Abstract
Objective. To define clinical phenotypes of postamputation pain and identify markers of risk for the development of chronic pain.
Design. Cross-sectional study of military service members enrolled 3-18 months after traumatic amputation injury.
Setting. Military Medical Center
Subjects. 124 recent active duty military service members
Methods. Study subjects completed multiple pain and psychometric questionnaires to assess the qualities of phantom and residual limb pain. Medical records were reviewed to determine the presence/absence of a regional catheter near the time of injury. Subtypes of residual limb pain (somatic, neuroma, and complex regional pain syndrome) were additionally analyzed and associated with clinical risk factors.
Results. A majority of enrolled patients (64.5%) reported clinically significant pain (pain score ≥3 averaged over previous week). 61% experienced residual limb pain and 58% experienced phantom pain. When analysis of pain subtypes was performed in those with residual limb pain, we found evidence of a sensitized neuroma in 48.7%, somatic pain in 40.8%, and complex regional pain syndrome in 19.7% of individuals. The presence of clinically significant neuropathic residual limb pain was associated with symptoms of PTSD and depression. Neuropathic pain of any severity was associated with symptoms of all four assessed clinical risk factors: depression, PTSD, catastrophizing, and the absence of regional analgesia catheter.
Conclusions. Most military service members in this cohort suffered both phantom and residual limb pain following amputation. Neuroma was a common cause of neuropathic pain in this group. Associated risk factors for significant neuropathic pain included PTSD and depression. PTSD, depression, catastrophizing, and the absence of a regional analgesia catheter were associated with neuropathic pain of any severity.
Keywords: Amputation, Nerve Injury, Residual Limb Pain, Phantom Pain, Neuroma, Regional Catheter, Depression, Catastrophizing, Post-traumatic Stress Disorder
Introduction
Chronic pain after trauma and surgery has a direct and lasting impact on the quality of life of thousands of injured military service members, including the over 1,573 individuals suffering battlefield amputation injury since 2001 [1]. In addition, more than 100,000 patients undergo amputation each year in the United States due to trauma or medical conditions including diabetes and peripheral vascular disease [2,3]. A significant percentage of these patients will suffer long-term morbidity from chronic pain with an incidence ranging from 50 to 80% [4,5].
The treatment of persistent, residual limb or phantom limb pain with existing analgesics has proven difficult [6] as is true for most types of persistent neuropathic pain. This failure has produced growing interest in identifying strategies to prevent chronic postsurgical pain (CPSP) of all types [7]. Unfortunately, most prospective, blinded and randomized trials using regional analgesia or perioperative pharmacologic analgesics have failed to show significant efficacy [8,9]. Promisingly, however, two recent Cochrane systematic reviews concluded that, when these trials are combined in a meta-analysis, some types of perioperative regional analgesia and pharmacologic analgesic therapy may indeed reduce the risk of chronic pain after surgery. Nonetheless, the overall efficacy was described as “modest” [10,11]. The reasons behind the “modest” and equivocal ability of these preventive strategies to reduce the incidence of CPSP are unknown. Several contributing factors have been considered including inadequate classification of chronic pain syndromes and the inability to identify patients most at risk of CPSP [12–14].
The importance of systematic evaluation of symptoms and signs in pain medicine has been highlighted in recent years [15]. Diagnostic enhancements have led to improvements in the treatment of medical illness such as leukemia and lymphoma [16], and granular analysis of disease subtypes is increasingly believed to be pivotal if we are to develop personalized pain therapies [17,18]. Though methods for clinical diagnosis in neuropathic pain have improved [19,20], significant limitations continue to exist, especially in the diagnostic classification of postamputation pain subtypes. Although neuroma is frequently observed and complex regional pain syndrome has been described after amputation, these separate entities are often lumped together as one diagnostic entity [21,22]. These separate subtypes likely result from distinct pathophysiologic mechanisms, may be associated with unique biomarkers and likely require unique approaches to treatment and prevention. For instance, a patient with a painful residual limb secondary to infection, heterotopic ossification or a poorly fitting prosthesis will require a significantly different therapy than a patient with a sensitized neuroma.
Identifying effective preventive therapies for amputation and surgical nerve injury requires an understanding of predisposing clinical factors [2,23,24]. Studies attempting to identify psychosocial variables as risk factors or correlates of pain have become more numerous in the past decade, but results have been inconsistent [25]. Nonetheless, there have been studies associating psychological conditions such as post-traumatic stress disorder (PTSD), depression, and pain catastrophizing with chronic pain [26–28]. The identification of clinical factors that predispose patients to CPSP is critical for adequate patient risk stratification and will increase the power of future trials to identify preventive therapies.
We report here results from VIPER (Veterans Integrated Pain Evaluation Research), a study of 124 recent active military traumatic amputees designed to discriminate pain phenotypes, evaluate clinical risk factors, discover biomarkers of chronic pain, and identify novel pain pathways. Subjects were enrolled three to 18 months after amputation, and extensive questionnaires were completed, assessing demographic data, surgical procedure, and screening for post-traumatic stress disorder (PTSD), depression, and pain catastrophizing. In order to more precisely categorize the distinct pain conditions occurring after amputation we used a diagnostic algorithm, the Duke postamputation pain algorithm (DUKE-PAPA), modified from our original algorithm [29], to differentiate the subtypes of postamputation pain. Using this phenotypic data and algorithm-based grouping of patients into diagnostic subgroups, we attempted to determine whether PTSD, depression, and pain catastrophizing were associated with chronic pain or the predetermined pain subtypes following amputation.
Given the equivocal literature on pain prevention with peripheral nerve catheters [30,31], we additionally evaluated the incidence of chronic pain after amputation in patients with and without peripheral nerve catheters placed soon after the time of injury.
Methods
Study Design
The Defense and Veterans Center for Integrative Pain Management (DVCIPM – DVCIPM.org) obtained Institutional Review Board approval at Walter Reed National Military Medical Center (WRNMMC) to enroll 124 recent active duty military post-traumatic amputees receiving care 3–18 months after amputation. We implemented a 3-month minimum given that chronic pain is usually defined as lasting 3 months or greater in most study populations. An 18-month cutoff was employed as the time-course of recovery/rehabilitation at WRNMMC is usually complete by this point in time, and few military service members would be present and eligible for enrollment after this period. Multiple pain and psychometric questionnaires were administered to each individual and completed with minimal guidance by a DVCIPM healthcare provider.
