Abstract
Background:
Immunomodulatory drugs (IMiDs) and proteasome inhibitors have dramatically changed management of multiple myeloma (MM). While MM remains incurable, consolidation and maintenance therapy aimed at improving duration of response can potentially improve survival outcomes. A majority of randomized controlled trials (RCTs) have demonstrated benefit of IMiD-based maintenance therapy in delaying disease progression; however, whether this therapy can lead to improved survival remains controversial.
Methods:
PubMed and abstract databases of major hematology and/or oncology meetings were searched for RCTs that studied maintenance therapy with IMiDs in MM. A meta-analysis was conducted to systematically evaluate the impact of IMiD-based maintenance therapy on survival outcomes and serious adverse events associated with the therapy. All statistical tests were two-sided.
Results:
Eighteen phase 3 RCTs enrolling 7730 patients were included. IMiD-based maintenance therapy statistically significantly prolonged progression-free survival (PFS; hazard ratio (HR) = 0.62, 95% confidence interval (CI) = 0.57 to 0.67, P < .001) but failed to improve overall survival (OS; HR = 0.93, 95% CI = 0.85 to 1.01, P = .082). Stratified analyses demonstrated that both thalidomide and lenalidomide provided PFS but not OS benefit in transplantation as well as nontransplantation settings. IMiD-based maintenance therapy in MM led to a higher risk of grade 3–4 thromboembolism (risk ratio = 2.52, 95% CI = 1.41 to 4.52, P = .002). Thalidomide maintenance therapy increased the risk of peripheral neuropathy; lenalidomide maintenance therapy increased the risks of myelosuppression and second primary hematological malignancies.
Conclusions:
Thalidomide- or lenalidomide-based maintenance therapy improves PFS but not OS in MM and increases risks of grade 3–4 adverse events, including thromboembolism, peripheral neuropathy, neutropenia, and infection.
Multiple myeloma (MM) is a B-cell malignancy characterized by the aberrant expansion of clonal plasma cells and a complex array of clinical manifestations that typically include hypercalcemia, renal dysfunction, anemia, and bone lesions. MM accounts for approximately 10% of all hematological malignancies and is responsible for approximately 2% of cancer-related mortalities ( 1 , 2 ). Historically, the combination of melphalan and prednisone was established as a standard of care in the early 1960s and remained the cornerstone of MM therapy for decades ( 3 ). High-dose chemotherapy (HDC), eg, melphalan 200mg/m 2 , followed by autologous stem cell transplantation (ASCT) was developed in the 1980s. This regimen represented another important advance in MM therapy and greatly improved the progression-free survival (PFS) and overall survival (OS) of MM patients ( 3 ). A better understanding of the interaction between MM cells and the bone marrow microenvironment and its role in disease progression has promoted the development of novel agents for MM therapy over the past two decades ( 4 ). Immunomodulatory drugs (IMiDs) including thalidomide, lenalidomide, and pomalidomide and the proteasome inhibitors bortezomib and carfilzomib have been increasingly incorporated into MM therapy regimens in recent years ( 4–6 ). Emergence of these newer therapeutic agents has led to a dramatic change in the paradigm of MM therapy and has resulted in further improved clinical outcomes for MM patients ( 4 ).
Initial therapy for MM patients depends to a certain extent on their eligibility for ASCT. Patients considered to be transplantation candidates usually receive two to four cycles of induction therapy and subsequent myeloablative high-dose therapy (HDT) immediately followed by ASCT ( 2 ). Transplantation-ineligible patients are also first treated with induction therapy; however, the therapy duration is often extended ( 2 ). Despite the tremendous advance achieved by the incorporation of novel agents, including bortezomib, thalidomide, and lenalidomide, into induction regimens, a majority of patients experience disease progression or relapse and die within 10 years of treatment initiation ( 7 ). Therefore, the development of effective strategies to better control the disease after initial therapy is imperative to increasing the long-term survival of MM patients. The role of consolidation and maintenance therapies following initial induction therapy has been actively investigated in the field. Consolidation therapy is used in the transplantation setting and typically consists of a short course of more intensive therapy, eg, two to four cycles of combination regimens such as bortezomib, lenalidomide, and dexamethasone, with the primary goal of deepening the response achieved by induction therapy and HDT/ASCT ( 5 , 7 ). Maintenance therapy is usually administered continuously for a prolonged period of time, typically with a single agent, with the goal of preventing or delaying disease progression ( 5 , 7 ). Both approaches have been shown to delay disease relapse and the need for second-line intensive therapy ( 8 ).
Interferon-α and glucocorticoids were the first agents studied for MM maintenance therapy; however, these agents have not been widely adopted because of concerns of long-term efficacy, tolerability, and/or side effects ( 7–11 ). Newer agents are more attractive candidates for use in the maintenance setting because of improved efficacy and better tolerability. While a few clinical studies on the role of bortezomib in MM maintenance therapy are available, the most well-studied agents in this setting are IMiDs by far, including thalidomide and lenalidomide ( 7 , 12 ). Randomized controlled trials (RCTs) studying the role of thalidomide and lenalidomide in MM maintenance therapy either after HDT/ASCT in transplant-eligible patients or after conventional induction therapy in transplant-ineligible patients have shown relatively consistent results regarding PFS prolongation. However, whether maintenance therapy with these agents ultimately improves the OS of MM patients remains controversial. Although thalidomide and lenalidomide are relatively well tolerated, long-term use may still lead to higher risks of cytopenia, infection, thromboembolism, neuropathy, and even second primary malignancies. Therefore, a comprehensive and in-depth analysis to further evaluate the survival benefits of thalidomide and lenalidomide maintenance therapy is necessary because these results may help establish the best algorithms for MM management.
Here, we report the results of a meta-analysis of RCTs that studied the efficacy and safety of thalidomide and lenalidomide in MM maintenance therapy in both the transplantation and nontransplantation settings and discuss the roles of IMiD maintenance therapy in MM management in the context of available literature and our comprehensive meta-analysis.
Methods
Data Sources and Searches
PubMed, Web of Science, ASH, ASCO, EHA, and ESMO databases were searched for eligible RCTs that studied the outcome of MM patients who received thalidomide- or lenalidomide-based maintenance therapy. The search terms used were “thalidomide OR lenalidomide,” “multiple myeloma,” and “maintenance therapy.” The search was limited to publications in English. Reference lists of included articles were manually screened to retrieve eligible studies that may have been missed during the initial online search. The final update of the search was conducted on April 3, 2015.
Study Selection and Endpoints
Studies eligible for inclusion in this meta-analysis were required to meet all the following criteria: 1) they were published before April 2015 and written in English; 2) they were RCTs; 3) the study population was comprised of patients with newly diagnosed or previously treated MM; 4) the study had an intervention group: IMiD-containing maintenance therapy and a control group: IMiD-free or no maintenance therapy; and 5) they provided sufficient information on hazard ratios (HRs) of at least one of the following survival outcomes: PFS, event-free survival (EFS), time to progression (TTP), and OS. We included both studies in the transplantation setting and those in the nontransplantation setting. The publication with the most updated results was chosen if multiple publications were available for a given study. Two investigators (FY and YS) independently conducted the literature search and study selection, and two additional investigators (YW and WZ) resolved any discrepancies, reaching a final list agreed upon by all four investigators. Our efficacy endpoints of interest were survival outcomes, including PFS (or EFS/TTP) and OS. The safety endpoints were treatment-related grade 3–4 adverse events.
