Abstract
Background
Self-reported smoking is the principal measure used to assess lung cancer risk in epidemiological studies. We evaluated if circulating cotinine—a nicotine metabolite and biomarker of recent tobacco exposure—provides additional information on lung cancer risk.
Methods
The study was conducted in the Lung Cancer Cohort Consortium (LC3) involving 20 prospective cohort studies. Pre-diagnostic serum cotinine concentrations were measured in one laboratory on 5364 lung cancer cases and 5364 individually matched controls. We used conditional logistic regression to evaluate the association between circulating cotinine and lung cancer, and assessed if cotinine provided additional risk-discriminative information compared with self-reported smoking (smoking status, smoking intensity, smoking duration), using receiver-operating characteristic (ROC) curve analysis.
Results
We observed a strong positive association between cotinine and lung cancer risk for current smokers [odds ratio (OR ) per 500 nmol/L increase in cotinine (OR500): 1.39, 95% confidence interval (CI): 1.32–1.47]. Cotinine concentrations consistent with active smoking (≥115 nmol/L) were common in former smokers (cases: 14.6%; controls: 9.2%) and rare in never smokers (cases: 2.7%; controls: 0.8%). Former and never smokers with cotinine concentrations indicative of active smoking (≥115 nmol/L) also showed increased lung cancer risk. For current smokers, the risk-discriminative performance of cotinine combined with self-reported smoking (AUCintegrated: 0.69, 95% CI: 0.68–0.71) yielded a small improvement over self-reported smoking alone (AUCsmoke: 0.66, 95% CI: 0.64–0.68) (P = 1.5x10–9).
Conclusions
Circulating cotinine concentrations are consistently associated with lung cancer risk for current smokers and provide additional risk-discriminative information compared with self-report smoking alone.
Keywords: Cotinine, biomarker, lung cancer, consortium, case-control, prospective
Key Messages
Among current smokers, circulating cotinine concentrations are consistently associated with lung cancer risk in a dose-response manner.
Among current smokers, cotinine combined with self-reported smoking provides a small but measurable risk-discriminative performance over self-reported smoking alone.
Cotinine as a biomarker for recent tobacco exposure reduces misclassification, particularly among self-reported former and never smokers.
Introduction
Lung cancer accounts for 1.69 million deaths every year,1 and 20% of all cancer deaths worldwide.2 Tobacco smoking is the main determinant of lung cancer risk,3 but is typically self-reported which may underestimate lung cancer risk estimates in epidemiological studies.4
Tobacco smoke contains more than 7000 chemical compounds, many of which are carcinogenic.2 Tobacco also contains nicotine, a highly addictive compound that is primarily metabolized to cotinine in the liver.5 Circulating cotinine has a half-life of 7–40 h.6,7 As such, circulating cotinine concentrations among active smokers likely reflect recent smoking intensity,8 whereas circulating cotinine concentrations in self-reported former or never smokers may indicate exposure to second-hand tobacco smoke or active smoking (i.e. misclassification in an epidemiological study). Cotinine can therefore serve as a biomarker for recent tobacco smoke exposure regardless of self-reported smoking status, and may provide improved estimates of lung cancer risk compared with exposure data derived from self-reported smoking alone. Second-hand smoke is classified as carcinogenic to humans,9 and nicotine is present in second-hand smoke.10 However, it is not yet known if self-reported never smokers with circulating cotinine concentrations consistent with second-hand smoke exposure are at increased lung cancer risk.
Only two prospective studies have evaluated the association between pre-diagnostic circulating cotinine concentrations and lung cancer risk for both sexes and across smoking categories.11,12 Both the European Prospective Investigation into Cancer and Nutrition (EPIC) study11 and a prospective study with data from the Janus biobank in Norway12 report positive linear associations between circulating cotinine concentrations and lung cancer risk for current smokers. Despite reasonably large sample sizes overall, neither previous study had sufficient power to provide robust risk estimates for never smokers (EPIC: n = 96 never smoking cases; Janus: n = 53 never smoking cases).
In the current study, we sought to comprehensively evaluate the association between circulating cotinine concentrations and lung cancer risk for self-reported current, former and never smokers. We used pre-diagnostic serum samples of 5364 case-control pairs from the Lung Cancer Cohort Consortium (LC3). The LC3 includes 20 prospective cohorts from the USA (US), Europe (EU), Australia (AU) and Asia. Lung cancer cases in never and former smokers were intentionally oversampled to allow for risk-stratified analysis by self-reported smoking status. We also aimed to evaluate the risk-discriminative performance of circulating cotinine alone, self-reported smoking alone and circulating cotinine combined with self-reported smoking among current and former smokers separately.
