Abstract
The goal of regenerative medicine is to restore function through therapy at levels such as the gene, cell, tissue, or organ. For many disorders, however, regenerative medicine approaches in isolation may not be optimally effective. Rehabilitation is a promising adjunct therapy given the beneficial impact that physical activity and other training modalities can offer. Accordingly, “regenerative rehabilitation” is an emerging concentration of study, with the specific goal of improving positive functional outcomes by enhancing tissue restoration following injury. This article focuses on one emerging example of regenerative rehabilitation—namely, the integration of clinically based protocols with stem cell technologies following central nervous system injury. For the purposes of this review, the state of stem cell technologies for the central nervous system is summarized, and a rationale for a synergistic benefit of carefully orchestrated rehabilitation protocols in conjunction with cellular therapies is provided. An overview of practical steps to increase the involvement of physical therapy in regenerative rehabilitation research also is provided.
Regenerative medicine can be defined as the use of materials, such as cells or molecules, to ultimately restore function in damaged systems.1 Significant developments in gene therapy,2 cell replacement,3,4 artificial prostheses (eg, indwelling electrodes),5,6 and tissue or organ engineering7,8 are bringing regenerative medicine toward judicious clinical implementation. This advancement reflects a large fiscal investment in relevant technologies. More than $4 billion has been directed at regenerative medicine to date, and spending is projected to exceed $100 billion in the next 5 to 10 years.9 The state of California alone has invested $3 billion over a 10-year period just on stem cell technologies.10
Physical therapy as an adjunct to regenerative approaches is a timely consideration given that a major goal of ongoing research efforts is to investigate therapies that can complement regenerative tools and thereby maximize functional outcomes. Avant-garde examples include movement therapy following hand transplantation,11 augmentation of motor function after spinal cord injury (SCI) via physical therapist–administered intermittent hypoxia,12 and peripheral muscle loading to augment skeletal muscle regeneration following stem cell transplantation.13,14 To continue this progress, important questions must be addressed through systematic investigation regarding the effective combination of rehabilitation with regenerative medicine. Will aggressive rehabilitation following introduction of a biological device (eg, a scaffold or cell) help or hinder regenerative approaches? What is the optimal window of opportunity for delivering and dosing such therapies? Can they be successfully applied to systems with limited regeneration, such as the central nervous system (CNS)? The answers may lie in a disciplinary interface between regenerative medicine and rehabilitation—namely, “regenerative rehabilitation.”15,16
As a complement to recent publications,15–17 the present article will discuss the rationale for rehabilitation as an essential component of and promising partner to regenerative medicine. Our primary purpose is to focus on one of the more challenging goals—CNS repair or regeneration. As daunting as this goal may seem, the nervous system's capability for substantial plasticity18 (ie, physiological or morphological changes that could underlie “regeneration”) is well known. Here, we specifically discuss CNS stem cell therapy as one such intervention. Information on stem cells, CNS-derived stem cells, and challenges to their efficacy will be presented—in particular with regard to transplantation. This information is followed first by evidence that activation of the nervous system can positively affect stem cell biology and then by a discussion of the ongoing integration of rehabilitation with regenerative medicine approaches. We conclude by broadening our focus to proposed action items that support the critical involvement of physical therapy as a major player in the development of regenerative rehabilitation approaches.
