Abstract
A recently described mouse homolog of the human roseoloviruses, murine roseolovirus (MRV), causes loss of peripheral and thymic CD4+ cells during neonatal infection of BALB/c mice. Despite significant disruptions to the normal adaptive immune response, infected BALB/c mice reproducibly recover from infection, consistent with prior studies on a related virus, mouse thymic virus (MTV). Herein we show that in contrast to published studies on MTV, MRV appears to robustly infect neonatal C57BL/6 (B6) mice causing severe depletion of thymocytes and peripheral T-cells. Moreover, B6 mice recovered from infection. We investigated the mechanism of thymocyte and T-cell loss, determining that the major thymocyte subsets were infected with MRV, however CD4+ and CD4+CD8− showed increased apoptosis during infection. We found that CD8+ T-cells populated MRV-infected thymi. These CD8+ T cells expressed markers of activation, had restricted T-cell receptor repertoire and accumulated intracellular effector proteins, consistent with a cytotoxic lymphocyte phenotype, and suggesting their involvement in viral clearance. Indeed, absence of CD8+ T-cells prevented recovery from MRV infection and led to lethality in infected animals whereas B-cell deficient mice showed CD4+ T-cell loss but recovered from infection without lethality. Thus these results demonstrate that CD8+ T-cells are required for protective immunity against a naturally occurring murine pathogen that infects the thymus, and establish a novel infection model for MRV in B6 mice, providing the foundation for detailed future studies on MRV with the availability of innumerable mutant mice on the B6 background.
Introduction
Infection with the roseoloviruses, HHV6A, HHV6B, and HHV7, is virtually ubiquitous amongst humans (1). Epidemiological and cross-sectional studies have identified a myriad of conditions associated with viremia or seropositivity (2–12). However, these studies are hampered by the paucity of appropriate uninfected control individuals.
Primary HHV6 infection usually resolves and the virus persists as either a latent virus or as a chronic viral infection (13). HHV6 can also reactivate and cause viremia during immune suppression. Both the humoral and cytotoxic T-lymphocyte responses to HHV6 have been characterized (14). Neutralizing IgM antibodies toward HHV6 develop during acute exantham subitum (15). Virus-specific CD4+ and CD8+ T-cells are present in adults without HHV6 viremia, presumably due to prior exposure to the virus (16, 17). However, the relative importance of these cell types during acute HHV6 infection or reactivation is unclear. Several clinical conditions arising after immune suppression are associated with HHV6 reactivation, suggesting that active anti-HHV6 surveillance contributes to controlling latent or chronic HHV6 infection. A clearer understanding of the specific roles of each aspect of the immune system may aid in prevention of HHV6-dependent pathological consequences but it has been challenging to study the pathogenesis of human roseolovirus infections because these viruses are highly species-specific and require manipulation of the host to infect mice for detailed experimental analysis (1).
We recently identified that a naturally occurring murine betaherpesvirus, murine roseolovirus (MRV), is a murine homolog of the human roseoloviruses (18). MRV phenotypically resembled mouse thymic virus (MTV, also known as mouse T-lymphotropic virus [MTLV], Murid Herpesvirus 3 [MuHV-3]) as both viruses caused thymic “necrosis” in neonatal mice but genome sequence data for MTV are not publically available so we cannot definitely make conclusions about the relationships between these two viruses. Phylogenetic analyses showed that MRV is more closely related to human roseoloviruses than to another naturally occurring murine betaherpesvirus, murine cytomegalovirus (MCMV). Since MRV infects the neonatal thymus (18) and HHV6 can directly infect and lyse CD4+ T-cells (19–22), MRV and human roseoloviruses may potentially share common pathological mechanisms, potentially allowing insight into the human viruses by studying MRV.
Prior studies of BALB/c mice infected with MTV indicate that recovery from infection can occur but the mechanisms contributing to these findings remained unknown (23). Here, we found that neonatal infection of BALB/c mice with MRV leads to severe CD4+ thymocyte and T-cell depletion, followed by recovery, consistent with prior studies on MTV. In addition, in contrast to prior studies of MTV where C57BL mice were relatively resistant to infection (24), we found that C57BL/6 (B6) mice are susceptible to infection, allowing us to address issues of viral tropism and potential pathogenic mechanisms by use of genetic mutants available on the B6 genetic background. We studied immune mechanisms controlling MRV infection and found that CD8+ T-cells, but not mature B-cells, are crucial for control of MRV infection.
Materials and Methods
Mice
C57BL/6 mice were purchased from Charles River Laboratories. Rag1−/−, CD8−/− and µMT mice were purchased from The Jackson Laboratory. Infected and uninfected mice were independently housed to avoid horizontal viral transmission.
Viral Isolation and infection
Viral stocks were produced by in vivo passaging as previously described (18, 23, 25). A single viral stock was used for all experiments. For all infections, P0 mice were intraperitoneally inoculated with 100µl of a 1:10 dilution of thawed MRV viral stock.