Methods
Study population
All prospective cohort studies with frozen baseline plasma or serum samples, which were members of the US National Cancer Institute (NCI) Cohort Consortium in 2009, were invited to participate in the Lung Cancer Cohort Consortium (LC3). A total of 20 NCI cohorts met the eligibility criteria and agreed to participate in the LC3. The combined LC3 cohort population included more than 2 million study participants from the USA, EU, AU and Asia. The LC3 project was approved by the institutional review boards of each participating cohort. All LC3 participants provided written informed consent. Further information about individual LC3 cohorts, including follow-up procedures, has been previously published.13
Selection of cases and controls
We defined lung cancer cases as all invasive cancers coded C34.0 to C34.9 in the International Classification of Diseases for Oncology, Second Edition (ICD-O-2). In all, 11 399 incident lung cancer cases were identified, from which 5545 cases were selected for the current study. Former and never smokers were intentionally oversampled to improve statistical power for smoking-stratified analyses. Cases and controls were individually matched (1 case: 1 control) by cohort, sex (male or female), age (initially ± 1 year, subsequently relaxed to ± 3 years), ethnicity (US cohorts only), date of blood draw (initially ± 1 month, subsequently relaxed to ± 3 months) and smoking status (current, former or never). Former smokers were matched by time since quitting (<10 years or ≥10 years) for those cohorts with available information. Current smokers were matched by smoking intensity (<15 cigarettes per day (CPD) or ≥ 15 CPD) for those cohorts with available information. The self-reported smoking status variable used in the current analysis was a composite variable including tobacco use from cigarettes, cigars and pipes. Variables used for smoking intensity and smoking duration were also composite variables including tobacco use from cigarettes, cigars and pipes. After matching, a total of 5364 case-control pairs remained and were included in the current analysis.
Circulating cotinine concentrations
Centralized biochemical analysis of free circulating cotinine concentrations was performed by mass spectrometry (LC-MS/MS)14 at BEVITAL Laboratory in Bergen, Norway [www.bevital.no]. The lower limit of detection was 1 nmol/L, within-day coefficient of variation (CV) was 2–3%, and between-day CV was 6%. The intra-class correlation coefficient of cotinine is 0.89–0.95.15 In the current study, exposure to second-hand smoke among non-smokers (former and never) was defined as circulating cotinine concentrations between 5 and 115 nmol/L (∼1 ng/ml and ∼20 ng/ml, respectively).8,16 We considered cotinine concentrations <5 nmol/L as unexposed, and cotinine concentrations ≥115 nmol/L as indicative of active smoking.8,16 Due to the declining prevalence of smoking in some European countries and in the USA, the most commonly reported cut-point for second-hand smoke exposure is <85 nmol/L (∼15 ng/ml).8 Given that the LC3 includes cohorts from Asia where the smoking epidemic is on the rise,2 and taking into consideration potential differences in metabolic efficiency of study participants, we chose to use the uppermost threshold of circulating cotinine which has been previously used to define exposure to second-hand smoke (115 nmol/L ∼20 ng/ml).8 We did, however, repeat all analyses using cotinine concentrations <85 nmol/L as a cut-point, and observed no substantial change in any final results reported herein (data not shown).
Statistical analyses
We used analysis of variance to test if mean circulating cotinine concentrations differed among cohorts and between cases and controls after stratification by self-reported smoking status. The association between circulating cotinine concentration and lung cancer risk was evaluated using conditional logistic regression to calculate odds ratios (ORs) for each 500 nmol/L increase in cotinine concentration (OR500), without further adjustment for additional smoking variables or other risk factors. Risk analyses were performed for the LC3 study population overall, and stratified by self-reported smoking status (current, former or never), region (USA, EU/AU, Asia) and sex (male or female). We assessed heterogeneity by region, and interaction by sex with the likelihood-ratio test. To provide relative risk estimates for specific categories of cotinine concentration among current smokers, we calculated ORs for six categories of cotinine (nmol/L: 115 < 500, 500 < 1000, 1000 < 1500, 1500 < 2000, 2000 < 2500, ≥2500) with 0 < 115 nmol/L as the reference category. We separately adjusted these ORs for smoking intensity at time of blood draw [continuous, number of cigarettes per day (CPD)]. Where available, the CPD variable also included data on number of times per day the participant smoked tobacco from cigars or pipes.
To evaluate the association between exposure to second-hand smoke and lung cancer risk, we estimated ORs for former and never smokers separately by comparing participants with circulating cotinine concentrations between 5 < 115 nmol/L (exposed) to participants with circulating cotinine concentrations below 5 nmol/L (unexposed).
We used receiver operating characteristic (ROC) curves to evaluate the extent to which circulating cotinine could discriminate between future lung cancer cases and controls among current and former smokers. We evaluated the area under the curve (AUC) for three separate risk discrimination models: (i) cotinine alone (AUCcot); (ii) self-reported smoking alone (AUCsmoke); and (iii) cotinine combined with self-reported smoking in an integrated model (AUCintegrated). We used nonparametric methods according to DeLong et al.17 to evaluate differences in AUC estimates. The risk models based on self-reported smoking information included smoking status at time of blood draw (current or former), smoking duration at time of blood draw (continuous, number of years participant regularly smoked tobacco from cigarettes, pipes or cigars), and smoking intensity at time of blood draw (continuous, number of cigarettes, pipes or cigars per day (CPD)).
All statistical analyses were conducted using Stata version 14.2,18 and RStudio version 3.3.0
Results
Baseline characteristics
The current study population included 5364 lung cancer cases and 5364 individually matched controls (Table 1). The overall male-to-female ratio was similar, with some regional variation by participant sex and self-reported smoking status. US cohorts contributed the most women and the most former smokers, whereas Asian cohorts contributed the most men and the most never smokers. Current smokers accounted for nearly half of the overall study population (current: 47%, 2519 case-control pairs) with former and never smokers contributing approximately one-quarter each (former: 28%, 1518 case-control pairs; never: 25%, 1327 case-control pairs). Median age at cohort recruitment was 60 years, and median age at lung cancer diagnosis was 70 years.