Data Extraction
Data from the studies were collected by two independent reviewers (FY and YS), including the first author’s name, year of publication, trial design, ASCT status, maintenance therapy agents, and number of patients. Survival data were directly extracted from the text, table(s), or figure(s) of the publications or were estimated from Kaplan-Meier curve(s) where applicable.
Previously published meta-analyses ( 13–15 ) were also used as references of the data source when appropriate. Two additional authors (YW and WZ) resolved any discrepancies regarding the extraction/calculation of quantitative data. Adverse event data were directly extracted from the publications.
Statistical Analysis
Hazard ratios with 95% confidence intervals (CIs) of time-to-event data (PFS/EFS/TTP, OS) for the intervention arm vs control arm were used to represent the effect of treatment for each study, whereas the risk ratio (RR) with a 95% confidence interval was used to describe the effect size of dichotomous safety outcome. Data were combined into meta-analysis using the Comprehensive MetaAnalysis (v2), Stata (v13), and RevMan (v5.3) programs. The random effects model was utilized to calculate the pooled hazard ratio, risk ratio, and 95% confidence interval. Forest plots were generated using Comprehensive MetaAnalysis (v2). Statistical heterogeneity assessment was conducted using the Cochran’s Q test and I2 statistic, and differences between subgroups were assessed using methods described by Deeks et al ( 16 ). I2 describes the percentage of total variation across studies that is because of heterogeneity rather than chance. Statistically significant heterogeneity was defined as a P value of less than .1 or an I2 statistic greater than 50% ( 16 ). Sensitivity analysis was conducted by excluding one study at a time to explore the influence of each individual study on the pooled estimates. Publication bias was evaluated using Begg’s rank correlation test ( 17 ) and Egger’s regression test ( 18 ). Funnel plots were generated using Comprehensive MetaAnalysis (v2). All statistical tests were two-sided, and statistical significance was defined at P values of less than .05.
Results
Trial Selection
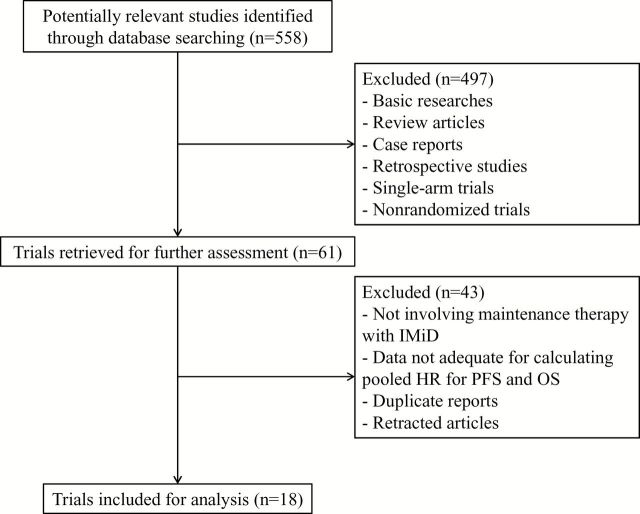
The search and selection process is illustrated in Figure 1 . The initial search resulted in 558 potentially relevant studies. During the screening process, we excluded a total of 497 ineligible studies, including basic research studies, reviews, nonclinical studies, case reports, retrospective studies, and nonrandomized controlled trials. Upon further review of the remaining 61 studies, additional 43 were excluded for one the following reasons: no IMiD maintenance therapy, duplicated publication of the same clinical study, retracted publication, or no adequate information on (or for calculation of) the hazard ratio for survival outcomes. Finally, a total of 18 randomized clinical trials (enrolling 7730 patients) that studied IMiD maintenance therapy in MM were included in the present meta-analysis ( 19–36 ).
Figure 1.
Flowchart showing study search, screening and selection. IMiD = immunomodulatory drug; HR = hazard ratio; OS = overall survival; PFS = progression-free survival.
Trial Characteristics
All 18 included studies were phase 3 RCTs, with a total of 7730 patients enrolled. Basic information and characteristics of the RCTs are presented in Table 1 . IMiDs used in the maintenance therapy regimen included thalidomide in 12 trials ( 19–30 ) and lenalidomide in six trials ( 31–36 ). IMiD-containing maintenance therapy was provided after ASCT in seven trials ( 19 , 20 , 23 , 27 , 30 , 32 , 33 ). In nine trials ( 21 , 22 , 24–26 , 28 , 31 , 34 , 35 ), the IMiD-containing regimen was administered in the nontransplantation setting. Two trials enrolled both transplantation-eligible and -ineligible patients ( 29 , 36 ).
Table 1.
Basic information of the eighteen included phase 3 randomized controlled trials
| Study | Trial name | IMiD used | ASCT status | No. of patients | Maintenance therapy regimen | Survival endpoint |
|---|---|---|---|---|---|---|
| Attal (2006) (19) | IFM 99 02 | Thalidomide | with | 201 | Pamidronate + thalidomide | EFS, OS |
| 196 | Pamidronate* | |||||
| 200 | No maintenance* | |||||
| Barlogie (2008) (20) | TT2 | Thalidomide | with | 323 | Thalidomide† + IFN-α + dexamethasone | EFS, OS |
| 345 | IFN-α + dexamethasone | |||||
| Palumbo (2008) (21) | GISMM2001-A | Thalidomide | without | 167 | Thalidomide† | PFS, OS |
| 164 | No maintenance | |||||
| Rajkumar (2008) (22) | THAL-MM-003 | Thalidomide | without | 234 | Thalidomide + dexamethasone | TTP, PFS, OS |
| 232 | Placebo + dexamethasone | |||||
| Lokhorst (2010) (23) | HOVON-50 | Thalidomide | with | 268 | Thalidomide† | PFS, OS |
| 268 | IFN-α | |||||
| Ludwig (2010) (24) | 01-002-0601 | Thalidomide | without | 64 | Thalidomide + IFN-α | PFS, OS |
| 64 | IFN-α | |||||
| Waage (2010) (25) | NMSG #12 | Thalidomide | without | 182 | Thalidomide† | PFS, OS |
| 175 | Placebo | |||||
| Wijermans (2010) (26) | HOVON-49 | Thalidomide | without | 165 | Thalidomide† | EFS, PFS, OS |
| 168 | No maintenance | |||||
| Maiolino (2012) (27) | GBRAM0001 | Thalidomide | with | 56 | Thalidiomide + dexamethasone | PFS, OS |
| 52 | Dexamethasone | |||||
| Mateos (2012) (28) | GEM2005MAS65 | Thalidomide | without | 91 | Bortezomib + thalidomide† | PFS, OS |
| 87 | Bortezomib + prednisone | |||||
| Morgan (2012) (29) | MRC Myeloma IX | Thalidomide | with | 245 | Thalidomide | PFS, OS |
| 247 | No maintenance | |||||
| without | 163 | Thalidomide | ||||
| 163 | No maintenance | |||||
| Stewart (2013) (30) | NCIC CTG MY.10 | Thalidomide | with | 166 | Thalidomide + prednisone | PFS, OS |
| 166 | No maintenance | |||||
| Zonder (2010) (31) | S0232 | Lenalidomide | without | 97 | Lenalidomide† + dexamethasone | PFS, OS |
| 95 | Placebo + dexamethasone | |||||
| Attal (2012) (32) | IFM 2005-02 | Lenalidomide | with | 307 | Lenalidomide | PFS, EFS, OS |
| 307 | Placebo | |||||
| McCarthy (2012) (33) | CALGB 100104 | Lenalidomide | with | 231 | Lenalidomide | TTP, OS |
| 229 | Placebo | |||||
| Palumbo (2012) (34) | MM-015 | Lenalidomide | without | 152 | Lenalidomide‡ | PFS, OS |
| 153 | Placebo‡ | |||||
| 154 | Placebo§ | |||||
| Benboubker (2014) (35) | FIRST | Lenalidomide | without | 535 | Lenalidomide + dexamethasoneǁ | PFS, OS |
| 541 | No maintenanceǁ | |||||
| 547 | No maintenance¶ | |||||
| Palumbo (2014) (36) | RV-MM-PI-209 | Lenalidomide | with | 57 | Lenalidomide | PFS, OS |
| 59 | No maintenance | |||||
| without | 59 | Lenalidomide | ||||
| 56 | No maintenance |
* Pamidronate and No maintenance arms were combined as control arm. ASCT = autologous stem cell transplantation; EFS = event-free survival; IMiD = immunomodulatory drug; OS = overall survival; PFS = progression-free survival; TTP = time to progression.