Flow Cytometry
Single cell lymphocyte suspensions were prepared from thymi or spleens from infected animals and processed by manual disruption through a 70µM filter. 106 to 107 cells were stained with fixable viability dye (eBioscience, San Diego, CA), before incubation in 2.4G2 supernatant (anti-FcRII/III). Cells were stained with surface antibodies (Affymetrix, Santa Clara, CA): anti-CD3ε (145-2C11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-TCRβ (H57-597), anti-CD45.2 (104) CD24 (M1/69), CD8β (eBioH35-17.2), CD62L (MEL-14), CD44 (IM7), CD69 (H1.2F3), KLRG1 (2F1), IFNγ (XMG1.2), Granzyme B (GB11) and mouse TCR Vβ screening panel (BD Biosciences, San Diego, CA). Annexin V staining was done following manufacturer recommendations (Biolegend, San Diego, CA). Intracellular cytokine staining was conducted using Fixation/Permeabilization Solution Kit (BD Cytofix/Cytoperm) (Biolegend, San Diego, CA). Samples were run by flow cytometry using a FACSCanto (BD Biosciences, San Jose, CA) and analyzed using FloJo X (TreeStar, Ashland, OR). Statistical analysis was conducted in GraphPad Prism 6.01 (GraphPad Software, La Jolla, CA)
Transmission electron microscopy
Ultrastructural analysis was performed by the Molecular Microbiology Imaging Facility at Washington University. Cells were fixed in 2% paraformaldehyde/2.5% glutaraldehyde in 100 mM phosphate buffer, pH 7.2 for 1 hr at room temperature. Samples were washed in sodium cacodylate buffer and postfixed in 1% osmium tetroxide (Polysciences Inc.) for 1 hr. Samples were then rinsed extensively in distilled H2O (dH2O) prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 hr. Following several rinses in dH2O, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections of 95 nm were cut with a Leica Ultracut UCT ultramicrotome (Leica Microsystems Inc., Bannockburn, IL), stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA Inc., Peabody, MA) equipped with an AMT 8 megapixel digital camera and AMT Image Capture Engine V602 software (Advanced Microscopy Techniques, Woburn, MA).
Viral genome copy quantification
Genomic DNA was prepared from mouse tissues using the Puregene extraction kit (Qiagen, Valencia, CA) following manufacturer recommendations. Each qPCR reaction utilized TaqMan Universal PCR Master Mix, No AmpErase UNG (Life Technologies, Grand Island, NY), as per manufacturer recommendations. MRV and Actin specific primers were as previously described (18). Reactions were run in triplicate and absolute quantification was determined by a standard curve.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism (GraphPad Software). Cell number and frequency were compared using unpaired, two-tailed t tests. Viral titers were compared using unpaired, two tailed t tests on log10 relative genome copies. Survival analyses were conducted using the log-rank Mantel-Cox test. Statistical significance defined as * p < 0.05; ** p < 0.01; *** p < 0.001, **** p < 0.0001
Results
MRV infection and recovery in C57BL/6 mice
Prior studies investigating infection of B6 with MTV have shown that neonatal B6 mice, in contrast to BALB/c mice, were resistant to thymic “necrosis” (24). We repeated similar experiments in B6 neonates, infecting them with MRV at P0, within 24 hours after birth (Fig. 1A, 1B). Infected B6 neonates had severe thymic “necrosis,” demonstrating near complete loss of single positive CD4 (CD4 SP) and double positive (DP) CD4+CD8+ thymocytes. Splenic T-cell numbers were also significantly reduced with near complete loss of CD4+ T-cells and reductions in CD8+ T-cell number. Furthermore, we assayed various organs at day 7 post-infection (p.i.) for viral copy number (Fig. 1C). As in BALB/c mice (18), viral titers were highest in the thymus of infected animals but MRV genomes also could be found in the spleen, liver, CNS, lung, kidney, and salivary gland. These results indicated that MRV-dependent thymocyte depletion can reproducibly occur in B6 mice.
FIGURE 1.
C57BL/6 mice have CD4+ T-cell depletion after neonatal MRV infection. P0 neonatal C57BL/6 mice were infected i.p. with MRV. (A and B) Flow cytometry on thymus and spleen 10 days p.i. with MRV. Thymus cells were gated on live lymphocytes and spleen cells were gated on live, CD3+, TCRβ+, lymphocytes with dot plots (A), frequency, and absolute quantification (B). (C) MRV genome copy numbers expressed as a ratio to mouse actin genome copy numbers at 1 week p.i. Open circles represent uninfected mice; filled circles represent infected mice. Data in A and B are representative of three independent experiments with N=6–8 per experiment. Data in C are representative of two independent experiments N=4–6 per experiment. Cell number and frequency were compared using unpaired, two-tailed t tests. Statistical significance defined as **** p < 0.0001
Remarkably, we found that B6 mice infected with MRV recovered CD4 SP and DP thymocyte percentage and absolute number at seven weeks p.i. (Fig. 2A, 2B). At this time, we also observed that peripheral CD4+ and CD8+ T-cell numbers were not significantly different between infected and uninfected control animals (Fig. 2A, 2B). We surmised this recovery was the result of control of viral titers because the observed viral titers at 7 weeks p.i. were significantly lower in the thymus compared with 1 week p.i. (Fig. 2C). We observed a similar trend in the spleens of infected animals, however the degree of reduction in viral titers was more modest (Fig. 2D).
