Summary:
In this study we interrogated the metabolome of human acute myeloid leukemia (AML) stem cells to elucidate properties relevant to therapeutic intervention. We demonstrate that amino acid uptake, steady-state levels, and catabolism are all elevated in the leukemia stem cell (LSC) population. Furthermore, LSCs isolated from de novo AML patients are uniquely reliant on amino acid metabolism for oxidative phosphorylation and survival. Pharmacological inhibition of amino acid metabolism reduces oxidative phosphorylation and induces cell death. In contrast, LSCs obtained from relapsed AML patients are not reliant on amino acid metabolism due to their ability to compensate through increased fatty acid metabolism. These findings indicate that clinically relevant eradication of LSCs can be achieved with drugs that target LSC metabolic vulnerabilities.
In Brief:
By interrogating metabolic properties of human acute myeloid leukemia, Jones et al. reveal increased amino acid metabolism in leukemia stem cells (LSCs), which rely on amino acids for oxidative phosphorylation and survival. Venetoclax with azacitidine induces LSC toxicity by decreasing amino acid uptake.
Introduction:
Conventional chemotherapy for AML patients often eliminates the majority of proliferating bulk tumor cells; however, at least some of the disease-initiating LSC population is spared (Guan and Hogge, 2000; Hope et al., 2004; Ishikawa et al., 2007; Reya et al., 2001; Terpstra et al., 1996) leading to disease progression and relapse. Targeting LSCs would likely result in better outcomes or even curative therapy for AML patients (Dick, 2005; Guzman and Jordan, 2004; Konopleva and Jordan, 2011; Pollyea and Jordan, 2017).
Since the 1950s, it has been documented that cancer cells rely on glycolysis for energy production instead of oxidative phosphorylation (OXPHOS) (Warburg, 1956), a more efficient process of producing ATP. In contrast, several more recent studies have demonstrated that cancer stem cells (CSCs) from multiple tumor types (AML, glioblastoma, melanoma, and pancreatic cancer) are dependent on OXPHOS, and have lower glycolytic reserves than more mature cancer cells (Janiszewska et al., 2012; Lagadinou et al., 2013; Roesch et al., 2013; Viale et al., 2014). Furthermore, we previously demonstrated OXPHOS is targeted in vitro by BCL2 inhibition in LSCs (Lagadinou et al., 2013). Recent studies have also shown increased levels of OXPHOS in CSCs can promote chemotherapy resistance (Lee et al., 2017). In addition to a selective dependence on OXPHOS, several studies have documented other LSC-specific metabolic properties. These include relatively low levels of reactive oxygen species and increased levels of glutathione (Lagadinou et al., 2013), activated AMPK signaling (Pei et al., 2018; Saito et al., 2015), sensitivity to disruption of electron transport chain components (Chan et al., 2015), dependence on amino acid transaminase 1 (Raffel et al., 2017), increased activation of branched-chain amino acid metabolism (Hattori et al., 2017), preferential reliance on mitochondrial translation and respiratory components (Cole et al., 2015; Skrtic et al., 2011), and elevated levels of fatty acid oxidation in some LSC subpopulations (Ye et al., 2016). Together these data demonstrate that understanding the unique metabolic properties of malignant stem cells may be central to improved therapy for AML and other cancers.
A recent AML clinical trial has shown promising results using a combination of the BCL-2 inhibitor drug venetoclax with the hypomethylating agent azacitidine. Elderly previously untreated AML patients had high overall response rates with deep and durable remissions, suggesting effective targeting of the LSC population (DiNardo et al., 2018a). A detailed molecular analysis of patients from this trial identified inhibition of oxidative phosphorylation as a key determinant of LSC eradication (Pollyea et al., 2018). In the present study, we have extended analysis of these patients to further elucidate the mechanism by which venetoclax with azacitidine targets the LSC population in both de novo and relapsed AML patients.
Results:
LSCs have increased amino acids
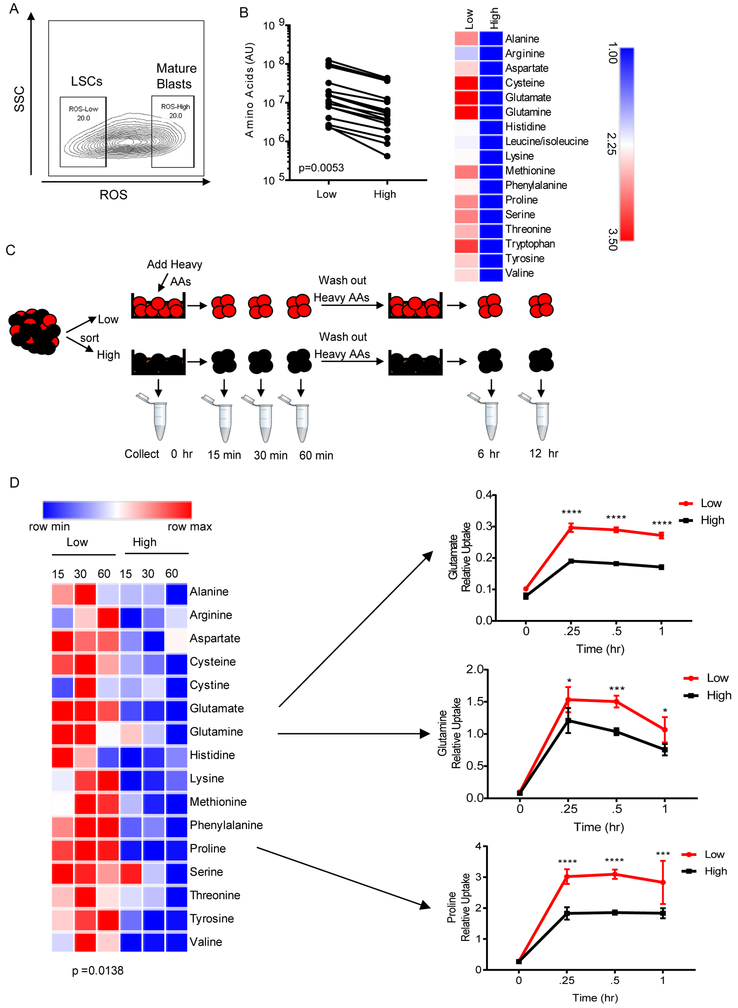
To identify LSCs, we employed relative levels of reactive oxygen species (ROS) as a means to isolate enriched populations of functionally-defined LSCs (ROS-low) and AML blasts (ROS-high) from primary human AML specimens (Figure 1A). This method has been validated for use in AML (Lagadinou et al., 2013; Pei et al.), as well as in other stem cell populations (Diehn et al., 2009; Jang and Sharkis, 2007; Smith et al., 2000), and obviates the need to rely on immunophenotypic markers, which are inherently heterogeneous in LSC populations (Eppert et al., 2011; Ho et al., 2016; Sarry et al., 2011). Furthermore, in the majority of patients the ROS-low population is enriched for phenotypic stem cells (CD34+, CD38−, CD123+), which makes the data obtained in this study comparable to other LSC studies (Figure S1A). We performed global metabolic profiling of ROS-low LSCs compared to the ROS-high AML blasts cells from fifteen primary AML specimens by mass spectrometry (Figure S1B) (patient samples 1-15 Table S1). Due to limited cell number, only ~100 metabolites were reliably detected in this analysis. Principle component analysis revealed that the metabolome of ROS-low LSCs and ROS-high AML blasts was largely similar, with heterogeneity among patients being greater than the differences between ROS-low and ROS-high cells (Figure S1C). Overall, thirty-nine metabolites were significantly increased in ROS-low LSCs compared to ROS-high AML blasts (Table S2) including 16 amino acids, five glutathione homeostasis metabolites, and two TCA cycle intermediates, which are all related to amino acid metabolism (Lu, 2009; Owen et al., 2002). Furthermore, pathway analysis revealed that amino acid metabolism was significantly enriched in ROS-low LSCs compared ROS-high AML blasts (Figure S1D). Consistently, total amino acids were the only class of metabolites significantly differentially elevated in comparing ROS-low LSCs to ROS-high blasts (Figure 1B, p=0.0053).
Figure 1: ROS-low LSCs have more amino acids than ROS-high blasts.
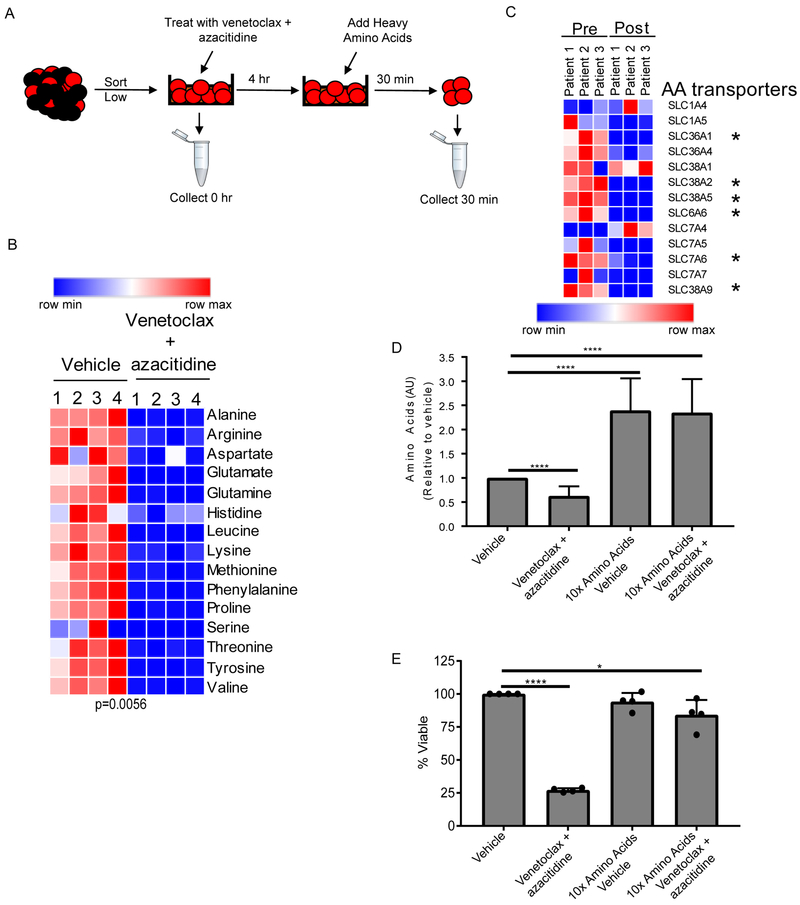
A. Representative flow plot of ROS-low enriched LSCs and ROS-high AML blasts from primary human specimens. B. Amino acid levels (arbitrary units-AU) based on mass spectrometry ROS-low LSCs and ROS-high AML blasts isolated from 15 primary AML specimens. Specimens 1-15 from Table S1 were used in this analysis. Significance was determined using a paired two-tailed student's t-test. C. Diagram showing experiment design. Stable isotope-labeled amino acids were pulsed into sorted ROS-low LSCs and ROS-high blasts for 15, 30 or 60 min and subsequently washed out for 6 or 12 hr. D. Heatmap of amino acid uptake in ROS-low LSCs and ROS-high AML blasts after a 15, 30, or 60 min pulse with stable isotope-labeled amino acids with examples of glutamine, glutamate and proline uptake from specimen 2. Data are represented as mean abundance ± standard deviation (SD) (n=4). Paired two-tailed student’s t-test was performed to identify statistical differences between amino acid uptake in ROS-low LSCs and ROS-high cells. * p<0.05, *** p<0.005, **** p<0.001
To investigate the role of amino acids, we measured uptake of stable isotope labeled amino acids and subsequent metabolism in cultured ROS-low LSCs and ROS-high AML blasts. (Figure 1C). Analysis of amino acid levels at 15, 30, and 60 min following exposure to isotope labeled reagents showed significantly faster uptake of amino acids in ROS-low LSCs compared to ROS-high cells (Figures 1D and S1E). This was particularly evident for glutamine and glutamate, amino acids that have been explored as potential therapeutic targets in cancer due to their role in glutathione and α-ketoglutarate synthesis (Pei et al., 2013) (Lagadinou et al., 2013). Increased proline uptake by LSCs was also highly significant (Figure 1D). Interestingly, proline and glutamine are interconvertible amino acids (Phang et al., 2015) further supporting the potential importance of glutamine metabolism in LSCs. Upon removal of heavy amino acids, we analyzed subsequent metabolism and observed a significantly higher percentage of amino acids utilization in ROS-low LSCs (Figure 1F). Again, this was particularly evident for amino acids glutamine, glutamate, and proline. These findings demonstrate that amino acid metabolism is substantially more active in the ROS-low population and suggest that glutamine, glutamate, and proline metabolism may be particularly important for LSCs.
