnancy period,63 particularly among women who are overweight or obese, need to be addressed with urgency. For this to occur, it is essential to recognize that randomized postpartum interventional trials should continue to follow women into a subsequent pregnancy and should be adequately funded and resourced.
Supplementary Material
Acknowledgments
Author contributions. J.M.D., A.R.D., C.M.O’B., D.A.J.M.S., and A.P. searched for studies and agreed on inclusion and exclusion. J.M.D. and A.R.D. extracted data and performed the meta-analysis. J.M.D., S.P., and A.G. drafted the manuscript. All authors revised and provided intellectual input into the final manuscript.
Funding/support. J.M.D. is supported through National Health and Medical Research Council (NHMRC) Practitioner Fellowship (ID 627005). S.P. is supported through a National Institutes of Health grant (DK087889).
Declaration of interest. J.M.D., A.R.D., C.M.O’B., D.A.J.M.S., A.P., and A.G. have no relevant interests to declare. S.P. received a research grant (not in field of women’s health) from Weight Watchers International, Inc.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 PRISMA checklist
Figure S1 Combined dietary and physical activity intervention for the outcome weight loss at the completion of the intervention; sensitivity analysis by adequate processes of randomization
Figure S2 Combined dietary and physical activity intervention for the outcome weight loss at the completion of the intervention; sensitivity analysis by low rates of postrandomization loss to follow-up or withdrawals
Table S1 Sources searched
Table S2 MEDLINE search strategy
Table S3 Characteristics of ongoing studies
Table S4 Characteristics of excluded studies
References
- 1. Chu SY, Kim SY, Bish CL.. Prepregnancy obesity prevalence in the United States, 2004 − 2005. Matern Child Health J. 2009;13:614–620. [DOI] [PubMed] [Google Scholar]
- 2. Dodd JM, Grivell RM, Nguyen A-M et al. , . Maternal and perinatal health outcomes by body mass index category. Aust N Z J Obstet Gynaecol. 2011;51:136–140. [DOI] [PubMed] [Google Scholar]
- 3. Dodd JM, Cramp C, Sui Z et al. , . The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med. 2014;12:161. doi:10.1186/s12916-014-0161-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dodd JM, Turnbull DA, McPhee AJ et al. , . Antenatal lifestyle advice for women who are overweight or obese: the LIMIT randomised trial. BMJ. 2014;348:g1285. doi:10.1111/1471-0528.13796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poston L, Bell R, Croker H et al. , . Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diab Endocrinol. 2015;3:767–777. [DOI] [PubMed] [Google Scholar]
- 6. International Weight Management in Pregnancy Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ. 2017;358:j3119. doi:10.1136/bmj.j3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poston L, Caleyachetty R, Cnattingius S et al. , . Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diab Endocrinol. 2016;4:1025–1036. [DOI] [PubMed] [Google Scholar]
- 8. American College of Obstetricians and Gynecologists. ACOG Committee Opinion number 315, September 2005. Obesity in pregnancy. Obstet Gynecol. 2005;106:671–675. [DOI] [PubMed] [Google Scholar]
- 9. Hanson MA, Bardsley A, De-Regil LM et al. , . The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int J Gynaecol Obstet. 2015;131(suppl 4):S213–S253. [DOI] [PubMed] [Google Scholar]
- 10. Mannan M, Doi SA, Mamun AA.. Association between weight gain during pregnancy and postpartum weight retention and obesity: a bias-adjusted meta-analysis. Nutr Rev. 2013;71:343–352. [DOI] [PubMed] [Google Scholar]
- 11. Gunderson EP, Abrams B.. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 2000;22:261–274. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt NM, Nicholson WK, Schmitt J.. The association of pregnancy and the development of obesity—results of a systematic review and meta-analysis on the natural history of postpartum weight retention. Int J Obes. 2007;31:1642–1651. [DOI] [PubMed] [Google Scholar]
- 13. Walls HL, Magliano DJ, Stevenson CE et al. , . Projected progression of the prevalence of obesity in Australia. Obesity (Silver Spring). 2012;20:872–878. [DOI] [PubMed] [Google Scholar]
- 14. Cnattingius S, Villamor E, Lagerros YT et al. , . High birth weight and obesity—a vicious circle across generations. Int J Obes. 2012;36:1320–1324. [DOI] [PubMed] [Google Scholar]
- 15. Cnattingius S, Villamor E.. Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet. 2016;387:558–565. [DOI] [PubMed] [Google Scholar]
- 16. Villamor E, Cnattingius S.. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–1170. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2011]. The Cochrane Collaboration; 2011. [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J et al. , . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 20. Jean Hailes Foundation for Women’s Health on behalf of the PCOS Australian Alliance. Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Melbourne, Australia: Jean Hailes Foundation for Women’s Health; 2011. [Google Scholar]