Subjects were included if they were a military health care system beneficiary aged 18 years or older and undergoing treatment at WRNMMC with a diagnosis of postinjury amputation of all or part of one limb. Amputation injury must also have occurred between 3 and 18 months prior to enrollment.
Patients were excluded if they were afflicted with severe traumatic brain injury, significant cognitive deficits, substantial hearing loss, spinal cord injury with permanent or persistent deficits, ongoing tissue damage that might cause pain, infection, heterotrophic ossification, poorly fitting prosthesis, or hip disarticulation.
Data Collection
From November 2011 to July 2013, demographic data were collected and multiple previously validated questionnaires were administered to quantify and qualitatively stratify each subject's pain. We utilized the Brief Pain Inventory (BPI) Short Form for assessment of pain and function [32], the Defense Veterans Pain Rating Scale (DVPRS) for additional symptomatic evaluation given its validity in this patient population [33], the Self-Report Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) to discriminate between nociceptive and neuropathic pain [34], Post Traumatic Stress Disorder Checklist-Military (PCL-M) normalized for a military population to screen for PTSD [35], Pain Catastrophizing Scale (PCS) to assess catastrophizing and its subscales of rumination, magnification and helplessness [36], Pain Health Questionnaire-9 (PHQ-9) to detect symptoms of depression [37], and Complex Regional Pain Syndrome (CRPS) Questionnaire using the Budapest Clinical Criteria. In the absence of a validated assessment tool for the detection of CRPS in an amputation population, we chose to apply the “Budapest Criteria” that is commonly used in existing-limb populations. Given that physical exam findings and therefore sensitivity of detection of CRPS in a missing-limb population might be diminished, we utilized the more sensitive “clinical” criteria as our detection tool [38]. Phantom sensation, phantom pain, residual limb pain and the presence of a sensitized neuroma were assessed through questions from the Groningen Questionnaire Problems After Arm/Leg Amputation [4] (Table 1).
Table 1.
Asssessment tools
| Symptom | Assessment Tool |
|---|---|
| Pain and function | Brief Pain Inventory (BPI) Short Form |
| Pain and function | Defense Veterans Pain Rating Scale (DVPRS), |
| Neuropathic symptoms | Self-Report Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS), |
| Post-traumatic stress disorder | Post-traumatic Stress Disorder Military Checklist (PCL-M) |
| Pain Catastrophizing | Pain Catastrophizing Scale (PCS), |
| Depression | Pain Health Questionnaire-9 (PHQ-9) |
| Complex Regional Pain Syndrome | Budapest Complex Regional Pain Syndrome (CRPS) Questionnaire |
| Phantom sensation, phantom pain, residual limb pain, neuroma | Questions from Groningen Questionnaire Problems After Leg Arm Amputation |
A physical exam was also performed at the time of assessment, documenting allodynia/hyperalgesia, presence/absence of a sensitized neuroma (Tinel's sign), temperature/color asymmetry, wound status, edema and skin/hair changes outside of the injury. The use of regional anesthesia catheter infusion was determined through a comprehensive review of each patient's chart from the time of traumatic injury and through the period of subsequent care. Although we were able to confirm catheter placement through review of records, many of these catheters were placed under battlefield conditions, and we were not able to always confirm the exact location of catheter placement. We therefore do not report on this finding.
Pain and Assessment Tool Interpretation
We defined clinically significant pain (cases) as an average pain score over the past week of greater than or equal to 3/10 on a numeric rating scale (NRS). This cutoff value has been used in previous pain literature to distinguish “mild” pain from “moderate/severe” pain, and has been used in prior studies of postamputation pain [8,38,39]. Those patients with clinically significant pain were further adjudicated into pain subtypes. Those subjects with pain less than 3/10 but greater than 0/10 completed all the data collection questionnaires, but subtypes were not analyzed. This case/control methodology was chosen to facilitate our parallel analyses where we are using case status to correlate with genetic polymorphisms and biomarkers of risk in susceptible individuals.
To examine potential, associated psychological comorbidities we defined diagnostic cutoff values for the assessment instruments that we utilized. Pain catastrophizing was defined as a PCS score of 15 or greater based on the work of Sullivan et al. [3], and consistent with other studies of risk factors for chronic postsurgical pain [40]. We defined possible depression as a PHQ-9 score of 10 or greater based on a meta-analysis by Manea et al. who found PHQ-9 scores between 8 and 11 were able to detect depression with reasonable sensitivity and specificity [41]. The presence of possible PTSD was defined by a PCL-M score of 50 or greater, normalized for a military population [42].
Pain Subtype Adjudication
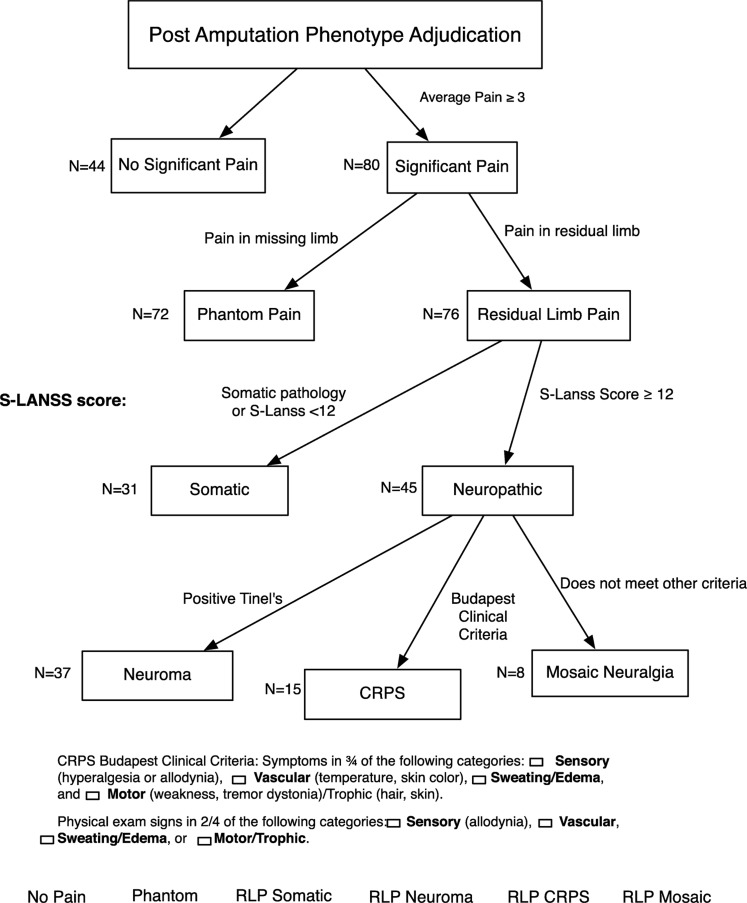
Between May 15 2012 and June 16 2014, three or more members of the VIPER Adjudication Committee (composed of Drs. Buchheit, Vandeven, Buckenmaier, Hsia, Macleod, and Shaw) met on six occasions to review data and adjudicate the study subjects into pain subtype using the previously described Duke-PAPA algorithm (Figure 1). This algorithm systematically characterizes postamputation pain into multiple discrete phenototypes that include phantom pain and subtypes of residual limb pain such as somatic, neuroma, complex regional pain syndrome, and mosaic (not otherwise specified) [29].