LSCs are dependent on amino acids for survival
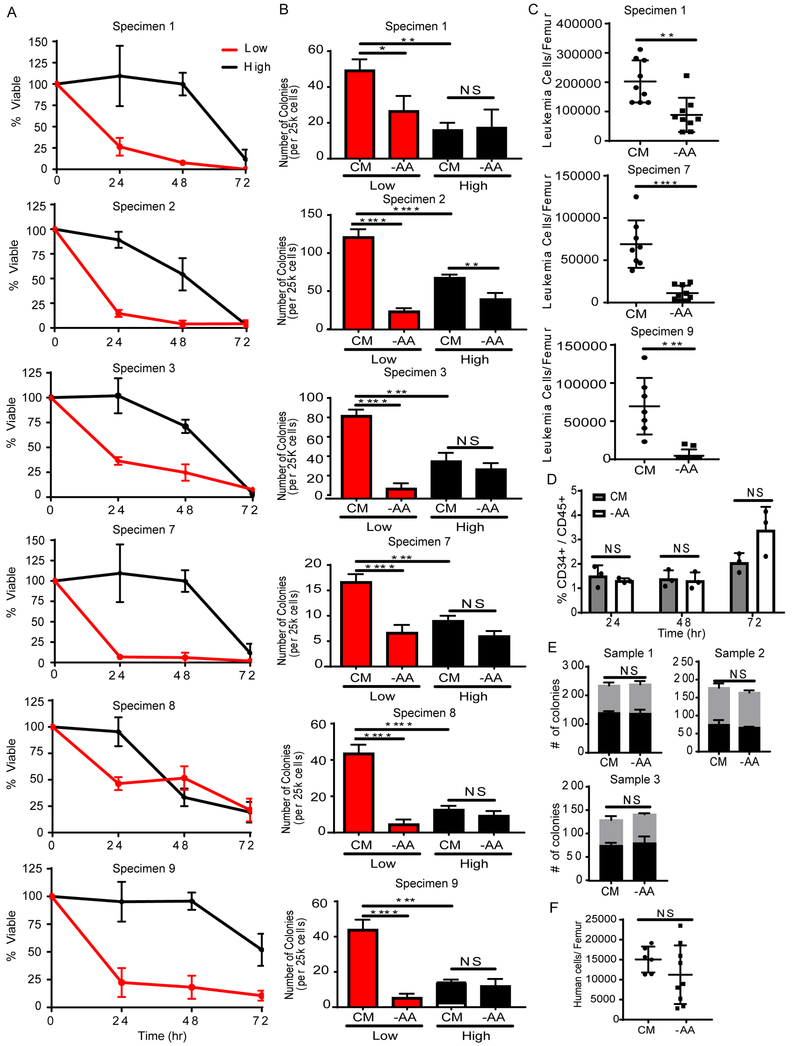
To determine if amino acid metabolism is functionally relevant for LSC survival, we measured cell viability and colony-forming potential of ROS-low LSCs and ROS-high AML cells after 24-72 hr of culture in the absence of exogenous amino acids. ROS-low LSC viability significantly decreased after 24 hr of amino acid depletion relative to control media. ROS-high cells were not substantially affected by amino acid deprivation until 72 hr of culture (Figure 2A). As we have previously demonstrated, ROS-high blasts have significantly less colony-forming potential compared to ROS-low LSCs (Lagadinou et al., 2013) (Figure 2B), consistent with the enrichment of AML stem/progenitor cells in the ROS-low compartment. Amino acid depletion decreased colony formation of ROS-low LSCs but had no significant effect on the colony-forming ability of ROS-high blasts (Figure 2B). These data indicate amino acid metabolism is preferentially important for ROS-low LSCs. To confirm that amino acid depletion targets the LSC compartment, we measured the ability of primary AML specimens to engraft immune deficient NSG-S mice after being cultured with or without amino acids. After 24 hr of culture without amino acids, we observed a 15%, 34%, and 38% decrease in bulk leukemia cell viability in specimens 1, 7, and 9 respectively (data not shown). Upon transplantation of these cells into NSG-S mice, we observed a significant decrease in engraftment for cells cultured without amino acids (Figure 2C), indicating that amino acid depletion directly targets functionally-defined LSCs. The primary xenograft model is considered the best available assay for determining functional LSCs and importantly does not rely on any phenotypic pre-selection of LSCs.
Figure 2: ROS-low LSCs are dependent on amino acids.
A. Viability of ROS-low LSCs (red line) and ROS-high cells (black line) after culturing without amino acids for 24, 48, and 72 hr relative to ROS-low LSCs and ROS-high cells cultured in media with amino acids. (n=3). B. Colony-forming ability of ROS-low LSCs (red bars) and ROS-high cells (black bars) after culturing without amino acids for 24 hr compared to cells cultured in media containing amino acids, complete media (CM). Statistical analysis was performed using two-way ANOVA. (n=3). C. Engraftment of unsorted primary AML specimens (1 and 7) after culturing with or without amino acids for 24 hr. Each dot represents the leukemia cells/femur of an individual animal. Statistical analysis was performed using an unpaired two-tailed Student’s t-test. D. Percentage of CD34+/CD45+ cells after culturing normal mobilized peripheral blood cells with or without amino acids for 24, 48, and 72 hr. Each dot represents an individual patient sample. Statistical analysis was performed using two-way ANOVA. (n=3). E. Colony forming ability of mobilized peripheral blood cells after culturing with or without amino acids for 24 hr. CFU-GM = colony forming unit - granulocyte/monocyte, BFU-E = burst forming unit - erythroid. Statistical analysis was performed using an unpaired two-tailed Student’s t-test. (n=3). F. Engraftment of mobilized peripheral blood sample 1 after culturing with or without amino acids for 24 hr. Each dot represents the human cells/femur of an individual animal. Statistical analysis was performed using an unpaired two-tailed Student’s t-test. Graphs represent the mean ± SD. * p<0.05, ** p<0.01, *** p<0.005, **** p<0.001, NS = not significant.
See also Figure S2
We also measured cell viability and colony-forming potential of ROS-low and ROS-high cells upon depletion of other metabolites. As glutamine was taken up and metabolized at particularly high rates in ROS-low LSCs, we measured the effect of its depletion on LSCs (Figures S2A and S2B). Glutamine depletion did not affect the viability or colony forming potential of ROS-low LSCs and had a minimal impact on ROS-high cells in a subset of patient samples. Compensation from other metabolites such as proline likely contributes to this result and makes targeting glutamine in CSCs potentially challenging. Glucose is one of the main metabolic fuels for many cancer cells (Warburg, 1956); therefore, we also measured the effect of glucose deprivation on LSCs. Glucose depletion did not affect the viability or colony forming potential of the ROS-low LSCs (Figures S2C and S2D). In contrast, ROS-high cells were highly dependent on glucose (Figure S2C). Notably, this indicates a fundamental difference in energy metabolism mechanisms between LSCs versus bulk tumor cells in the ROS-high population. Finally, β-oxidation of lipids is a major source of energy production and has been shown to be important in chemotherapy resistance in LSCs (Ye et al., 2016); however, neither ROS-low LSC nor ROS-high AML cell viability and colony-forming potential was affected by lipid depletion (Figure S2E and S2F).
To assess the role of amino acid metabolism in normal hematopoiesis, we measured the effect of amino acid depletion on primary human hematopoietic stem and progenitor cells (HSPCs). Three specimens derived from mobilized peripheral blood were cultured with and without amino acids for 24-72 hr. No significant differences in HSPC percentages were observed (Figure 2D). Furthermore, we measured the colony-forming potential of normal HSPCs after being cultured without amino acids for 24 hr and observed no significant change in the number or type of colonies formed (Figure 2E). To confirm that amino acid depletion does not target normal hematopoietic stem cells (HSCs), we measured the ability of a mobilized peripheral blood sample to engraft into immune deficient NSG-S mice after being cultured with or without amino acids for 24 hr. We observed no significant decrease in engraftment when cells were cultured without amino acids (Figure 2F), indicating that functionally-defined normal HSCs are less reliant on amino acids for survival. Overall, these data demonstrate that LSCs are uniquely dependent on amino acids for survival.