† Thalidomide, or lenalidomide, was also used during induction and/or consolidation therapy phases in experimental arms.
‡ Melphalan, prednisone, and thalidomide for nine cycles followed by lenalidomide or placebo maintenance.
§ Melphalan and prednisone for nine cycles, no maintenance therapy, data not used in this meta-analysis.
ǁ Lenalidomide and dexamethasone continuously or for 18 cycles only (No maintenance arm).
¶ Melphalan, prednisone, and thalidomide for 12 cycles, no maintenance therapy, data not used in this meta-analysis.
Survival Outcomes
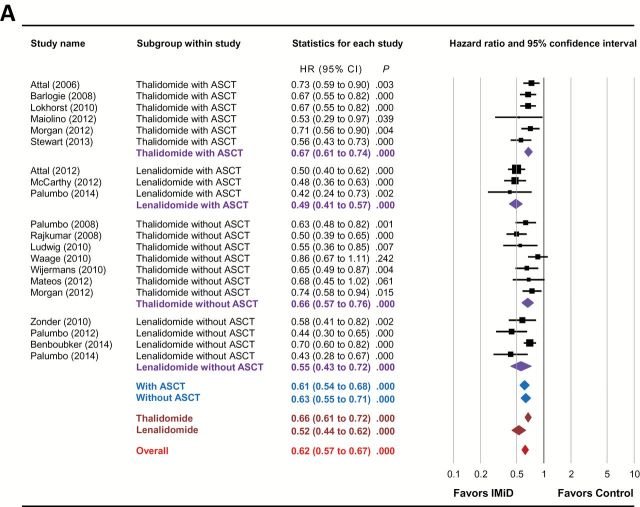
As shown in Figure 2A and Table 2 , IMiD-based maintenance therapy statistically significantly improved the PFS (HR = 0.62, 95% CI = 0.57 to 0.67, P < .001) in patients with MM. Subgroup analyses showed that both thalidomide- (HR = 0.66, 95% CI = 0.61 to 0.72, P < .001) and lenalidomide (HR = 0.52, 95% CI = 0.44 to 0.62, P < .001)-based maintenance therapies improved PFS. In addition, the PFS benefits of IMiD-based maintenance therapy were observed in both transplantation (HR = 0.61, 95% CI = 0.54 to 0.68, P < .001) and nontransplantation (HR = 0.63, 95% CI = 0.55 to 0.71, P < .001) settings. Stratification based on both IMiD agent and transplantation status confirmed that the PFS benefits of IMiD-based maintenance therapy in MM were independent of IMiD type and ASCT status ( Table 2 ).
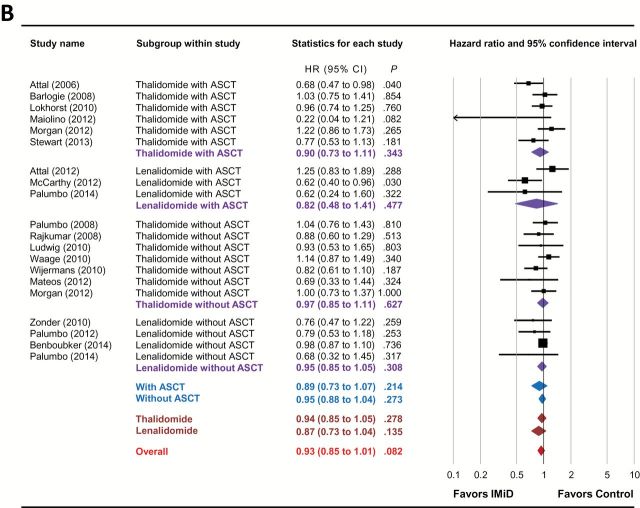
Figure 2.
Forest plots of hazard ratios for (A) progression-free survival and (B) overall survival of immunomodulatory drug-containing groups vs control groups. Hazard ratios (HRs) for each trial are represented by the squares , where the size of the square represents the weight of the trial in the meta-analysis and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the subgroup and overall summary HR estimates and 95% CIs. All statistical tests were two-sided. ASCT = autologous stem cell transplantation; IMiD = immunomodulatory drug.
Table 2.
Effects of immunomodulatory drug-based maintenance therapy on progression-free survival and overall survival in multiple myeloma*
| Immunomodulatory drug | ASCT status | No. of trials | PFS | OS | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Thalidomide/Lenalidomide | combined | 18 | 0.62 (0.57 to 0.67) | <.001 | 0.93 (0.85 to 1.01) | .082 |
| with ASCT | 9 | 0.61 (0.54 to 0.68) | <.001 | 0.89 (0.73 to 1.07) | .214 | |
| without ASCT | 11 | 0.63 (0.55 to 0.71) | <.001 | 0.95 (0.88 to 1.04) | .273 | |
| Thalidomide | combined | 12 | 0.66 (0.61 to 0.72) | <.001 | 0.94 (0.85 to 1.05) | .278 |
| with ASCT | 6 | 0.67 (0.61 to 0.74) | <.001 | 0.90 (0.73 to 1.11) | .343 | |
| without ASCT | 7 | 0.66 (0.57 to 0.76) | <.001 | 0.97 (0.85 to 1.11) | .627 | |
| Lenalidomide | combined | 6 | 0.52 (0.44 to 0.62) | <.001 | 0.87 (0.73 to 1.04) | .135 |
| with ASCT | 3 | 0.49 (0.41 to 0.57) | <.001 | 0.82 (0.48 to 1.41) | .477 | |
| without ASCT | 4 | 0.55 (0.43 to 0.72) | <.001 | 0.95 (0.85 to 1.05) | .308 | |
* All statistical tests were two-sided. ASCT = autologous stem cell transplantation; CI = confidence interval; HR = hazard ratio; PFS = progression-free survival; OS = overall survival.