Figure 1.
Phenotype adjudication algorithm.
The adjudication process started with classifying study subjects into cases and controls. At the time of assessment, if the patient experienced an average pain score over the past week of greater than or equal to 3/10 on a numeric rating scale, they were considered a “case” and were therefore adjudicated into pain subtypes. The presence/absence of phantom and residual limb pain were then assessed using a subset of questions from the Groningen assessment [4]. Phantom pain was distinguished from nonpainful phantom sensation, and defined by a positive response to the following question: “do you experience pain in any part of the amputated arm and/or leg?”. Residual limb pain was defined by a positive response to the question: “do you have any pain in the stump?”. If residual limb pain was present, it was then classified as “somatic” or “neuropathic” using the S-LANSS questionnaire with a cutoff value of 12 or greater defining pain as neuropathic. Those with neuropathic pain were then further characterized as having either neuroma or complex regional pain syndrome (CRPS) using both questionnaire and physical exam data. Pain elicited by tapping pressure applied at a specific point (Tinel's sign) or patient reported pain with focal point pressure was defined as neuroma. Budapest Clinical Criteria were used to define CRPS, requiring three out of four clinical symptoms and two out of four physical exam findings. If patients had an SLANSS score of 12 or greater but did not meet diagnostic criteria for CRPS or neuroma, the patient was classified as experiencing a not otherwise specified “mosaic neuralgia.”
All patients with pain greater than 0/10 were evaluated with the assessment tools noted above. Those with pain less than 3/10 were considered part of the “control” cohort for future biomarker analysis. These subjects were not adjudicated into pain subtypes but were included along with the rest of the study sample in an exploratory analysis examining risk factors for postamputation pain. Pain was considered “severe” if NRS pain score >5.
Statistical Analysis
All questionnaire data were entered into the Research Electronic Data Capture (REDCap)TM database and adjudication data were entered into a spreadsheet that was stored on the Duke secure servers only accessible by research staff. Data analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and R (version 3.1.2) statistical software programs. For the patient demographic data, the Wilcoxon rank sum test was used for the analysis of continuous variables and the Pearson's chi-square test was used to analyze categorical variables. For the clinical risk factors and chronic pain subtypes, odds ratios with 95% confidence intervals were calculated for each categorical variable and statistical significance was calculated with Fisher's exact test or the likelihood ratio chi-squared test. P values from Fisher's exact test are presented where appropriate (any expected cell counts <5 in contingency tables). A P value <0.05 was considered significant for all analyses. No adjustments were made for multiple testing in this exploratory investigation.
Results
Patient Demographics and Pain Incidence
The demographic characteristics of patients defined as cases versus those defined as controls are reported in Table 2. There were no significant differences in age, body mass index, time as amputation, ethnicity, amputation site or presence of regional analgesia catheter around the time of amputation between patients who had clinically significant pain at the time of enrollment compared to those who did not. When we analyzed the demographic characteristics of those who received or did not receive a regional analgesia catheter, we observed no difference.
Table 2.
Patient demographic data
| Control (N = 44) | Case (N = 80) | ||
|---|---|---|---|
| Demographic | Mean (SD) | Mean (SD) | P value |
| Age | 25.4 (5) | 26.9 (6.8) | 0.1562* |
| Body Mass Index | 26.1 (3.6) | 26.6 (3) | 0.2345* |
| Time since amputation (months) | 8.4 (3.9) | 8.9 (5.2) | 0.9457* |
| N (%) | N (%) | ||
| Male | 44 (100) | 78(97.5) | 0.9577 ˆ |
| Regional catheter | 22 (50) | 31 (38.8) | 0.2260 ˆ |
| Ethnicity | N (%) | N (%) | 0.5700 ˆ |
| American Indian/Alaska Native | 0 (0) | 2 (2.5) | |
| Asian | 2 (4.5) | 1 (1.3) | |
| Native Hawaiian or Other Pacific Islander | 0 (0) | 1 (1.3) | |
| Black or African American | 3 (6.8) | 5 (86.3 | |
| White | 39 (88.6) | 70 (88.6) | |
| Amputation Site | N (%) | N (%) | 0.8478 ˆ |
| Left upper extremity | 2 (4.5) | 5 (6.3) | |
| Right upper extremity | 1 (2.3) | 3 (3.8) | |
| Left lower extremity | 22 (50) | 34 (42.5) | |
| Right lower extremity | 19 (43.2) | 38 (47.5) |
*P value generated from Wilcoxon Rank-Sum test. ˆP value generated from chi-squared test.