Amino acids are essential for OXPHOS in LSCs
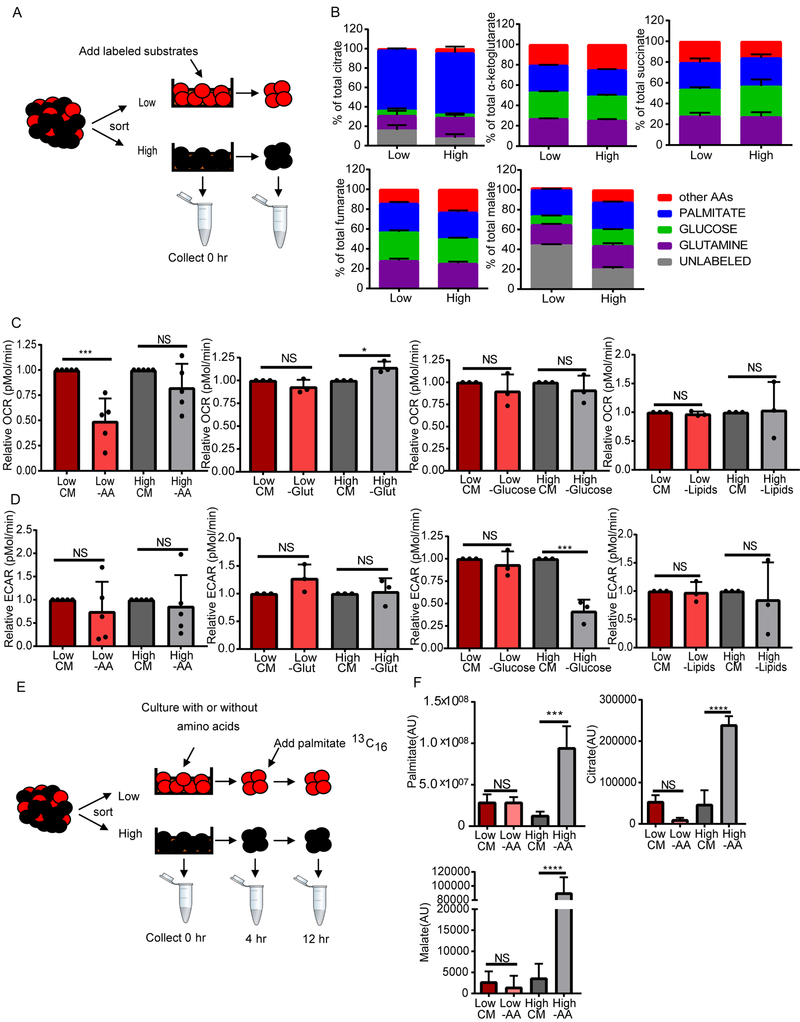
To investigate the role of amino acids in LSC physiology, we first examined the contribution of amino acids to protein translation in ROS-low LSCs compared to ROS-high blasts. We observed decreased levels of protein translation in ROS-low LSCs compared to ROS-high blasts (Figure S3A), suggesting that the increased level of amino acid metabolism is not related to metabolic requirements for protein synthesis. Furthermore, inhibition of protein translation did not preferentially kill ROS-low LSCs (Figure S3B). Since the selective killing of LSCs upon amino acid depletion did not appear to be related to protein translation, we next determined the contribution of amino acids to the LSC metabolome in comparison with other metabolic fuels. To do this we measured incorporation of 13C and 15N into metabolites in cells cultured in the presence of stable isotope-labeled amino acids without glutamine, glutamine, glucose or palmitic acid (Figure 3A). We found that amino acids, glucose and fatty acids each contribute to TCA cycle intermediate synthesis (Figure 3B). Interestingly, no significant differences between metabolite contribution to TCA cycle intermediates were observed between the ROS-low LSCs and ROS-high blasts. This finding indicates that some degree of amino acid catabolism into the TCA cycle may occur for energy production in both ROS-low LSCs and ROS-high blasts.
Figure 3: Amino acid depletion decreases OXPHOS specifically in ROS-low LSCs.
A. Diagram showing experimental design. ROS-low LSCs were cultured with metabolic substrates labeled with stable isotopes; including, glucose, glutamine, amino acids without glutamine, or palmitic acid for 24 hr. B. Graph shows enrichment of heavy atoms (13C and 15N) from the stable isotopes from each substrate into TCA cycle intermediates in ROS-low LSCs and ROS-high cells isolated from specimen 7. (n=4) C. ROS-low LSCs and ROS-high cells isolated from primary AML samples were cultured in complete media (CM) or without amino acids (-AA), glutamine (glut), glucose, or lipids for 4 hr. After culture, oxygen consumption rate (OCR) was measured on a XF24 Seahorse Analyzer. Relative OCR was determined for each patient relative to CM. Statistical analysis was performed using two-way ANOVA. Each dot represents a different patient specimen. Patient specimens used in this analysis are 2, 7, 8, 9, and 10 (n=5). D. ROS-low LSCs and ROS-high cells isolated from primary AML samples were cultured without amino acids (-AA), glutamine (glut), glucose, or lipids for 4 hr. After culture, glycolytic rate (extracellular acidification rate; ECAR) was measured on a XF24 Seahorse Analyzer. Relative ECAR was determined for each patient relative to CM. Statistical analysis was performed using two-way ANOVA. Each dot represents a different patient sample. Patient specimens used in this analysis include 2, 7, 8, 9, and 10. (n=3). E. Diagram showing experimental design. ROS-low LSCs and ROS-high AML cells were cultured with or without amino acids for 4 hr. Stable isotope-labeled palmitic acid was fluxed into cells for 8 hr. F. Graph shows levels of 13C palmitic acid and enrichment (arbitrary units-AU) of heavy atom (13C) from the labeled palmitate into TCA cycle intermediates (citrate and malate). Statistical analysis was performed using two-way ANOVA. Patient specimen 7 was used for this analysis. (n=4). Graphs represent the mean ± SD. * p<0.05, *** p<0.005, **** p<0.001, NS = not significant
See also Figure S3
Next, to determine if amino acid catabolism is important for OXPHOS in LSCs and AML blasts, we measured changes in oxygen consumption in ROS-low LSCs and ROS-high cells grown in multiple conditions. Cultures were performed in the absence of all 20 amino acids, without glutamine alone, without glucose alone, or without lipids for 4 hr. ROS-low LSCs grown without amino acids had significantly less oxygen consumption; however, no change in oxygen consumption was observed in ROS-high cells (Figure 3C). These data indicate that ROS-low LSCs are preferentially reliant on amino acids to fuel OXPHOS compared to ROS-high AML blasts. Furthermore, neither ROS-low LSCs nor ROS-high cells grown without glutamine, glucose, or lipids had any significant changes in oxygen consumption (Figure 3C). This is consistent with our findings that only amino acid depletion specifically targeted LSCs (Figure 2 and Figure S2). No consistent differences in basal OXPHOS were observed between ROS-low LSCs and ROS-high AML blasts (Figure S3C).
We next measured the effect of amino acid, glutamine, glucose, or lipid depletion on glycolysis in ROS-low LSCs and ROS-high blasts. Glucose deprivation in the ROS-high blasts but not ROS-low LSCs resulted in decreased glycolysis (Figure 3D). This is consistent with our finding that depletion of glucose only effected the viability of ROS-high cells (Figure S2C). Furthermore, we have previously shown that ROS-low LSCs have low levels of glycolytic reserves (Lagadinou et al., 2013). Altogether these data highlight the lack of LSC dependence on glucose for energy production, which is in stark contrast to the dependence of normal HSCs on glycolysis (Suda et al., 2011). Amino acid, glutamine, or lipid depletion did not significantly affect glycolysis rates (Figure 3D). No significant difference in glycolysis was observed between ROS-low LSCs and ROS-high AML blasts (Figure S3D).
We hypothesized that OXPHOS in LSCs and not AML blasts is selectively sensitive to loss of amino acids because LSCs are less metabolically flexible and therefore cannot compensate for amino acid loss. To test this hypothesis, we isolated ROS-low LSCs and ROS-high AML blasts, cultured them with or without amino acids for 4 hr and then added 13C16 palmitate or 13C6 glucose for 8 hr and measured the incorporation of these stable isotope labeled metabolites into TCA cycle intermediates (Figure 3E and Figure S3E). We observed that ROS-high AML blasts but not ROS-low LSCs significantly upregulated palmitate uptake, as well as catabolism into TCA cycle intermediates citrate and malate (Figure 3F). These data suggest that ROS-high AML blasts but not ROS-low LSCs are able to compensate for amino acid loss by increasing the contribution of fatty acid metabolism to the TCA cycle. Next, we measured the ability of glucose to compensate for loss of amino acids in ROS-low LSCs and ROS-AML blasts (Figure S3E). Amino acid depletion did not change the levels of glucose uptake in either ROS-low LSCs or ROS-high AML blasts (Figure S3F). Furthermore, we did not observe a difference in glucose uptake between the two populations (Figure S3F). In addition, ROS-high AML blasts produced significantly more of the glycolytic products, pyruvate and lactate, upon amino acid loss compared to ROS-low LSCs (Figure S3F). However, neither ROS-low LSCs nor ROS-high AML blasts increased the contribution of glucose into the TCA cycle upon amino acid loss. Overall, our data demonstrates that LSCs from de novo AML patients are less metabolically flexible than AML blasts, which likely contributes to the selective sensitivity of ROS-low LSCs to amino acid depletion.
Venetoclax with azacitidine decreases amino acid levels in AML patients.