FIGURE 2.
C57BL/6 mice recover from MRV infection. P0 neonatal C57BL/6 mice were infected i.p. with MRV. (A and B) Flow cytometry on thymus and spleen 7 weeks p.i. with MRV. Thymus cells were gated on live lymphocytes and spleen cells were gated on live, CD3+, TCRβ+, lymphocytes with dot plots (A), frequency, and absolute quantification (B). (C and D) MRV genome copy numbers (expressed as a ratio to mouse actin genome copy numbers) at 1 week or 7 weeks p.i. in the thymus(C) or the spleen (D) Open circles represent uninfected mice; filled circles represent infected mice. Data are representative of three independent experiments with N=5–6 per experiment. Cell number and frequency were compared using unpaired, two-tailed t tests. Statistical significance defined as *** p < 0.001, * p < 0.05
Acute infection of thymocyte subsets
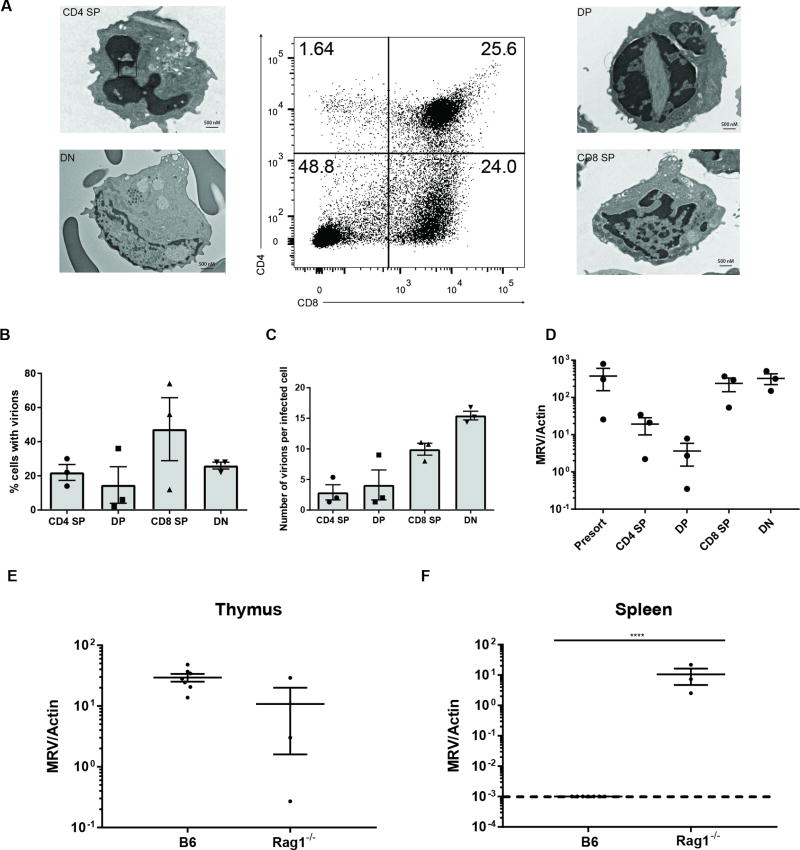
Since we observed that MRV viral copies were highest in the acutely infected thymus, we investigated which thymocyte subsets were directly infected with MRV. Given the near complete reduction of CD4+ cells during infection, we hypothesized that CD4+ cells were the major substrate for acute infection. Perhaps once all the CD4+ cells were lost during infection, there would be no more permissive cells to infect, leading to reduced titers and recovery from infection. To test this hypothesis, we harvested thymi from mice at six days p.i., as by seven days p.i. there were very few CD4 SP thymocytes. We utilized FACS sorting of the CD4 SP, DP, CD8+ (CD8 SP), and CD4−CD8− (DN) fractions followed by transmission electron microscopy (TEM) for identification of cells with visible herpesvirus virions (Fig. 3A). From single sections of sorted cells, we found approximately 20% of CD4 SP cells and 15% of DP cells had visible virions (Fig. 3B). Surprisingly, about 40% of CD8 SP and 25% of DN cells also had virions. We extended this analysis by quantifying the number of virions per infected cell and observed that CD4 SP and DP cells had less than 5 virions per cell, but CD8 SP and DN cells had 10 and 15 virions per infected cell, respectively (Fig. 3C). We confirmed these findings by conducting quantitative PCR on the thymocyte subsets from day 5–6 MRV infected neonates (Fig. 3D). MRV genome copies were present in CD4 SP and DP cells of infected thymi, but much higher levels were found in the CD8 SP and DN subsets. In sum, these data suggest that the high viral burden in MRV infected thymi is not due solely to high levels of MRV directly infecting CD4 SP or DP cells, rather CD8 SP and DN thymocytes appeared to carry more MRV per cell.
FIGURE 3.