Table 1.
Baseline and clinical characteristics of study participants overall and by continent in the Lung Cancer Cohort Consortium (LC3)
| US cohorts |
EU/AU cohorts |
Asian cohorts |
Overall |
|||||
|---|---|---|---|---|---|---|---|---|
| No. (%) of participants in each group |
No. (%) of participants in each group |
No. (%) of participants in each group |
No. (%) of participants in each group |
|||||
| Cases | Matched controls | Cases | Matched controls | Cases | Matched controls | Cases | Matched controls | |
| (n = 2400) | (n = 2400) | (n = 1189) | (n = 1189) | (n = 1775) | (n = 1775) | (n = 5364) | (n = 5364) | |
| Sex | ||||||||
| Men | 991 (41.3%) | 991 (41.3%) | 688 (57.9%) | 688 (57.9%) | 1229 (69.2%) | 1229 (69.2%) | 2908 (54.2%) | 2908 (54.2%) |
| Women | 1409 (58.7%) | 1409 (58.7%) | 501 (42.1%) | 501 (42.1%) | 546 (30.8%) | 546 (30.8%) | 2456 (45.8%) | 2456 (45.8%) |
| Smoking status | ||||||||
| Never | 569 (23.7%) | 569 (23.7%) | 156 (13.1%) | 156 (13.1%) | 602 (33.9%) | 602 (33.9%) | 1327 (24.7%) | 1327 (24.7%) |
| cotinine <5 nmol/L | 509 (89.5%) | 516 (90.7%) | 130 (83.3%) | 124 (79.5%) | 430 (71.4%) | 425 (70.6%) | 1069 (80.6%) | 1065 (80.2%) |
| cotinine 5-115 nmol/L | 46 (8.1%) | 50 (8.8%) | 22 (14.1%) | 28 (17.9%) | 153 (25.4%) | 174 (28.9%) | 221 (16.7%) | 252 (19.0%) |
| cotinine ≥115 nmol/L | 14 (2.4%) | 3 (0.5%) | 4 (2.6%) | 4 (2.6%) | 19 (3.2%) | 3 (0.5%) | 37 (2.7%) | 10 (0.8%) |
| Former | 1007 (42.0%) | 1007 (42.0%) | 335 (28.2%) | 335 (28.2%) | 176 (9.9%) | 176 (9.9%) | 1518 (28.3%) | 1518 (28.3%) |
| cotinine <5 nmol/L | 720 (71.5%) | 785 (78.0%) | 209 (62.4%) | 230 (68.7%) | 113 (64.2%) | 116 (65.9%) | 1042 (68.7%) | 1131 (74.5%) |
| cotinine 5-115 nmol/L | 136 (13.5%) | 142 (14.1%) | 64 (19.1%) | 62 (18.5%) | 54 (30.7%) | 44 (25.0%) | 254 (16.7%) | 248 (16.3%) |
| cotinine ≥115 nmol/L | 151 (15.0%) | 80 (7.9%) | 62 (18.5%) | 43 (12.8%) | 9 (5.1%) | 16 (9.1%) | 222 (14.6%) | 139 (9.2%) |
| cigarettes per day,a mean (SD) | 26 (15) | 21 (14) | 23 (16) | 18 (15) | 23 (16) | 16 (13) | 25 (15) | 19 (15) |
| Current | 824 (34.3%) | 824 (34.3%) | 698 (58.7%) | 698 (58.7%) | 997 (56.2%) | 997 (56.2%) | 2519 (47.0%) | 2519 (47.0%) |
| cotinine <5 nmol/L | 31 (3.8%) | 47 (5.7%) | 6 (0.9%) | 5 (0.7%) | 15 (1.5%) | 15 (1.5%) | 52 (2.1%) | 67 (2.7%) |
| cotinine 5<115 nmol/L | 14 (1.7%) | 29 (3.5%) | 5 (0.7%) | 16 (2.3%) | 20 (2.0%) | 50 (5.0%) | 39 (1.5%) | 95 (3.8%) |
| cotinine ≥115 nmol/L | 779 (94.5%) | 748 (90.8%) | 687 (98.4%) | 677 (97.0%) | 962 (96.5%) | 932 (93.5%) | 2428 (96.4%) | 2357 (93.5%) |
| cigarettes per day,a mean (SD) | 22 (12) | 21 (12) | 18 (9) | 17 (9) | 20 (10) | 16 (9) | 20 (11) | 18 (10) |
| Serum cotinine, nmol/L, mean (SD) | 542 (789) | 446 (731) | 804 (731) | 685 (670) | 710 (781) | 536 (646) | 655 (781) | 529 (696) |
| Never | 36 (236) | 5 (51) | 23 (131) | 40 (237) | 25 (140) | 9 (59) | 29 (187) | 11 (97) |
| Former | 169 (484) | 101 (451) | 225 (539) | 156 (467) | 40 (165) | 75 (264) | 166 (474) | 110 (438) |
| Current | 1345 (703) | 1172 (705) | 1257 (515) | 1083 (511) | 1241 (651) | 936 (602) | 1280 (636) | 1054 (623) |
| Age at recruitment, years, median (5th–95th percentile) | 60 (42-74) | 60 (42-74) | 60 (45-70) | 60 (45-70) | 60 (46-72) | 60 (46-72) | 60 (44-72) | 60 (44-72) |
| Age at diagnosis, years, median (5th – 95th percentile) | 70 (55-83) | 69 (54-79) | 70 (52-80) | 70 (54-82) | ||||
Where available, the cigarettes per day variable also included data on number of times per day the participant smoked cigars or pipes.