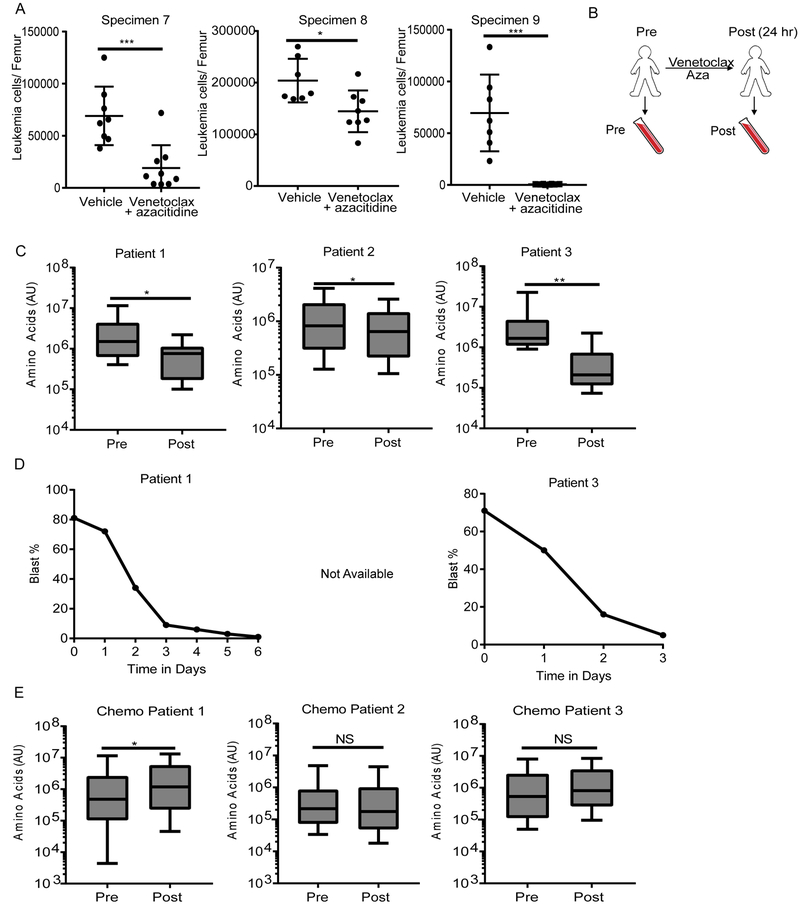
The findings outlined in Figures 1-3 indicate that therapeutic strategies capable of inhibiting amino acid metabolism may be effective for eradication of LSCs. To examine this concept in more detail, we evaluated AML patient specimens from a recent study in which the clinical data indicate targeting of the LSC population. The trial utilized a combination of the BCL2 inhibitor, venetoclax, in combination with either decitabine or azacitidine (hypomethylating agents)(DiNardo et al., 2018a) for the treatment of de novo AML patients. Patients enrolled on this study at a single institution who received venetoclax + azacitidine had a 91% overall response rate, with deep and durable remissions, suggesting that this regimen is targeting LSCs in patients (Pollyea et al., 2018). Importantly, we have previously shown that BCL2 knockdown or pharmacological inhibition results in decreased OXPHOS and selectively targets LSCs in preclinical models (Lagadinou et al., 2013). We confirmed that venetoclax in combination with azacitidine is able to target functional LSCs by measuring engraftment ability of primary AML specimens after 24 hr of ex vivo treatment (Figure 4A). Based on these data, we hypothesized that inhibition of BCL2 may act to reduce amino acid metabolism and thereby suppress OXPHOS.
Figure 4: Venetoclax in combination with azacitidine decreases amino acids in AML patients.
A. Engraftment of an unsorted primary AML specimens (7 and 8) after culturing with 500 nM venetoclax with 2.5 μM azacitidine or vehicle control for 24 hr. Each dot represents the leukemia cells/femur of an individual animal. Graphs represent the mean ± SD. Statistical analysis was performed using an unpaired two-tailed Student’s t-test. B. Diagram showing peripheral blood sampling from patients before therapy (pre) and 24 hr after venetoclax + azacitidine (post). C. Amino acid levels (measuring relative abundance) in ROS-low LSCs isolated from three patients on the venetoclax with azacitidine trial before (pre) and 24 hr post treatment (post) with venetoclax + azacitidine. Box plots represent the minimum to maximum values with the center line representing the mean. Error bars represent minimum and maximum. Statistical analysis was performed using an unpaired two-tailed Student’s t-test. D. Peripheral blast percentages (percentage of leukemia cells in the blood) determined by manual complete blood counts CBC in two patients treated with venetoclax + azacitidine. Day 0 is the day pretreatment, day 1 is 24 hr post treatment, etc. E. Amino acid levels in ROS-low LSCs isolated from three patients before (pre) and 24 hr post conventional chemotherapy cytarabine + an anthracycline (7+3) (post). Box plots represent the minimum to maximum values with the center line representing the mean. Error bars represent minimum and maximum. * p<0.05, ** p<0.01, *** p<0.005, NS = not significant
See also Figure S4
To test our hypothesis, we isolated ROS-low LSC populations from patients immediately before and 24 hr after initiating treatment with venetoclax and azacitidine and analyzed metabolic changes (Figure 4B). Analysis of these specimens showed that the drug combination induced a significant decrease in total levels of amino acids in ROS-low LSCs (Figure 4C). Importantly, this therapy did not globally change the LSC metabolome suggesting that venetoclax with azacitidine is selectively targeting amino acid metabolism (Figure S4A and S4B). While only a slight reduction in leukemic burden was detected one-day post therapy, a much greater reduction was seen two to six days posttherapy (Figure 4D), indicating that the metabolic changes observed at the one-day time point precede the onset of overt leukemic cell death in patients. Interestingly, venetoclax with azacitidine treatment did not reduce amino acid levels in ROS-high AML blasts (Figure S4C), suggesting that the drugs specifically affect amino acid metabolism in ROS-low LSCs. To further investigate the hypothesis that amino acid reduction is part of the mechanism by which venetoclax with azacitidine target LSCs, we measured amino acid levels pre and 24 hr post treatment with conventional chemotherapy. The data show that amino acids levels were not reduced in ROS-low LSCs upon treatment with chemotherapy (Figure 4E). Furthermore, we did not observe any changes in cell cycle upon venetoclax with azacitidine treatment (data not shown), suggesting that the drug combination does not preferentially target any cell cycle state but rather is equally cytotoxic regardless of cycle status. These findings demonstrate that venetoclax with azacitidine, but not conventional therapy, reduces amino acid levels in ROS-low LSCs. Further, the data indicate that venetoclax with azacitidine treatment reduces amino acid metabolism in LSCs in vivo.
To confirm these results in a metabolically controlled environment, we modeled venetoclax with azacitidine treatment in a patient derived xenograft (PDX) system. As expected, treatment with the combination of venetoclax with azacitidine for two weeks significantly decreased leukemia burden (Figure S4D). Importantly, as observed in patients, venetoclax and azacitidine decreased amino acid levels (Figure S4E). Furthermore, the majority of metabolic pathways altered by venetoclax with azacitidine were directly related to amino acid metabolism (Figure S4F). Overall, this data strongly supports the hypothesis that venetoclax with azacitidine decreases amino acid levels in ROS-low LSCs in vivo.
Venetoclax with azacitidine decreases amino acid uptake.
The levels of amino acid uptake are higher in ROS-low LSCs than ROS-high cells (Figure 1D), therefore we hypothesized that venetoclax with azacitidine may decrease amino acid levels by impairing the ability of LSCs to import amino acids. To test this hypothesis, we measured changes in stable isotope labelled amino acid uptake in human LSCs after a 4 hr in vitro treatment with venetoclax with azacitidine (Figure 5A). Amino acid uptake was significantly reduced in LSCs after drug treatment (Figure 5B). To corroborate this data in patients, gene expression of known amino acid transporters was assessed in ROS-low LSCs after 5-7 hr of treatment. A significant decrease in six of the 13 amino acid transporters expressed in our patient samples was detected (Figure 5C). We corroborated these findings in vitro by treating LSCs isolated from three patients with venetoclax, azacitidine, or venetoclax + azacitidine (Figure S5A). The drug combination significantly reduced amino acid transporter expression; however, neither venetoclax nor azacitidine alone reduced expression of amino acid transporters (Figure S5A). This data suggests that the combination of venetoclax with azacitidine is needed to effectively target amino acid metabolism in LSCs. Importantly, the changes in amino acid transporter gene expression occur before amino acid levels changes (Figure S5B). We also observed that azacitidine potentiates the effect of venetoclax on ROS-low LSC and ROS-high AML blast viability in vitro (Figure S5C). Furthermore, both venetoclax and azacitidine are necessary to reduce amino acid levels in ROS-low LSCs in the majority of patient specimens (Figure S5D). Overall, these data demonstrate the combination of venetoclax with azacitidine results in decreased amino acid uptake in LSCs.
Figure 5: Venetoclax in combination with azacitidine decreases amino acid uptake.
A. Diagram showing experiment design. ROS-low LSCs were sorted, treated with venetoclax (500 nM) and azacitidine (2.5 μM) for 4 hr and then stable isotope-labeled amino acids were added for 30 min. Samples were then washed, collected, and analyzed for intracellular stable isotope amino acids. B. Heatmap of amino acid uptake in ROS-low LSCs upon vehicle or venetoclax with treatment. Specimen 2 was used for this analysis. (n=4) C. Heatmap of amino acids transporters RNA expression from 3 samples from patient on the clinical trial prior to (pre) and following (post) treatment with venetoclax + azacitidine for 5-7 hr. D. Amino acid levels in ROS-low LSCs were cultured in media containing ten times the levels of amino acids found in normal human plasma for 4 hr. Cells were then treated with vehicle or venetoclax (500 nM) and azacitidine (2.5 μM) for 4 hr and amino acid levels were determined relative to vehicle control. Specimens 7, 9, and 10 were used for this analysis. (n-3). E. ROS-low LSCs were cultured in media containing ten times the levels of amino acids found in normal human plasma for 4 hr. Cells were then treated with vehicle or venetoclax (500 nM) and azacitidine (2.5 μM) and cell viability was measured at 24 hr as a percentage relative to vehicle control (normal amino acid levels and no drug treatment). Each dot represents an individual patient sample treated in vitro. Specimens 7, 9, 10, and 11 were used for this analysis. Graphs represent the mean ± SD. For each graph statistical analysis was performed using Anova analysis. * p<0.05, **** p<0.001
See also Figure S5
Next, we sought to determine if decreased levels of amino acid uptake were an essential part of the mechanism by which venetoclax with azacitidine targets LSCs. LSCs were pretreated with 10 times the level of amino acids compared to normal human plasma for 4 hr before treatment with venetoclax with azacitidine. The data show that pretreatment with excess amino acids strongly elevates intracellular amino acid levels, which are maintained even upon treatment with venetoclax and azacitidine (Figure 5D). Importantly, the viability of LSCs pretreated with excess amino acids was also rescued (Figure 5E). Next, we determined whether culturing ROS-low LSCs in high levels of amino acids rescued cell viability by increasing amino acid transporter expression (Figure S5E). We observed that transporter expression was not altered by culturing LSCs with high levels of amino acids (Figure S5E). This finding suggests that pretreatment with high amino acids levels allows LSCs to uptake extra amino acids, which in turn protects from decreased amino acid transporter expression upon venetoclax with azacitidine treatment. Together, these data demonstrate that decreased amino acids levels upon treatment with venetoclax and azacitidine is functionally linked to the mechanism by which venetoclax with azacitidine eradicates LSCs.
Venetoclax with azacitidine reduces OXPHOS in an amino acid dependent manner.