Thymocytes are directly infected with MRV. (A–D) P0 neonatal C57BL/6 mice were infected i.p. with Murine Roseolovirus (MRV). (A–C) Transmission Electron Microscopy (TEM) of FACS sorted fractions. Thymocytes were pregated on live lymphocytes (A) Representative images of sorted fractions from day 5–6 p.i.. (B) Percentage of cells scored positive for presence of herpesvirus virions (N=100 per fraction) (C) Number of scored virions per infected cell (D) MRV genome copy number expressed as a ratio to mouse actin genome copy numbers from the indicated sorted fraction of thymus from mice 5–6 days p.i.. (E and F) MRV genome copy numbers (expressed as a ratio to mouse actin genome copy numbers) from B6 or Rag1−/− mice at 10 days p.i. in the thymus (E) or the spleen (F) Data are pooled from three independent experiments. Cell number and frequency were compared using unpaired, two-tailed t tests. Viral titers were compared using unpaired, two tailed t tests on log10 relative genome copies. Statistical significance defined as ** p < 0.01; *** p < 0.001, **** p < 0.0001
We also infected Rag1−/− mice in which thymocytes are arrested at the DN3 stage of development due to lack of productive TCRβ rearrangements and thus they do not possess DP, CD4 SP, or CD8 SP cells (Fig. 3E–3F). This experiment allowed us to test if MRV could infect cells other than these cell types. We found that at day 10 p.i. infected thymi from Rag1−/− mice had MRV levels comparable to B6 control mice. Even though Rag1−/− mice lack peripheral T cells, they also had significantly higher viral titers in the spleen compared with B6 control mice. These findings support the notion that MRV has tropism beyond thymocytes and T-cells.
These results in WT B6 mice were rather unexpected as absolute numbers of CD8 SP and DN cells appeared only modestly impacted during acute MRV infection, and effectively rules out the possibility that MRV infection resolves because of complete depletion of CD4-expressing cells. We then sought to investigate the mechanism by which CD4 SP cells and DP cells were lost during MRV infection. We hypothesized these cells may be dying of apoptosis. Indeed, CD4+, CD4+CD8+, and CD4−CD8− fractions of the MRV infected thymi had increased percentages of early and late apoptotic cells (Fig. 4A–4D). By contrast, CD8 SP cells were relatively spared of apoptosis, even though they harbored virions by TEM and viral genome copy number (Fig 3A–D).
FIGURE 4. CD4 expressing thymocytes undergo increased apoptosis after MRV infection.
(A–C) Flow cytometry from thymi of mice day 5.5 p.i.. Early apoptotic cells defined as Annexin V+ Viability Dye−. Late apoptotic cells defined as Annexin V+ Viability Dye+. Open circles represent uninfected mice; filled circles represent infected mice (D) Representative flow cytometry. Cell frequency was compared using unpaired, two-tailed t tests. Statistical significance defined as ** p < 0.01; *** p < 0.001, **** p < 0.0001
Accumulation of SP CD8+ thymocytes in MRV-infected thymi
Reproducibly at day 10 p.i. in WT B6 mice, we observed that infected thymi have CD8 SP thymocytes that disproportionately populated the thymus, as compared to the other major thymocyte subsets. We hypothesized that these cells may possess unique properties that distinguish them from typical CD8 SP thymocytes. In the absence of their immediate developmental precursor, DP cells, we reasoned these cells might be CD8+ intermediate single positive (CD8 ISP) cells. However, by flow cytometry, we found that the cells were HSAlow, suggesting they were not CD8 ISPs (Fig. 5A–5B). We also hypothesized these cells might express TCRγδ because of the prevalence of these cells during the neonatal and perinatal period. Instead, we found that these thymic CD8 SP cells were CD8αβ+ TCRβ+ resembling mature CD8+ T-cells (Fig. 5C).
FIGURE 5.
