Abstract
Objective. This study investigated interscalene block for shoulder arthroplasty with various ropivacaine concentrations in the presence of clonidine, dexamethasone, and buprenorphine. The goal was prolonged analgesia with minimal motor blockade.
Design. Prospective, double-blind, randomized controlled trial.
Setting. University-affiliated orthopedic hospital.
Methods. Patients (20/group) received acetaminophen, ketorolac, pregabalin, opioids, and “Control”; interscalene block, 0.375% ropivacaine, intravenous additives (buprenorphine, clonidine, dexamethasone); “High Dose”; 0.375% ropivacaine, perineural additives; “Medium Dose”; 0.2% ropivacaine, perineural additives; and “Low Dose”; 0.1% ropivacaine, perineural additives.
Results. Pain with movement at 24 hours was 4.9 ± 2.5 (mean ± standard deviation [SD]) (Control), 4.5 ± 3.0 (High Dose), 3.4 ± 1.8 (Medium Dose), 4.2 ± 2.4 (Low Dose). The difference between Medium Dose and Control was −1.5 (95% CI: −2.9, −0.1) (P = 0.040). Median time until need for opioids was 16.1 hours (Control) vs 23.7 hours (High Dose); hazard ratio 0.37 [95% CI: 0.17, 0.79]. High Dose had less pain with movement the morning after surgery, vs Control; 2.9 ± 2.5 vs 4.9 ± 2.7; P = 0.027. Pain with movement in the Post-Anesthesia Care Unit was higher in Low Dose, vs Control; 0.9 ± 1.4 vs 0 ± 0, P = 0.009. Low Dose had superior hand strength in the Post-Anesthesia Care Unit (mean ± SD of pre-operative strength: 44.0 ± 20.3%) compared to Control (27.5 ± 24.5%) (P = 0.031).
Conclusions. For maximum pain reduction, combining perineural additives with ropivacaine 0.375% or 0.2% is suggested. To minimize motor blockade, perineural additives can be combined with ropivacaine, 0.1%.
Keywords: Buprenorphine, Clonidine, Dexamethasone, Interscalene Nerve Block, Ropivacaine, Total Shoulder Arthroplasty
Introduction
Interscalene nerve blockade (ISB) can provide analgesia after major shoulder surgery including total shoulder arthroplasty (TSA) [1]. The ideal analgesic ISB would give prolonged analgesia, reduce opioid use, and cause minimal motor blockade. Local anesthetic by itself does not meet these criteria. Pain scores after ISB for TSA are low until the block wears off. However, ISB reduces motor strength and typically ISB does not provide sufficient duration of analgesia unless significant systemic opioids are also administered [2].
Many compounds have been added to local anesthetic in the hopes of prolonging analgesia after peripheral nerve blockade. Addition of dexamethasone [3] or clonidine [4] to perineural local anesthetic appears to increase duration of analgesia and motor blockade. Prolonged analgesia is typically valued. However, excessive duration of motor blockade may be undesirable as this can hinder postoperative evaluation of nerve function and may delay identification of nerve injury. Buprenorphine prolongs analgesia after blockade with local anesthetic in several contexts [5–8].
Interestingly, local anesthetic is not necessary to obtain analgesia after ISB as ISB with clonidine alone has analgesic and opioid-sparing effects [9]. Similarly, perineural clonidine with buprenorphine, without local anesthetic, provides analgesia of long duration but no motor blockade [10]. Combining local anesthetics with multiple adjuvants (“multimodal perineural analgesia”) may prolong analgesia after single injection peripheral nerve blockade and may allow use of lower concentrations of local anesthetic [10–13].
The purpose of this study was to determine if a combination of medications can be delivered during ISB to provide prolonged analgesia with minimal motor blockade. Varying concentrations of ropivacaine were given with three perineural additives (clonidine, dexamethasone, and buprenorphine). This prospective randomized blinded dose-response trial addressed the following hypotheses about ISB performed for TSA, in conjunction with multimodal analgesia. 1) Addition of perineural additives to ISB with 0.375% ropivacaine reduces pain with movement at 24 hours after the nerve block (compared to plain ropivacaine ISB with systemic controls for the additives). 2) Lower concentrations of ropivacaine (0.2% and 0.1%, both with additives) also provide improved analgesia at 24 hours. 3) Postoperative motor blockade, after ISB with local anesthetic + additives, will be less with 0.1% or 0.2% ropivacaine (compared to 0.375% ropivacaine). The primary outcome for the power analysis was pain with movement at 24 hours.
Methods
After Institutional Review Board approval and informed written consent, 80 patients with osteoarthritis scheduled for a primary TSA, with planned use of general anesthesia and ISB, age 18–80 years, entered the study. The study was registered at clinicaltrials.gov (NCT01782872). Exclusion criteria included allergy or intolerance to one of the study medications, American Society of Anesthesiologists physical classification of IV, insulin-dependent diabetes, hepatic or renal failure, bradycardia (heart rate < 50), or hypotension (systolic pressure < 90 mm Hg), use of opioids for longer than 3 months, active diagnosis of chronic regional pain syndrome, inability to follow the study protocol, and lack of English fluency.
Prior to surgery, an ultrasound-guided in-plane single-injection ISB (20 mL) was performed with the goal of injecting between C5 and C6. Prior to study initiation, a research assistant not otherwise associated with the study prepared opaque numbered envelopes containing group assignment; each envelope was opened after the patient signed consent. The intraoperative anesthesiologist was not blinded to group assignment. The patient and the research assistant collecting the data were both blinded to group assignment.
Groups consisted of 1) “Control”; ISB with 0.375% ropivacaine, 20 mL, and IV administration of buprenorphine (150 mcg) + clonidine (100 mcg) + dexamethasone (4 mg); 2) “High Dose”; 0.375% ropivacaine with perineural buprenorphine + clonidine + dexamethasone; 3) “Medium Dose”; 0.2% ropivacaine with perineural buprenorphine + clonidine + dexamethasone; and 4) “Low Dose”; 0.1% ropivacaine with perineural buprenorphine + clonidine + dexamethasone.
The analgesic ED95 (95th percentile for effective dose) for a ropivacaine ISB is approximately 20 mL of 0.34% ropivacaine [1]. We adopted the recommended [1] 20 mL of 0.375% ropivacaine. ED50s for both volume and concentration were much less. EDs with added clonidine, dexamethasone, and buprenorphine may differ. Doses of additives were chosen based on previous studies. Buprenorphine doses in some studies consist of 300 mcg [5–7], but opioid side effects have been noted. Other reports use reduced doses of 150–200 mcg perineural buprenorphine [10], due to concerns that perineural administration of 300 mcg buprenorphine was associated with nausea and vomiting. A recent interscalene study used 150 mcg buprenorphine in a total volume of 30 mL [14]. Although perineural buprenorphine dose-response studies are not available, we chose 150 mcg as this appears to be an effective dose, and higher doses may cause excessive opioid-related side effects. An early report that dexamethasone prolonged ISB used 8 mg (in 20 mL) [15], but 4 mg had similar effects to 8 mg (both in 40 mL) [16]. We chose 4 mg, as this dose has been shown to be effective but is unlikely to have major systemic analgesic effect [17]. A recent study found similar prolongation of analgesic effect after addition of 1, 2, or 4 mg dexamethasone to bupivacaine for brachial plexus nerve block [18]. The clonidine dose (100 mcg) has been used in previous studies [19] and is thought to be a supramaximal dose without major systemic side effects [20]. A second perineural clonidine dose-response study concluded that the best dose was between 30 and 90 mcg [21], which further indicates that 100 mcg is an adequate clonidine dose.
Patients received intravenous sedation for the nerve block (2–5 mg midazolam and 0–20 mg ketamine). A standardized general anesthetic included induction with propofol and placement of a laryngeal mask airway (endotracheal intubation was performed if clinically indicated). Indications for endotracheal intubation included patient characteristics (such as gastro-esophageal reflux disease) and technical inability to readily obtain a good fit with the laryngeal mask airway (LMA). Anesthesia was maintained with nitrous oxide (50–70%), isoflurane (0.2–0.8%), and a propofol infusion as needed. Patients received ketamine (intraoperative total of 50 mg), ondansetron (4 mg), and ketorolac (15–30 mg; 30 mg unless age > 70 or weight < 60 kg).
Postoperative analgesia consisted of standing acetaminophen (650 mg q 6 hours × 3 days), pregabalin (75–100 mg po q 8 hours × 3 days; 100 mg unless age > 70 or weight < 60 kg), and ketorolac (15–30 mg q 8 hours, last dose postoperative day (POD)2 am; 30 mg unless age > 70 or weight < 60 kg). Patients received on-demand intravenous patient-controlled analgesia (PCA) (hydromorphone 0.2 mg/mL, no basal rate, 1.3 mL/activation, 10 minute lock-out period) and oral oxycodone (5–10 mg q 3 hours prn). Patients received a one-page set of instructions about their pain management; they were asked to take oxycodone for moderate or severe pain and to use the intravenous hydromorphone only for severe pain that has not been relieved by the oral oxycodone (see Appendix B).
Outcomes
The primary outcome was pain with movement at 24 hours after ISB (Numerical Rating Scale, NRS, of 0–10). Pain with movement refers to pain during middle deltoid strength assessment; if the patient could not move due to motor block, the arm was abducted passively and pain assessed.
Middle deltoid strength and handgrip strength were assessed with a dynamometer on the operative arm preoperatively, shortly postoperatively (i.e., in the Post-Anesthesia Care Unit, PACU), and twice daily after surgery (POD 1 and 2) while patients were in the hospital. Sensory function and pain scores were obtained twice daily.