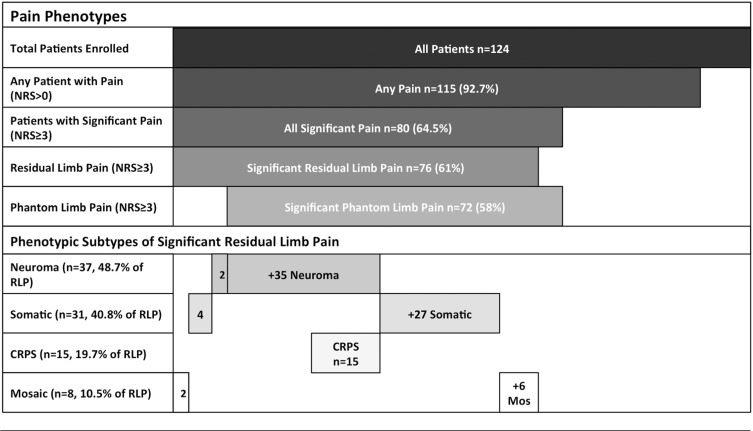
A majority of enrolled patients (80/124 or 64.5%) reported clinically significant pain scores (pain score ≥3 averaged over the previous week, Table 3) while 17% of patients reported experiencing severe pain (pain score > 5). The distribution of patients noting clinically significant pain and severe pain in this cohort is consistent with previously published studies of chronic pain after amputation [4,5].
Table 3.
Distribution of pain subtypes
|
Subtypes of Chronic Postamputation Pain
Of the 80 subjects who described significant postamputation pain (NRS ≥3/10), the majority experienced both residual limb pain (76/80) and phantom limb pain (72/80) (Table 3). Analysis of significant residual limb pain subtypes revealed that 37/76 (48.7%) demonstrated evidence of a sensitized neuroma. Symptoms and exam evidence of complex regional pain syndrome was observed in 15/76 (19.7%). It was notable that all subjects with CRPS also had evidence of a symptomatic neuroma. Somatic pain was identified in 31/76 patients (40.8%) with residual limb pain. Only eight patients (10.5%) with neuropathic residual limb pain could not be classified as having either neuroma or CRPS and received the mosaic neuralgia classification.
Association Between Clinical Risk Factors and Subtypes of Chronic Postamputation Pain
Each enrolled patient completed PHQ-9, PCL-M, and PCS questionnaires assessing the presence of depression, post-traumatic stress disorder (PTSD) and pain catastrophizing, respectively. Odds ratios were calculated for these clinical risk factors in association with chronic postamputation pain (see Table 4).
Table 4.
Clinical risk factors for development of postamputation chronic pain subtypes
| Chronic Pain (cases) N = 80 | Phantom Pain (cases) N = 72 | RLP (cases) N = 76 | RLP-Somatic (cases) N = 31 | RLP-Neuro pathic (cases) N = 45 | RLP-Neuroma (cases) N = 37 | RLP-CRPS (cases) N = 15 | RLP-Mosaic (cases) N = 8 | Neuropathic Pain (All) N = 61 | |
|---|---|---|---|---|---|---|---|---|---|
| OR (CI) | OR (CI) | OR (CI) | OR (CI) | OR (CI) | OR (CI) | OR (CI) | OR (CI) | OR (CI) | |
| [P] | [P] | [P] | [P] | [P] | [P] | [P] | [P] | [P] | |
| Clinical Factor | |||||||||
| PCS | 13.39 (1.73−103.87) | 6.20 (0.34–112.49) | 3.05 (0.16–59.29) | 0.48 (0.15–1.50) | 2.71 (0.87–8.45) | 2.47 (0.85–7.14) | 1.82 (0.53–6.20) | 1.08 (0.20–5.85) | 3.78 (1.28–11.18) |
| [P value] | [0.0016]* | [0.1876] | [0.5676] | [0.194] | [0.0735] | [0.0899] | [0.3474] | [1.0000] | [0.0103]* |
| PCL-M | 10.08 (1.28–79.14) | 1.72 (0.20–15.14) | 2.31 (0.12–45.15) | 0.19 (0.04–0.89) | 6.92 (1.44–33.17) | 6.67 (1.71–26.04) | 7.13 (1.98–25.70) | 0.58 (0.07–5.12) | 9.28 (2.01–42.88) |
| [P value] | [0.0099]* | [1.0000] | [1.0000] | [0.0373]* | [0.0088]* | [0.0038]* | [0.0029]* | [1.0000] | [0.0010]* |
| PHQ-9 | 2.40 (0.94–6.13) | 9.13 (0.51–164.62) | 4.46 (0.23–86.00) | 0.38 (0.13–1.09) | 3.53 (1.22–10.19) | 3.72 (1.36–10.15) | 4.59 (1.42–14.91) | 0.71 (0.13–3.79) | 4.46 (1.81–10.99) |
| [P value] | [0.055] | [0.0520] | [0.3036] | [0.0624] | [ 0.0142]* | [0.0081]* | [0.010]* | [1.0000] | [0.0006]* |
| Regional Catheter | 0.63 (0.30–1.33) | 5.00 (0.58–42.80) | 1.96 (0.19–19.70) | 1.24 (0.50–3.12) | 0.91 (0.37–2.25) | 1.42 (0.58–3.51) | 2.09 (0.67–6.49) | 0.20 (0.02–1.71) | 0.44 (0.21–0.92) |
| [P value] | [0.227] | [0.142] | [1.0000] | [0.6423] | [0.8397] | [0.4443] | [0.2034] | [0.142] | [0.0269]* |
The odds-ratios for development of chronic pain (all types), neuropathic pain, phantom limb pain, all types of residual limb pain, somatic residual limb pain, residual limb pain from presence of neuroma, complex regional pain syndrome, mosaic neuropathic residual limb pain and all neuropathic residual limb pain are reported above with P values in brackets. Factors associated with significant risk of a specific pain subtype (P value <0.05) are marked with an asterisk and italicized in bold. The data presented in the final column include total patients with neuropathic pain regardless of case or control status.
When we analyzed subjects with clinically significant residual limb pain (RLP) (NRS ≥3/10, N = 76), without regard to subtype, no associations with clinical risk factors were observed. However, when neuropathic RLP was analyzed (N = 45), significant associations were noted with PTSD and depression. When we further discriminated subtypes of neuropathic RLP, we found that the RLP subtypes of neuroma (N = 37) and CRPS (N = 15) were additionally associated with PTSD and depression. Non-neuropathic residual limb pain (somatic RLP) was negatively associated with the presence of PTSD only.