Mature CD8+ T-cells with effector phenotype infiltrate MRV infected thymi. P0 neonatal C57BL/6 mice were infected i.p. with Murine Roseolovirus (MRV). Thymi analyzed by flow cytometry. Thymocytes were pregated on live lymphocytes (A and B) Representative flow cytometry, absolute number and frequency of CD8ISP (CD8+CD4− CD24+ TCRβlow) cells or mature CD8+ T-cells (CD8+CD4− CD24− TCRβhigh) in infected or control thymi. (C) Representative flow cytometry for surface CD8β on CD8+ T-cells (D) Geometric mean fluorescence intensity of TCRβ from infected or control thymi. (E and F) Representative flow cytometry (E) and absolute number (F) of CD62L, CD44, CD69 and KLRG1 expression on CD8+ T-cells Open circles represent uninfected mice; filled circles represent infected mice. Data are representative of two or more independent experiments with N=3–5 per experiment. Cell number and frequency were compared using unpaired, two-tailed t tests. Statistical significance defined as ** p < 0.01; *** p < 0.001, **** p < 0.0001
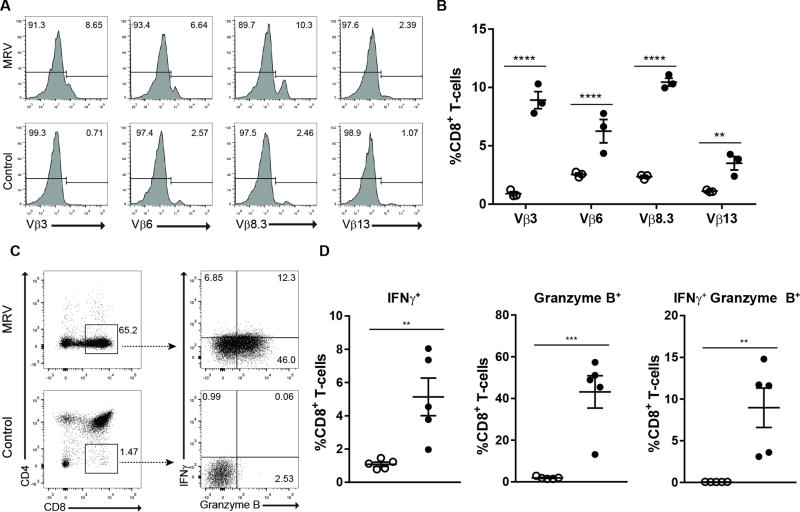
We next explored whether these CD8 SP T cells could be effector cells. The mean fluorescence intensity (MFI) of TCRβ on CD8 SP T-cells was reduced in infected thymi, consistent with activation-induced TCR downregulation (Fig. 5D) (26). We further found that these cells were positive for KLRG1, CD69, and CD44 while having decreased expression of CD62L, as compared to CD8 SP thymocytes from uninfected mice, consistent with effector T cells (Fig. 5E–5F). There was a stark reduction in naïve CD8+ T-cells and increase in the number of effector T-cells in MRV infected thymi. We also observed enrichment of CD8 SP cells with TCRVβ3, 6, 8.3, and 13, consistent with an antigen-restricted polyclonal response (Fig. 6A–6B). The CD8 SP T-cells also had increased expression of effector molecules, interferon γ (IFNγ) and granzyme B, compared with uninfected control mice (Fig. 6C–6D). Collectively, these data suggest that MRV infection elicits an effector CD8+ T-cell response in infected thymi, and their accumulation may help explain why there was no apparent depletion of CD8 SP cells, despite infection of these cells and depletion of their DP thymocyte precursors (Fig 3).
FIGURE 6.
Thymic CD8+ T-cells with a restricted TCR Vβ repertoire and intracellular effector molecules accumulate in MRV infected mice.(A and B) Representative flow cytometry (A) and frequency (B) of TCR Vβ3, 6, 8.3 and 13 on CD8+ T-cells (C and D) Representative flow cytometry (C) and frequencies (D) from intracellular staining of unstimulated thymocytes for Interferon γ (IFNγ) and Granzyme B. Open circles represent uninfected mice; filled circles represent infected mice. Data are representative of two or more independent experiments with N=3–5 per experiment. Cell number and frequency were compared using unpaired, two-tailed t tests. Statistical significance defined as ** p < 0.01; *** p < 0.001, **** p < 0.0001
CD8+ T cells protect against MRV infection
The uncontrolled MRV infection in RAG1–/– mice and the presence of activated CD8+ T-cells, with effector phenotype, in the thymus of infected WT B6 mice strongly suggested they may play a role in antiviral immunity. We found that CD8−/− neonates could be infected with MRV with CD4+ T-cell depletion occurring by one week p.i. However, unlike B6 and µMT mice, CD8−/− animals were unable to recover peripheral CD4+ T-cells and MRV infection was lethal to 80% of animals by day 50 p.i. (Fig. 7A–7B). In contrast, neonatal mice deficient in mature B-cells (µMT) infected with MRV showed near complete loss of peripheral blood CD4+ cells at one and two weeks p.i. Yet, by three weeks p.i. µMT mice had recovered normal complements of CD4+ T-cells (Fig. 7C). Additionally, infection of µMT mice was not lethal, as 100% of infected animals survived 5 months p.i. (Fig. 7D).
FIGURE 7.