Secondary outcomes were based on the IMMPACT (Initiative on Methods, Measurement and Pain Assessment in Clinical Trials) recommendations [22]. Pain was assessed with 1) NRS pain scores (at rest and with movement), preoperatively, PACU, 24 hours post-block, twice daily on POD 1 and 2; and 2) opioid use (oral and intravenous), POD 0, 1, and 2. Physical functioning was assessed with the BPI (Brief Pain Inventory), POD1 pm. Emotional functioning was assessed with the POMS (Profile of Mood States), reported as Total Mood Disturbance ranging from −24 to 177, where lower scores indicate more stable mood profiles (POD1 pm) [23]. Patient global impression of change was assessed by querying about satisfaction (1–10 scale), POD1 pm. Side effects were assessed with 1) spontaneously reported symptoms and adverse events, POD1 pm (“Have you noticed any problems with the pain management? If yes, what are they?”); and 2) the Opioid Related Symptom Distress Scale (ORSDS, which has been validated for orthopedic patients [24]), POD1 pm.
Duration of analgesia from the ISB was determined by asking four questions on POD1 and POD2: 1) “At what time did your shoulder start having pain?”; 2) “When did the nerve block stop providing pain relief?”; 3) “When did you first need to use the PCA and/or take oxycodone?”; 4) “When did the nerve block entirely wear off?” Quality of sleep was assessed the morning of POD 1, 2 regarding the preceding night (“Did you have difficulty sleeping last night because of pain?”; “Did you awaken last night because of pain?”; If “yes” then: “How many times did you awaken last night because of pain?”).
Patients were deemed to have responded if they had mild or no shoulder pain on arrival to the PACU (NRS < 3 with shoulder movement) and minimal need for PCA (defined as activation of the PCA no more than four times in the first 24 hours after the operation) [2].
Study data were collected and managed using REDCap electronic data capture tools hosted at Hospital for Special Surgery [25].
Statistical Analysis
Pain with movement at 24 hours was selected as the primary outcome because of its clinical importance. ISB with bupivacaine and clonidine was associated with pain scores at 24 hours of 6 which were reduced to 3 by addition of perineural dexamethasone [15]—suggesting that perineural additives can reduce pain at 24 hours. Median pain scores with movement after TSA were 0 on the POD0, but changed to 3.8 by POD1 am [2], which further indicates that an effective ISB will dramatically reduce pain with movement after TSA. We anticipated the mean ± standard deviation (SD) NRS score with movement at 24 hours from end of block administration to be 3.8 ± 2.7 in the control group, with this estimate obtained from a previous study [2]. We determined that a sample size of 16 patients in each of the four treatment groups would provide 80% power at a two-sided alpha of 0.05 to detect a pairwise difference in the mean NRS score of 2.8, assuming a common within-group standard deviation of 2.7, using two-sample t-tests. This reduction (to a pain score of 1) was assumed because in a previous study, pain scores on the day of surgery in the PACU were 0.625 ± 1.25 [2]. To account for drop-outs, we planned to enroll 20 patients per group for a total of 80 patients in the study (four treatment groups).
The primary outcome (pain with movement at 24 hours from the nerve block) was compared between each of the three experimental groups and the control group (i.e., three pairwise comparisons) using two-sample t-tests. Continuous and ordinal outcomes were compared between each of the three experimental groups and the control group using two-sample t-tests or Wilcoxon rank-sum tests, depending upon the distribution of the data, with absolute effect size presented as difference in means or Hodges-Lehmann estimate of location shift, respectively, with 95% confidence intervals. Categorical variables were compared between groups using chi-square or Fisher’s exact test, as appropriate, with effect sizes presented as risk difference with 95% confidence intervals. The generalized estimating equations (GEE) method [26,27] with an autoregressive [AR(1)] correlation structure was used to compare mean NRS pain and handgrip strength between groups at postoperative time points after adjustment for corresponding preoperative value. The GEE method accounts for the correlation between repeated measurements on the same patient, where the AR(1) correlation structure assumes a greater degree of correlation among measurements recorded closer in time. The Kaplan-Meier method was used to estimate the median duration of analgesia in the presence of censored data. Censored data occurs when patients had not experienced the event by the time of last interview. In this context, “Total” indicates the number of patients who either provided an endpoint time or indicated that the endpoint had not yet occurred by last interview. Patients missing from the total could not answer the question, did not recall, or were lost to follow-up. Cox proportional hazards modeling was used to compare analgesic duration between groups, with effect sizes presented as hazard ratios with 95% confidence intervals. The proration method was used to permute missing POMS questionnaire data when missing answers did not exceed 20%. All tests were two-sided, with statistical significance defined as P < 0.05. Adjustment for multiple comparisons was not performed because each of the experimental group/control group comparisons were defined a priori. However, an alpha level of 0.05 divided by three pairwise comparisons (i.e., 0.05/3 = 0.02) was also used to assess the robustness of any observed statistically significant differences. Statistical analyses were performed with SAS Version 9.3 (SAS Institute, Cary, NC, USA).
Data were analyzed after 10 patients/group to apply the stopping rule. Randomization with a blocking factor of two ensured that the numbers of patients in each group were equal. The predetermined stopping rule stated if four (of 10) patients in an experimental group had NRS pain in the PACU ≥ 2.3 at rest, then enrollment in that limb would be stopped. The NRS score of 2.3 was chosen based on the observation that pain at rest in the PACU with a control block was 0 (0, 0) (median [25%, 75%]) but pain at rest on POD1am was 2.3 (0, 3.8) [2]. No group met the stopping rule so the study was completed as designed.
Results
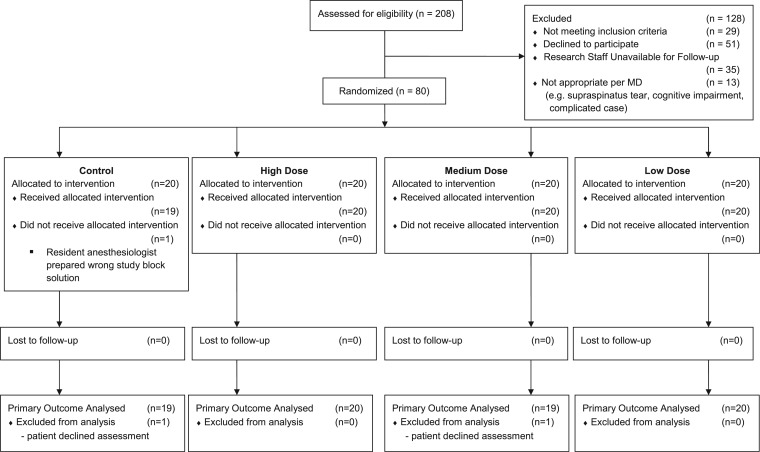
Eighty patients were enrolled (Figure 1, Table 1), from October 16, 2012 to January 28, 2014.
Figure 1.
CONSORT flow diagram.
Table 1.
Patient characteristics
| Control | High dose | P vs control | Medium dose | P vs control | Low dose | P vs control | |
|---|---|---|---|---|---|---|---|
| Age (years) (mean ± SD) | 68 ± 7 | 66 ± 8 | 0.237 | 64 ± 9 | 0.073 | 69 ± 7 | 0.73 |
| Male/female (%) | 50%/50% | 35%/65% | 0.337 | 55%/45% | 0.752 | 25%/75% | 0.103 |
| ASA status 1/2/3 | 1/15/4 | 0/16/4 | 0.999 | 3/14/3 | 0.55 | 0/18/2 | 0.999 |
| BMI (mean ± SD) | 31 ± 6 | 28 ± 4 | 0.098 | 25 ± 3 | <0.001 | 27 ± 4 | 0.029 |
| Preoperative opioid usage | |||||||
| yes (n [%]) | 1 (5%) | 3 (15%) | 0.605 | 2 (10%) | 0.999 | 0 (0%) | 0.999 |
| if yes, daily dose (oral morphine eq.) (median, IQR) | 7.5 (7.5, 7.5) | 5 (5,10) | N/A | 50 (50,50) | N/A | 0 (0,0) | N/A |
| Midazolam dose during ISB (median, IQR) | 5 (5,5) | 5 (5,5) | 0.395 | 5 (5,5) | 0.999 | 5 (2.5,5) | 0.485 |
| Ketamine dose during ISB (median, IQR) | 20 (10,20) | 20 (10,20) | 0.938 | 20 (10,20) | 0.787 | 20 (10,20) | 0.879 |
| Intraoperative propofol infusion (n [%]) | 16 (80%) | 15 (75%) | 0.999 | 15 (75%) | 0.999 | 14 (70%) | 0.716 |
| Endotracheal tube usage (n [%]) | 5 (25%) | 4 (20%) | 0.999 | 3 (15%) | 0.695 | 4 (20%) | 0.999 |
| Length of surgery (min) (mean ± SD) | 107 ± 20 | 104 ± 15 | 0.622 | 100 ± 20 | 0.302 | 105 ± 13 | 0.795 |
ASA = American society of anesthesiologists physical status; BMI = body mass index; ISB = interscalene nerve blockade; IQR = interquartile range; SD = standard deviation. Control = interscalene block with 0.375% ropivacaine, 20 mL, and IV administration of buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); High Dose = 0.375% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); Medium Dose = 0.2% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); Lose Dose = 0.1% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); N/A=Not Applicable (data presented for description only).