Exploratory Analysis of the Association Between Clinical Risk Factors and Postamputation Pain
Because there were multiple patients with some degree of neuropathic pain in the control group that we did not want to completely exclude, we conducted an exploratory analysis examining risk factors for postamputation pain in all subjects. When all patients (NRS>0/10) with neuropathic symptoms (SLANSS ≥12) were included, we found neuropathic pain to be significantly associated with all four clinical risk factors (catastrophizing, PTSD, depression, and regional analgesia catheter) (see right hand column of Table 4). Thus, for the study sample as a whole, patients who met criteria for PTSD, who scored higher on depression or pain catastrophizing, or had the absence of a regional analgesia catheter were much more likely to report higher levels of neuropathic pain.
Additional analysis of opioid pain medication (oxycodone, morphine, hydrocodone, methadone, and fentanyl) use was performed. There were 30 study subjects that were taking an opioid at the time of assessment. As one might anticipate, patients who were cases (average pain ≥3) were more likely to use opioids (27/30, 90%) than those who were controls (3/30, 10%).
Discussion
The Veterans Integrated Pain Evaluation Research (VIPER) study included young, active duty soldiers who suffered traumatic battlefield amputation within 3–18 months of enrollment. The VIPER study was designed to distinguish pain subtypes, analyze associations between pain and a number of clinical risk factors, and discover novel biomarkers unique to each pain subtype. Relative youth and lack of medical comorbidity makes this patient population unique. This relatively homogeneous patient population may also provide a less noisy background when comparing genetic, epigenetic, and proteomic signatures between patients with and without chronic residual limb pain as we attempt to identify biomarkers of pain susceptibility in the future.
Data on pain characteristics were collected using four distinct pain evaluation scales for each enrolled patient including a visual analogue scale (VAS) score, S-LANSS score for evaluation of neuropathic pain, BPI, and DVPRS. DVPRS and VAS are well-validated pain measurement tools which record pain.
Using the average pain score over the past week as our method to categorize patients as either control (<3) or case (≥3), we found that the overall incidence of significant chronic pain after amputation to be 65%, agreeing well with previously published data [2,43–45]. We additionally observed that 90% of patients receiving opioid analgesics at the time of study enrollment described significant pain (≥3), and therefore, were analyzed as cases. Given that only 3/10 (10%) of those receiving opioid medications were part of the control group (average pain <3), these medications appear to have minimally affected the case/control status of study subjects.
Pain phenotype adjudication using the Duke-PAPA algorithm [29] revealed that the majority of amputees with significant chronic pain were experiencing both phantom limb pain sensations and residual limb pain (68 out of 80). Almost 60% of patients with residual limb pain were determined to have chronic neuropathic pain (45 out of 76) as defined by the S-LANSS scoring system while the remaining 40% were found to have somatic pain in the residual limb.
Although the coexistence of phantom and RLP has been noted previously, it has not been reported to the degree observed in this cohort [5,43]. Literature in recent decades has increasingly supported central causes of phantom pain as a dominant paradigm, with decreasing emphasis on the role of peripheral neurologic input in maintaining phantom limb pain [46,47]. However, the peripheral contributions to phantom limb pain have again been questioned with the publication of successful treatment of phantom pain with intraforaminal blockade of the dorsal root ganglion [48]. The strong diagnostic coexistence of sensitized neuromas, residual limb pain, and phantom pain in this study further supports a likely important interplay between these pathologic processes. However, we must also exercise caution, as causation cannot be implied from this observational study.
It is also notable that there is a high prevalence of neuromas in this postamputation population (48.7% of those with residual limb pain had evidence of a neuroma). This prevalence is higher than previously reported incidences [5,49] and further strengthens the importance of neuroma in the comprehensive treatment of the patient with postamputation pain.
Though previous epidemiological analysis of CRPS patients showed that 24% of CRPS patients had surgery as the inciting injury [50], and CRPS has been observed after amputation [6,51] it is unclear how many postsurgical patients develop CRPS after amputation or other surgical procedures involving injury to a major peripheral nerve. Using the Budapest Criteria, we found 15 out of 76 (19.7%) patients with chronic residual limb pain were defined as having CRPS in this cohort. Although significantly less common than neuroma in our study, we believe it is important to distinguish this diagnostic group given the therapeutic treatment implications.
In the past 10 years, multiple chronic postsurgical pain studies have also collected data on patient psychosocial variables as potential risk factors for development of pain [25,52]. Many of these have shown a positive correlation between the presence of CPSP and depression, anxiety, PTSD, and pain catastrophizing [53,54]. In patients experiencing chronic postmastectomy pain, for example, anxiety and pain catastrophizing were significantly associated with the presence of pain [55]. There are very few studies, however, specifically looking at an association between the presence of pain and PTSD, depression or pain catastrophizing in amputees [44,56]. Additionally, it is unknown if there are stronger associations with any of the subtypes of postamputation pain.
In our study, we found a significant association between symptoms of catastrophizing and PTSD and clinically significant (NRS ≥ 3/10) chronic postamputation pain. It is notable that when we analyzed risk factors for cases of residual limb pain without regard to subtype discrimination, we found no significant associations. However, when we examined the risk factor associations for the defined subtypes of residual limb pain, we found significant associations between PTSD and depression and all three subtypes of neuropathic RLP (RLP neuropathic, RLP neuroma, RLP CRPS). These findings imply that risk factors (and therefore potentially treatments) do not affect all pain conditions equally. Interestingly, in an exploratory analysis that included all study patients (including those with NRS<3) we found that all four risk factors were related to neuropathic pain. Taken together, these findings suggest that risk factors such as psychosocial variables and regional analgesia catheter placement may play a role in postamputation pain.
As all data were collected at one time point months after amputation, it is not possible to determine whether these psychosocial factors were present before surgery (acting as risk factors for development of pain) or if they developed subsequent to injury as a result of the traumatic experience. There is some evidence that pre-operative depression and anxiety are associated with a higher incidence of pain after surgery. Brander et al. assessed pre-operative depression and anxiety in patients about to undergo total knee replacement and found a significant increase in chronic pain incidence at one year in patients with high Beck-Depression Inventory and State-Trait Anxiety Index scores [57]. A number of other studies have assessed psychosocial variables as predictive risk factors but most have been in patients undergoing lumbar spine surgery [58,59]. Both total knee arthroplasty patients and lumbar spine surgery patients often have a long history of chronic pain before surgery so it is unclear whether these findings can be applied to CPSP that occurs in patients without pre-existing pain conditions.