Circulating cotinine concentrations
We observed substantial regional variation in mean circulating cotinine concentrations (Table 1). The distribution of cotinine concentrations in current, former and never smoking study participants are depicted in Supplementary Figure 1, available as Supplementary data at IJE online. For current smokers, we observed slightly higher mean cotinine concentrations in both cases and controls among US participants (cases: 1346 nmol/L; controls: 1172 nmol/L) compared with participants from the EU/AU (cases: 1257 nmol/L; controls: 1083 nmol/L) and Asia (cases: 1241 nmol/L; controls: 936 nmol/L) (P < 0.001). Former smoking cases from the EU/AU cohorts had higher mean circulating cotinine concentrations (225 nmol/L) than former smoking cases from the US (169 nmol/L) or Asia (40 nmol/L) ( P < 0.001). For never smoking controls, we observed higher mean cotinine concentrations in EU/AU cohorts (40 nmol/L) compared with never smoking controls from the US (5 nmol/L) and Asian (9 nmol/L) cohorts (P < 0.001). Circulating cotinine concentrations consistent with active smoking (≥115 nmol/L) were common in self-reported former smokers (cases: 14.6%; controls: 9.2%), and rare in self-reported never smokers (cases overall: 2.7%; controls overall: 0.8%) (Table 1; Supplementary Figure 1, available as Supplementary data at IJE online). A sensitivity analysis, whereby we considered circulating cotinine concentrations consistent with active smoking to be ≥85 nmol/L, did not substantially change the results for former smokers (cases: 15.5%; controls: 9.4%) or never smokers (cases: 2.9%; controls: 0.9%).
Cotinine and lung cancer risk in current smokers
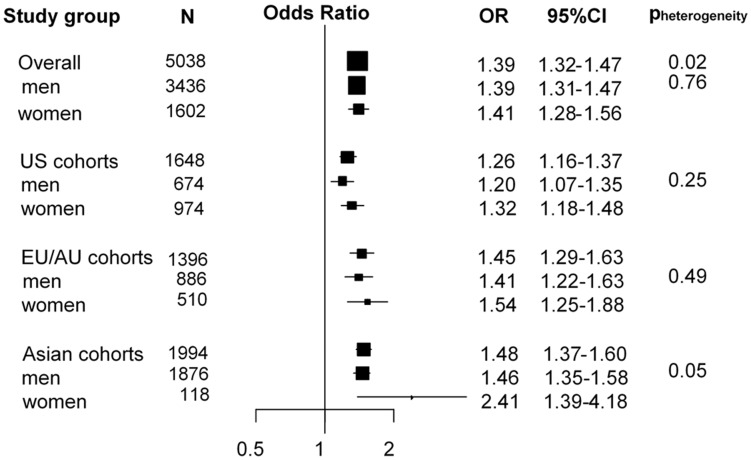
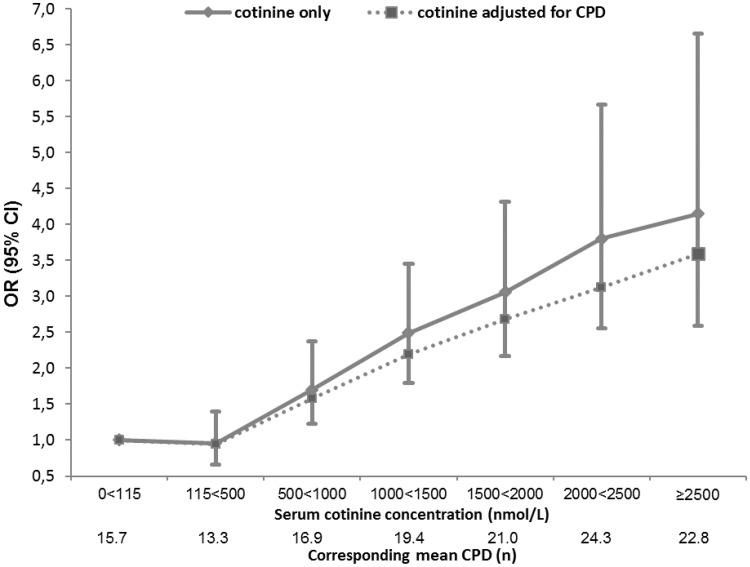
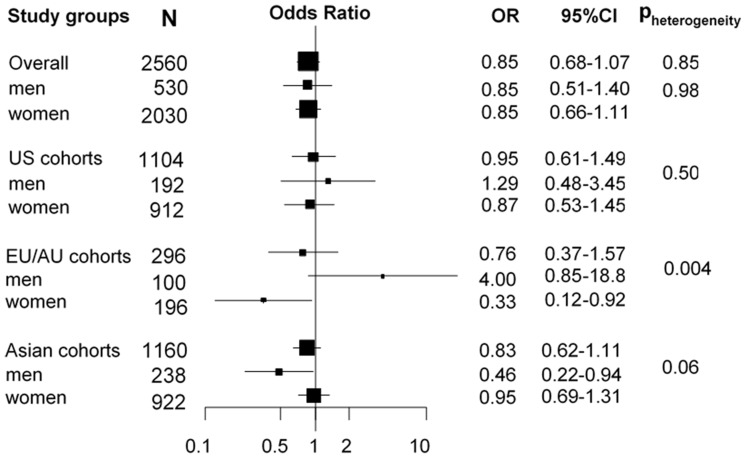
For current smokers, higher circulating cotinine concentrations were consistently associated with increased lung cancer risk (OR per 500 nmol/L increase in cotinine [OR500]: 1.39, 95% CI: 1.32–1.47) (Figure 1), with a stronger association observed for current smoking women from Asian cohorts (OR: 2.41, 95% CI: 1.39–4.18) (Pheterogeneity for continent = 0.02; Pinteraction for sex = 0.05). We observed a strong dose-response relationship between cotinine and lung cancer risk for current smokers, with no apparent plateau in OR at higher cotinine concentrations (Figure 2). The OR reached 4.15 (95% CI: 2.59–6.66) for current smokers with cotinine concentrations ≥2500 nmol/L compared with current smokers with cotinine concentrations between 0 and 115 nmol/L (Figure 2). These ORs were only moderately impacted by adjustment for self-reported smoking intensity (CPD) (Figure 2).