Primary Outcome
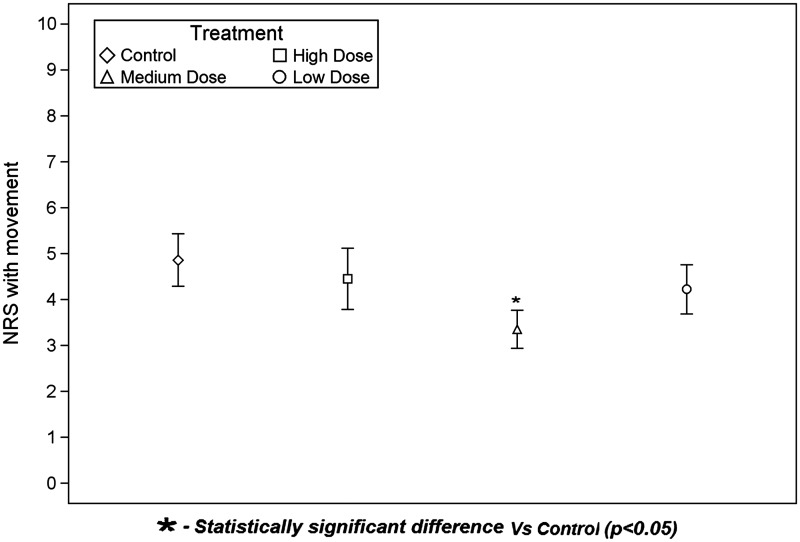
The primary outcome, NRS pain score with movement at 24 hours was lower (better) in the Medium Dose group compared to the Control group (Figure 2). The NRS pain score at 24 hours was 4.9 ± 2.5 (mean ± SD) for Control. For Medium Dose, pain scores were 3.4 ± 1.8, with a difference vs Control of −1.5 (95% CI: −2.9, −0.1) (P = 0.040). However, there were no differences between Control and either Low Dose or High Dose. Pain scores were 4.5 ± 3.0 for High Dose, with a difference vs Control of −0.4 (95% CI: −2.2, 1.4) (P = 0.646). For Low Dose, pain scores were 4.2 ± 2.4, with a difference vs Control of −0.6 (95% CI: −2.2, 1.0) (P = 0.423).
Figure 2.
Pain scores with movement at 24 hours. Numeric Rating Scale pain scores were determined at 24 hours during shoulder abduction. The diamond, square, triangle, and circle represent the group means. The bottom and top of each rectangle indicate the first and third quartiles, respectively. The horizontal line within each rectangle indicates the median. The lines extending out of the bottom and top of each rectangle represent the minimum and maximum values that lie within 1.5 times the interquartile range below and above the first and third quartiles, respectively. Outliers are not shown. NRS = Numeric Rating Scale.
Significant Secondary Pain Outcomes
Rebound pain was defined as maximum pain with movement after the block both stopped providing pain relief and completely wore off [12]. The following rebound NRS pain scores were obtained: 6.1 ± 2 (control; mean ± SD, n = 11), 5.2 ± 1.6 (High Dose, n = 7), 4.2 ± 1.6 (Medium Dose, n = 12), 6.1 ± 2.6 (Low Dose, n = 7). Regression analysis, adjusted for baseline pain, gave the following (means [95% CI]) for comparisons to control: High Dose: −0.7 (−2.7, 1.3, P = 0.501), Medium Dose: −1.8 (−3.4, −0.1, P = 0.034), Low Dose: 0 (−1.75, 1.76, P = 0.966).
Additional pain scores were obtained in the PACU on day of surgery, at POD1 am and POD1 pm. Some patients were not evaluated in the PACU due to excessive sedation (n = 3; 2 in Control and 1 in High Dose). Other than those three excluded for sedation, the remainder (n = 77) were alert and oriented. The 24-hour visit coincided with the POD1 am visit for 28 patients and coincided with the POD1 pm visit for 2 patients (most study patients received operations early in the day).
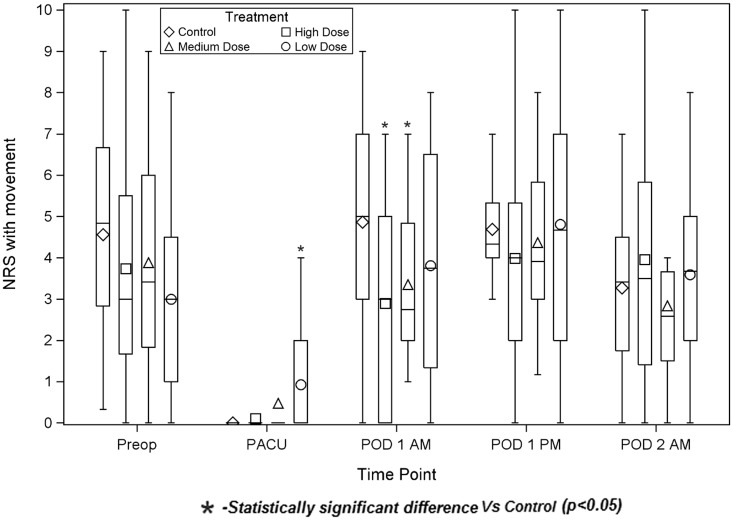
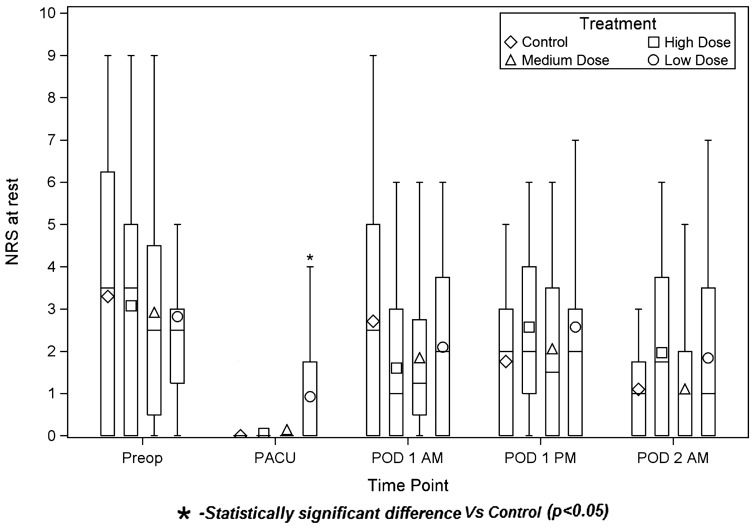
The High Dose group had less pain with movement on the morning of POD1 than Control (2.9 ± 2.5 vs 4.9 ± 2.7, respectively; P = 0.027), (Figure 3). (Average times from ISB placement to POD1 am assessment were [Control] 21 hours 27 minutes, [High Dose 2] 21 hours 40 minutes, [Medium Dose] 22 hours 37 minutes, [Low Dose] 22 hours 13 minutes.) Conversely, the Low Dose group had more pain in the PACU than did Control, both at rest (Figure 4) (0.9 ± 1.5 vs 0 ± 0, P = 0.012) and with movement (0.9 ± 1.4 vs 0 ± 0, P = 0.009). (Average times from ISB placement to PACU assessment were [Control] 4 hours 17 minutes, [High Dose] 4 hours 4 minutes, [Medium Dose] 4 hours 14 minutes, [Low Dose] 4 hours 8 minutes.)
Figure 3.
Pain scores with movement. Numeric Rating Scale pain scores were determined during shoulder abduction. The diamond, square, triangle, and circle represent the group means. The bottom and top of each rectangle indicate the first and third quartiles, respectively. The horizontal line within each rectangle indicates the median. The lines extending out of the bottom and top of each rectangle represent the minimum and maximum values that lie within 1.5 times the interquartile range below and above the first and third quartiles, respectively. Outliers are not shown. NRS = Numeric Rating Scale; PACU = Post-Anesthesia Care Unit; POD = postoperative day.
Figure 4.
Pain scores at rest. Numeric Rating Scale pain scores were determined at rest. The diamond, square, triangle, and circle represent the group means. The bottom and top of each rectangle indicate the first and third quartiles, respectively. The horizontal line within each rectangle indicates the median. The lines extending out of the bottom and top of each rectangle represent the minimum and maximum values that lie within 1.5 times the interquartile range below and above the first and third quartiles, respectively. Outliers are not shown. NRS = Numeric Rating Scale; PACU = Post-Anesthesia Care Unit; POD = postoperative day.
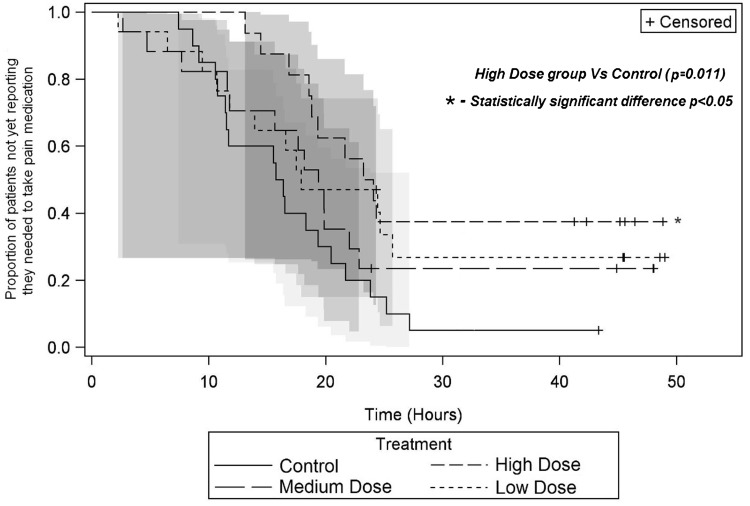
The median time until a patient needed to take opioid pain medication was 16.1 hours (Control), 23.7 hours (High Dose), 19.4 hours (Medium Dose), and 17.9 hours (Low Dose) (Figure 5). There was evidence of a lower hazard rate (i.e., reduced chance of patient needing to take pain medication at the next moment) in the High Dose group compared to Control (hazard ratio [95% CI]: 0.37 [0.17, 0.79], P = 0.011). Upper bound of 95% CI was incalculable because a large proportion of patients did not experience the event by the end of the assessment period.
Figure 5.
Block duration. Kaplan-Meier plot showing the proportion of patients in each group not yet reporting need for pain medication by time elapsed after the end of block administration. Survival curves are shown with 95% Hall-Wellner bands. Comparison of the High Dose group to control demonstrated P = 0.011.
Thus, there was a statistically significant difference between the Control and High Dose groups for the time until a patient needed opioids, but exploratory pairwise comparisons between the experimental groups did not find statistically significant differences. Other indicators of block duration were not significantly different among groups (Table 2).
Table 2.