CD8+ T-cells but not mature B-cells are required for recovery after MRV infection. P0 neonatal C57BL/6 mice were infected i.p. with Murine Roseolovirus (MRV). (A) Time course from flow cytometry on peripheral blood from CD8−/− mice. (B) Kaplan Meier curve comparing infected with uninfected CD8−/− animals. (C) Time course from flow cytometry on peripheral blood from µMT mice. (D) Kaplan Meier curve comparing infected with uninfected µMT animals. (E and F) MRV genome copy number from thymus (E) or spleen (F) of P0 infected mice harvested on day 7 p.i. from B6 mice or CD8−/− mice. (G and H) MRV genome copy number from thymus (G) or spleen (H) of P0 infected mice harvested on day 21 p.i. from B6 mice or CD8−/− mice. (I) Kaplan Meier curve comparing infected with uninfected TCRβ−/− animals. Gray points and lines represent uninfected mice; black points and lines represent infected mice. Data in A and C are representative of two independent experiments with N=3–6 in each group. Data in B and D are pooled from two independent experiments with N=12 and N=10 respectively. Data in E–H are pooled from two independent experiments N=4–7. Data in I are pooled from two independent experiments N=6–9. Viral titers were compared using unpaired, two tailed t tests on log10 relative genome copies. Survival analyses were conducted using the log-rank Mantel-Cox test. Statistical significance defined as ** p < 0.01; *** p < 0.001, **** p < 0.0001
Since CD4+ T-cell depletion occurred in CD8−/− mice, it appeared that this depletion was not dependent on direct killing by CD8+ T-cells, suggesting that there was enhanced proliferation of MRV. Indeed, we found that there was increased viral burden in the thymus and spleen at one week p.i. in CD8−/− animals (Fig. 7E– 7F). By three weeks p.i., we observed that CD8−/− mice had four-log increase in MRV genome copies in the thymus and at least five-log increase in the spleen (Fig. 7G–7H). Interestingly, when we infected TCRβ−/− mice, that already have severe loss of CD4+ cells before infection, we found that infected mice died at similar kinetics to infection of CD8−/− mice, strongly suggesting that the loss of CD4+ T-cells during infection of CD8−/− mice did not enhance lethal pathology (Fig. 7I). These data argue that other cell populations are infected and extend pathology associated with MRV infection.
Discussion
In this study, we established neonatal MRV infection in the B6 mouse strain. Previous reports on MTV suggested that neonatal B6 mice were relatively resistant to gross thymic “necrosis,” but in our studies with MRV, the viral titers and CD4+ cell loss were comparable between BALB/c and B6 mice. The basis for these differences between MRV and MTV is unclear but the original studies characterizing MTV utilized pooled viral stocks harvested from the thymus, spleen, kidney and pancreas from day 7 p.i. Webster Swiss neonates. By contrast, we used viral stocks isolated from BALB/c thymi alone because our analyses showed that viral copy numbers are much higher in infected thymi compared with other peripheral organs. Although these differences could be due to viral titer differences or genetic drift of the viral genome, these findings raise the possibility that MRV and MTV are not identical. Nonetheless, we showed that MRV infected neonatal B6 mice can control viral copy number and robustly recover CD4+ cells in the thymus and periphery after infection.
The major phenotypes followed in studies on MTV were thymic “necrosis” and specific loss of CD4+ T-cells. Several studies suggested these phenotypes were consistent with MTV having preferential tropism for CD4+ cells. Here we showed that MRV indeed can directly infect CD4+ developing thymocytes, but can be found at higher frequencies in thymocytes not expressing CD4. Infection of the DN subset could indicate that MRV has tropism for cells recently entering the thymus from the bone marrow or perhaps even more immature hematopoietic precursors. HHV6 was initially identified as human B cell lymphotropic virus (HBLV), but in our studies we did not identify B-cell tropism for MRV (data not shown). Moreover, our studies of RAG1−/− animals strongly suggest that MRV infects cells other than mature T- and B-cells, especially in the spleen where viral titers were very high. Future identification of infected cells will be critical to more comprehensively characterize MRV tropism.
An important outstanding question is why CD4 expressing cells appear to be selectively susceptible to MRV mediated depletion with increased apoptosis. We hypothesize that MRV may have evolved to temporarily hamper the immune system during acute infection in order to more effeciently disseminate and/or establish latency. For example, MRV may express proteins which interact with molecules specifically expressed in CD4+ T cells, ultimately affecting susceptibility to apoptosis following viral infection. Another potential mechanism of CD4 thymocyte depletion includes the infection of parenchymal or antigen-presenting cells, which interact with CD4+ T cells. Specifically, infection of thymic stromal cells could alter presentation of peptides to CD4+ thymocytes leading to their deletion but this would not totally explain loss of mature CD4+ T cells already present in the spleen. Finally, it is possible that CD8+ T cells may be uniquely resistant to lytic infection by MRV, even though we showed that they can be infected. This hypothesis would suggest that virus-specific CD8+ T cells would need to expand from the small pool of mature CD8+ T cells already present in neonatal mice. Thus, the myriad potential mechanisms for selective CD4+ T cell loss will require additional experimental analysis.
We also determined that activated CD8+ T-cells infiltrated the thymus of infected animals. These cells expressed effector molecules and possessed restricted TCR repertoires. Infection of mice lacking CD8+ T-cells prevented recovery of peripheral CD4+ T-cells and caused lethality, associated with elevated viral titers in both the thymi and spleens of CD8−/− animals as compared with B6 controls. We also found that the lethality observed was not dependent on acute CD4+ T-cell loss, as mice deficient in TCRβ−/− mice, lacking CD4+ cells before infection, were similarly susceptible to MRV as CD8−/ mice. Taken together, these experiments indicate that MRV infection is controlled by CD8+ T cells.