Conversely, IMiD-based maintenance therapy failed to statistically significantly improve OS (HR = 0.93, 95% CI = 0.85 to 1.01, P = .082) in MM ( Fig 2B and Table 2 ), although a clear trend was observed for longer OS in the IMiD arm. Subgroup analysis based on different types of IMiDs showed that neither thalidomide (HR = 0.94, 95% CI = 0.85 to 1.05, P = .278) nor lenalidomide (HR = 0.87, 95% CI = 0.73 to 1.04, P = .135) statistically improved OS in the maintenance setting. In addition, IMiD maintenance therapy did not improve OS in either transplantation (HR = 0.89, 95% CI = 0.73 to 1.07, P = .214) or nontransplantation (HR = 0.95, 95% CI = 0.88 to 1.04, P = .273) settings. On further stratification, both thalidomide- and lenalidomide-based maintenance therapy failed to confer a statistically significant OS benefit regardless of ASCT status ( Table 2 ).
Notably, while the results described above were based on analysis performed with Comprehensive MetaAnalysis (v2), we obtained identical results with Stata (v13) and RevMan (v5.3) (data not shown).
Heterogeneity and Sensitivity Analyses
We assessed the heterogeneity of data from the included trials using Cochran’s Q test and I2 statistic. Interestingly, statistical results indicated the presence of heterogeneity for PFS data ( P = .018, I2 = 44.2%) but not OS data ( P = .273, I2 = 14.5%). The study population in any given trial is essentially the same; therefore, these results indicate the presence of data heterogeneity across the included studies. In addition, apparent heterogeneities were also present regarding trial design, therapy regimen, calculation of survival outcomes, statistical methods, and other factors. Therefore, we decided to use the random effects model to conduct the meta-analysis of survival outcomes. Notably, using the fixed effects model for analysis does not change the major conclusions reported in this study (data not shown).
We ran the analyses repeatedly, removing one different study for each iteration to observe the change of pooled hazard ratios. The pooled hazard ratios ranged from 0.61 to 0.63 for PFS and 0.91 to 0.95 for OS, indicating that the survival outcome meta-analysis results were stable.
Publication Bias
Funnel plots are shown in Supplementary Figure 1 (available online). The results of Begg’s rank correlation test and Egger’s regression test of PFS ( PBegg = .048, PEgger = .020) and OS ( PBegg = .041, PEgger = .023) suggested potential publication bias for the present study.
Adverse Events
We conducted a meta-analysis to compare grade 3–4 adverse events in IMiD-containing arms and control arms and identified events that were potentially attributable to IMiD ( Table 3 ). We found that IMiD maintenance therapy statistically significantly increased the risks for developing grade 3–4 vascular events, including deep vein thrombosis (DVT) and pulmonary embolism (PE) (RR = 2.52, 95% CI = 1.41 to 4.52, P = .002) and peripheral neuropathy (RR = 2.27, 95% CI = 1.35 to 3.84, P = .002). We also observed higher risks for developing grade 3–4 hematological adverse events including neutropenia (RR = 2.73, 95% CI = 1.63 to 4.55, P < .001), thrombocytopenia (RR = 2.14, 95% CI = 1.34 to 3.40, P = .001), and anemia (RR = 1.43, 95% CI = 1.13 to 1.80, P = .003) in IMiD-containing arms. Maintenance therapy regimens containing IMiDs also increased the risks for developing grade 3–4 infection (RR = 1.64, 95% CI = 1.40 to 1.92, P < .001), fatigue (RR = 1.48, 95% CI = 1.08 to 2.03, P = .016), and constipation (RR = 1.82, 95% CI = 1.03 to 3.21, P = .039).
Table 3.
Meta-analysis of grade 3–4 adverse events*
| Adverse event | No. of trials |
Events in
IMiD arm |
Events in control arm | RR (95% CI) | P |
|---|---|---|---|---|---|
| Vascular events | 10 | 123/1958 | 54/2157 | 2.52 (1.41 to 4.52) | .002 |
| Peripheral neuropathy | 6 | 53/1419 | 28/1610 | 2.27 (1.35 to 3.84) | .002 |
| Neutropenia | 9 | 484/1894 | 255/2090 | 2.73 (1.63 to 4.55) | <.001 |
| Thrombocytopenia | 10 | 159/1886 | 89/2084 | 2.14 (1.34 to 3.40) | .001 |
| Anemia | 10 | 149/1955 | 114/2153 | 1.43 (1.13 to 1.80) | .003 |
| Infection | 9 | 297/1654 | 223/1863 | 1.64 (1.40 to 1.92) | <.001 |
| Fatigue | 10 | 130/1969 | 106/2166 | 1.48 (1.08 to 2.03) | .016 |
| Constipation | 7 | 36/1502 | 18/1692 | 1.82 (1.03 to 3.21) | .039 |
| Second primary malignancies | 7 | 93/1593 | 72/1589 | 1.33 (0.81 to 2.19) | .257 |
* All statistical tests were two-sided. CI = confidence interval; IMiD = immunomodulatory drug; RR = risk ratio.
Notably, an increased risk of grade 3–4 peripheral neuropathy was found with thalidomide (RR = 2.83, 95% CI = 1.26 to 6.35, P = .012) but not lenalidomide (RR = 1.94, 95% CI = 0.66 to 5.77, P = .231) maintenance therapy. In contrast, an increased risk of myelosuppression was only prominent with lenalidomide but not thalidomide (for neutropenia, RR = 3.38, 95% CI = 1.78 to 6.40, P < .001, vs RR = 1.61, 95% CI = 0.89 to 2.94, P = .118; for thrombocytopenia, RR = 2.05, 95% CI = 1.27 to 3.33, P = .004, vs RR = 4.53, 95% CI = 0.59 to 34.58, P = .146; and for anemia, RR = 1.38, 95% CI = 1.08 to 1.77, P = .009, vs RR = 1.83, 95% CI = 0.90 to 3.70, P = .095). Increased risks of thromboembolic events, infection, and fatigue were observed with both thalidomide and lenalidomide (data not shown).
We did not observe an increased risk of developing second primary malignancies in patients randomly assigned to IMiD-containing arms (RR = 1.33, 95% CI = 0.81 to 2.19, P = .257). However, when we specifically analyzed the risks of developing second primary hematological or solid malignancies, we found that lenalidomide was associated with a higher risk of developing secondary primary hematological malignancies (RR = 2.10, 95% CI = 1.08 to 4.09, P = .029) but not solid tumors (RR = 1.23, 95% CI = 0.54 to 2.80, P = .628).
Discussion
IMiDs are attractive agents for maintenance therapy of MM because of their impressive antimyeloma activity, predictable and mostly manageable side effects, and availability as oral medications. In the past decade, thalidomide and lenalidomide have been extensively studied in RCTs for maintenance therapy of MM, both following ASCT and in transplantation-ineligible patients. IMiD-based maintenance therapy has consistently demonstrated an increased response rate, which translates into the relatively consistent improvement of TTP/EFS/PFS; however, whether these benefits can also translate into an improvement in OS remains controversial. Our meta-analysis clearly showed that IMiD-based maintenance therapy can statistically significantly improve PFS but not OS in MM regardless of ASCT status.
Thalidomide in the Transplantation Setting
Meta-analysis of the six included studies ( 19 , 20 , 23 , 27 , 29 , 30 ) showed that thalidomide maintenance therapy significantly improved the PFS of MM patients after ASCT (HR = 0.67, 95% CI = 0.61 to 0.74, P < .001). The PFS prolongation was observed in all six studies (HR = 0.56–0.73), demonstrating an unequivocal benefit of thalidomide maintenance therapy after ASCT in delaying disease progression.