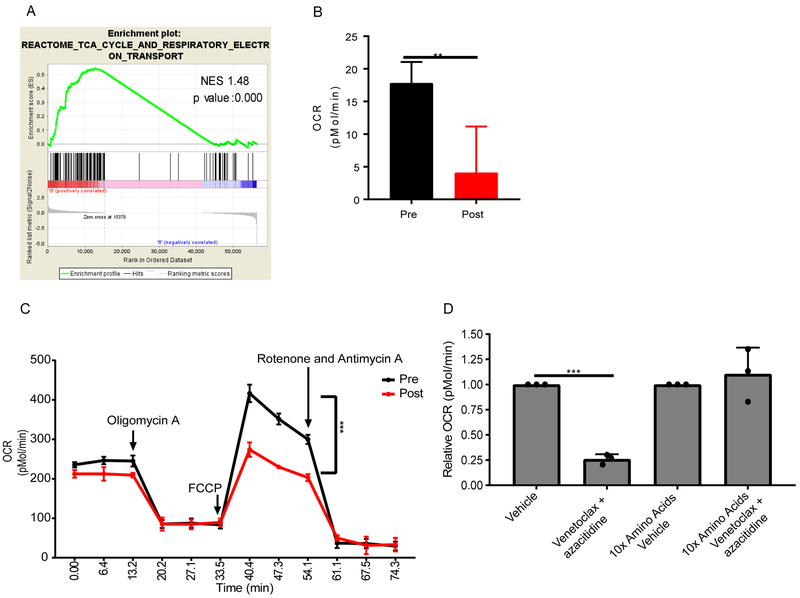
We have previously shown that BCL2 knockdown or pharmacological inhibition results in decreased OXPHOS in preclinical models (Lagadinou et al., 2013). In addition, we have shown in this report that LSCs are reliant on amino acid catabolism for OXPHOS (Figure 3) Therefore, we hypothesized that venetoclax with azacitidine targets OXPHOS by decreasing levels of amino acids. To test this hypothesis, we performed an enrichment map analysis to identify pathways altered in three patients treated with venetoclax with azacitidine (Figure S6A). Three pathways were significantly decreased by venetoclax with azacitidine treatment include protein translation, TCA cycle, and adenylyl cyclase activity. We have shown that protein translation does not account for the differential sensitivity to amino acid loss between the ROS-low and ROS-high cells (Figure S3). Additionally, there was not strong rationale to connect adenylyl cyclase to amino acid metabolism; therefore, we focused on the TCA cycle. To confirm that the TCA cycle was altered, we performed GSEA (gene set enrichment analysis) analysis on data from transcriptome sequencing of ROS-low LSCs isolated from three patients pre and 5-7 hr post venetoclax with azacitidine treatment. We observed a significant decrease in the TCA cycle reactome signature post treatment (Figure 6A), suggesting that OXPHOS was decreased by venetoclax with azacitidine treatment in LSCs isolated from AML patients. To confirm that OXPHOS was reduced by venetoclax with azacitidine, we directly measured OXPHOS in patient samples from the venetoclax with azacitidine clinical trial. After a 24 hr treatment with venetoclax and azacitidine blasts from patient 3 had a significant decrease in OXPHOS (Figure 6B). In AML cells isolated from patient 2, baseline OXPHOS levels were minimally decreased post treatment; however, OCR spare capacity was significantly reduced post treatment, as illustrated by the decreased induction of OCR upon electron transport chain uncoupling via FCCP treatment (Figure 6C). These data indicate that the capacity of the mitochondria to produce energy after venetoclax with azacitidine treatment is reduced. Next, we confirmed our findings in a PDX model by treating mice transplanted with human AML with venetoclax, azacitidine, or venetoclax with azacitidine for 24 hr, harvesting human leukemic cells from the bone marrow of the mice, and measuring OXPHOS. We observed a significant decrease in OXPHOS upon treatment with the combination of venetoclax with azacitidine but not with venetoclax or azacitidine alone (Figure S6B). This is consistent with previous findings that both venetoclax and azacitidine are needed to decrease amino acid levels and amino acid transporter expression in LSCs. These data demonstrate that venetoclax with azacitidine decreases OXPHOS and suppresses the ability of leukemic cells to up-regulate OXPHOS in AML patients.
Figure 6: Venetoclax in combination with azacitidine decreases OXPHOS in AML patients.
A. Gene set enrichment analysis of RNA-sequencing data for OXPHOS related genes in ROS-low LSCs pre and 5-7 hr post venetoclax + azacitidine treatment. This analysis was done using the three patients receiving venetoclax with azacitidine on the clinical trial. B. Basal oxygen consumption levels measured by seahorse assay in AML blasts isolated from patient 3 pre and 24 hr post venetoclax with azacitidine treatment. Statistical analysis was performed using an unpaired two-tailed Student's t-test (n=5). C. Basal and stressed oxygen consumption rate (OCR) in AML blasts isolated from patient 2 pre and 24 hr post venetoclax + azacitidine treatment Statistical analysis was performed using an unpaired two-tailed Student's t-test (n=5). D. Oxygen consumption measured in sorted ROS-low LSCs cultured in normal media or in media containing 10 times the levels of amino acids found in human serum for 4 hr and then treated with venetoclax (500 nM) and azacitidine (2.5 μM) for 4 hr. Relative OCR was determined for each patient compared to ROS-low LSCs cultured in media with normal levels of amino acids without drug treatment (vehicle). Each dot represents an individual patient sample treated in vitro. Specimens 7, 9, and 10 were used for this analysis. Statistical analysis was performed using two-way ANOVA. Graphs represent the mean ± SD. ** p<0.01, *** p<0.005
See also Figure S6
Finally, to determine if decreased amino acid levels upon venetoclax with azacitidine treatment contributed to decreased OXPHOS levels, ROS-low LSCs were cultured in high levels of amino acids, as in figure 5d, before treatment with venetoclax and azacitidine. Consistent with patient observations, venetoclax with azacitidine significantly decreased OXPHOS in ROS-low LSCs (Figure 6D). Furthermore, pretreatment with high levels of amino acids rescued OXPHOS levels upon venetoclax with azacitidine treatment (Figure 6D). This data demonstrates that venetoclax with azacitidine reduces OXPHOS by decreasing the levels of amino acids.
Relapse LSCs escape amino acid loss by increasing fatty acid metabolism.
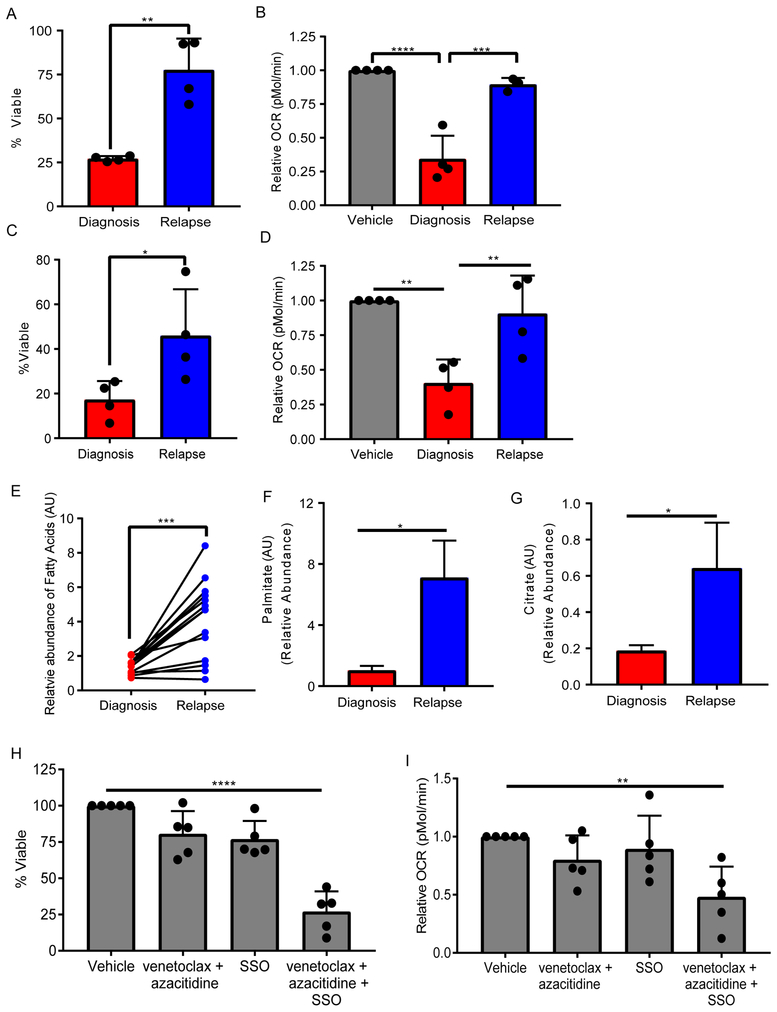
The overall response rate of older de novo AML patients to venetoclax with azacitidine is very high(DiNardo et al., 2018a). However, initial studies using venetoclax as a single agent or in combination with azacitidine for relapsed/refractory AML patients have not shown strong efficacy(DiNardo et al., 2018b; Konopleva et al., 2016). Therefore, we sought to determine whether the differential response to venetoclax with azacitidine at relapse was due to the inability to effectively target LSCs. We hypothesized that relapse LSCs would be resistant to the amino acid loss induced by venetoclax and azacitidine. To test this hypothesis, ROS-low LSCs from de novo and relapse AML patients were treated with venetoclax and azacitidine in vitro to determine whether relapse LSCs are more resistant to the drug combination. As shown in Figure 7A, LSCs isolated from de novo patients had a significantly greater reduction in viability upon treatment compared to relapse LSCs. Furthermore, OXPHOS levels were not reduced in relapse LSCs upon venetoclax with azacitidine treatment, unlike de novo LSCs (Figure 7B). These data indicate that venetoclax with azacitidine is not able to affect the metabolism of relapse LSCs in the same manner as shown for LSCs from de novo patients. To further explore this observation, we determined OXPHOS levels and survival of de novo vs relapse LSCs cultured without amino acids. Relapse LSCs were more resistant to amino acid depletion compared to de novo LSCs (Figure 7C). In addition, OXPHOS in relapse LSCs was not affected by amino acid depletion unlike LSCs isolated from de novo patients (Figure 7D).
Figure 7: Relapse LSCs upregulate fatty acid metabolism to escape amino acid depletion.