- 21. Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen, Denmark: The Nordic Centre; 2014. [Google Scholar]
- 22. Ryan D, Heaner M.. Guidelines (2013) for managing overweight and obesity in adults. Preface to the full report. Obesity (Silver Spring). 2014;22(suppl 2):S1–S3. [DOI] [PubMed] [Google Scholar]
- 23. Berry D, Verbiest S, Hall EG et al. , . A postpartum community-based weight management intervention designed for low-income women: feasibility and initial efficacy testing. J Natl Black Nurses Assoc. 2015;26:29–39. [PubMed] [Google Scholar]
- 24. Bertz F, Brekke HK, Ellegard L et al. , . Diet and exercise weight-loss trial in lactating overweight and obese women. Am J Clin Nutr. 2012;96:698–705. [DOI] [PubMed] [Google Scholar]
- 25. Chang MW, Brown R, Nitzke S.. Results and lessons learned from a prevention of weight gain program for low-income overweight and obese young mothers: Mothers in Motion. BMC Public Health. 2017;17:182. doi:10.1186/s12889-017-4109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colleran HL, Wideman L, Lovelady CA.. Effects of energy restriction and exercise on bone mineral density during lactation. Med Sci Sports Exerc. 2012;44:1570–1579. [DOI] [PubMed] [Google Scholar]
- 27. Craigie AM, Macleod M, Barton KL et al. , . Supporting postpartum weight loss in women living in deprived communities: design implications for a randomised control trial. Eur J Clin Nutr. 2011;65:952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. deRosset L, Berry DC, Sanchez-Lugo L et al. , . Mama Sana … Usted Sana: lessons learned from a postpartum weight loss intervention for Hispanic women with infants six months or less. Hisp Health Care Int. 2013;11:78–86. [DOI] [PubMed] [Google Scholar]
- 29. Dewey KG, Lovelady CA, Nommsen-Rivers LA et al. , . A randomized study of the effects of aerobic exercise by lactating women on breast-milk volume and composition. N Engl J Med. 1994;330:449–453. [DOI] [PubMed] [Google Scholar]
- 30. Gilmore LA, Klempel MC, Martin CK et al. , . Personalized mobile health intervention for health and weight loss in postpartum women receiving women, infants, and children benefit: a randomized controlled pilot study. J Womens Health (Larchmt). 2017;26:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haire-Joshu DL, Schwarz CD, Peskoe SB et al. , . A group randomized controlled trial integrating obesity prevention and control for postpartum adolescents in a home visiting program. Int J Behav Nutr Phys Act. 2015;12:88. doi:10.1186/s12966-015-0247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herring SJ, Cruice JF, Bennett GG et al. , . Using technology to promote postpartum weight loss in urban, low-income mothers: a pilot randomized controlled trial. J Nutr Educ Behav. 2014;46:610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang TT, Yeh CY, Tsai YC.. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery. 2011;27:257–264. [DOI] [PubMed] [Google Scholar]
- 34. Huseinovic E, Bertz F, Leu Agelii M et al. , . Effectiveness of a weight loss intervention in postpartum women: results from a randomized controlled trial in primary health care. Am J Clin Nutr. 2016;104:362–370. [DOI] [PubMed] [Google Scholar]
- 35. Keller C, Ainsworth B, Records K et al. , . A comparison of a social support physical activity intervention in weight management among post-partum Latinas. BMC Public Health. 2014;14:971. doi:10.1186/1471-2458-14-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krummel D, Semmens E, MacBride AM et al. , . Lessons learned from the Mothers' Overweight Management Atudy in 4 West Virginia WIC offices. J Nutr Educ Behav. 2010;42(3 suppl):S52–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leermakers EA, Anglin K, Wing RR.. Reducing postpartum weight retention through a correspondence intervention. Int J Obes Relat Metab Disord. 1998;22:1103–1109. [DOI] [PubMed] [Google Scholar]
- 38. Lovelady CA, Garner KE, Moreno KL et al. , . The effect of weight loss in overweight, lactating women on the growth of their infants. N Engl J Med. 2000;342:449–453. [DOI] [PubMed] [Google Scholar]
- 39. Maturi MS, Afshary P, Abedi P.. Effect of physical activity intervention based on a pedometer on physical activity level and anthropometric measures after childbirth: a randomized controlled trial. BMC Pregnancy Childbirth. 2011;11:103. doi:10.1186/1471-2393-11-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCrory MA, Nommsen-Rivers LA, Molé PA et al. , . Randomized trial of the short-term effects of dieting compared with dieting plus aerobic exercise on lactation performance. Am J Clin Nutr. 1999;69:959–956. [DOI] [PubMed] [Google Scholar]