Block duration
| Control |
High dose |
Medium dose |
Low dose |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Censored/total | Median time to event (95% CI) | Censored/total | Median time to event (95% CI) | Hazard ratio (95% CI) | P value* | Censored/total | Median time to event (95% CI) | Hazard ratio (95% CI) | P value* | Censored/total | Median time to event (95% CI) | Hazard ratio (95% CI) | P value* |
| Shoulder started having pain | 0/20 | 17.4 (10.6, 22.7) | 0/19 | 20.3 (18.9, 21.7) | 0.73 (0.39, 1.38) | 0.338 | 0/20 | 18.5 (15.7, 20.9) | 1.03 (0.55, 1.93) | 0.918 | 2/19 | 17.5 (13.5, 22.9) | 0.68 (0.35, 1.32) | 0.251 |
| Nerve block stopped providing pain relief | 0/13 | 18.9 (10.6, 24.4) | 1/10 | 21.4 (14.9, 24.3) | 0.68 (0.29, 1.60) | 0.379 | 1/14 | 18.6 (15.7, 21.3) | 0.99 (0.45, 2.15) | 0.976 | 1/11 | 17.9 (12.9, 22.9) | 1.09 (0.48, 2.50) | 0.837 |
| Pain medication needed to be taken | 1/20 | 16.1 (10.8, 20.5) | 6/16 | 23.7 (18.6, a) | 0.37 (0.17, 0.79) | 0.011 | 4/17 | 19.4 (11.6, 22.9) | 0.65 (0.32, 1.31) | 0.228 | 5/17 | 17.9 (10.7, 25.7) | 0.53 (0.25, 1.09) | 0.085 |
| Nerve block entirely wore off | 0/14 | 21.6 (11.8, 24.9) | 2/15 | 24.6 (20.3, 27.1) | 0.61 (0.29, 1.30) | 0.202 | 0/17 | 18.9 (16.2, 22.4) | 1.33 (0.65, 2.74) | 0.433 | 1/10 | 21.9 (8.9, 32.9) | 0.69 (0.30, 1.61) | 0.395 |
*P values from Cox proportional hazards models.
aUpper bound of 95% CI was incalculable because a large proportion of patients did not experience the event by the end of the assessment period.
Control = interscalene block with 0.375% ropivacaine, 20 mL, and IV administration of buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); High Dose = 0.375% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); Medium Dose = 0.2% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); Lose Dose = 0.1% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg).
Motor Strength
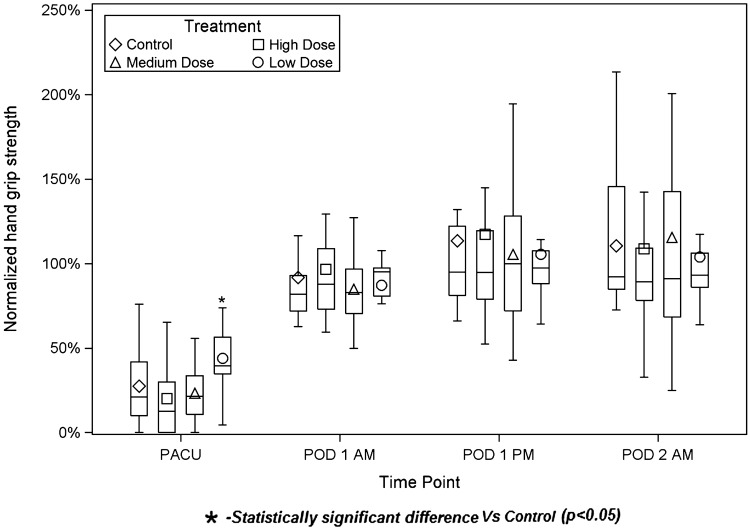
The Low Dose group had superior normalized handgrip strength in the PACU (mean ± SD of preoperative strength: 44.0 ± 20.3%) compared to Control (27.5 ± 24.5%) (P = 0.038) (Figure 6). At other time points (POD1 am, POD1 pm, POD2 am), comparisons of experimental groups to Control group did not reach statistical significance. Pairwise exploratory comparisons between experimental revealed the following statistically significant comparisons: High Dose vs Low Dose (PACU), P = 0.002; Medium Dose vs Low Dose (PACU), P = 0.002. Middle deltoid strength was not different among groups (Table 3).
Figure 6.
Normalized handgrip strength. The diamond, square, triangle, and circle represent the group means. The bottom and top of each rectangle indicate the first and third quartiles, respectively. The horizontal line within each rectangle indicates the median. The lines extending out of the bottom and top of each rectangle represent the minimum and maximum values that lie within 1.5 times the interquartile range below and above the first and third quartiles, respectively. Outliers are not shown.
Table 3.
Other outcomes
| Control |
High dose |
Medium dose |
Low dose |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timepoint | N | Median (Q1, Q3) or percentage | N | Median (Q1, Q3) or percentage | Difference in medians or percentages (95% CI) | P value | N | Median (Q1, Q3) or percentage | Difference in medians or percentages (95% CI) | P value | N | Median (Q1, Q3) or percentage | Difference in medians or percentages (95% CI) | P value | |
| Total opioid use (mg oral morphine equivalents) | DOS + POD 1 | 20 | 65.1 (38.1, 89.3) | 20 | 58.8 (21.2, 95.2) | −9.4 (−35.7, 24.0) | 0.546 | 18 | 61.1 (22.5, 99.6) | −4.5 (−37.5, 23.4) | 0.695 | 19 | 45.9 (15.3, 126.0) | −15.6 (−43.2, 28.2) | 0.407 |
| ORSDS composite score | POD 1 PM | 19 | 0.4 (0.2, 0.7) | 19 | 0.6 (0.2, 0.8) | 0.1 (−0.2, 0.3) | 0.460 | 17 | 0.6 (0.2, 0.8) | 0.2 (−0.2, 0.5) | 0.242 | 19 | 0.5 (0.3, 0.9) | 0.1 (−0.2, 0.4) | 0.524 |
| Responder status | 24 hours | 20 | 60.0% | 20 | 50.0% | −10.0% (37.0, 19.3) | 0.525 | 20 | 55.0% | −5.0% (−32.6, 23.7) | 0.749 | 20 | 40.0% | −20.0% (−45.6, 10.2) | 0.206 |
| Difficulty sleeping | POD 1 AM | 19 | 21.1% | 19 | 10.5% | −10.5% (−34.1, 13.8) | 0.660 | 20 | 15.0% | −6.1% (−30.0, 18.5) | 0.695 | 20 | 10.0% | −11.1% (−34.5, 12.6) | 0.408 |
| Length of stay (days) | 20 | 2.1 (2.0, 2.2) | 20 | 2.1 (2.0, 2.6) | 0 (−0.1, 0.1) | 0.503 | 20 | 2.1 (2.0, 3.1) | 0.1 (−0.1, 0.4) | 0.414 | 20 | 2.1 (2.0, 2.2) | 0 (−0.1, 0.1) | 0.969 | |
| Physical functioning | |||||||||||||||
| BPIinterferencescore | POD 1 PM | 19 | 1.3 (0, 2.2) | 19 | 1.0 (0, 2.3) | −0.2 (−1.3, 0.5) | 0.452 | 16 | 0.3 (0, 1.3) | −0.7 (−1.3, 0) | 0.177 | 19 | 1.3 (0, 3.3) | 0 (−0.8, 1.2) | 0.930 |
| Emotional functioning | |||||||||||||||
| POMS TMD | POD 1 PM | 19 | −5 (−12, 12) | 19 | 9 (−12, 13) | 6.0 (−10.9, 20.0) | 0.488 | 16 | −1 (−7.5, 13.5) | 4.0 (−12.0, 15.0) | 0.544 | 19 | 8 (−11, 25) | 5.0 (−9.0, 22.0) | 0.397 |
| Middle deltoid strength | 24 hours | 19 | 1.8 (1.4, 2.2) | 18 | 2.8 (1.7, 4.2) | 0.7 (−0.1, 1.7) | 0.137 | 17 | 1.5 (1.3, 2.2) | −0.1 (−0.6, 0.7) | 0.604 | 18 | 2.2 (2.0, 3.2) | 0.5 (−0.1, 1.3) | 0.122 |
| Satisfaction | POD 1 PM | 19 | 10 (8, 10) | 19 | 10 (9, 10) | 0 (0, 0) | 0.906 | 17 | 10 (9, 10) | 0 (0, 0) | 0.619 | 19 | 9 (8, 10) | 0 (−1, 0) | 0.214 |
BPI = brief pain inventory; ORSDS = opioid-related symptom distress scale; POD = postoperative day; POMS TMD = profile of mood states total mood disturbance.
Control = interscalene block with 0.375% ropivacaine, 20 mL, and IV administration of buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); High Dose = 0.375% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); Medium Dose = 0.2% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg); Lose Dose = 0.1% ropivacaine with perineural buprenorphine (150 μg) + clonidine (100 μg) + dexamethasone (4 mg).
Other Secondary Outcomes
No differences between groups were observed in total opioid use, opioid side effects (see Appendix A), responder status, difficulty sleeping, length of stay, physical functioning, emotional functioning, and overall satisfaction (Table 3). There were no unexpected spontaneously reported adverse events.
Discussion
The study searched for a nerve block mixture that would provide prolonged analgesia with minimal motor blockade. We identified groups with either improved pain outcomes or greater strength when compared to Control. However, varying the concentrations of ropivacaine in the presence of perineural buprenorphine/clonidine/dexamethasone did not identify a group with both greater strength and reduced pain, when compared to ropivacaine alone with intravenous administration of additives. The addition of buprenorphine, clonidine, and dexamethasone to ropivacaine for ISB reduced pain with movement at 24 hours in the Medium Dose group. Rebound pain scores were lower in the Medium Dose group, compared to control. The High Dose group had less pain on the morning of POD1 and an increased time to first opioid use, compared to Control. However, the High Dose group did not have statistically different pain scores from Control at 24 hours, nor were the rebound pain scores different from Control. The Low Dose group had greater handgrip strength in the PACU, but also more pain in the PACU as compared to Control. Greater handgrip strength does not directly correlate with clinical outcomes, but it is possible that improved postoperative hand movement avoids anxiety that is sometimes associated with prolonged dense nerve blockade of the hand. The potential benefit of increased strength in the Low Dose group must be weighed against the modestly elevated pain levels noted in the PACU. The NRS pain score difference was 0.9 on a 0–10 NRS scale, which may have limited clinical import.