A. Viability of LSCs isolated from de novo or relapse/refractory AML patients and treated with 500 nM venetoclax with 2.5 μM azacitidine for 24 hr relative to vehicle control for each patient. Statistical analysis was performed using an unpaired two-tailed Student's t-test. B. Relative OXPHOS levels based on oxygen consumption rate (OCR) from LSCs isolated from de novo or relapse/refractory AML patients and treated with 500 nM venetoclax with 2.5 μM azacitidine for 4 hr relative to vehicle control for each patient. Statistical analysis was performed using two-way ANOVA. C. Viability of LSCs isolated from de novo or relapse/refractory AML patients cultured without amino acids for 24 hr relative to vehicle control for each patient. Statistical analysis was performed using an unpaired two-tailed Student's t-test. D. Relative OXPHOS levels from LSCs isolated from de novo or relapse/refractory AML patients and cultured without amino acids for 4 hr relative to vehicle control for each patient. Statistical analysis was performed using two-way ANOVA. The specimens used in A-D include, de novo: 3, 7, 9, and 10; relapse/refractory: 1, 2, 8, and 16. E. Fatty acid levels from LSCs isolated from de novo or relapse/refractory AML patients and cultured without amino acids for 4 hr. Each dot and the connecting lines represent the mean of the abundance of a fatty acid in the de novo and relapse patients. Statistical analysis was performed using a paired two-tailed Student's t-test. The specimens used are 7, 9, 10, 1, 2, and 8. F. Palmitate 13C16 levels in LSCs isolated from de novo (specimen 7) or relapse/refractory (specimen 8) AML patients and cultured without amino acids for 4 hr and cultured with palmitate 13C16 for an additional 8 hr. Relative abundance was determine upon amino acid depletion compared to cells cultured in amino acid containing media. Statistical analysis was performed using an unpaired two-tailed Student's t-test. G. Citrate 13C3 levels in LSCs isolated from de novo or relapse/refractory AML patients and cultured without amino acids for 4 hr and cultured with palmitate 13C16 for an additional 8 hr. Relative abundance was determine upon amino acid depletion compared to cells cultured in amino acid containing media. Statistical analysis was performed using an unpaired two-tailed Student's t-test. H. Viability of LSCs isolated from relapse/refractory AML patients and treated with 500 nM venetoclax with 2.5 μM azacitidine, 50 μM SSO, or venetoclax with azacitidine and SSO for 24 hr relative to vehicle control for each patient. Statistical analysis was performed using two-way ANOVA. I. Oxygen consumption rate was measured in LSCs isolated from relapse/refractory AML patients and treated with 500 nM venetoclax with 2.5 μM azacitidine, 50 μM SSO, or venetoclax with azacitidine and SSO for 4 hr relative to vehicle control for each patient. Statistical analysis was performed using two-way ANOVA. Specimens 1, 2, 8, 16 and 17 were used for analysis in H and I. Each dot represents an individual patient sample treated in vitro. Graphs represent the mean ± SD. * p<0.05, ** p<0.01, *** p<0.005, **** p<0.001
Also see Figure S7
We hypothesized that relapse LSCs escape amino acid depletion by upregulating other metabolic pathways. To test this hypothesis, metabolic changes in de novo and relapse LSCs were examined after a 4hr culture without amino acids. A significant increase in fatty acid levels after amino acid depletion was observed, specifically in relapse LSCs (Figure 7E). To determine whether the increased fatty acids in relapsed LSCs can compensate for amino acids in driving energy metabolism, the flux of palmitate upon amino acid depletion was assessed. As shown in Figure 7F, palmitate uptake was significantly increased in relapse LSCs compared to de novo LSCs. Furthermore, the levels of palmitate metabolized to TCA cycle intermediate citrate were significantly increased in relapse LSCs as well (Figure 7G). These data indicate that relapse LSCs can compensate for amino acid loss by increasing the contribution of fatty acid metabolism into the TCA cycle and thereby rescuing any reductions in OXPHOS. Finally, we determined if inhibiting fatty acid uptake using CD36 inhibitor, sorbitan sesquioleate (SSO), could re-sensitize relapse LSCs to venetoclax with azacitidine. Relapse LSCs treated with SSO and venetoclax with azacitidine or cultured in amino acid depleted media were significantly less viable than any of the treatment conditions alone (Figure 7H and Figure S7A). Furthermore, SSO treatment in combination with venetoclax with azacitidine or amino acid depleted media resulted in a significant decrease in OXPHOS (Figure 7I, and Figure S7B). Importantly, venetoclax with azacitidine, SSO, and the combination did not affect normal HSPCs viability (Figure S7C) or colony forming ability (Figure S7D). These data suggest that targeting both fatty acid uptake and amino acid metabolism may be a therapeutic strategy to eradicate relapse LSCs.
Discussion:
To identify metabolic vulnerabilities of LSCs, we performed an extensive study of metabolomic differences between functional LSCs and bulk AML cells. Importantly, all studies described in this report were performed with primary AML cells, thereby avoiding any confounding effects that may arise from using established cell line models. We show that LSCs have higher levels of amino acids, and that amino acid metabolism is essential for survival of de novo LSCs. Metabolism of specific amino acids including cysteine, glutamine, and branched chain amino acids has been shown to be important in multiple hematologic malignancies (Cramer et al., 2017; Gregory et al., 2016; Raffel et al., 2017). However, a role for amino acid metabolism in driving oxidative phosphorylation has not previously been reported. Importantly, our data show that amino acid metabolism is less relevant to the survival of normal HSPCs. This finding is in contrast to a previous report (Taya et al., 2016). We believe the discrepancy between our work and Taya et al is likely due to the duration of the experiments; we deprived HSPCs of amino acids for 3 days, whereas Taya et al. reported a 7 day analysis.
We determined that amino acids are metabolized in the TCA cycle in LSCs, and that LSCs from de novo AML patients were selectively dependent on amino acid metabolism for OXPHOS. We have previously shown that LSCs have a differential reliance on OXPHOS compared to AML blasts or normal HSCs (Lagadinou et al., 2013). This is because AML blasts and HSCs can up-regulate glycolysis to compensate for loss of OXPHOS, but LSCs do not have this capacity (Lagadinou et al., 2013). These data were confirmed in our present study through glucose tracing experiments, and are consistent with our finding that AML blasts but not LSCs are highly dependent on glucose for survival and glycolysis. We also showed in de novo AML that blasts but not LSCs can increase their utilization of fatty acid metabolism which directly contributes to the TCA cycle. This differential metabolic flexibility likely explains why LSCs are more sensitive to amino acid loss compared to AML blasts. Finally, we demonstrated that venetoclax with azacytidine treatment can target amino acid metabolism in LSCs in AML patients. Decreased amino acid uptake upon venetoclax with azacitidine treatment contributes to a reduction in OXPHOS, which targets LSCs. Altogether; we propose that LSCs in de novo AML patients selectively rely on amino acid metabolism to fuel OXPHOS. Furthermore, targeting amino acid uptake results in decreased OXPHOS and selective targeting of LSCs in AML patients. The proposed mechanism is summarized in figure 8.
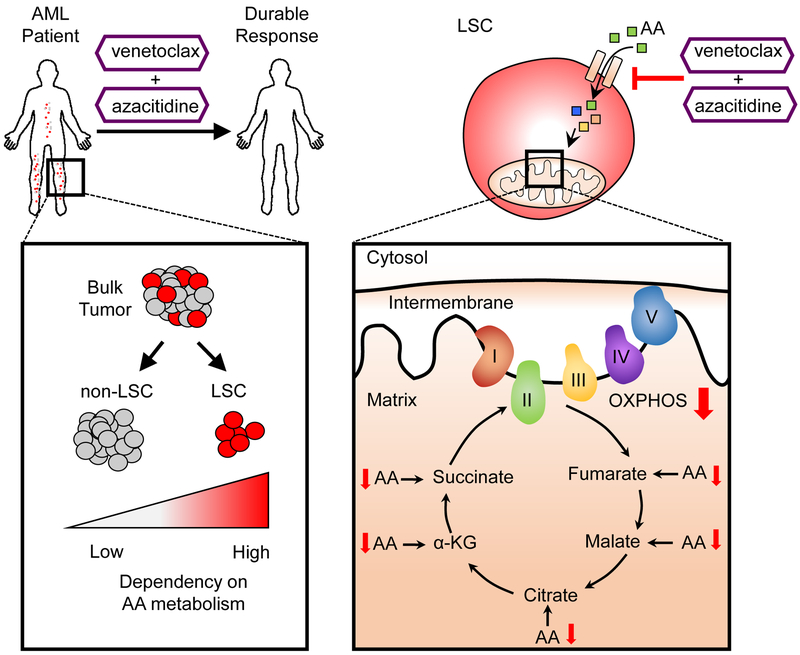
Figure 8: Summary of LSC targeting by venetoclax with azacitidine in AML patients.
LSCs catabolize amino acids into TCA cycle intermediates, making LSCs highly dependent on amino acid metabolism for OXPHOS and survival. Targeting amino acid uptake with venetoclax with azacitidine decreased OXPHOS resulting in LSC killing. Importantly, targeting amino acid metabolism in AML patients via venetoclax with azacitidine treatment results in durable remissions.
The combination of venetoclax with azacitidine is an exciting therapeutic regimen, with patients achieving high overall response rates in clinical trials (DiNardo et al., 2018a; Pollyea et al., 2018). These findings validate the potential of targeting metabolic vulnerabilities of cancer stem cells in patients. Notably, the initial trials of venetoclax and azacitidine were limited to de novo patients otherwise ineligible for conventional chemotherapy. An obvious extension of the initial work is to employ venetoclax and azacitidine for relapsed/refractory patients; however, initial reports indicate venetoclax-based regimens are much less effective in relapsed patients than observed for previously untreated patients (DiNardo et al., 2018b; Konopleva et al., 2016). In analyzing LSCs from relapsed patients, we observed acquisition of the ability to metabolically compensate for amino acid loss up upregulating fatty acid metabolism, which was not observed in treatment naive LSCs. These findings demonstrate that LSCs evolve during initial therapeutic challenge and subsequent progression of primary disease. We previously showed LSCs at relapse dramatically increase their frequency and phenotypic diversity (Ho et al., 2016). The data reported here extend those findings to the level of LSC metabolism. Notably, we show proof of concept studies demonstrating that venetoclax and azacitidine therapy can be modified by inclusion of drugs that inhibit fatty acid metabolism, thereby restoring LSC sensitivity. Future studies will focus on translating these preclinical findings into a clinically actionable strategy for relapsed AML patients.
Together, these findings highlight the importance of understanding and monitoring metabolic properties in LSCs or other cancer stems cells in patients. Of particular relevance to the present study, CSCs from brain, breast, and pancreatic cancer have also been reported to rely on OXPHOS (Janiszewska et al., 2012; Lee et al., 2017; Sancho et al., 2015; Viale et al., 2014). We propose that targeting amino acid metabolism and therefore OXPHOS in CSCs from these forms of cancer may provide therapeutic benefit.
STAR Methods:
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Craig T. Jordan (Craig.Jordan@ucdenver.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human Specimens:
AML specimens were obtained from apheresis product, peripheral blood, or bone marrow from AML patients, or mobilized peripheral blood from healthy donors who gave informed consent for sample procurement on the University of Colorado tissue procurement protocol. See Table S1 for additional details on the human AML specimens.