In contrast to the consistent conclusions reached in RCTs regarding the PFS advantage, whether thalidomide maintenance therapy yields an OS benefit after ASCT remains controversial. The IFM 99 02 trial was the first study that showed an OS benefit of thalidomide maintenance therapy after ASCT ( 19 ). The investigators attributed the survival benefit partly to the best quality of response in the thalidomide arm, which was supported by the observation that thalidomide benefited patients who failed to achieve a very good partial response (VGPR) but had a limited effect among patients who did achieve VGPR. However, the thalidomide arm also included treatment with pamidronate, which confounds the interpretation of the results from this study because other intravenously administered bisphosphonates have been shown to improve OS ( 37 ). Two subsequent studies, TT2 ( 20 , 38 ) and HOVON-50 ( 23 ), had a similar design in that thalidomide was used throughout induction, consolidation, and maintenance therapies. However, both studies failed to demonstrate an OS benefit of thalidomide maintenance therapy. One explanation may be shorter survival after relapse in the thalidomide groups, which was observed in both trials ( 20 , 23 , 38 ), raising the concern that extended exposure to thalidomide may lead to the selection of drug-resistant clones that challenge salvage therapies. Another explanation is the use of thalidomide as salvage therapy upon relapse in control arms, which may prolong survival after disease progression, compensating for the shorter initial EFS ( 20 , 38 ). Three more recent trials investigating maintenance therapy with thalidomide after ASCT, alone or in combination with corticosteroids, did not reveal any statistically significant OS benefit ( 27 , 29 , 30 ). In this context, our meta-analysis demonstrated that the prolonged PFS did not translate into improved OS, although a trend for longer survival in patients receiving thalidomide maintenance therapy was observed (HR = 0.90, 95% CI = 0.73 to 1.11, P = .343).
We excluded a potentially relevant Australian trial in which continuous prednisolone with or without 12 months of thalidomide was used after ASCT because the investigators defined the one-year use of thalidomide as consolidation therapy in this setting ( 39 ). In this trial, incorporation of thalidomide after ASCT statistically significantly improved OS compared with prednisolone alone for maintenance therapy. Notably, patients who progressed on the arm without thalidomide did not cross over to receive treatment with thalidomide, with most patients not receiving this IMiD. Because the majority of studies failed to demonstrate an OS benefit of thalidomide in the transplantation setting, the addition of this study to the meta-analysis may potentially alter the result. In fact, two earlier meta-analyses with fewer trials included this study, and both analyses demonstrated that thalidomide after ASCT prolonged OS in MM ( 13 , 29 ). However, when we added this Australian study into our meta-analysis, we still did not observe a convincing OS benefit of thalidomide after ASCT (HR = 0.83, 95% CI = 0.64 to 1.06, P = .128).
However, this result does not concretely demonstrate whether thalidomide maintenance therapy after ASCT leads to improved OS in MM; therefore, performing an updated meta-analysis when updates of the above trials with extended follow-up and more eligible studies become available may be worthwhile. In addition to the effect of therapy administered after disease progression, the lack of statistical power and insufficient follow-up time may have obscured OS outcomes in prior clinical trials ( 30 ). In fact, longer follow-up has already resulted in opposite conclusions in terms of the OS outcome in both the IFM 99 02 and TT2 studies ( 27 , 40 ).
Lenalidomide in the Transplantation Setting
Three trials have investigated the role of lenalidomide maintenance therapy after ASCT ( 32 , 33 , 36 ). All three studies demonstrated superior PFS in the lenalidomide arms (HR = 0.42–0.50). Our meta-analysis confirmed a PFS benefit of lenalidomide maintenance therapy following transplantation (HR = 0.49, 95% CI = 0.41 to 0.57, P < .001).
Similar to thalidomide, the OS benefit of lenalidomide maintenance therapy after ASCT is controversial. The CALGB 100104 trial demonstrated statistically significantly improved OS in patients receiving lenalidomide maintenance therapy compared with patients who received the placebo ( 33 ). However, the IFM 2005-02 trial published at the same time revealed a similar OS between the lenalidomide and placebo groups ( 32 ). This discrepancy may have resulted from differences in induction and consolidation therapies. Specifically, in the IFM 2005-02 trial, both arms received two cycles of lenalidomide as consolidation therapy after ASCT; however, the consolidation therapy was not included in the CALGB 100104 trial ( 33 ). In addition, patients randomly assigned to lenalidomide in the IFM 2005-02 trial only continued maintenance treatment for a limited time, whereas in the CALG 100104 trial maintenance therapy was continued until disease progression. A more recent trial compared lenalidomide maintenance with no maintenance after ASCT, and both arms received induction therapy with lenalidomide plus dexamethasone (Len/Dex) prior to ASCT ( 36 ). In this study, no OS advantage was observed in the lenalidomide arm. The investigators suggested that a longer follow-up is necessary to better evaluate the survival benefit of a delayed clinical relapse ( 36 ). Our meta-analysis of the above three studies indicated that lenalidomide maintenance therapy after ASCT did not prolong OS in MM (HR = 0.82, 95% CI = 0.48 to 1.41, P = .477). If excluding the Palumbo study ( 36 ), which might be too early to see a survival benefit, meta-analysis of the IFM 2005-02 and CALGB 100104 trials also showed no OS benefit of lenalidomide maintenance therapy after ASCT (HR = 0.88, 95% CI = 0.44 to 1.76, P = .723). Because the data on lenalidomide maintenance therapy after ASCT are limited at this time, an updated analysis is warranted when extended follow-up data of the above studies and additional relevant trials become available.
Thalidomide in the Nontransplantation Setting
A majority of the included studies showed a superior PFS with thalidomide maintenance therapy in MM patients not receiving ASCT; however, in two studies no statistically significant difference was found between the thalidomide and control arms ( 25 , 28 ). Meta-analysis of all seven studies clearly demonstrated that thalidomide maintenance therapy statistically significantly improved the PFS in the nontransplantation setting (HR = 0.66, 95% CI = 0.57 to 0.76, P < .001). In contrast, all seven studies were consistent in finding that thalidomide maintenance therapy in the nontransplantation setting did not improve OS, which was further confirmed by our meta-analysis (HR = 0.97, 95% CI = 0.85 to 1.11, P = .627).
Melphalan plus prednisone (MP) has been the standard of care in the past for MM patients ineligible for ASCT; however, with the introduction of IMiDs, a series of clinical trials was launched to compare MP plus thalidomide (MPT) with MP alone for this patient population, three of which contained maintenance therapy with thalidomide ( 21 , 25 , 26 ). Two French studies without a maintenance phase demonstrated that MPT statistically significantly improved both PFS and OS in patients both younger and older than 75 years ( 41 , 42 ). However, a Turkish study did not reveal any DFS or OS benefit comparing MPT with MP ( 43 ). With thalidomide maintenance following MPT induction therapy, investigators of the HOVON-49 trial reported both improved PFS (13 months vs 9 months, P ˂ .001) and OS (40 months vs 31 months, P = .05), although multivariable Cox regression only revealed a statistical significance for PFS ( P = .006) but not OS ( P = .19) ( 26 ). With similar designs, the Italian study found that thalidomide maintenance improved PFS/EFS but not OS ( 21 ), and the Nordic study did not show any PFS or OS benefit of thalidomide maintenance ( 25 ). Inconsistent results may have arisen from different patient populations (eg, performance status) and dose and schedule variations ( 25 ). A meta-analysis of five of the above studies (full data of Turkish study not yet available) showed a clear advantage of MPT vs MP in terms of response rate, PFS, and OS ( 44 ). Another meta-analysis of individual patient data of all six studies confirmed the same conclusions ( 45 ).