Although dose-dependency was not clearly shown, these results suggest that perineural use of adjuncts reduces postoperative pain in the High Dose and Medium Dose groups, compared to Control. For the primary outcome of pain at 24 hours, only the Medium Dose group was significantly lower vs Control. Similarly, for rebound pain only, the Medium Dose group was significantly lower vs Control. These findings, if confirmed in other studies, could indicate that there is an optimal concentration of ropivacaine (when administered with the additives) for provision of postoperative analgesia. One could hypothesize that using too little ropivacaine does not provide sufficient nerve blockade for prolonged analgesia, but that using too much ropivacaine provokes transient hyperalgesia [28] that also reduces postoperative analgesia. However, the block duration question of time until need for analgesics revealed longer duration for High Dose but not Medium Dose, which is not supportive of this hypothesis. Further investigations are needed to clarify local anesthetic dose-response in the presence of multiple adjuvants.
ISB can substantially improve pain control after shoulder surgery [29]. However, despite use of multimodal analgesia, ISB with plain ropivacaine (0.375%) does not provide ideal postoperative analgesia on POD1 [2]. For Goon et al [2], median pain scores were 0 on the day of TSA surgery (POD0), but rose by morning of POD1 to 2.3 at rest and 3.8 with movement. Patients in Goon et al [2] used the equivalent of 62 ± 49 mg oral morphine (mean ± SD) in the first 24 hours after surgery, despite multimodal analgesia with acetaminophen, pregabalin, and meloxicam. Nerve blockade with ropivacaine 0.375% was associated with decreased strength in Goon et al [2]; median normalized deltoid strength was 0% on POD0, which returned to 86% on the morning of POD1. The current study was intended to possibly identify a better injectate for ISB that would provide more prolonged analgesia, with reduced opioid use and reduced motor blockade.
Median ORSDS scores among TSA patients on POD1 ranged from 0.4 to 0.6. For comparison, previous work found that median ORSDS scores were lower for outpatient distal upper extremity surgery (0.19), in the same range for both outpatient anterior cruciate ligament reconstruction (0.52) and inpatient total knee arthroplasty (0.51), and higher for inpatient lumbar spine fusion (0.94) [24].
Single-shot peripheral nerve blocks are very common at the authors’ institution, but continuous peripheral nerve blockade is uncommon and is decreasing in frequency. Indisputable advantages of continuous nerve blockade include the ability to titrate the level of analgesia (with the concomitant motor and sensory blockade), as well as the possibility of indefinite duration of nerve block analgesia. Some operational disadvantages include longer time for placement, secondary block failure (potentially related to catheter dislodgment or migration), and the need to monitor and manage a nerve block catheter. Theoretical risks of peripheral nerve catheters include the possibility of increased risks of infection and nerve injury. Nerve injury could be associated with the use of a larger needle, presence of a foreign body near the nerve, and lengthy exposure of nerves to local anesthetics.
Off-label perineural use has been advocated for buprenorphine [7], clonidine [4], and dexamethasone [30]. Perineural use of each of these drugs has been extensively studied, but limited information is available about combinations of additives for peripheral nerve blockade.
Several studies found prolonged analgesia from perineural dexamethasone for ISB [16,17,31]. However, perineural (ISB) dexamethasone (10 mg) did not provide longer analgesia than systemic dexamethasone (10 mg) [32]. A recent systemically controlled study [33] found that perineural, but not systemic, dexamethasone (4 mg) substantially prolonged analgesia after ropivacaine ISB. A meta-analysis [4] showed that clonidine prolongs analgesia and motor block after nerve blocks by about 2 hours. Moreover, interscalene clonidine provides analgesia in the absence of local anesthetic [9]. The addition of buprenorphine to local anesthetic for brachial plexus blockade can prolong analgesia from 5 to 17 hours [5]. Both systemic and perineural (sciatic) buprenorphine reduced postoperative pain, but perineural buprenorphine provided longer analgesia [7]. Intramuscular buprenorphine provides analgesia, but the mean duration of postoperative analgesia after 200 mcg buprenorphine was 5.6 hours [34].
The current study used three analgesic adjuncts. This has been termed multimodal perineural analgesia [10,11,35,36]. Rat safety study of combined perineural bupivacaine-clonidine-buprenorphine-dexamethasone demonstrated absence of irreversible behavioral effects and absence of histologic changes in the nervous tissue [37], supporting the safety of this approach. Quality assurance data also support efficacy and safety of multiple perineural adjuncts, with block duration of 33–37 hours [13].
Randomized clinical trials of perineural local anesthetic plus three additives are lacking. Local anesthetic dose-response studies in the presence of multiple perineural adjuncts have not been reported. Unlike the current study, neither of the Williams studies [12,13] are clinical randomized controlled trials.
The strengths of this study include randomized trial design with a systemic control group. Comprehensive outcome measures covered pain, analgesic consumption, physical and emotional functioning, satisfaction, and adverse events. There are also limitations to the study. Some of the questionnaires used for secondary outcomes (BPI and POMS) were only administered postoperatively (in order to lessen the time commitment for the patient); the absence of a baseline may hinder interpretation of these results. Generalizability of the study is affected by the vigorous use of multimodal analgesia (nerve blockade, intraoperative ketamine, acetaminophen, ketorolac, pregabalin, oral and intravenous opioids); different results may occur in other anesthetic and analgesic contexts. Because the study focus was on multimodal perineural analgesia, it is not feasible to ascribe the results to a particular additive. Different doses or combinations of additives would likely have different effects. Study drugs were administered to sedated patients by unblinded anesthesiologists, but data collection was performed by blinded research coordinators. Block duration was assessed at 24 and 48 hours, but by some measures some of the blocks were still in effect at that time; it would have been desirable to obtain subsequent data points.
Interpretation of these results would be clearer if we included additional negative controls, such as a group of patients who did not receive clonidine, dexamethasone, or buprenorphine. However, previous work [Goon et al.] done in preparation for the current study showed that multimodal analgesia plus ISB by itself worked well but did not meet our goals of prolonged pain relief with minimal opioid use and minimal motor blockade. During study design it was decided not to include this negative control, but rather to use a control group that received systemic additives.
The combination of bupivacaine-clonidine-buprenorphine-dexamethasone is compatible and stable in solution [35]. However, similar data are lacking for ropivacaine combined with three adjuvants. It is not known if the combined adjuvants alter ropivacaine solutions in a way that could affect block onset or spread. Patients in this study all received general anesthesia immediately after performance of ISB, so block onset and rate of spread were not assessed.
The additives reduced pain on the morning after surgery (High Dose Group) in comparison with the control group, and the duration until first opioid dose was also longer (median 23.7 vs 16.1, P = 0.011) in the High Dose group. This illustrates that at this specific time interval, “Rebound Pain” [38,12] as it was evolving was attenuated by the adjuvants. Once the blocks completely resolved, the peak pain scores with movement were 6.1 (Control) vs 5.2 (High Dose) vs 4.2 (Medium Dose, P = 0.034 vs control), vs 6.1 (Low Dose). We were unable to delineate whether the attenuation of rebound pain was related to the additional duration or related to the presence of the CBD adjuvants. Further research could attempt to elucidate selection and dosing of adjuncts to local anesthetics for optimal postoperative analgesia. Our anesthesia group has not reached a consensus on the best anesthetic/analgesic regimen for TSA. Some practitioners use multimodal analgesia and add dexamethasone to the nerve blocks, but others do not.
Conclusions
In summary, if pain reduction is the main priority, then combining perineural additives (buprenorphine, clonidine, dexamethasone) with either ropivacaine 0.375% (High Dose) or 0.2% (Medium Dose) is supported by this study. Pain with movement was reduced at 24 hours in the Medium Dose group, compared to Control. The High Dose group had reduced pain on the morning after surgery, as well as prolonged time until first need for opioid analgesics. Alternatively, if the priority is minimizing motor blockade, then perineural additives can be combined with Low Dose ropivacaine (0.1%). The Low Dose group had greater handgrip strength in the PACU, compared to Control, but also had more pain in the PACU.
Acknowledgments
We would like to thank Paul J. Christos, DrPH, MS, for assistance with the sample size calculation, as well as Heather Reel, MA, and Sumudu Dehipawala, BS, for assistance with data collection.
Funding sources: This study was funded by Hospital for Special Surgery Anesthesiology Department Research and Education Fund, New York, NY. The REDCap electronic data capture tools are funded by the CTSC grant (grant number UL1 TR000457-06) from the National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD. Dr. Paul Christos (see Acknowledgments) was partially supported by grant UL1-TR000457-06 to the Clinical and Translational Science Center at Weill Cornell Medical College.
Disclosure and conflicts of interest: Lawrence V. Gulotta, MD: Biomet, Inc. – Consultant. David M. Dines, MD: Biomet, Inc. – Royalties. Edward V. Craig, MD, MPH: Biomet, Inc. – Royalties and Consultant. The remaining authors have no conflicts of interest to report. This study was previously presented, in part, at the 39th Annual Regional Anesthesiology and Acute Pain Medicine Meeting in Chicago, IL, in April 2014.
Appendix
Appendix A.