Patient Derived Xenograft Models:
To determine the effect of amino acid depletion on LSCs function a primary AML specimen was cultured with or without amino acids for 24 hr. Female NSG-S mice were then transplanted with human AML leukemia cells via tail vein injection (0.8 million/mouse). Three weeks after transplantation the composition of the leukemia cells within the bone marrow was examined. To determine the effect of venetoclax + azacitidine in vivo NSGS mice were transplanted with human AML leukemia cells via tail vein injection (1 million/mouse). Six weeks after transplantation, mice were treated with 100 mg/kg/day venetoclax (Abbvie) and 3mg/kg/M,W,F azacitidine (SelleckChem, S1782) or saline for two weeks, and then the composition of the residual leukemia cells within the bone marrow was examined. All animal experiments were approved by the University of Colorado Denver (OLAR).
METHOD DETAILS
Human Specimen Culturing:
When culturing was required, all samples were cultured in a base media of MEM without amino acids and 5.5 mM glucose (My BioSource, MBS752807) supplemented with physiologic levels of amino acids (Carolina, 84-3700) as previously reported (Schug et al.) and 10nM human cytokines SCF (PEPROTech, 300-07), IL3 (PEPROTech, 200-03), and FLT3 (PEPROTech, 300-19). In addition, the media was supplemented with low density lipoprotein (Millipore, 437744), β-ME (Gibco, 21985-023), penicillin/streptomycin (Gibco, 15140122), and 20% BIT9500 serum substitute (Stem Cell Technology, 09500). Indicated metabolites were removed from the base media when appropriate.
Cell Sorting:
Primary AML specimens were thawed, stained with CD45 (BD, 571875) to identify the blast population, CD19 (BD, 555413) and CD3 (BD, 557749) to exclude the lymphocyte populations, DAPI (EMD Millipore, 278298), and CellROX deep red (Thermo Fisher, C10422), and sorted using a BD FACSARIA. ROS-low LSCs were identified as the cells with the 20% lowest ROS levels and the ROS-high blasts were identified as the cells with the highest 20% ROS levels, as previously described(Lagadinou et al., 2013).
Global UHPLC-MS Metabolomics:
Approximately 100,000-200,000 ROS-Low LSCs or ROS-high blasts or total AML cells were sorted and metabolomics analyses were performed via ultra-high pressure-liquid chromatography-mass spectrometry (UHPLC-MS – Vanquish and Q Exactive, Thermo Fisher) as previously reported (Nemkov et al., 2015). Briefly, cells were extracted in ice cold methanol:acetonitrile:water (5:3:2 v/v) at a concentration of 1 million cells/ml of buffer. After vortexing for 30 min at 4°C, samples were centrifuged at 15,000 g for 10 min at 4°C and supernatants processed for metabolomics analyses. Ten microliters of sample extracts were loaded onto a Kinetex XB-C18 column (150 × 2.1 mm i.d., 1.7 μm – Phenomenex). A 3 min isocratic run (5% B) and a 9 min gradient from 5-95% B (phase A: water and B: acetonitrile with + 0.1% formic acid for positive ion mode or with 10 mM ammonium acetate for negative ion mode) were used to elute metabolites. The mass spectrometer scanned in Full MS mode (3 min method) or performed acquisition independent fragmentation (AIF - MS/MS analysis – 9 min method) at 70,000 resolution in the 70-900 m/z range, 4 kV spray voltage, 15 sheath gas and 5 auxiliary gas, operated in negative and then positive ion mode (separate runs). Metabolite assignment was performed against an in-house standard library, as reported (Nemkov et al., 2015). Metabolite levels were then normalized to protein quantification.
Metabolic Flux:
200,000-500,000 ROS-Low LSCs or ROS-high blasts were sorted and incubated with stable isotope substrates including uniformly 13C,15N-labeled amino acids (Cambridge Isotope Laboratories, MSK-A2-US-1.2), 13C5,15N2 glutamine (Sigma-Aldrich, 707983), 13C6 glucose (Sigma-Aldrich, 389374), or 13C16 palmitic acid (Sigma-Aldrich, 705573) for 8 or 24 hr where indicated. Percent contribution of each 13C,15N-labeled to TCA cycle intermediates was calculated in independent experiments. Substrates were added at physiologically relevant concentrations (5.5 mM glucose 13C6, 100 μM Palmitate 13C16, 100μM amino acids 13C,15N -glutamine, 650μM glutamine13C5,15N2). Media formulations remained consistent across all experiments, though each iteration contained a different single stable isotope-labeled tracer. From each independent experiment we calculated the labeled isotopologues as percentage of the total (labeled + unlabeled), normalized to the relative abundance of each substrate. Metabolomics analyses were performed via UHPLC-MS using the 3 min method as described above and previously (Nemkov et al., 2015).
Viability Assays:
Patient samples were sorted and cultured without indicated metabolites or drugs for 24-72 hr. Viability was assessed by trypan blue (Gibco, 15250-071) staining and manual cell counting.
Normal HSC Analysis:
Mobilized peripheral blood samples from three individuals was thawed, cultured in indicated conditions for 24-72 hr and CD34+ (BD, 572577) and CD45+ (BD, 571875), double positive percentages were quantified by flow cytometry (FACsCaliber, BD).
CFU Assays:
Primary AML samples or normal mobilized peripheral blood samples were cultured without indicated metabolites or with indicated drugs for 24 hr before being plated in human methylcellulose (R&D systems HSC003). Colonies were counts 10-17 days after the initial plating.
OP-Puro Staining:
Patient samples were sorted for ROS-low LSCs and ROS-high AML cells and stained with OP-Puro according to the manufactures protocol (Thermo-Fisher C10459). The stained cells were quantified by flow cytometry (FACsCaliber, BD).
Seahorse Assays:
XF24 (Agilent Technologies, 100850-001) or XF96 (Agilent Technologies, 102417-100) extracellular flux assay kits were used to measure oxygen consumption (OCR) and glycolytic flux (ECAR). ROS-low LSCs or ROS-high blasts were sorted, grown in metabolite depleted media, drug treated for four hr, or directly plated in a XF24 cell culture microplate. OCR and ECAR was measured according to the manufacture protocol and as previously described (Lagadinou et al., 2013). Briefly, five replicate wells of 5 × 105 ROS-low LSCs, ROS-high blasts, or total AML blast per well were seeded in a Cell-Tak (Corning, 324240) coated 24-well XF24 well plate. Thirty min prior to analysis, the medium was replaced with Seahorse XF media (Agilent Technologies, 102353-100) and the plate was incubated at 37°C. Analyses were performed both at basal conditi ons and after injection of 5 μg/ml oligomycin (Sigma-Aldrich, 871744), 2 μM FCCP (Sigma-Aldrich, C2920), 5 μM Antimycin A (Sigma-Aldrich, A8774), and 5 μM rotenone (Sigma-Aldrich, R8875).
RNA sequencing and data analysis:
Peripheral blood samples were taken from AML patients with peripheral disease burden pre and 5-7 hr post treatment with Venetoclax with azacitidine. ROS-low LSCs were isolated as described above. RNA was isolated (Qiagen RNeasy mini plus kit 74134). The TruSeq RNA Sample Preparation Kit V2 (Illumina) was used for next generation sequencing library construction per the manufacturer's protocols. Amplicons are ~200–500 bp in size. The amplified libraries were hybridized to the Illumina single end flow cell and amplified using the cBot (Illumina). Single end reads of 100 nucleotides were generated for each sample and aligned to the organism specific reference genome. Raw reads generated from the Illumina HiSeq2500 sequencer were de-multiplexed using configurebcl2fastq.pl version 1.8.4. Quality filtering and adapter removal was performed using Trimmomatic version 0.32 with the following parameters: “SLIDINGWINDOW:4:20 TRAILING:13 LEADING:13 ILLUMINACLIP:adapters.fasta:2:30:10 MINLEN:15”. Processed/cleaned reads were then mapped to the UCSC hg19 genome build with SHRiMP version 2.2.3 with the following setting: “–qv-offset 33 -all-contigs.” GSEA (Subramanian et al., 2005) analysis and enrichment map (Merico et al., 2010) was performed as previously described. Data is available in GEO (accession number: GSE116567).
QUANTIFICATION AND STATISTICAL ANALYSIS
Error bars represent a standard deviation (SD). Biological factors were investigated for their significance using two-way Anova, two-tailed Student’s t test with paired or unpaired analysis. Specific analysis used is indicated in the figure legend of each figure. P value less than 0.05 was considered significant. Data with statistical significance (* p<0.05, ** p<0.01, *** p<0.005, **** p<0.001) are shown in Figures.
DATA AND SOFTWARE AVAILABILITY
RNA-seq data from AML samples is available in GEO (accession number: GSE116567).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD45-FITC | BD Biosciences | Cat# 564585; RRID:AB_2732068 |
| CD3-PeCy7 | BD Biosciences | Cat# 557749; RRID:AB_396855 |
| CD19-Pe | BD Biosciences | Cat# 555413; RRID:AB_395813 |
| Cell ROX deep red | Thermo Fisher | Cat# C10422; RRID:N/A |
| CD34-PeCy7 | BD Biosciences | Cat# 560710; RRID:AB_1727470 |
| OP-puro | Thermo Fisher | Cat# C10459; RRID: N/A |
| Biological Samples | ||
| Human acute myeloid leukemia specimens | University of Colorado Hematologic Malignancies Tissue Bank | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Amino Acids | Carolina | Cat# 84-3700; RRID: N/A |
| Human SCF | PEPROtect | Cat# 300-07; RRID: N/A |
| Human IL3 | PEPROtect | Cat# 200-03; RRID: N/A |
| Human FLT3 | PEPROtect | Cat# 300-19; RRID: N/A |
| Low Density lipoprotein | Millipore | Cat# 437744; RRID: N/A |
| BIT9500 Serum Substitute | Stem Cell Technology | Cat# 09500; RRID: N/A |
| 13C,15N-labeled amino acids | Cambridge Isotope Laboratories | Cat# MSK-A2-US-1.2; RRID: N/A |
| 13C5,15N2 glutamine | Sigma-Aldrich | Cat# 707983; RRID: N/A |
| 13C6 glucose | Sigma-Aldrich | Cat# 389374; RRID: N/A |
| 13C16 palmitic acid | Sigma-Aldrich | Cat# 705573; RRID: N/A |
| Cell-Tak | Corning | Cat# 324240; RRID: N/A |
| Human methylcellulose | R&D systems | Cat# HSC003; RRID: N/A |
| Venetoclax | Abbvie | N/A |
| Azacitidine | SelleckChem | Cat# S1782; RRID: N/A |
| Sorbitan sesquioleate (SSO) | Sigma-Aldrich | Cat#S3386; RRIS: N/A |
| Oligomycin | Sigma-Aldrich | Cat# 871744; RRID: N/A |
| FCCP | Sigma-Aldrich | Cat# C2920; RRID: N/A |
| Antimycin A | Sigma-Aldrich | Cat# A8774; RRID: N/A |
| Rotenone | Sigma-Aldrich | Cat# R8875; RRID: N/A |
| Critical Commercial Assays | ||
| XF24 extracellular flux assay kits | Agilent Technologies | Cat# 100850-001 |
| XF96 extracellular flux assay kits | Agilent Technologies | Cat# 102417-100 |
| RNeasy mini plus kit | Qiagen | Cat# 74134; RRID: N/A |
| TruSeq RNA Sample Preparation Kit V2 | Illumina | N/A |
| Deposited Data | ||
| Transcriptome Sequencing | this paper | GSE116567 |
| Experimental Models: Organisms/Strains | ||
| NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ | The Jackson Laboratory | Cat# JAX:013062, RRID:IMSR_JAX:013062 |
| Software and Algorithms | ||
| Flowjo | Flowjo | N/A |
| Metaboanalyst | http://www.metaboanalyst.ca/ | N/A |
| Morpheus | https://software.broadinstitute.org/morpheus/ | N/A |
| Gene Expression Enrichment Analysis | http://software.broadinstitute.org/gsea/index.jsp | N/A |
| Enrichment Map Analysis | http://baderlab.org/Software/EnrichmentMap/ | N/A |
Significance:
We demonstrate that LSCs are reliant on amino acid metabolism for survival and that combination of venetoclax with azacitidine acts to inhibit amino acid uptake and catabolism, thereby providing a molecular mechanism for targeting of LSCs. Recent clinical studies have demonstrated that venetoclax with azacitidine mediates direct in vivo targeting of LSCs in previously untreated AML patients, leading to deep and durable remissions for a majority of patients. Importantly, LSCs derived from relapsed patients demonstrate a more complex metabolic profile and are less sensitive to venetoclax and azacitidine treatment. These data indicate that metabolic targeting of LSCs is a promising therapeutic strategy, but that such approaches must be tailored to distinct LSC properties that arise during the course of therapy/pathogenesis.