- 41. McIntyre HD, Peacock A, Miller YD et al. , . Pilot study of an individualised early postpartum intervention to increase physical activity in women with previous gestational diabetes. Int J Endocrinol. 2012;2012:892019. doi:10.1155/2012/892019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nicklas JM, Zera CA, England LJ et al. , . A web-based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2014;124:563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ostbye T, Krause KM, Lovelady CA et al. , . Active mothers postpartum: a randomized controlled weight-loss intervention trial. Am J Prev Med. 2009;37:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ostbye T, Krause KM, Stroo M et al. , . Parent-focused change to prevent obesity in preschoolers: results from the KAN-DO study. Prev Med. 2012;55:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Toole ML, Sawicki MA, Artal R.. Structured diet and physical activity prevent postpartum weight retention. J Womens Health. 2003;12:991–998. [DOI] [PubMed] [Google Scholar]
- 46. Phelan S, Hagobian T, Brannen A et al. , . Effect of an internet-based program on weight loss for low-income postpartum women: a randomized clinical trial. JAMA. 2017;317:2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reinhardt JA, van der Ploeg HP, Grzegrzulka R et al. , . lmplementing lifestyle change through phone-based motivational interviewing in rural-based women with previous gestational diabetes mellitus. Health Promot J Aust. 2012;23:5–9. [DOI] [PubMed] [Google Scholar]
- 48. Tripette J, Murakami H, Gando Y et al. , . Home-based active video games to promote weight loss during the postpartum period. Med Sci Sports Exerc. 2014;46:472–478. [DOI] [PubMed] [Google Scholar]
- 49. Walker LO, Sterling BS, Latimer L et al. , . Ethnic-specific weight-loss interventions for low-income postpartum women: findings and lessons. West J Nurs Res. 2012;34:654–676. [DOI] [PubMed] [Google Scholar]
- 50. Rosal MC, Haughton CF, Estabrook BB et al. , . Fresh Start, a postpartum weight loss intervention for diverse low-income women: design and methods for a randomized clinical trial. BMC Public Health. 2016;16:953. doi:10.1186/s12889-016-3520-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. ClinicalTrials.gov. Identifier NCT02763150. Pre-pregnancy lifestyle intervention to prevent the recurrence of gestational diabetes in overweight and obese women. Bethesda, MD: US National Library of Medicine; 2016. https://clinicaltrials.gov/ct2/show/NCT02763150?term=weight+loss&cond=pre-pregnancy&rank=2. Accessed August 15, 2017.
- 52. Assaf-Balut C, Garcia de la Torre N, Duran A et al. , . A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): a randomized controlled trial: the St. Carlos GDM prevention study. PLoS One. 2017;12:e0185873. doi:10.1371/journal.pone.0185873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davenport MH, Giroux I, Sopper MM et al. , . Postpartum exercise regardless of intensity improves chronic disease risk factors. Med Sci Sports Exerc. 2011;43:951–958. [DOI] [PubMed] [Google Scholar]
- 54. Kinnunen T, Pasanen M, Aittasalo M et al. , . Reducing postpartum weight retention—a pilot trial in primary health care. Nutr J. 2007;6:21. doi:10.1186/1475-2891-6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Martin J, MacDonald-Wicks L, Hure A et al. , . Reducing postpartum weight retention and improving breastfeeding outcomes in overweight women: a pilot randomised controlled trial. Nutrients. 2015;7:1464–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peccei A, Blake-Lamb T, Rahilly D et al. , . Intensive prenatal nutrition counseling in a community health setting: a randomized controlled trial. Obstet Gynecol. 2017;130:423–432. [DOI] [PubMed] [Google Scholar]
- 57. Taveras EM, Blackburn K, Gillman MW et al. , . First Steps for Mommy and Me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wilkinson SA, van der Pligt P, Gibbons KS et al. , . Trial for reducing weight retention in new mums: a randomised controlled trial evaluating a low intensity, postpartum weight management programme. J Hum Nutr Diet. 2015;28:15–28. [DOI] [PubMed] [Google Scholar]
- 59. Zourladani A, Tsaloglido A, Tzetzis G et al. , . The effect of a low impact exercise training programme on the well-being of Greek postpartum women: a randomised controlled trial. Int Sportmed J. 2012;12:30–38. [Google Scholar]
- 60. Flynn AC, Dalrymple K, Barr S et al. , . Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev. 2016;74:312–328. [DOI] [PubMed] [Google Scholar]
- 61. Poston L, Briley AL, Barr S et al. , . Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth. 2013;13:148. doi:10.1186/1471-2393-13-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Willis K, Sheiner E.. Bariatric surgery and pregnancy: the magical solution? J Perinat Med. 2013;41:133–140. [DOI] [PubMed] [Google Scholar]
- 63. Hanson MA, Barker M, Bustreo F et al. , . Interventions to prevent preconception and maternal obesity. Lancet Diab Endocrinol. 2017;5:65–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.