Opioid-related symptom distress scale
| Control | High dose |
Medium dose |
Low dose |
||||
|---|---|---|---|---|---|---|---|
| (n = 19) | (n = 19) | P value | (n = 17) | P value | (n = 19) | P value | |
| Nausea | |||||||
| Count (%) | 0.999 | 0.999 | 0.999 | ||||
| No | 15 (78.9) | 15 (78.9) | 13 (76.5) | 14 (73.7) | |||
| Yes | 4 (21.1) | 4 (21.1) | 4 (23.5) | 5 (26.3) | |||
| Frequency, count (%) | |||||||
| N/A | 15 (78.9) | 15 (78.9) | 13 (76.5) | 14 (73.7) | |||
| Rarely | 3 (15.8) | 1 (5.3) | 2 (11.8) | 2 (10.5) | |||
| Occasionally | 1 (5.3) | 2 (10.5) | 2 (11.8) | 2 (10.5) | |||
| Frequently | 0 (0) | 1 (5.3) | 0 (0) | 1 (5.3) | |||
| Almost constantly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severity, count (%) | |||||||
| N/A | 15 (78.9) | 15 (78.9) | 13 (76.5) | 14 (73.7) | |||
| Slight | 1 (5.3) | 0 (0) | 1 (5.9) | 2 (10.5) | |||
| Moderate | 3 (15.8) | 1 (5.3) | 1 (5.9) | 3 (15.8) | |||
| Severe | 0 (0) | 3 (15.8) | 2 (11.8) | 0 (0) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 15 (78.9) | 15 (78.9) | 13 (76.5) | 14 (73.7) | |||
| Not at all | 0 (0) | 2 (10.5) | 1 (5.9) | 1 (5.3) | |||
| A little bit | 2 (10.5) | 0 (0) | 2 (11.8) | 2 (10.5) | |||
| Somewhat | 1 (5.3) | 0 (0) | 0 (0) | 0 (0) | |||
| Quite a bit | 1 (5.3) | 1 (5.3) | 1 (5.9) | 2 (10.5) | |||
| Very much | 0 (0) | 1 (5.3) | 0 (0) | 0 (0) | |||
| Score, median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0.871 | 0 (0, 0) | 0.812 | 0 (0, 0.9) | 0.698 |
| Vomiting, count (%) | |||||||
| Count | 0.999 | 0.999 | 0.487 | ||||
| No | 17 (89.5) | 17 (89.5) | 16 (94.1) | 19 (100) | |||
| Yes | 2 (10.5) | 2 (10.5) | 1 (5.9) | 0 (0) | |||
| Frequency, count (%) | |||||||
| N/A | 17 (89.5) | 17 (89.5) | 16 (94.1) | 19 (100) | |||
| Rarely | 1 (5.3) | 1 (5.3) | 1 (5.9) | 0 (0) | |||
| Occasionally | 1 (5.3) | 1 (5.3) | 0 (0) | 0 (0) | |||
| Frequently | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Almost constantly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severity, count (%) | |||||||
| N/A | 17 (89.5) | 17 (89.5) | 16 (94.1) | 19 (100) | |||
| Slight | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Moderate | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severe | 2 (10.5) | 2 (10.5) | 1 (5.9) | 0 (0) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 17 (89.5) | 17 (89.5) | 16 (94.1) | 19 (100) | |||
| Not at all | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| A little bit | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Somewhat | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Quite a bit | 2 (10.5) | 1 (5.3) | 0 (0) | 0 (0) | |||
| Very much | 0 (0) | 1 (5.3) | 0 (0) | 0 (0) | |||
| Score, median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0.999 | 0 (0, 0) | 0.600 | 0 (0, 0) | 0.171 |
| Constipation | |||||||
| Count (%) | 0.232 | 0.717 | 0.999 | ||||
| No | 13 (68.4) | 17 (89.5) | 13 (76.5) | 13 (68.4) | |||
| Yes | 6 (31.6) | 2 (10.5) | 4 (23.5) | 6 (31.6) | |||
| Frequency, count (%) | |||||||
| N/A | 13 (68.4) | 17 (89.5) | 13 (76.5) | 13 (68.4) | |||
| No answer | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Rarely | 1 (5.3) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Occasionally | 1 (5.3) | 0 (0) | 1 (5.9) | 2 (10.5) | |||
| Frequently | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Almost constantly | 4 (21.1) | 2 (10.5) | 1 (5.9) | 3 (15.8) | |||
| Severity, count (%) | |||||||
| N/A | 13 (68.4) | 17 (89.5) | 13 (76.5) | 13 (68.4) | |||
| No answer | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Slight | 2 (10.5) | 0 (0) | 1 (5.9) | 4 (21.1) | |||
| Moderate | 3 (15.8) | 1 (5.3) | 0 (0) | 1 (5.3) | |||
| Severe | 1 (5.3) | 1 (5.3) | 2 (11.8) | 1 (5.3) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 13 (68.4) | 17 (89.5) | 13 (76.5) | 13 (68.4) | |||
| No answer | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Not at all | 4 (21.1) | 0 (0) | 1 (5.9) | 2 (10.5) | |||
| A little bit | 0 (0) | 1 (5.3) | 0 (0) | 1 (5.3) | |||
| Somewhat | 2 (10.5) | 1 (5.3) | 1 (5.9) | 2 (10.5) | |||
| Quite a bit | 0 (0) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Very much | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Score, median (Q1, Q3) | 0 (0, 1.8) | 0 (0, 0) | 0.178 | 0 (0, 0) | 0.509 | 0 (0, 1.3) | 0.958 |
| Difficulty passing urine | |||||||
| Count (%) | 0.658 | 0.688 | 0.999 | ||||
| No | 14 (73.7) | 16 (84.2) | 12 (70.6) | 15 (78.9) | |||
| Yes | 3 (15.8) | 2 (10.5) | 4 (23.5) | 3 (15.8) | |||
| Catheter | 2 (10.5) | 1 (5.3) | 1 (5.9) | 1 (5.3) | |||
| Frequency, count (%) | |||||||
| N/A | 16 (84.2) | 17 (89.5) | 13 (68.4) | 16 (84.2) | |||
| Rarely | 0 (0) | 1 (5.3) | 1 (5.9) | 1 (5.3) | |||
| Occasionally | 1 (5.3) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Frequently | 0 (0) | 1 (5.3) | 1 (5.9) | 0 (0) | |||
| Almost constantly | 2 (10.5) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Severity, count (%) | |||||||
| N/A | 16 (84.2) | 17 (89.5) | 13 (68.4) | 16 (84.2) | |||
| Slight | 1 (5.3) | 0 (0) | 2 (11.8) | 1 (5.3) | |||
| Moderate | 1 (5.3) | 1 (5.3) | 2 (11.8) | 2 (10.5) | |||
| Severe | 1 (5.3) | 1 (5.3) | 0 (0) | 0 (0) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 16 (84.2) | 17 (89.5) | 13 (68.4) | 16 (84.2) | |||
| Not at all | 1 (5.3) | 0 (0) | 1 (5.9) | 0 (0) | |||
| A little bit | 2 (10.5) | 2 (10.5) | 2 (11.8) | 1 (5.3) | |||
| Somewhat | 0 (0) | 0 (0) | 1 (5.9) | 2 (10.5) | |||
| Quite a bit | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Very much | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Score, median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0.610 | 0 (0, 0.6) | 0.726 | 0 (0, 0) | 0.822 |
| Difficulty concentrating | |||||||
| Count (%) | 0.405 | 0.055 | 0.660 | ||||
| No | 17 (89.5) | 14 (73.7) | 10 (58.8) | 15 (78.9) | |||
| Yes | 2 (10.5) | 5 (26.3) | 7 (41.2) | 4 (21.1) | |||
| Frequency, count (%) | |||||||
| N/A | 17 (89.5) | 14 (73.7) | 10 (58.8) | 15 (78.9) | |||
| Rarely | 0 (0) | 0 (0) | 2 (11.8) | 0 (0) | |||
| Occasionally | 2 (10.5) | 5 (26.3) | 4 (23.5) | 1 (5.3) | |||
| Frequently | 0 (0) | 0 (0) | 0 (0) | 2 (10.5) | |||
| Almost constantly | 0 (0) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Severity, count (%) | |||||||
| N/A | 17 (89.5) | 14 (73.7) | 10 (58.8) | 15 (78.9) | |||
| Slight | 2 (10.5) | 1 (5.3) | 4 (23.5) | 2 (10.5) | |||
| Moderate | 0 (0) | 4 (21.1) | 3 (17.6) | 1 (5.3) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 17 (89.5) | 14 (73.7) | 10 (58.8) | 15 (78.9) | |||
| Not at all | 0 (0) | 2 (10.5) | 0 (0) | 1 (5.3) | |||
| A little bit | 1 (5.3) | 0 (0) | 2 (11.8) | 0 (0) | |||
| Somewhat | 1 (5.3) | 2 (10.5) | 5 (29.4) | 1 (5.3) | |||
| Quite a bit | 0 (0) | 1 (5.3) | 0 (0) | 2 (10.5) | |||
| Very much | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Score, median (Q1, Q3) | 0 (0, 0) | 0 (0, 1.6) | 0.196 | 0 (0, 1.8) | 0.035 | 0 (0, 0) | 0.352 |
| Drowsiness/difficulty staying awake | |||||||
| Count (%) | 0.022 | 0.102 | 0.097 | ||||
| No | 14 (73.7) | 7 (36.8) | 8 (47.1) | 9 (47.4) | |||
| Yes | 5 (26.3) | 12 (63.2) | 9 (52.9) | 10 (52.6) | |||
| Frequency, count (%) | |||||||
| N/A | 14 (73.7) | 7 (36.8) | 8 (47.1) | 9 (47.4) | |||
| Rarely | 0 (0) | 1 (5.3) | 1 (5.9) | 0 (0) | |||
| Occasionally | 3 (15.8) | 5 (26.3) | 2 (11.8) | 2 (10.5) | |||
| Frequently | 1 (5.3) | 5 (26.3) | 3 (17.6) | 6 (31.6) | |||
| Almost constantly | 1 (5.3) | 1 (5.3) | 3 (17.6) | 2 (10.5) | |||
| Severity, count (%) | |||||||
| N/A | 14 (73.7) | 7 (36.8) | 8 (47.1) | 9 (47.4) | |||
| Slight | 1 (5.3) | 3 (15.8) | 3 (17.6) | 1 (5.3) | |||
| Moderate | 3 (15.8) | 8 (42.1) | 3 (17.6) | 5 (26.3) | |||
| Severe | 1 (5.3) | 1 (5.3) | 3 (17.6) | 4 (21.1) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 14 (73.7) | 7 (36.8) | 8 (47.1) | 9 (47.4) | |||
| Not at all | 3 (15.8) | 7 (36.8) | 1 (5.9) | 3 (15.8) | |||
| A little bit | 1 (5.3) | 4 (21.1) | 3 (17.6) | 3 (15.8) | |||
| Somewhat | 1 (5.3) | 0 (0) | 3 (17.6) | 0 (0) | |||
| Quite a bit | 0 (0) | 1 (5.3) | 1 (5.9) | 3 (15.8) | |||
| Very much | 0 (0) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Score, median (Q1, Q3) | 0 (0, 1.3) | 1.5 (0, 1.9) | 0.053 | 1.2 (0, 2.5) | 0.083 | 1.5 (0, 2.2) | 0.072 |