Our meta-analysis focused on the benefit of thalidomide maintenance therapy, and the three relevant trials ( 21 , 25 , 26 ) collectively showed that MPT followed by thalidomide maintenance has clear benefits for patients ineligible for ASCT, ie, increased response quality and duration, which translates into improved PFS. Whether this also leads to improved OS remains unclear and may warrant longer follow-up and/or further study.
In the MRC Myeloma IX trial, single-agent thalidomide maintenance therapy following MP or attenuated cyclophosphamide, thalidomide, dexamethasone (CTDa) induction therapy in nontransplantation patients improved PFS but not OS ( 29 ). The initial low availability of novel salvage therapy agents may have confounded the OS result. In addition, survival after progression was worse in the thalidomide maintenance group, which was similar to earlier reports ( 20 , 23 ) and may partially explain the lack of OS benefit in this trial. A European trial found that when added to IFN-α maintenance therapy following MP or thalidomide plus dexamethasone (Thal/Dex) induction therapy, thalidomide improved PFS but not OS, although the power of OS analysis was limited in this study ( 24 ). In another study, when compared with dexamethasone alone, continuous Thal/Dex for newly diagnosed MM statistically significantly improved the overall response rate (ORR), TTP, and PFS; however, the OS was similar in two arms, which is again possibly because of insufficient power ( 22 ).
Bortezomib is another emerging novel agent in MM therapy and has been used in both induction and maintenance settings. In the GEM2005MAS65 trial, compared with bortezomib plus prednisone (VP), bortezomib plus thalidomide (VT) maintenance therapy in patients who received induction therapy with bortezomib, melphalan, prednisone (VMP) or bortezomib, thalidomide, prednisone (VTP) improved both PFS and OS, albeit without statistical significance ( 28 ), indicating a favorable role of thalidomide in maintenance therapy. In an Italian study, VT maintenance therapy following VMP plus thalidomide (VMPT) induction was superior to VMP induction without maintenance, leading to statistically significantly improved PFS and OS ( 46 ). We did not include this study in our meta-analysis because both bortezomib and thalidomide were used in the intervention arm and determining the contribution to improved survival would be impossible. However, if we include this study, the results for PFS (HR = 0.64, 95% CI = 0.57 to 0.73, P < .001) and OS (HR = 0.91, 95% CI = 0.80 to 1.04, P = .168) remain unchanged.
Lenalidomide in the Nontransplantation Setting
We included four RCTs of lenalidomide maintenance therapy for MM in the nontransplantation setting in this study. All trials were consistent in concluding that in patients not receiving ASCT lenalidomide-based maintenance therapy prolonged PFS but did not statistically significantly improve OS. The meta-analysis confirmed the impact of lenalidomide maintenance on both PFS (HR = 0.55, 95% CI = 0.43 to 0.72, P < .001) and OS (HR = 0.95, 95% CI = 0.85 to 1.05, P = .308) in this setting.
Similar to thalidomide, when added to MP for newly diagnosed MM, lenalidomide led to favorable response and survival outcomes. In the MM-015 study, MP plus lenalidomide (MPR) induction statistically significantly improved PFS when compared with MP alone, and lenalidomide maintenance following MPR induction further improved PFS ( 34 ). OS was similar in all three arms in this study; however, the OS analysis may have been confounded by the crossover of patients to open-label Len/Dex at progression ( 34 ). In a recently published trial, lenalidomide maintenance following Len/Dex induction and MPR consolidation statistically significantly improved PFS but not OS ( 36 ). Whether the improved PFS will eventually translate into longer OS can be further addressed when longer follow-up data are available.
Again, similar to the case of thalidomide, when compared with dexamethasone alone, continuous Len/Dex statistically significantly improved ORR and PFS but not OS ( 31 ). The lack of OS benefit in this SWOG study can be attributed to the crossover design and insufficient statistical power because of early study closure ( 31 ). In the more recent FIRST trial, continuous Len/Dex was superior to limited Len/Dex (18 cycles), leading to prolonged PFS but not OS ( 35 ). This suggests a benefit of lenalidomide-based maintenance therapy in MM patients ineligible for ASCT.
Adverse Events
Although largely safe, prolonged use of IMiDs for maintenance therapy of MM can lead to increased risks of several adverse events. One of the prominent side effects is thromboembolism, which warrants routine use of aspirin and/or anticoagulants such as warfarin and low molecular weight heparin. Peripheral neuropathy is another major concern of extended IMiD use. In this meta-analysis, an increased risk of grade 3–4 peripheral neuropathy was found with thalidomide but not lenalidomide maintenance therapy. The incidence of peripheral neuropathy was also much lower with lenalidomide use compared with thalidomide as found in this study, consistent with the current practice experience. Myelosuppression was found to be more prominent with lenalidomide according to our meta-analysis, which is also consistent with the present practice experience. Reports have described that the extended use of lenalidomide increases the risk of second primary malignancies ( 32–34 ). A meta-analysis of individual patient data has demonstrated that lenalidomide is associated with an increased risk of second primary malignancies, primarily hematological malignancies ( 47 ). Notably, the increased risk may be primarily driven by the combination of melphalan and lenalidomide ( 47 ). Our meta-analysis of four trials ( 32–34 , 36 ) found that lenalidomide was associated with a higher risk of developing secondary primary hematological malignancies but not solid tumors. Patients in these four trials should all have had exposure to melphalan, which might be contributing to the increased incidence of second primary hematological malignancies. Our analysis was limited by the data available in the included trials, in which the potential risk of other second primary malignancies may not be fully displayed in limited follow-up. While measures can be taken to prevent thromboembolism and other common adverse events that are largely manageable, increased risk of peripheral neuropathy with thalidomide, and more importantly increased risk for developing second primary malignancies with lenalidomide, warrant serious consideration when starting maintenance therapy with these agents.
Whether lenalidomide is more effective and less toxic than thalidomide is an important question but is beyond the scope of our study. A previous study attempted an indirect meta-analysis approach and showed interesting results ( 48 ). Indirect comparison of lenalidomide vs thalidomide maintenance after ASCT showed a PFS advantage but no OS difference when using observation/placebo as the common comparator. Indirect comparison of MPR followed by lenalidomide maintenance (MPR-R) vs MPT followed by thalidomide maintenance (MPT-T) in the nontransplantation setting showed a statistically significant PFS but not OS advantage for MPR-R when using MP alone as the common comparator. Regarding toxicity, consistent with practice experience, our meta-analysis showed that peripheral neuropathy is primarily a concern for thalidomide; however, concerns for second primary malignancies have been raised for lenalidomide but are less reported for thalidomide. Notably, a head-to-head RCT of MPR-R vs MPT-T is pending, which may provide additional insight into this issue.