During acute MRV infection, neonatal mice have severely impaired immune responses. The near complete loss of CD4+ cells eliminates important T helper subsets from enhancing the antiviral immune response. Specifically, these deficient mice need to properly polarize, activate, and prime the CD8+ T-cells without T-helper cell-derived cytokines. Perhaps not surprisingly, we found that mature B-cells and presumably antibodies were not required for control of neonatal MRV infection. In the absence of CD4+ T-cells in the periphery, B-cells may not garner appropriate T-cell help for class switching and somatic hypermutation. Thus, during neonatal thymus infection that causes severe CD4+ T cell loss, CD8+ T-cells appear to be especially important for clearing MRV and preventing death.
While MRV infection of adult mice does not lead to similar reductions of CD4+ T-cells, we can detect viral genome copies in the thymus of adult animals infected with MRV (Patel and Yokoyama, unpublished). Many aspects of the innate and adaptive immune system mature as mice age and a combination of these factors likely protect adult mice from CD4+ T-cell depletion (27, 28). However, while we hypothesize that CD8+ T-cells may also be important in the control of MRV in adult mice, CD8+ T cell control may not be as dominant in adult mice, as they are in neonatal mice. Further study on MRV infection of adult mice will clarify these issues.
Only a small fraction of humans with acute primary HHV6 infection present with symptoms of exantham subitum. The mechanisms explaining why some patients present with disease and others appear to acquire HHV6 via subclinical infection are unknown. Our studies suggest that roseoloviruses are controlled by CD8+ T-cells and that impairment of the responsiveness of CD8+ T-cells can enhance viral pathogenesis. Perhaps patients with exantham subitum have impaired CD8+ T-cell responses and this accounts for their disease presentation. A subset of children with primary HHV6 have persistent viral DNA in their peripheral blood and this too may be due to impaired CD8+ T-cell function (29). Human roseolovirus reactivation occurs during immune suppression, another scenario where CD8+ T-cell surveillance is curtailed. Autologous T-cell therapy for prophylaxis against HHV6 reactivation has focused on activation and transfer of CD4+ T-cells (30). Our studies suggest that CD8+ T-cells may provide enhanced protection.
Acknowledgments
We thank Wandy Beatty and Jeff Elsner at the Washington University Molecular Microbiology Imaging Facility for assistance with TEM imaging and the Yokoyama lab for excellent discussions.
This study was supported by NIH T32GM008200 (SJP), the Howard Hughes Medical Institute (WMY), and the Barnes-Jewish Hospital Foundation (WMY)
Abbreviations
- MRV
murine roseolovirus
- MTV
mouse thymic virus
- B6
C57BL/6
- HHV6A
Human Herpesvirus 6A
- HHV6B
Human Herpesvirus 6B
- HHV7
Human Herpesvirus 7
- MTLV
mouse T-lymphotropic virus
- MuHV-3
Murid Herpesvirus 3
- MCMV
murine cytomegalovirus
- CD4 SP
single positive CD4
- DP
CD4+ CD8+ double positive
- p.i.
post-infection
- CD8 SP
CD8 single positive
- DN
CD4−CD8−
- CD8 ISP
CD8 intermediate single positive
- MFI
mean fluorescence intensity
- IFNγ
Interferon γ
References
- 1.Braun DK, Dominguez G, Pellett PE. Human herpesvirus 6. Clinical microbiology reviews. 1997;10:521–567. doi: 10.1128/cmr.10.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blazsek A, Sillo P, Ishii N, Gergely P, Jr, Poor G, Preisz K, Hashimoto T, Medvecz M, Karpati S. Searching for foreign antigens as possible triggering factors of autoimmunity: Torque Teno virus DNA prevalence is elevated in sera of patients with bullous pemphigoid. Exp Dermatol. 2008;17:446–454. doi: 10.1111/j.1600-0625.2007.00663.x. [DOI] [PubMed] [Google Scholar]
- 3.Shimazu Y, Kondo T, Ishikawa T, Yamashita K, Takaori-Kondo A. Human herpesvirus-6 encephalitis during hematopoietic stem cell transplantation leads to poor prognosis. Transplant infectious disease : an official journal of the Transplantation Society. 2013;15:195–201. doi: 10.1111/tid.12049. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita N, Morishima T. HHV-6 and seizures. Herpes : the journal of the IHMF. 2005;12:46–49. [PubMed] [Google Scholar]
- 5.Kondo K, Nagafuji H, Hata A, Tomomori C, Yamanishi K. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J Infect Dis. 1993;167:1197–1200. doi: 10.1093/infdis/167.5.1197. [DOI] [PubMed] [Google Scholar]
- 6.Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, Rhoads MP, Nguy L, Bornemann R, Morrow RA, Corey L. A population-based study of primary human herpesvirus 6 infection. The New England journal of medicine. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 7.Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 8.Ablashi DV, Eastman HB, Owen CB, Roman MM, Friedman J, Zabriskie JB, Peterson DL, Pearson GR, Whitman JE. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J Clin Virol. 2000;16:179–191. doi: 10.1016/s1386-6532(99)00079-7. [DOI] [PubMed] [Google Scholar]