Figure 1.
Forest plot showing odds ratios (OR) and 95% confidence intervals (95% CI) of lung cancer risk per 500 nmol/L increase in cotinine concentrations among all current smokers in the Lung Cancer Cohort Consortium (LC3). Pheterogeneity indicates differences in OR estimates between regions in the overall analysis and between men and women in the stratified analysis.
Figure 2.
Odds ratios (OR) and 95% confidence intervals (CI) for lung cancer risk by cotinine concentration before and after adjustment for self-reported cigarettes per day (CPD) among all current smokers in the Lung Cancer Cohort Consortium (LC3). Where available, the CPD variable also included data on number of times per day the participant smoked cigars or pipes. Corresponding mean CPD for each category of cotinine concentration is given. Current smokers with cotinine levels below 115 nmol/L were used as the reference group.
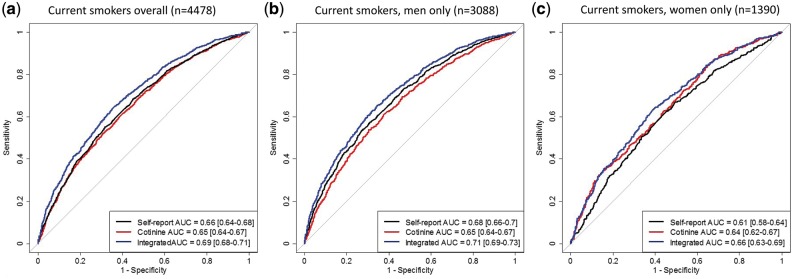
In discrimination analysis for current smokers, the cotinine only model yielded a similar area under the curve (AUC) (AUCcot: 0.65, 95% CI: 0.64–0.67) as the self-reported smoking model (status, duration, intensity) (AUCsmoke: 0.66, 95% CI: 0.64–0.68) (Figure 3a). We saw a small improvement in discrimination between future lung cancer cases and controls when cotinine was combined with self-reported smoking in an integrated model (AUCintegrated: 0.69, 95% CI: 0.68–0.71) (Figure 3a) (P = 1.5x10–9). The integrated model (cotinine model + self-reported smoking model) showed similar improvement for current smoking men (Figure 3b) and current smoking women (Figure 3c).
Figure 3.
Receiver operating characteristic (ROC) curve and area under the curve (AUC) for risk-discriminative performance of cotinine alone (AUCcot), self-reported smoking alone (AUCsmoke), and cotinine combined with self-reported smoking (AUCintegrated) in current smokers overall, and stratified by sex in the Lung Cancer Cohort Consortium (LC3). This analysis includes current smokers with complete data on cotinine as well as self-reported smoking status, smoking intensity and smoking duration.