Another important question that cannot be answered by our study because of the lack of necessary data is which patient population will benefit the most from IMiD maintenance therapy. Two trials on thalidomide maintenance therapy after ASCT showed that response prior to maintenance therapy can affect survival outcome. For example, patients who did not achieve VGPR or better with thalidomide maintenance therapy had prolonged EFS/PFS, but this benefit was not statistically significant in the patients who achieved at least VGPR ( 19 , 27 ). In the IFM 2005-02 trial, lenalidomide maintenance therapy after ASCT prolonged the three-year PFS rates regardless of whether they achieved at least VGPR prior to maintenance ( 32 ). Currently, whether patients not yet achieving VGPR or better after ASCT would benefit more from IMiD maintenance therapy remains unclear. Whether cytogenetic abnormalities predict potential survival benefits from IMiD maintenance therapy also remains controversial. In the IFM 99 02 trial, thalidomide maintenance after ASCT statistically significantly prolonged EFS in patients without chromosome 13 deletion but not in patients with this abnormality ( 19 ). In the TT2 study, thalidomide maintenance following ASCT statistically significantly improved PFS regardless of the absence or presence of cytogenetic abnormalities but extended OS only in patients with cytogenetic abnormalities ( 20 ). In the MRC Myeloma IX study, thalidomide maintenance after ASCT improved the PFS in patients with favorable iFISH but decreased the OS in patients with adverse iFISH ( 29 ). In the IFM 2005-02 trial, lenalidomide maintenance therapy after ASCT improved the three-year PFS rates regardless of del(13q) status ( 32 ). In patients with high-risk cytogenetic abnormalities (t(4;14), t(14;16), and/or del(17p)), lenalidomide maintenance therapy failed to improve PFS or OS according to two trials ( 28 , 36 ).
To our knowledge, our study is the first comprehensive meta-analysis investigating the benefits and risks of IMiD-based maintenance therapy in MM in both transplantation and nontransplantation settings. Our findings that both thalidomide and lenalidomide maintenance therapy statistically significantly prolonged PFS but did not confer a clear OS benefit regardless of ASCT status add valuable knowledge to the field. However, longer OS was observed in IMiD maintenance arms in our study, although no statistical significance was reached. In addition to concerns of possible insufficient follow-up time and statistical power, other explanations include effective salvage therapy, which may include IMiD after relapse in the control arms, and shorter survival after disease progression in IMiD maintenance arms because of emergence of resistant clones with extended exposure of IMiD. It should be noted that with more therapy options available currently, patients can often receive multiple lines of therapy and have longer OS. Therefore, it becomes more difficult to see an OS advantage in upfront therapy trials. While it is important to evaluate OS, perhaps PFS would be a better measure of success to primary therapy. Initial therapy might only benefit OS if the treatment increases the proportion of “cured” patients.
Our study has several limitations. First, this meta-analysis is based on abstracted data instead of individual patient data. Second, our analysis is limited by the high degree of variation among included studies, which had heterogeneity in trial design, inclusion, and exclusion criteria, patient characteristics including cytogenetic profile, therapy regimen including induction and salvage therapy modalities, definition and calculation of survival outcomes, follow-up length, etc. Third, a potential publication bias is present. The evidence of maintenance treatment effectiveness might be biased because of potential bias in selecting trials for inclusion; particularly, only published trials are included and some studies without sufficient data were excluded. In addition, the meta-analyses of lenalidomide maintenance therapy were limited by the low number of available studies. We advise cautious interpretation of the results.
In conclusion, our meta-analysis demonstrated that thalidomide- and lenalidomide-based maintenance therapy statistically significantly improved PFS but not OS in MM and increased the risks of developing grade 3–4 adverse events, including thromboembolism, peripheral neuropathy, neutropenia, and infection.
Funding
This work was done with no funding.
Supplementary Material
R. E. Champlain and M. L. Wang received research funding from Celgene. R. E. Champlain serves as consultant for Celgene.
References
- 1. Spicka I . Advances in multiple myeloma therapy during two past decades . Comput Struct Biotechnol J . 2014. ; 10 ( 16 ): 38 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vincent Rajkumar S . Multiple myeloma: 2014 Update on diagnosis, risk-stratification, and management . Am J Hematol . 2014. ; 89 ( 10 ): 999 – 1009 . [DOI] [PubMed] [Google Scholar]
- 3. Laubach J, Richardson P, Anderson K . Multiple myeloma . Annu Rev Med . 2011. ; 62 : 249 – 264 . [DOI] [PubMed] [Google Scholar]
- 4. Rollig C, Knop S, Bornhauser M . Multiple myeloma . Lancet . 2014. ; 385 ( 9983 ): 2197 – 2208 . [DOI] [PubMed] [Google Scholar]
- 5. Palumbo A, Anderson K . Multiple myeloma . N Engl J Med . 2011. ; 364 ( 11 ): 1046 – 1060 . [DOI] [PubMed] [Google Scholar]
- 6. Holstein SA, Liu H, McCarthy PL . Multiple Myeloma . Hematol Oncol Clin North Am . 2014. ; 28 ( 6 ): 1113 – 1129 . [DOI] [PubMed] [Google Scholar]
- 7. McCarthy PL, Palumbo A . Maintenance therapy for multiple myeloma . Hematol Oncol Clin North Am . 2014. ; 28 ( 5 ): 839 – 859 . [DOI] [PubMed] [Google Scholar]
- 8. Al-Mansour Z, Ramanathan M . Post-Autologous (ASCT) Stem Cell Transplant Therapy in Multiple Myeloma . Adv Hematol . 2014. ; 2014 : 652395 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fritz E, Ludwig H . Interferon-alpha treatment in multiple myeloma: meta-analysis of 30 randomised trials among 3948 patients . Ann Oncol . 2000. ; 11 ( 11 ): 1427 – 1436 . [DOI] [PubMed] [Google Scholar]
- 10. Berenson JR, Crowley JJ, Grogan TM, et al. . Maintenance therapy with alternate-day prednisone improves survival in multiple myeloma patients . Blood . 2002. ; 99 ( 9 ): 3163 – 3168 . [DOI] [PubMed] [Google Scholar]
- 11. Rajkumar SV . Treatment of multiple myeloma . Nat Rev Clin Oncol . 2011. ; 8 ( 8 ): 479 – 491 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shank BR, Brown VT, Schwartz RN . Multiple myeloma maintenance therapy: A review of the pharmacologic treatment . J Oncol Pharm Pract . 2015. ; 21 ( 1 ): 36 – 51 . [DOI] [PubMed] [Google Scholar]
- 13. Ye X, Huang J, Pan Q, et al. . Maintenance therapy with immunomodulatory drugs after autologous stem cell transplantation in patients with multiple myeloma: a meta-analysis of randomized controlled trials . PLoS One . 2013. ; 8 ( 8 ): e72635 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang B, Yu RL, Chi XH, et al. . Lenalidomide treatment for multiple myeloma: systematic review and meta-analysis of randomized controlled trials . PLoS One . 2013. ; 8 ( 5 ): e64354 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou Y, Sheng Z, Lu H, et al. . Continuous treatment with new agents for newly diagnosed multiple myeloma . Anticancer Drugs . 2013. ; 24 ( 5 ): 527 – 533 . [DOI] [PubMed] [Google Scholar]