- 9.Ablashi DV, Lapps W, Kaplan M, Whitman JE, Richert JR, Pearson GR. Human Herpesvirus-6 (HHV-6) infection in multiple sclerosis: a preliminary report. Multiple sclerosis. 1998;4:490–496. doi: 10.1177/135245859800400606. [DOI] [PubMed] [Google Scholar]
- 10.Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapenko S, Millers A, Nora Z, Logina I, Kukaine R, Murovska M. Correlation between HHV-6 reactivation and multiple sclerosis disease activity. J Med Virol. 2003;69:111–117. doi: 10.1002/jmv.10258. [DOI] [PubMed] [Google Scholar]
- 12.Goodman AD, Mock DJ, Powers JM, Baker JV, Blumberg BM. Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J Infect Dis. 2003;187:1365–1376. doi: 10.1086/368172. [DOI] [PubMed] [Google Scholar]
- 13.Knipe DM, Howley PM. Fields virology. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia, PA: 2013. [Google Scholar]
- 14.Becerra A, Gibson L, Stern LJ, Calvo-Calle JM. Immune response to HHV-6 and implications for immunotherapy. Current Opinion in Virology. 2014;9:154–161. doi: 10.1016/j.coviro.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibovitch EC, Brunetto GS, Caruso B, Fenton K, Ohayon J, Reich DS, Jacobson S. Coinfection of human herpesviruses 6A (HHV-6A) and HHV-6B as demonstrated by novel digital droplet PCR assay. PLoS One. 2014;9:e92328. doi: 10.1371/journal.pone.0092328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel LN, Detjen AK. Integration of childhood TB into guidelines for the management of acute malnutrition in high burden countries. Public health action. 2017;7:110–115. doi: 10.5588/pha.17.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel P, Raju NJ, Reddy B, Suresh U, Sankar DB, Reddy TVK. Heavy metal contamination in river water and sediments of the Swarnamukhi River Basin, India: risk assessment and environmental implications. Environmental geochemistry and health. 2017 doi: 10.1007/s10653-017-0006-7. [DOI] [PubMed] [Google Scholar]
- 18.Yao K, Peng Z, Fan X. Preparation of nanoparticles with an environment-friendly approach. Journal of environmental sciences. 2009;21:727–730. doi: 10.1016/s1001-0742(08)62331-1. [DOI] [PubMed] [Google Scholar]
- 19.Ron R, Zbaida D, Kafka IZ, Rosentsveig R, Leibovitch I, Tenne R. Attenuation of encrustation by self-assembled inorganic fullerene-like nanoparticles. Nanoscale. 2014;6:5251–5259. doi: 10.1039/c3nr06231g. [DOI] [PubMed] [Google Scholar]
- 20.Gobbi A, Stoddart CA, Malnati MS, Locatelli G, Santoro F, Abbey NW, Bare C, Linquist-Stepps V, Moreno MB, Herndier BG, Lusso P, McCune JM. Human herpesvirus 6 (HHV-6) causes severe thymocyte depletion in SCID-hu Thy/Liv mice. J Exp Med. 1999;189:1953–1960. doi: 10.1084/jem.189.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner A, Carlson SA, Nukui M, Murphy EA, Berges BK. Human Herpesvirus 6A Infection and Immunopathogenesis in Humanized Rag2−/− c−/− Mice. Journal of Virology. 2013;87:12020–12028. doi: 10.1128/JVI.01556-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvat B, Berges BK, Lusso P. Recent developments in animal models for human herpesvirus 6A and 6B. Current Opinion in Virology. 2014;9:97–103. doi: 10.1016/j.coviro.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morse SS, Sakaguchi N, Sakaguchi S. Virus and autoimmunity: induction of autoimmune disease in mice by mouse T lymphotropic virus (MTLV) destroying CD4+ T cells. J Immunol. 1999;162:5309–5316. [PubMed] [Google Scholar]
- 24.Rowe WP, Capps WI. A new mouse virus causing necrosis of the thymus in newborn mice. J Exp Med. 1961;113:831–844. doi: 10.1084/jem.113.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morse SS, Valinsky JE. Mouse thymic virus (MTLV). A mammalian herpesvirus cytolytic for CD4+ (L3T4+) T lymphocytes. J Exp Med. 1989;169:591–596. doi: 10.1084/jem.169.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucke-Wold B, Misra R, Patel TG. Risk factors for low high-density lipoprotein among Asian Indians in the United States. World journal of diabetes. 2017;8:297–303. doi: 10.4239/wjd.v8.i6.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marodi L. Neonatal innate immunity to infectious agents. Infect Immun. 2006;74:1999–2006. doi: 10.1128/IAI.74.4.1999-2006.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature reviews. Immunology. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 29.Butt Z, Patel L, Das MK, Mecoli CA, Ramji A. NXP-2 Positive Dermatomyositis: A Unique Clinical Presentation. Case reports in rheumatology. 2017;2017:4817275. doi: 10.1155/2017/4817275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel HG, Tabassum S, Shaikh S. E. coli Sepsis: Red Flag for Colon Carcinoma-A Case Report and Review of the Literature. Case reports in gastrointestinal medicine. 2017;2017:2570524. doi: 10.1155/2017/2570524. [DOI] [PMC free article] [PubMed] [Google Scholar]