Cotinine and lung cancer risk in former smokers
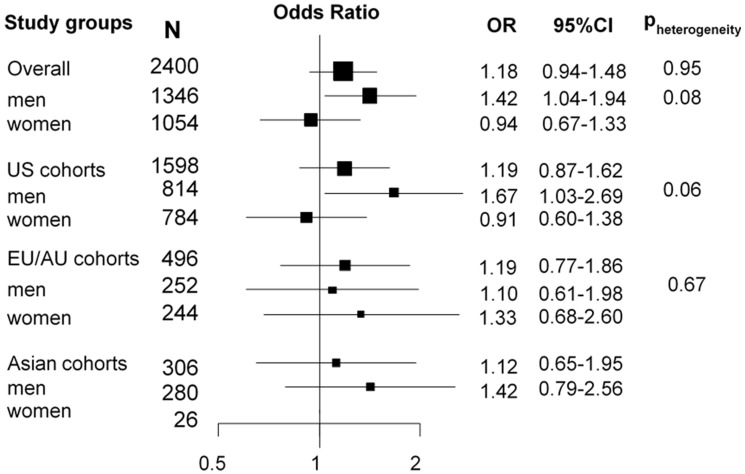
For former smokers, we observed a positive association between increasing cotinine concentrations and lung cancer risk (OR500: 1.17, 95% CI: 1.07–1.28) (Supplementary Figure 2, available as Supplementary data at IJE online), which was likely a reflection of the higher proportion of former smoking cases (14.6%) than former smoking controls (9.2%) with cotinine concentrations indicative of active smoking (Table 1). However, when we excluded former smoking participants with cotinine concentrations ≥115 nmol/L (Figure 4), the confidence interval widened (ORrestricted: 1.18, 95% CI: 0.94–1.48), even though the odds ratio was not attenuated. This association appeared to be driven by former smoking men from US cohorts (ORrestricted US men: 1.67, 95% CI: 1.03–2.69) (Figure 4).
Figure 4.
Forest plot showing odds ratios (OR) and 95% confidence intervals (95% CI) of lung cancer risk for former smokers, comparing participants having circulating cotinine concentrations 5-115 nmol/L with participants having circulating cotinine concentration below 5 nmol/L. Pheterogeneity indicates differences in OR estimates between regions in the overall analysis and between men and women in the stratified analysis.
In discrimination analysis for former smokers, an integrated model combining circulating cotinine concentration and self-reported smoking (status, duration, intensity) showed no improved discrimination between future lung cancer cases and controls (AUCintegrated: 0.74, 95% CI: 0.71–0.76) when compared with the model based on self-reported smoking alone (AUCsmoke: 0.73, 95% CI: 0.71–0.75) (Supplementary Figure 3, available as Supplementary data at IJE online).
Cotinine and lung cancer risk in never smokers
For never smokers, we observed a positive association between circulating cotinine concentration and lung cancer risk (OR500: 1.64, 95% CI: 1.17–2.30) (Supplementary Figure 4, available as Supplementary data at IJE online), but this association was driven by never smoking US men with cotinine concentrations indicative of active smoking (cotinine ≥ 115 nmol/L) (OR500 in US men: 2.25, 95% CI: 0.98–5.18) (Supplementary Figure 4, available as Supplementary data at IJE online). When we excluded never smoking participants with cotinine concentrations ≥115 nmol/L, no association between cotinine and lung cancer risk was observed (ORrestricted: 0.85, 95% CI: 0.68–1.07) (Figure 5).
Figure 5.
Forest plot showing odds ratios (OR) and 95% confidence intervals (95% CI) of lung cancer risk for never smokers, comparing participants with circulating cotinine concentrations 5-115 nmol/L with participants with circulating cotinine concentrations below 5 nmol/L. Pheterogeneity indicates differences in OR estimates between regions in the overall analysis and between men and women in the stratified analysis.
Discussion
We comprehensively evaluated the association between circulating cotinine concentration—an objective measure of recent tobacco exposure—and lung cancer risk within 20 prospective cohorts from the USA, Europe, Australia and Asia. Higher cotinine concentrations were consistently associated with increased lung cancer risk for current smokers in a dose-response manner. In former and never smokers, cotinine was primarily associated with lung cancer risk for participants with cotinine concentrations indicative of active smoking. We further assessed the risk-discriminative performance of circulating cotinine compared to, and combined with, self-reported smoking. In current but not former smokers, combining cotinine with self-reported smoking information in an integrated model yielded a small improvement in discrimination between future lung cancer cases and controls compared with a self-reported smoking model.
Cotinine and misclassification by self-reported smoking
Any risk factor based on self-reported information is prone to misclassification in epidemiological studies, including self-reported smoking. As our results indicate, circulating cotinine—as an objective biomarker for recent tobacco exposure—has the potential to identify misclassified self-reported non-smokers (former and never smokers) in epidemiological studies, and to refine lung cancer risk estimates.
In our study, cotinine concentrations indicative of active smoking (cotinine ≥115 nmol/L) were rare in never smokers, but relatively common in former smokers. In particular, we observed that nearly 15% of former smoking cases and 9% of former smoking controls had cotinine concentrations ≥115 nmol/L. We cannot determine whether former smokers with high cotinine concentrations were misclassified active smokers, or if high cotinine concentrations in former smokers reflect short-term abstinence from smoking, the use of nicotine substitutes such as nicotine patches, or unreported use of smokeless tobacco, for which future research could provide a more detailed account. Additional information on time since last cigarette would also be useful for future studies. Furthermore, circulating cotinine concentrations depend on individual differences in both smoking behaviour and metabolism;19 additional nicotine metabolites, such as 3-hydroxycotinine, may provide more accurate information on smoking exposure.