| Feeling light-headed or dizzy | |||||||
| Count (%) | 0.999 | 0.332 | 0.999 | ||||
| No | 12 (63.2) | 12 (63.2) | 8 (47.1) | 12 (63.2) | |||
| Yes | 7 (36.8) | 7 (36.8) | 9 (52.9) | 7 (36.8) | |||
| Frequency, count (%) | |||||||
| N/A | 12 (63.2) | 12 (63.2) | 8 (47.1) | 12 (63.2) | |||
| Rarely | 4 (21.1) | 2 (10.5) | 4 (23.5) | 2 (10.5) | |||
| Occasionally | 3 (15.8) | 4 (21.1) | 5 (29.4) | 5 (26.3) | |||
| Frequently | 0 (0) | 1 (5.3) | 0 (0) | 0 (0) | |||
| Almost constantly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severity, count (%) | |||||||
| N/A | 12 (63.2) | 12 (63.2) | 8 (47.1) | 12 (63.2) | |||
| Slight | 2 (10.5) | 3 (15.8) | 6 (35.3) | 3 (15.8) | |||
| Moderate | 2 (10.5) | 4 (21.1) | 2 (11.8) | 3 (15.8) | |||
| Severe | 2 (10.5) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Very severe | 1 (5.3) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 12 (63.2) | 12 (63.2) | 8 (47.1) | 12 (63.2) | |||
| Not at all | 0 (0) | 3 (15.8) | 2 (11.8) | 0 (0) | |||
| A little bit | 2 (10.5) | 2 (10.5) | 4 (23.5) | 4 (21.1) | |||
| Somewhat | 1 (5.3) | 1 (5.3) | 2 (11.8) | 0 (0) | |||
| Quite a bit | 3 (15.8) | 1 (5.3) | 1 (5.9) | 2 (10.5) | |||
| Very much | 1 (5.3) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Score, median (Q1, Q3) | 0 (0, 1.9) | 0 (0, 1.3) | 0.789 | 0.9 (0, 1.5) | 0.730 | 0 (0, 1.9) | 0.933 |
| Feeling confused | |||||||
| Count (%) | 0.999 | 0.650 | 0.999 | ||||
| No | 17 (89.5) | 18 (94.7) | 14 (82.4) | 18 (94.7) | |||
| Yes | 2 (10.5) | 1 (5.3) | 3 (17.6) | 1 (5.3) | |||
| Frequency, count (%) | |||||||
| N/A | 17 (89.5) | 18 (94.7) | 14 (82.4) | 18 (94.7) | |||
| Rarely | 2 (10.5) | 1 (5.3) | 1 (5.9) | 0 (0) | |||
| Occasionally | 0 (0) | 0 (0) | 2 (11.8) | 0 (0) | |||
| Frequently | 0 (0) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Almost constantly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severity, count (%) | |||||||
| N/A | 17 (89.5) | 18 (94.7) | 14 (82.4) | 18 (94.7) | |||
| Slight | 1 (5.3) | 1 (5.3) | 2 (11.8) | 0 (0) | |||
| Moderate | 1 (5.3) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 17 (89.5) | 18 (94.7) | 14 (82.4) | 18 (94.7) | |||
| Not at all | 1 (5.3) | 1 (5.3) | 0 (0) | 0 (0) | |||
| A little bit | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Somewhat | 1 (5.3) | 0 (0) | 2 (11.8) | 0 (0) | |||
| Quite a bit | 0 (0) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Very much | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Score, median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0.557 | 0 (0, 0) | 0.531 | 0 (0, 0) | 0.621 |
| Feelings of general fatigue or weakness | |||||||
| Count (%) | 0.999 | 0.550 | 0.999 | ||||
| No | 13 (68.4) | 13 (68.4) | 10 (58.8) | 13 (68.4) | |||
| Yes | 6 (31.6) | 6 (31.6) | 7 (41.2) | 6 (31.6) | |||
| Frequency, count (%) | |||||||
| N/A | 13 (68.4) | 13 (68.4) | 10 (58.8) | 13 (68.4) | |||
| No answer | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Rarely | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Occasionally | 3 (15.8) | 4 (21.1) | 1 (5.9) | 1 (5.3) | |||
| Frequently | 2 (10.5) | 1 (5.3) | 1 (5.9) | 3 (15.8) | |||
| Almost constantly | 1 (5.3) | 1 (5.3) | 4 (23.5) | 2 (10.5) | |||
| Severity, count (%) | |||||||
| N/A | 13 (68.4) | 13 (68.4) | 10 (58.8) | 13 (68.4) | |||
| No answer | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Slight | 1 (5.3) | 1 (5.3) | 1 (5.9) | 0 (0) | |||
| Moderate | 4 (21.1) | 5 (26.3) | 4 (23.5) | 4 (21.1) | |||
| Severe | 1 (5.3) | 0 (0) | 1 (5.9) | 2 (10.5) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 13 (68.4) | 13 (68.4) | 10 (58.8) | 13 (68.4) | |||
| No answer | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Not at all | 4 (21.1) | 2 (10.5) | 1 (5.9) | 0 (0) | |||
| A little bit | 0 (0) | 2 (10.5) | 2 (11.8) | 3 (15.8) | |||
| Somewhat | 2 (10.5) | 2 (10.5) | 2 (11.8) | 0 (0) | |||
| Quite a bit | 0 (0) | 0 (0) | 0 (0) | 2 (10.5) | |||
| Very much | 0 (0) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Score, median (Q1, Q3) | 0 (0, 1.6) | 0 (0, 1.9) | 0.972 | 0 (0, 2.1) | 0.510 | 0 (0, 2.2) | 0.739 |
| Itchiness | |||||||
| Count (%) | 0.405 | 0.999 | 0.405 | ||||
| No | 14 (73.7) | 17 (89.5) | 13 (76.5) | 17 (89.5) | |||
| Yes | 5 (26.3) | 2 (10.5) | 4 (23.5) | 2 (10.5) | |||
| Frequency, count (%) | |||||||
| N/A | 14 (73.7) | 17 (89.5) | 13 (76.5) | 17 (89.5) | |||
| Rarely | 1 (5.3) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Occasionally | 3 (15.8) | 2 (10.5) | 2 (11.8) | 1 (5.3) | |||
| Frequently | 0 (0) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Almost constantly | 1 (5.3) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Severity, count (%) | |||||||
| N/A | 14 (73.7) | 17 (89.5) | 13 (76.5) | 17 (89.5) | |||
| Slight | 3 (15.8) | 2 (10.5) | 3 (17.6) | 1 (5.3) | |||
| Moderate | 1 (5.3) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Severe | 1 (5.3) | 0 (0) | 0 (0) | 0 (0) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 14 (73.7) | 17 (89.5) | 13 (76.5) | 17 (89.5) | |||
| Not at all | 1 (5.3) | 1 (5.3) | 1 (5.9) | 0 (0) | |||
| A little bit | 2 (10.5) | 0 (0) | 2 (11.8) | 0 (0) | |||
| Somewhat | 1 (5.3) | 1 (5.3) | 1 (5.9) | 2 (10.5) | |||
| Quite a bit | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Very much | 1 (5.3) | 0 (0) | 0 (0) | 0 (0) | |||
| Score, median (Q1, Q3) | 0 (0, 1.3) | 0 (0, 0) | 0.218 | 0 (0, 0) | 0.869 | 0 (0, 0) | 0.278 |
| Dry mouth | |||||||
| Count (%) | 0.283 | 0.387 | 0.141 | ||||
| No | 7 (36.8) | 4 (21.1) | 4 (23.5) | 3 (15.8) | |||
| Yes | 12 (63.2) | 15 (78.9) | 13 (76.5) | 16 (84.2) | |||
| Frequency, count (%) | |||||||
| N/A | 7 (36.8) | 4 (21.1) | 4 (23.5) | 3 (15.8) | |||
| Rarely | 0 (0) | 1 (5.3) | 1 (5.9) | 2 (10.5) | |||
| Occasionally | 3 (15.8) | 2 (10.5) | 2 (11.8) | 5 (26.3) | |||
| Frequently | 3 (15.8) | 5 (26.3) | 3 (17.6) | 4 (21.1) | |||
| Almost constantly | 6 (31.6) | 7 (36.8) | 7 (41.2) | 5 (26.3) | |||
| Severity, count (%) | |||||||
| N/A | 7 (36.8) | 4 (21.1) | 4 (23.5) | 3 (15.8) | |||
| Slight | 1 (5.3) | 3 (15.8) | 6 (35.3) | 5 (26.3) | |||
| Moderate | 7 (36.8) | 9 (47.4) | 0 (0) | 7 (36.8) | |||
| Severe | 3 (15.8) | 3 (15.8) | 4 (23.5) | 4 (21.1) | |||
| Very severe | 1 (5.3) | 0 (0) | 3 (17.6) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 7 (36.8) | 4 (21.1) | 4 (23.5) | 3 (15.8) | |||
| Not at all | 2 (10.5) | 5 (26.3) | 3 (17.6) | 5 (26.3) | |||
| A little bit | 6 (31.6) | 3 (15.8) | 3 (17.6) | 4 (21.1) | |||
| Somewhat | 2 (10.5) | 2 (10.5) | 2 (11.8) | 4 (21.1) | |||
| Quite a bit | 2 (10.5) | 4 (21.1) | 5 (29.4) | 2 (10.5) | |||
| Very much | 0 (0) | 1 (5.3) | 0 (0) | 1 (5.3) | |||
| Score, median (Q1, Q3) | 1.9 (0, 2.8) | 2.3 (1.3, 2.7) | 0.587 | 1.9 (0.9, 3.4) | 0.382 | 1.9 (0.9, 2.5) | 0.629 |
| Headache | |||||||
| Count (%) | 0.604 | 0.999 | 0.604 | ||||
| No | 16 (84.2) | 18 (94.7) | 14 (82.4) | 18 (94.7) | |||
| Yes | 3 (15.8) | 1 (5.3) | 3 (17.6) | 1 (5.3) | |||
| Frequency, count (%) | |||||||
| N/A | 16 (84.2) | 18 (94.7) | 14 (82.4) | 18 (94.7) | |||
| Rarely | 2 (10.5) | 1 (5.3) | 3 (17.6) | 0 (0) | |||
| Occasionally | 1 (5.3) | 0 (0) | 0 (0) | 0 (0) | |||
| Frequently | 0 (0) | 0 (0) | 0 (0) | 1 (5.3) | |||
| Almost constantly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severity, count (%) | |||||||
| N/A | 16 (84.2) | 18 (94.7) | 14 (82.4) | 18 (94.7) | |||
| Slight | 2 (10.5) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Moderate | 1 (5.3) | 1 (5.3) | 2 (11.8) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Very severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Bothersomeness, count (%) | |||||||
| N/A | 16 (84.2) | 18 (94.7) | 14 (82.4) | 18 (94.7) | |||
| Not at all | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| A little bit | 0 (0) | 1 (5.3) | 1 (5.9) | 0 (0) | |||
| Somewhat | 2 (10.5) | 0 (0) | 1 (5.9) | 0 (0) | |||
| Quite a bit | 1 (5.3) | 0 (0) | 1 (5.9) | 1 (5.3) | |||
| Very much | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Score, median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0.305 | 0 (0, 0) | 0.923 | 0 (0, 0) | 0.357 |
| Composite score, median (Q1, Q3) | 0.4 (0.2, 0.7) | 0.6 (0.2, 0.8) | 0.460 | 0.6 (0.2, 0.8) | 0.242 | 0.5 (0.3, 0.9) | 0.524 |
Appendix B.