- 16. Deeks JJ, Higgins JP, Altman DG . Analysing data and undertaking meta-analyses . In: Higgins JP, Green S , (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 ed : The Cochrane Collaboration; ; 2011. . [Google Scholar]
- 17. Begg CB, Mazumdar M . Operating characteristics of a rank correlation test for publication bias . Biometrics . 1994. ; 50 ( 4 ): 1088 – 1101 . [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, et al. . Bias in meta-analysis detected by a simple, graphical test . BMJ . 1997. ; 315 ( 7109 ): 629 – 634 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Attal M, Harousseau JL, Leyvraz S, et al. . Maintenance therapy with thalidomide improves survival in patients with multiple myeloma . Blood . 2006. ; 108 ( 10 ): 3289 – 3294 . [DOI] [PubMed] [Google Scholar]
- 20. Barlogie B, Pineda-Roman M, van Rhee F, et al. . Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities . Blood . 2008. ; 112 ( 8 ): 3115 – 3121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palumbo A, Bringhen S, Liberati AM, et al. . Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial . Blood . 2008. ; 112 ( 8 ): 3107 – 3114 . [DOI] [PubMed] [Google Scholar]
- 22. Rajkumar SV, Rosinol L, Hussein M, et al. . Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma . J Clin Oncol . 2008. ; 26 ( 13 ): 2171 – 2177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lokhorst HM, van der Holt B, Zweegman S, et al. . A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma . Blood . 2010. ; 115 ( 6 ): 1113 – 1120 . [DOI] [PubMed] [Google Scholar]
- 24. Ludwig H, Adam Z, Tothova E, et al. . Thalidomide maintenance treatment increases progression-free but not overall survival in elderly patients with myeloma . Haematologica . 2010. ; 95 ( 9 ): 1548 – 1554 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waage A, Gimsing P, Fayers P, et al. . Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma . Blood . 2010. ; 116 ( 9 ): 1405 – 1412 . [DOI] [PubMed] [Google Scholar]
- 26. Wijermans P, Schaafsma M, Termorshuizen F, et al. . Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study . J Clin Oncol . 2010. ; 28 ( 19 ): 3160 – 3166 . [DOI] [PubMed] [Google Scholar]
- 27. Maiolino A, Hungria VT, Garnica M, et al. . Thalidomide plus dexamethasone as a maintenance therapy after autologous hematopoietic stem cell transplantation improves progression-free survival in multiple myeloma . Am J Hematol . 2012. ; 87 ( 10 ): 948 – 952 . [DOI] [PubMed] [Google Scholar]
- 28. Mateos MV, Oriol A, Martinez-Lopez J, et al. . Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial . Blood . 2012. ; 120 ( 13 ): 2581 – 2588 . [DOI] [PubMed] [Google Scholar]
- 29. Morgan GJ, Gregory WM, Davies FE, et al. . The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis . Blood . 2012. ; 119 ( 1 ): 7 – 15 . [DOI] [PubMed] [Google Scholar]
- 30. Stewart AK, Trudel S, Bahlis NJ, et al. . A randomized phase 3 trial of thalidomide and prednisone as maintenance therapy after ASCT in patients with MM with a quality-of-life assessment: the National Cancer Institute of Canada Clinicals Trials Group Myeloma 10 Trial . Blood . 2013. ; 121 ( 9 ): 1517 – 1523 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zonder JA, Crowley J, Hussein MA, et al. . Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232) . Blood . 2010. ; 116 ( 26 ): 5838 – 5841 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Attal M, Lauwers-Cances V, Marit G, et al. . Lenalidomide maintenance after stem-cell transplantation for multiple myeloma . N Engl J Med . 2012. ; 366 ( 19 ): 1782 – 1791 . [DOI] [PubMed] [Google Scholar]
- 33. McCarthy PL, Owzar K, Hofmeister CC, et al. . Lenalidomide after stem-cell transplantation for multiple myeloma . N Engl J Med . 2012. ; 366 ( 19 ): 1770 – 1781 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palumbo A, Hajek R, Delforge M, et al. . Continuous lenalidomide treatment for newly diagnosed multiple myeloma . N Engl J Med . 2012. ; 366 ( 19 ): 1759 – 1769 . [DOI] [PubMed] [Google Scholar]
- 35. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. . Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma . N Engl J Med . 2014. ; 371 ( 10 ): 906 – 917 . [DOI] [PubMed] [Google Scholar]
- 36. Palumbo A, Cavallo F, Gay F, et al. . Autologous transplantation and maintenance therapy in multiple myeloma . N Engl J Med . 2014. ; 371 ( 10 ): 895 – 905 . [DOI] [PubMed] [Google Scholar]
- 37. Morgan GJ, Davies FE, Gregory WM, et al. . Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment . Clin Cancer Res . 2013. ; 19 ( 21 ): 6030 – 6038 . [DOI] [PubMed] [Google Scholar]
- 38. Barlogie B, Tricot G, Anaissie E, et al. . Thalidomide and hematopoietic-cell transplantation for multiple myeloma . N Engl J Med . 2006. ; 354 ( 10 ): 1021 – 1030 . [DOI] [PubMed] [Google Scholar]
- 39. Spencer A, Prince HM, Roberts AW, et al. . Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure . J Clin Oncol . 2009. ; 27 ( 11 ): 1788 – 1793 . [DOI] [PubMed] [Google Scholar]
- 40. Barlogie B, Attal M, Crowley J, et al. . Long-term follow-up of autotransplantation trials for multiple myeloma: update of protocols conducted by the intergroupe francophone du myelome, southwest oncology group, and university of arkansas for medical sciences . J Clin Oncol . 2010. ; 28 ( 7 ): 1209 – 1214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Facon T, Mary JY, Hulin C, et al. . Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial . Lancet . 2007. ; 370 ( 9594 ): 1209 – 1218 . [DOI] [PubMed] [Google Scholar]
- 42. Hulin C, Facon T, Rodon P, et al. . Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial . J Clin Oncol . 2009. ; 27 ( 22 ): 3664 – 3670 . [DOI] [PubMed] [Google Scholar]
- 43. Beksac M, Haznedar R, Firatli-Tuglular T, et al. . Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group . Eur J Haematol . 2011. ; 86 ( 1 ): 16 – 22 . [DOI] [PubMed] [Google Scholar]
- 44. Kapoor P, Rajkumar SV, Dispenzieri A, et al. . Melphalan and prednisone versus melphalan, prednisone and thalidomide for elderly and/or transplant ineligible patients with multiple myeloma: a meta-analysis . Leukemia . 2011. ; 25 ( 4 ): 689 – 696 . [DOI] [PubMed] [Google Scholar]
- 45. Fayers PM, Palumbo A, Hulin C, et al. . Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials . Blood . 2011. ; 118 ( 5 ): 1239 – 1247 . [DOI] [PubMed] [Google Scholar]
- 46. Palumbo A, Bringhen S, Larocca A, et al. . Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival . J Clin Oncol . 2014. ; 32 ( 7 ): 634 – 640 . [DOI] [PubMed] [Google Scholar]
- 47. Palumbo A, Bringhen S, Kumar SK, et al. . Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data . Lancet Oncol . 2014. ; 15 ( 3 ): 333 – 342 . [DOI] [PubMed] [Google Scholar]
- 48. Zou Y, Sheng Z, Niu S, et al. . Lenalidomide versus thalidomide based regimens as first-line therapy for patients with multiple myeloma . Leuk Lymphoma . 2013. ; 54 ( 10 ): 2219 – 2225 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.