Cotinine and lung cancer risk in current smokers
This is the largest study to date assessing the association between cotinine and lung cancer risk, involving 2519 current smoking case-control pairs. For current smokers, we observed a strong dose-response relationship between circulating cotinine concentrations and lung cancer risk, a result that is consistent with previous smaller studies.11,12,20,21 Compared with self-reported smoking information, circulating cotinine concentrations among current smokers may account for other aspects of tobacco smoking that influence lung cancer risk, such as inhalation depth.22
By including circulating cotinine and self-reported smoking information (smoking status, smoking duration and smoking intensity) for all types of tobacco into an integrated model for current smokers, we demonstrated that it is possible to attain a small improvement in discrimination between future lung cancer cases and controls.
The US National Lung Screening Trial showed that lung cancer mortality can be reduced by 20% through low-dose computed tomography (CT).23,24 The current screening criterion for ever smokers has important negative aspects including a high false-positive rate and poor cost efficiency.23–26 Improving screening criteria is crucial if CT screening is to be implemented in a cost-effective manner. Risk biomarkers, including cotinine, may be useful for improving traditional risk prediction models and for refining eligibility criteria for CT screening. Whereas the use of objective risk biomarkers for lung cancer screening may seem appealing, our data indicate that the added benefit of cotinine alone or cotinine integrated with self-reporting smoking is limited. The impact of cotinine combined with other independent risk markers will need to be assessed.
Cotinine and lung cancer risk in former and never smokers
This is the largest study to date assessing the association between cotinine and lung cancer risk, involving 1518 former smoking and 1327 never smoking case-control pairs.
Second-hand smoke is classified as carcinogenic to humans,9 and an estimated 21 400 worldwide lung cancer deaths among non-smokers were attributed to second-hand smoke exposure in 2004.27 However, our results for non-smokers (former and never) suggest that circulating cotinine concentration—as measured in a single serum sample—may not capture increased lung cancer risk associated with second-hand smoking.
We hypothesized that cotinine may be used to assess lung cancer risk due to second-hand smoke in self-reported never smokers, but despite our relatively large sample of more than 1300 self-reported never smoker case-control pairs, we did not observe any risk increase for participants with circulating cotinine concentrations consistent with second-hand smoke exposure (cotinine concentration 5 < 115 nmol/L) compared to those with no nicotine exposure (cotinine concentration 0 < 5 nmol/L).
Strengths and limitations
This is the largest study to date (5364 smoking-matched case-control pairs) investigating the association between circulating cotinine concentration and lung cancer risk. Our study benefits from centralized biochemical analysis of pre-diagnostic serum/plasma cotinine concentrations. We had sufficient power to provide robust OR estimates for never and former smokers, and a diverse sample allowing for informative stratified analyses by sex and region. Oversampling of never and former smokers meant that individual cohorts provided varying proportions of participants by sex and self-reported smoking status. However, we believe this has a negligible impact on our findings considering that we observed no interactions by study region. Our data allowed for direct intra-individual comparisons between self-reported smoking status and circulating cotinine concentrations. We were therefore able to estimate the proportion of misclassified former and never smokers, which will be of much use for future studies. We lacked information on nicotine substitution use for the purpose of smoking cessation, and can therefore not confirm whether all former smokers with circulating cotinine concentrations indicative of active smoking were truly misclassified. For this reason, data on time since last cigarette could have provided additional important information among former smokers. Since we did not have access to questionnaire data on second-hand smoke exposure, our study is limited to using cotinine as a proxy for second-hand smoke. One limitation of cotinine as a biomarker is that it only provides information on recent smoking exposure. Other biomarkers, such as AHRR gene-methylation,28 may provide additional information on tobacco smoking over the previous 10-20 years. Our future work will include risk analyses for a panel of different biomarkers, including their relative and combined risk-discriminative performance.
Conclusions
We found higher cotinine concentrations to be consistently associated with lung cancer risk for current smokers, whereas associations for former and never smokers were driven by participants with cotinine concentrations consistent with active smoking. Cotinine may be useful for risk discrimination purposes among current smokers and to identify misclassified never and former smokers in epidemiological studies.
Funding and Acknowledgements
This work was supported by the Research Council of Norway (grant number 267776/H10). The work undertaken by T.L.L. for this paper was conducted during a postdoctoral placement at the International Agency for Research on Cancer, within the framework of an agreement between the Research Council of Norway and the Norwegian University of Science and Technology.
The Lung Cancer Cohort Consortium (LC3) was supported by the National Institutes of Health/National Cancer Institute (grant number 1U1CA155340–01) and National Health and Medical Research Council (NHMRC) (Grant ID: 1050198.
Collaboration with authors from the Women’s Health Study was supported by the National Institutes of Health (grant numbers CA047988, CA182913, HL043851, HL080467 and HL099355).
Cancer incidence data for the Campaign Against Cancer and Stroke (CLUE I) and the Campaign Against Cancer and Heart Disease (CLUE II) cohorts were provided by the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Department of Health and Mental Hygiene. The CLUE authors would like to thank the State of Maryland, the Maryland Cigarette Restitution Fund and the National Program of Cancer Registries of the Centers for Disease Control and Prevention for the funds that helped support the collection and availability of the cancer registry data. The CLUE authors would also like to thank the CLUE participants and staff at the George W. Comstock Centre for Public Health Research and Prevention.