There have been multiple previous attempts to use perioperative regional and neuraxial analgesia to prevent chronic postamputation pain. After some initial encouraging results [60–62] a number of small randomized trials of short term regional anesthesia did not show any effect on chronic phantom or residual pain incidence after amputation [8,31,63]. It is unclear if this lack of effect was due to underpowered studies, incomplete clinical pain syndrome classification, or the technique and duration of therapy. In our unique patient cohort, consisting of young men and women experiencing limb trauma and amputation, we found that patients who had a documented placement of peripheral nerve catheters near the time of traumatic injury were less likely to have neuropathic pain symptoms in the affected limb or stump. The presence of a peripheral nerve catheter at the time of injury or amputation was determined through an extensive review of each patient's military medical record. It is also interesting to note that in this cohort, the median duration of catheter treatment was 10 days with a maximum catheter duration of 29 days, considerably longer than many other studies of regional analgesia catheters [64]. This extended duration of catheter infusion may have a beneficial effect as has been previously demonstrated by Borghi et al. [65]. As understanding of the extended inflammatory response leading to nociceptor sensitization advances [66–71], and the importance of preventing abnormal nociceptor signaling throughout the course of this inflammatory response becomes more clear [72–76], it is intriguing that patients with a prolonged exposure to local anesthetics around a peripheral nerve may have a decreased incidence of neuropathic pain after amputation.
There are multiple limitations to this research. First of all, our small sample size (124 subjects) limits the analysis and conclusions, particularly in regards to the correlations between neuropathic pain subtypes, clinical risk factors, and the use of a regional analgesia catheter. Answering these questions would require either a larger study or a more focused clinical question. We also have limitations with our regional analgesia catheter use given the lack of more granular data on catheter location and drug doses, although it is reasonable to assume that the majority of these catheters were placed in proximity to the target nerves under ultrasound guidance. These are inherent challenges in the collection of information in the setting of active military conflict. The undefined role of traumatic brain injury (TBI) in this research cohort might also be considered a limitation of this study. Although severe TBI was an exclusion criterion, many of the service members most likely experienced mild to moderate TBI, affecting the experience of pain. A granular description of all comorbidities would have been ideal to capture in this population. We were acutely aware throughout the course of the study to avoid additional disruption in the lives of the service members and the negative effects of questionnaire burden. We therefore gathered only the most critical data necessary to accomplish the aims of this research. It is reasonable to assume that the majority of those injured experienced a mild to moderate TBI, maintaining a relatively homogeneous sample.
Additional limitations would include the lack of pre-injury pain assessment and longitudinal follow-up of study subjects. We do not know if any of the study subjects had pre-existing chronic pain conditions that might affect outcomes following traumatic injury. It is also unclear if pain severity, quality, and phenotype might evolve over the subsequent months in these military service members, especially in those enrolled earlier in their recovery (3–6 months).
Conclusions
In conclusion, the discrimination of pain subtypes in this observational study of young, healthy traumatic amputees reveals a similar prevalence of postamputation pain with current literature [21], but a significant phenotypic overlap of both phantom and residual limb pain. There is additionally a prominent representation of sensitized neuromas as a cause of chronic residual limb pain, identifying this condition as a potential target for future therapies. Utilizing the Duke-PAPA diagnostic algorithm, we were able to classify patients into known categories of pain syndromes including phantom and residual limb pain, and further into RLP subtypes such as somatic, neuroma, complex regional pain syndrome, and mosaic neuralgia. Furthermore, we were able to demonstrate a significant association between symptoms of PTSD and depression and all subtypes of neuropathic RLP. Though presence of a peripheral nerve catheter did not reduce the number of patients with significant neuropathic RLP, it did reduce the incidence of neuropathic pain of all severities. These risk factors did not associate as strongly with somatic pain syndromes, consistent with a distinct pathophysiology. From this work it is unclear whether these clinical factors are predictive of future chronic pain development. Future longitudinal studies are needed to examine this question, and an ideal intervention trial would simultaneously treat these medical and psychological risk factors to determine if the prevalence of chronic postinjury pain can be reduced.
Funding sources: This work was supported by the Department of Defense, Congressionally Directed Medical Research Programs (DM102142), Dr. Andrew Shaw, PI. Dr. Buchheit has additional funding from Department of Defense: Congressionally Directed Medical Research Program (PT1100575). Dr. Van de Ven has funding from T32: 5T32GM008600-19.
Disclosure and conflict of interest: Neither I, nor any of the co-authors have any conflicts of interest. We are not involved in any consulting, speaking, or financial capacities for any of the pharmaceutical agents or techniques named in the report. The manuscript was prepared in its entirety by the authors noted above.
Acknowledgments
The contents of this manuscript do not reflect the views of the Department of Defense, the Department of Veterans Affairs or the United States Government.
We would like to thank Kyung Nancy Kwon and the staff of DVCIPM for their research acumen and their tireless dedication to care of the wounded warrior. We also thank Mary Kirkley at Duke for her organizational skills and hard work, without which the research would not have been possible. Finally, we sincerely thank the wounded service members who gave to country and then to research in the hopes of improving the lives of those wounded in the future.
References
- 1. Fischer H. U.S. Military Casualty Statistics: Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom, Congressional Research Service Report for Congress, February 5, 2013;1–12.