Pain management instructions to the patient
|
References
- 1. Fredrickson MJ, Smith KR, Wong AC. Importance of volume and concentration for ropivacaine interscalene block in preventing recovery room pain and minimizing motor block after shoulder surgery. Anesthesiology 2010;112:1374–81. [DOI] [PubMed] [Google Scholar]
- 2. Goon AK, Dines DM, Craig EV, et al. A clinical pathway for total shoulder arthroplasty—a pilot study. HSS J 2014;10:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: A systematic review and meta-analysis of randomized trials. Br J Anaesth 2014;112:427–39. [DOI] [PubMed] [Google Scholar]
- 4. Pöpping DM, Elia N, Marret E, Wenk M, Tramèr MR. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: A meta-analysis of randomized trials. Anesthesiology 2009;111:406–15. [DOI] [PubMed] [Google Scholar]
- 5. Candido KD, Franco CD, Khan MA, Winnie AP, Raja DS. Buprenorphine added to the local anesthetic for brachial plexus block to provide postoperative analgesia in outpatients. Reg Anesth Pain Med 2001;26:352–6. [DOI] [PubMed] [Google Scholar]
- 6. Candido KD, Winnie AP, Ghaleb AH, Fattouh MW, Franco CD. Buprenorphine added to the local anesthetic for axillary brachial plexus block prolongs postoperative analgesia. Reg Anesth Pain Med 2002;27:162–7. [DOI] [PubMed] [Google Scholar]
- 7. Candido KD, Hennes J, Gonzalez S, et al. Buprenorphine enhances and prolongs the postoperative analgesic effect of bupivacaine in patients receiving infragluteal sciatic nerve block. Anesthesiology 2010;113:1419–26. [DOI] [PubMed] [Google Scholar]
- 8. Modi M, Rastogi S, Kumar A. Buprenorphine with bupivacaine for intraoral nerve blocks to provide postoperative analgesia in outpatients after minor oral surgery. J Oral Maxillofac Surg 2009;67:2571–6. [DOI] [PubMed] [Google Scholar]
- 9. Iskandar H, Benard A, Ruel-Raymond J, Cochard G, Manaud B. The analgesic effect of interscalene block using clonidine as an analgesic for shoulder arthroscopy. Anesth Analg 2003;96:260–2. [DOI] [PubMed] [Google Scholar]
- 10. Whiting DJ, Williams BA, Orebaugh SL, Toshok RR. Case report: Postoperative analgesia and preserved motor function with clonidine and buprenorphine via a sciatic perineural catheter. J Clin Anesth 2009;21:297–9. [DOI] [PubMed] [Google Scholar]
- 11. Ibinson JW, Mangione MP, Williams BA. Local anesthetics in diabetic rats (and patients): Shifting from a known slippery slope toward a potentially better multimodal perineural paradigm? Reg Anesth Pain Med 2012;37:574. [DOI] [PubMed] [Google Scholar]
- 12. Williams BA, Ibinson JW, Mangione MP, et al. Research priorities regarding multimodal peripheral nerve blocks for postoperative analgesia and anesthesia based on hospital quality data extracted from over 1,300 cases (2011-2014). Pain Med 2015;16:7–12. [DOI] [PubMed] [Google Scholar]
- 13. Williams BA, Ibinson JW, Mangione MP, Scanlan RL, Cohen PZ. Clinical benchmarks regarding multimodal peripheral nerve blocks for postoperative analgesia: Observations regarding combined perineural midazolam-clonidine-buprenorphine-dexamethasone. Pain Med 2015;16:1–6. [DOI] [PubMed] [Google Scholar]
- 14. Behr A, Freo U, Ori C, Westermann B, Alemanno F. Buprenorphine added to levobupivacaine enhances postoperative analgesia of middle interscalene brachial plexus block. J Anesth 2012;26:746–51. [DOI] [PubMed] [Google Scholar]
- 15. Vieira PA, Pulai I, Tsao GC, et al. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol 2010;27:285–8. [DOI] [PubMed] [Google Scholar]
- 16. Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: A prospective randomized trial. J Anesth 2011;25:704–9. [DOI] [PubMed] [Google Scholar]
- 17. De Oliveira GS, Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2011;115:575–88. [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Richman KA, Grodofsky SR, et al. Is there a dose response of dexamethasone as adjuvant for supraclavicular brachial plexus nerve block? A prospective randomized double-blinded clinical study. J Clin Anesth 2015;27:237–42. [DOI] [PubMed] [Google Scholar]
- 19. YaDeau JT, LaSala VR, Paroli L, et al. Clonidine and analgesic duration after popliteal fossa nerve blockade: Randomized, double-blind, placebo-controlled study. Anesth Analg 2008;106:1916–20. [DOI] [PubMed] [Google Scholar]
- 20. Singelyn FJ, Gouverneur JM, Robert A. A minimum dose of clonidine added to mepivacaine prolongs the duration of anesthesia and analgesia after axillary brachial plexus block. Anesth Analg 1996;83:1046–50. [DOI] [PubMed] [Google Scholar]
- 21. Bernard JM, Macaire P. Dose-range effects of clonidine added to lidocaine for brachial plexus block. Anesthesiology 1997;87:277–84. [DOI] [PubMed] [Google Scholar]
- 22. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- 23. McNair DM, Heuchert JWP. Profile of Mood States: Technical Update. New York: Multi-Health Systems; 2005:1–28. [Google Scholar]
- 24. YaDeau JT, Liu SS, Rade MC, Marcello D, Liguori GA. Performance characteristics and validation of the Opioid-Related Symptom Distress Scale for evaluation of analgesic side effects after orthopedic surgery. Anesth Analg 2011;113:369–77. [DOI] [PubMed] [Google Scholar]
- 25. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma Y, Mazumdar M, Memtsoudis SG. Beyond repeated-measures analysis of variance: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med 2012;37:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics 1988;44:1049–60. [PubMed] [Google Scholar]
- 28. Kolarczyk LM, Williams BA. Transient heat hyperalgesia during resolution of ropivacaine sciatic nerve block in the rat. Reg Anesth Pain Med 2011;36:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hadzic A, Williams BA, Karaca PE, et al. For outpatient rotator cuff surgery, nerve block anesthesia provides superior same-day recovery over general anesthesia. Anesthesiology 2005;102:1001–7. [DOI] [PubMed] [Google Scholar]
- 30. Parrington SJ, O'Donnell D, Chan VW, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med 2010;35:422–6. [DOI] [PubMed] [Google Scholar]
- 31. Cummings KC, 3rd, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth 2011;107:446–53. [DOI] [PubMed] [Google Scholar]
- 32. Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: A prospective, randomized, placebo-controlled study. Br J Anaesth 2013;111:445–52. [DOI] [PubMed] [Google Scholar]
- 33. Kawanishi R, Yamamoto K, Tobetto Y, et al. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: A prospective randomized trial. Local Reg Anesth 2014;7:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dobkin AB, Esposito B, Philbin C. Double-blind evaluation of buprenorphine hydrochloride for post-operative pain. Can Anaesth Soc J 1977;24:195–202. [DOI] [PubMed] [Google Scholar]
- 35. Williams BA, Schott NJ, Mangione MP, Ibinson JW. Perineural dexamethasone and multimodal perineural analgesia: How much is too much? Anesth Analg 2014;118:912–4. [DOI] [PubMed] [Google Scholar]
- 36. Williams BA, Hough KA, Tsui BY, et al. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med 2011;36:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams BA, Butt MT, Zeller JR, Coffee S, Pippi MA. Multimodal perineural analgesia with combined bupivacaine-clonidine-buprenorphine-dexamethasone: Safe in vivo and chemically compatible in solution. Pain Med 2015;16:186–98. [DOI] [PubMed] [Google Scholar]
- 38. Williams BA, Bottegal MT, Kentor ML, Irrgang JJ, Williams JP. Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: Retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med 2007;32:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]