The WHI programme is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the programme possible. A full listing of WHI investigators can be found at: [http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf].
The Health Professionals Follow-up Study and Nurses’ Health Study were supported by a grant from the National Institutes of Health (NIH) grants UM1CA186107, P50CA127003, P01CA87969, R01CA49449, and UM1 CA167552. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors would like to thank the participants and staff of the Health Professionals Follow-up Study and Nurses’ Health Study for their valuable contributions, as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
The MEC cohort acknowledges partial funding from NIH grant U01 CA164973.
Conflict of interest: None declared.
Supplementary Material
References
- 1. World Health Organization. Cancer Fact Sheet 2017 http://www.who.int/mediacentre/factsheets/fs297/en/ (February 2017, date last accessed).
- 2. Stewart BW, Wild CP (eds). World Cancer Report 2014 Lyon, France: International Agency for Research on Cancer, 2014. [PubMed]
- 3. Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P.. Risk factors for lung cancer worldwide. Eur Respir J 2016;48:889–902. [DOI] [PubMed] [Google Scholar]
- 4. Corbin M, Haslett S, Pearce N, Maule M, Greenland S.. A comparison of sensitivity-specificity imputation, direct imputation and fully Bayesian analysis to adjust for exposure misclassification when validation data are unavailable. Int J Epidemiol 2017;46:1063–72. [DOI] [PubMed] [Google Scholar]
- 5. Benowitz NL, Jacob P 3rd.. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther 1994;56:483–93. [DOI] [PubMed] [Google Scholar]
- 6. Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev 1996;18:188–204. [DOI] [PubMed] [Google Scholar]
- 7. Jaakkola MS, Jaakkola JJ.. Assessment of exposure to environmental tobacco smoke. Eur Respir J 1997;10:2384–97. [DOI] [PubMed] [Google Scholar]
- 8. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health 2016;13:1236.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Second-hand Tobacco Smoke. A Review of Human Carcinogens Part E: Personal Habits and Indoor Combustions Lyon, France: International Agency for Research on Cancer, 2012. [PMC free article] [PubMed]
- 10. Jarvis MJ, Feyerabend C, Bryant A, Hedges B, Primatesta P.. Passive smoking in the home: plasma cotinine concentrations in non-smokers with smoking partners. Tob Control 2001;10:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Timofeeva MN, McKay JD, Davey Smith G. et al. Genetic polymorphisms in 15q25 and 19q13 loci, cotinine levels, and risk of lung cancer in EPIC. CancerEpidemiol Biomarkers Prev 2011;20:2250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A.. Serum cotinine level as predictor of lung cancer risk. CancerEpidemiol Biomarkers Prev 2006;15:1184–88. [DOI] [PubMed] [Google Scholar]
- 13. Fanidi A, Muller D, Yuan J-M. et al. Circulating folate, vitamin B6 and methionine in relation to lung cancer risk in the Lung Cancer Cohort Consortium (LC3). J Natl Cancer Inst 2018;110. doi: 10.1093/jnci/djx119. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Midttun O, Hustad S, Ueland PM.. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2009;23:1371–79. [DOI] [PubMed] [Google Scholar]
- 15. Bevital. Summary Table Platform D- LC-MS/MS 2017 http://bevital.no/ (10 May 2017, date last accessed).
- 16. Theophilus EH, Coggins CR, Chen P, Schmidt E, Borgerding MF.. Magnitudes of biomarker reductions in response to controlled reductions in cigarettes smoked per day: a one-week clinical confinement study. Regul Toxicol Pharmacol 2015;71:225–34. [DOI] [PubMed] [Google Scholar]
- 17. DeLong ER, DeLong DM, Clarke-Pearson DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 18. StataCorp. Stata Statistical Software: Release 14 College Station, TX: StataCorp LP, 2015.
- 19. McIntyre A, Ganti AK.. Lung cancer - a global perspective. J Surg Oncol 2017;115:550–54. [DOI] [PubMed] [Google Scholar]
- 20. Ellard GA, de Waard F, Kemmeren JM.. Urinary nicotine metabolite excretion and lung cancer risk in a female cohort. Br J Cancer 1995;72:788–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan JM, Gao YT, Murphy SE. et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res 2011;71:6749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukumoto K, Ito H, Matsuo K. et al. Cigarette smoke inhalation and risk of lung cancer: a case–control study in a large Japanese population. Eur J Cancer Prev 2015;24:195–200. [DOI] [PubMed] [Google Scholar]
- 23. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD. et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinsky PF, Gierada DS, Hocking W, Patz EF Jr, Kramer BS.. National Lung Screening Trial findings by age: medicare-eligible versus under-65 population. Ann Intern Med 2014;161:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Black WC, Gareen IF, Soneji SS. et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014;371:1793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Black WC. Computed tomography screening for lung cancer in the National Lung Screening Trial: a cost-effectiveness analysis. J Thorac Imaging 2015;30:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss UA.. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011;377:139–46. [DOI] [PubMed] [Google Scholar]
- 28. Baglietto L, Ponzi E, Haycock P. et al. DNA methylation changes measured in pre-diagnostic peripheral blood samples are associated with smoking and lung cancer risk. Int J Cancer 2017;140:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.