- 2. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006;367(9522):1618–25. [DOI] [PubMed] [Google Scholar]
- 3. Van de Ven TJ, John Hsia H-L. Causes and prevention of chronic postsurgical pain. Curr Opin Crit Care 2012;18(4):366–71. [DOI] [PubMed] [Google Scholar]
- 4. Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: An epidemiological study. Pain 2000;87(1):33–41. [DOI] [PubMed] [Google Scholar]
- 5. Reiber GE, McFarland LV, Hubbard S, et al. Servicemembers and veterans with major traumatic limb loss from Vietnam war and OIF/OEF conflicts: Survey methods, participants, and summary findings. J Rehabil Res Dev. 2010; 47:275–97. [DOI] [PubMed] [Google Scholar]
- 6. Wolff A, Vanduynhoven E, van Kleef M, Huygen F, Pope JE, Mekhail N. 21. Phantom pain. Pain Pract 2011;11(4):403–13. [DOI] [PubMed] [Google Scholar]
- 7. Cohen SP, Raja SN. Prevention of chronic postsurgical pain: The ongoing search for the holy grail of anesthesiology. Anesthesiology 2013;118(2):241–3. [DOI] [PubMed] [Google Scholar]
- 8. Nikolajsen L, Ilkjær S, Christensen JH, Krøner K. Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet 1997;350:1353–7. [DOI] [PubMed] [Google Scholar]
- 9. Ypsilantis E, Tang TY. Pre-emptive analgesia for chronic limb pain after amputation for peripheral vascular disease: A systematic review. Ann Vasc Surg 2010;24(8):1139–46. [DOI] [PubMed] [Google Scholar]
- 10. Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: A Cochrane systematic review and meta-analysis. Br J Anaesth 2013;111(5):711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No.: CD008307. Chichester, UK: Wiley; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, et al. The ACTTION-American Pain Society Pain Taxonomy (AAPT): An evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain 2014;15(3):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dworkin RH, Turk DC. Accelerating the development of improved analgesic treatments: The ACTION public-private partnership. Pain Med 2011;12Suppl 3:S109–17. [DOI] [PubMed] [Google Scholar]
- 14. Dworkin RH, Turk DC, Peirce-Sandner S, et al. Assay sensitivity and study features in neuropathic pain trials: An ACTTION meta-analysis. Neurology 2013;81(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1-2):9–19. [DOI] [PubMed] [Google Scholar]
- 16. Cramer P, Hallek M. Hematological cancer in 2011: New therapeutic targets and treatment strategies. Nat Rev Clin Oncol 2012;9(2):72–4. [DOI] [PubMed] [Google Scholar]
- 17. de Jong TD, Vosslamber S, Verweij CL. Moving towards personalized medicine in rheumatoid arthritis. Arthritis Res Ther 2014;16:110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hehn von CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73(4):638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harden RN, Oaklander AL, Burton AW, et al. Complex regional pain syndrome: Practical diagnostic and treatment guidelines, 4th edition. Pain Med 2013;14(2):180–229. [DOI] [PubMed] [Google Scholar]
- 20. Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: Revised 2009. Eur J Neurol 2010;17(8):1010–8. [DOI] [PubMed] [Google Scholar]
- 21. Ehde DM, Czerniecki JM, Smith DG, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil 2000;81(8):1039–44. [DOI] [PubMed] [Google Scholar]
- 22. Wartan SW, Hamann W, Wedley JR, McColl I. Phantom pain and sensation among British veteran amputees. Br J Anaesth 1997;78(6):652–9. [DOI] [PubMed] [Google Scholar]
- 23. Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008;138(1):22–8. [DOI] [PubMed] [Google Scholar]
- 24. Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: Risk factors and protective factors. Exp Rev Neurother 2009;9(5):723–44. [DOI] [PubMed] [Google Scholar]
- 25. Hinrichs-Rocker A, Schulz K, Järvinen I, Lefering R, Simanski C, Neugebauer EAM. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP)—A systematic review. Eur J Pain 2012;13(7):719–30. [DOI] [PubMed] [Google Scholar]
- 26. Arnow BA, Blasey CM, Constantino MJ, et al. Catastrophizing, depression and pain-related disability. Gen Hosp Psychiatry 2011;33(2):150–6. [DOI] [PubMed] [Google Scholar]
- 27. Carroll I, Barelka P, Wang CKM, et al. A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg 2012;115(3):694–702. [DOI] [PubMed] [Google Scholar]
- 28. Magni G, Moreschi C, Rigatti-Luchini S, Merskey H. Prospective study on the relationship between depressive symptoms and chronic musculoskeletal pain. Pain 1994;56(3):289–97. [DOI] [PubMed] [Google Scholar]
- 29. Clarke C, Lindsay DR, Pyati S, Buchheit T. Residual limb pain is not a diagnosis: A proposed algorithm to classify postamputation pain. Clin J Pain 2013;29(6):551–62. [DOI] [PubMed] [Google Scholar]
- 30. Morey TE, Giannoni J, Duncan E, Scarborough MT, Enneking FK. Nerve sheath catheter analgesia after amputation. Clin Orthop Relat Res 2002;(397):281–9. [DOI] [PubMed] [Google Scholar]
- 31. Pinzur MS, Garla PG, Pluth T, Vrbos L. Continuous postoperative infusion of a regional anesthetic after an amputation of the lower extremity. A randomized clinical trial. J Bone Joint Surg Am 1996;78(10):1501–5. [DOI] [PubMed] [Google Scholar]
- 32. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 2004;20(5):309–18. [DOI] [PubMed] [Google Scholar]
- 33. Buckenmaier CC3, Galloway KT, Polomano RC, McDuffie M, Kwon N, Gallagher RM. Preliminary Validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a Military Population. Pain Med 2012;14:110–123. [DOI] [PubMed] [Google Scholar]
- 34. Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: Validation for use in clinical and postal research. J Pain 2005;6(3):149–58. [DOI] [PubMed] [Google Scholar]
- 35. Bliese PD, Wright KM, Adler AB, Cabrera O, Castro CA, Hoge CW. Validating the primary care posttraumatic stress disorder screen and the posttraumatic stress disorder checklist with soldiers returning from combat. J Consult Clin Psychol 2008;76(2):272–81. [DOI] [PubMed] [Google Scholar]
- 36. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: Development and validation. Psychological assessment. Am Psychol Assoc 1995;7:524–32. [Google Scholar]
- 37. Wittkampf K, van Ravesteijn H, Baas K, et al. The accuracy of Patient Health Questionnaire-9 in detecting depression and measuring depression severity in high-risk groups in primary care. Gen Hosp Psychiatry 2009;31(5):451–9. [DOI] [PubMed] [Google Scholar]