Highlights:
Leukemia stem cells (LSCs) rely on amino acids for survival.
LSCs use amino acids to fuel oxidative phosphorylation (OXPHOS).
Venetoclax with azacitidine inhibits OXPHOS in LSCs from leukemia patients.
Venetoclax with azacitidine targets OXPHOS by inhibiting amino acid metabolism.
Acknowledgements:
We gratefully acknowledge expert review from Dr. William Carroll. The authors thank the University of Colorado Hematology Clinical Trials Unit (HCTU) for help in acquisition of temporal patient samples. We also acknowledge the Molecular and Cellular Analytical Core within the Colorado Nutrition and Obesity Research Center for the use of the Seahorse Analyzer.
Funding: This work was supported by the American Cancer Society 25A5072 and the Colorado Clinical and Translational Sciences Institute AEF CCTSI YR9 CO 2301425 (C.L.J.); the University of Colorado Department of Medicine Outstanding Early Career Scholar Program (D.A.P); the V Foundation award T2017-012 (J.D.); the Ruth L. Kirschstein individual predoctoral national service award (F31CA196330-01) (B.A.); the Ruth and Ralph Seligman Chair in Hematology Research (C.S.); National Institutes of Health (NIH) National Cancer Institute (NCI) grant R01 5 R01 CA200707 (C.T.J). C.T.J. is supported by the Nancy Carroll Allen Endowed Chair.
Footnotes
Declaration of Interest: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, Zhao F, Medeiros BC, Tyvoll DA, and Majeti R (2015). Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med 21, 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A, Wang Z, Coyaud E, Voisin V, Gronda M, Jitkova Y, Mattson R, Hurren R, Babovic S, Maclean N, et al. (2015). Inhibition of the Mitochondrial Protease ClpP as a Therapeutic Strategy for Human Acute Myeloid Leukemia. Cancer Cell 27, 864–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, et al. (2017). Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med 23, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick JE (2005). Acute myeloid leukemia stem cells. Ann N Y Acad Sci 1044, 1–5. [DOI] [PubMed] [Google Scholar]
- Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al. (2009). Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, Arellano M, Frattini MG, Kantarjian H, Popovic R, et al. (2018a). Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 19, 216–228. [DOI] [PubMed] [Google Scholar]
- DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, Daver N, Covert W, Marx KR, Mace M, et al. (2018b). Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol 93, 401–407. [DOI] [PubMed] [Google Scholar]
- Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, et al. (2011). Stem cell gene expression programs influence clinical outcome in human leukemia. Nature Medicine 17, 1086. [DOI] [PubMed] [Google Scholar]
- Gregory MA, D'Alessandro A, Alvarez-Calderon F, Kim J, Nemkov T, Adane B, Rozhok AI, Kumar A, Kumar V, Pollyea DA, et al. (2016). ATM/G6PD-driven redox metabolism promotes FLT3 inhibitor resistance in acute myeloid leukemia. Proc Natl Acad Sci U S A 113, E6669–e6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, and Hogge DE (2000). Proliferative status of primitive hematopoietic progenitors from patients with acute myelogenous leukemia (AML). Leukemia 14, 2135–2141. [DOI] [PubMed] [Google Scholar]
- Guzman ML, and Jordan CT (2004). Considerations for targeting malignant stem cells in leukemia. Cancer control : journal of the Moffitt Cancer Center 11, 97–104. [DOI] [PubMed] [Google Scholar]
- Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, Glushka J, Tayyari F, McSkimming D, Kannan N, Tojo A, et al. (2017). Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature 545, 500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T-C, LaMere M, Stevens BM, Ashton JM, Myers JR, O’Dwyer KM, Liesveld JL, Mendler JH, Guzman M, Morrissette JD, et al. (2016). Evolution of acute myelogenous leukemia stem cell properties after treatment and progression. Blood 128, 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope KJ, Jin L, and Dick JE (2004). Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature immunology 5, 738–743. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, et al. (2007). Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25, 1315–1321. [DOI] [PubMed] [Google Scholar]
- Jang YY, and Sharkis SJ (2007). A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110, 3056–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiszewska M, Suva ML, Riggi N, Houtkooper RH, Auwerx J, Clement-Schatlo V, Radovanovic I, Rheinbay E, Provero P, and Stamenkovic I (2012). Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev 26, 1926–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, et al. (2016). Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer discovery 6, 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva MY, and Jordan CT (2011). Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol 29, 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer KM, et al. (2013). BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Giltnane JM, Balko JM, Schwarz LJ, Guerrero-Zotano AL, Hutchinson KE, Nixon MJ, Estrada MV, Sanchez V, Sanders ME, et al. (2017). MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell metabolism 26, 633–647 e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC (2009). Regulation of glutathione synthesis. Mol Aspects Med 30, 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merico D, Isserlin R, Stueker O, Emili A, and Bader GD (2010). Enrichment Map: A Network-Based Method for Gene-Set Enrichment Visualization and Interpretation. PLOS ONE 5, e13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemkov T, D'Alessandro A, and Hansen KC (2015). Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino acids 47, 2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, and Hanson RW (2002). The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277, 30409–30412. [DOI] [PubMed] [Google Scholar]
- Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, Mack SC, Lai S, Rich JN, Inguva A, Shannon KM, et al. AMPK/FIS1-Mediated Mitophagy Is Required for Self-Renewal of Human AML Stem Cells. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, Mack SC, Lai S, Rich JN, Inguva A, Shannon KM, et al. (2018). AMPK/FIS1-Mediated Mitophagy Is Required for Self-Renewal of Human AML Stem Cells. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei S, Minhajuddin M, Callahan KP, Balys M, Ashton JM, Neering SJ, Lagadinou ED, Corbett C, Ye H, Liesveld JL, et al. (2013). Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J Biol Chem 288, 33542–33558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang JM, Liu W, Hancock CN, and Fischer JW (2015). Proline metabolism and cancer: emerging links to glutamine and collagen. Current opinion in clinical nutrition and metabolic care 18, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollyea DA, and Jordan CT (2017). Therapeutic targeting of acute myeloid leukemia stem cells. Blood. [DOI] [PubMed] [Google Scholar]
- Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, D’Alessandro A, Culp-Hill R, Riemondy KA, Gillen AE, et al. (2018). Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in acute myeloid leukemia patients Nature Medicine In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffel S, Falcone M, Kneisel N, Hansson J, Wang W, Lutz C, Bullinger L, Poschet G, Nonnenmacher Y, Barnert A, et al. (2017). BCAT1 restricts alphaKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature 551, 384–388. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, and Weissman IL (2001). Stem cells, cancer, and cancer stem cells. Nature 414, 105–111. [DOI] [PubMed] [Google Scholar]
- Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Korbel C, Laschke MW, Gimotty PA, Philipp SE, et al. (2013). Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell 23, 811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Chapple RH, Lin A, Kitano A, and Nakada D (2015). AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow. Cell Stem Cell 17, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M, Barneda D, Sellers K, Campos-Olivas R, Grana O, et al. (2015). MYC/PGC-1alpha Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell metabolism 22, 590–605. [DOI] [PubMed] [Google Scholar]
- Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, Swider CR, Strzelecki AC, Cavelier C, Recher C, et al. (2011). Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest 121, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug Zachary T., Peck B, Jones ***Dylan T., Zhang Q, Grosskurth S, Alam Israt S., Goodwin Louise M., Smethurst E, Mason S, Blyth K, et al. Acetyl-CoA Synthetase 2 Promotes Acetate Utilization and Maintains Cancer Cell Growth under Metabolic Stress. Cancer Cell 27, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrtic M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, Hurren R, Jitkova Y, Gronda M, Maclean N, et al. (2011). Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 20, 674–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Ladi E, Mayer-Proschel M, and Noble M (2000). Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A 97, 10032–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takubo K, and Semenza GL (2011). Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9, 298–310. [DOI] [PubMed] [Google Scholar]
- Taya Y, Ota Y, Wilkinson AC, Kanazawa A, Watarai H, Kasai M, Nakauchi H, and Yamazaki S (2016). Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation Science (New York, NY: ) 354, 1152–1155. [DOI] [PubMed] [Google Scholar]
- Terpstra W, Ploemacher RE, Prins A, van Lom K, Pouwels K, Wognum AW, Wagemaker G, Lowenberg B, and Wielenga JJ (1996). Fluorouracil selectively spares acute myeloid leukemia cells with long-term growth abilities in immunodeficient mice and in culture. Blood 88, 1944–1950. [PubMed] [Google Scholar]
- Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, et al. (2014). Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514, 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O (1956). On the origin of cancer cells. Science 123, 309–314. [DOI] [PubMed] [Google Scholar]
- Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, Stevens B, Pei S, Balys M, Ashton JM, et al. (2016). Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell 19, 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.