- 38. Harden RN, Bruehl S, Perez RS, et al. validation of proposed diagnostic criteria (the “budapest criteria”) for complex regional pain syndrome. Pain 2010;150(2):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain 1997;72(1-2):95–7. [DOI] [PubMed] [Google Scholar]
- 40. Scott W, Wideman TH, Sullivan MJL. Clinically meaningful scores on pain catastrophizing before and after multidisciplinary rehabilitation: A prospective study of individuals with subacute pain after whiplash injury. Clin J Pain 2014;30(3):183–90. [DOI] [PubMed] [Google Scholar]
- 41. Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ 2012;184(3):E191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forbes D, Creamer M, Biddle D. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther 2001;39(8):977–86. [DOI] [PubMed] [Google Scholar]
- 43. Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch Phys Med Rehabil 2005;86(10):1910–9. [DOI] [PubMed] [Google Scholar]
- 44. Perkins ZB, De'Ath HD, Sharp G, Tai NRM. Factors affecting outcome after traumatic limb amputation. Br J Surg 2012;99Suppl 1:75–86. [DOI] [PubMed] [Google Scholar]
- 45. Sherman RA, Sherman CJ, Parker L. Chronic phantom and stump pain among american veterans: Results of a survey. Pain 1984;18(1):83–95. [DOI] [PubMed] [Google Scholar]
- 46. Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci 2001;21(10):3609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: A case of maladaptive CNS plasticity? Nat Rev Neurosci 2006;7(11):873–81. [DOI] [PubMed] [Google Scholar]
- 48. Vaso A, Adahan H-M, Gjika A, et al. Peripheral nervous system origin of phantom limb pain. Pain 2014;155(7):1384–91. [DOI] [PubMed] [Google Scholar]
- 49. Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: Incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain 1985;21(3):267–78. [DOI] [PubMed] [Google Scholar]
- 50. Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: A retrospective chart review of 134 patients. Pain 1999;80:539–44. [DOI] [PubMed] [Google Scholar]
- 51. Bosmans JC, Geertzen JHB, Post WJ, van der Schans CP, Dijkstra PU. Factors associated with phantom limb pain: A 31/2-year prospective study. Clin Rehabil 2010;24(5):444–53. [DOI] [PubMed] [Google Scholar]
- 52. VanDenKerkhof EG, Hopman WM, Goldstein DH, et al. Impact of perioperative pain intensity, pain qualities, and opioid use on chronic pain after surgery. Reg Anesth Pain Med 2012;37:19–27. [DOI] [PubMed] [Google Scholar]
- 53. Tasmuth T, Blomqvist C, Kalso E. Chronic post-treatment symptoms in patients with breast cancer operated in different surgical units. Eur J Surg Oncol 1999;25(1):38–43. [DOI] [PubMed] [Google Scholar]
- 54. Taillefer M-C, Carrier M, Bélisle S, et al. Prevalence, characteristics, and predictors of chronic nonanginal postoperative pain after a cardiac operation: A cross-sectional study. J Thorac Cardiovasc Surg 2006;131(6):1274–80. [DOI] [PubMed] [Google Scholar]
- 55. Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain in breast cancer survivors: Analysis of clinical, demographic, and psychosocial factors. J Pain 2013;14(10):1185–95. [DOI] [PubMed] [Google Scholar]
- 56. Darnall BD, Ephraim P, Wegener ST, et al. Depressive symptoms and mental health service utilization among persons with limb loss: Results of a national survey. Arch Phys Med Rehabil 2005;86(4):650–8. [DOI] [PubMed] [Google Scholar]
- 57. Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: A prospective, observational study. Clin Orthop Relat Res. 2003;(416):27–36. [DOI] [PubMed] [Google Scholar]
- 58. Schade V, Semmer N, Main CJ, Hora J, Boos N. The impact of clinical, morphological, psychosocial and work-related factors on the outcome of lumbar discectomy. Pain 1999;80(1-2):239–49. [DOI] [PubMed] [Google Scholar]
- 59. Iversen MD, Daltroy LH, Fossel AH, Katz JN. The prognostic importance of patient pre-operative expectations of surgery for lumbar spinal stenosis. Patient Educ Couns 1998;34(2):169–78. [DOI] [PubMed] [Google Scholar]
- 60. Fisher A, Meller Y. Continuous postoperative regional analgesia by nerve sheath block for amputation surgery–A pilot study. Anesth Analg 1991;72(3):300–3. [DOI] [PubMed] [Google Scholar]
- 61. Bach S, Noreng MF, Tjéllden NU. Phantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockade. Pain 1988;33(3):297–301. [DOI] [PubMed] [Google Scholar]
- 62. Jahangiri M, Jayatunga AP, Bradley JW, Dark CH. Prevention of phantom pain after major lower limb amputation by epidural infusion of diamorphine, clonidine and bupivacaine. Ann R Coll Surg Engl 1994;76(5):324–6. [PMC free article] [PubMed] [Google Scholar]
- 63. Karanikolas M, Aretha D, Tsolakis I, et al. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: A prospective, randomized, clinical trial. Anesthesiology 2011;114(5):1144–54. [DOI] [PubMed] [Google Scholar]
- 64. Ilfeld BM. Continuous peripheral nerve blocks. Anesth Analg 2011;113(4):904–25. [DOI] [PubMed] [Google Scholar]
- 65. Borghi B, D'Addabbo M, White PF, et al. The use of prolonged peripheral neural blockade after lower extremity amputation: The effect on symptoms associated with phantom limb syndrome. Anesth Analg 2010;111(5):1308–15. [DOI] [PubMed] [Google Scholar]
- 66. Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci 2007;10(11):1361–8. [DOI] [PubMed] [Google Scholar]
- 67. Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011;152(S):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014;13:533-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ji R-R, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain 2013:154;S10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ellis A, Bennett DLH. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 2013;111(1):26–37. [DOI] [PubMed] [Google Scholar]
- 71. Guo W, Wang H, Watanabe M, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci 2007;27(22):6006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009;196(1):115–28. [DOI] [PubMed] [Google Scholar]
- 73. Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain 2006;7:S3–12. [DOI] [PubMed] [Google Scholar]
- 74. Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52(1):77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139(2):267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kawasaki Y, Zhang L, Cheng J-K, Ji R-R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008;28(20):5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]