Stem Cells for Neural Regeneration
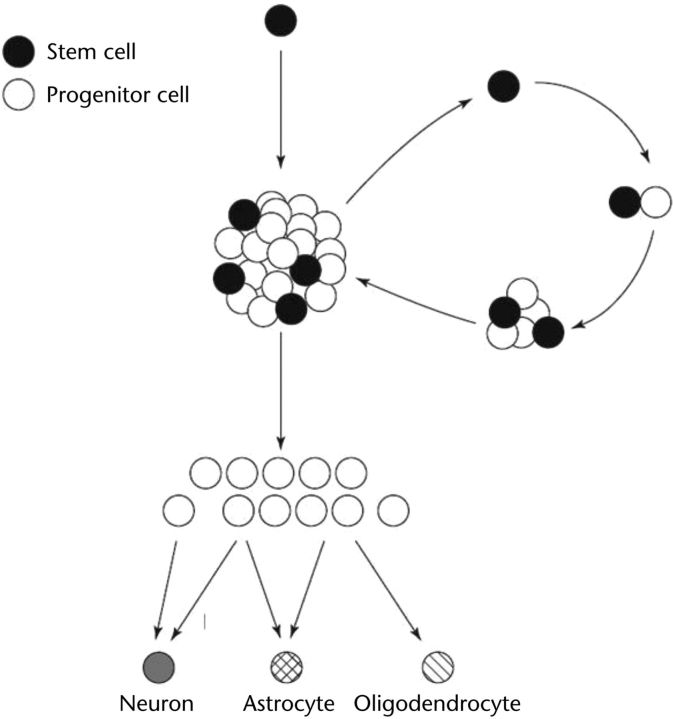
A fundamental goal of CNS regenerative medicine is to circumvent the inherent inability of CNS tissue to regenerate.19,20 Tools used for neural regeneration have been reviewed elsewhere,21 and here we expand on stem cells as one promising choice. The very biology of a stem cell makes it an attractive option for neural regeneration (Fig. 1). The term “stem cell” refers to a cell that can: (1) repopulate a given tissue through division into additional stem cells (self-renewal) and (2) progress or differentiate from an immature cell to a postmitotic, mature cell such as a neuron or astrocyte (multipotency). To further explain self-renewal, stem cells asymmetrically divide, generating one identical daughter stem cell and one daughter progenitor cell. The former daughter stem cell replenishes the original stem cell reservoir, thus “self-renewing” the population. The latter progenitor cell is more “committed” and thus closer to its final differentiative state. To further explain multipotency, daughter progenitors divide at least once and ultimately become the mature cell types of a given tissue (ie, neurons and glia in the CNS).
Figure 1.
General lineage of neural stem cells. Neural stem cells undergo asymmetric division periodically for normal maintenance or in response to injury or other stimuli. One daughter cell (black) is an identical stem cell that renews or repopulates stem cell numbers. The other daughter cell (white) is a more committed progenitor cell that also may divide, one or more times, to create an even greater number of presumptive progeny cells. In the neural system, progeny cells will become postmitotic and mature into the mature central nervous system cell types: neurons, astrocytes, and oligodendrocytes. Reprinted with permission from Elsevier Sciences Ltd from: Weiss S, Reynolds BA, Vescovi AL, et al. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393.
Stem cells in the CNS are robustly present during embryonic development. They also persist in the postnatal and adult CNS, albeit at much lower numbers22 and only in specific locations.23–26 The 2 most well-characterized adult stem cell regions of the CNS are the subgranular zone of the dentate gyrus in the hippocampus and the subventricular zones of the lateral ventricles.22,27–29 The hippocampal lineage produces neuronal progenitors that divide once and integrate into the dentate gyrus, likely contributing to hippocampal homeostasis.30 The subventricular zone lineage divides several times to replenish interneurons in the olfactory bulb to support the chemical sense of smell in lower mammals.31 In higher mammals, some evidence suggests the subventricular lineage may respond to injury32,33 by homing to tumor or stroke foci, although the significance of this phenomenon is unclear.
Self-renewal, multipotency, and a presence in the adult brain are central features of the therapeutic application of stem cells for CNS repair. First, through a sustained ability to divide (self-renewal)—stem cells can generate numerous new cells that replace lost cells or provide nutritive support (ie, secretion of molecules that promote healing) to residual tissue. Second, because they are multipotent, stem cells generate multiple cell types for any given tissue. Accordingly, stem cells from the CNS (or those that can be programmed to make CNS cell types) possess the added benefit of producing not just any cell, but neural cells in particular (ie, neurons, astrocytes, and oligodendrocytes). These qualities are particularly important when cell loss occurs, a characteristic of many CNS pathologies.* Third, the persistence of stem cells into adulthood raises possibilities relevant to rehabilitation. How do these pools contribute to recovery following injury, if at all? Can we use clinically relevant physical therapy protocols to augment neurogenesis in the injured or diseased CNS and promote functionally meaningful, endogenous cell replacement and self-repair? These are intriguing, yet elusive, issues that are currently being addressed by experimental strategies involving transplantation of stem cells from various sources in preclinical models of neurological disease and trauma.
Limitations Associated With Stem Cell Therapies in the CNS
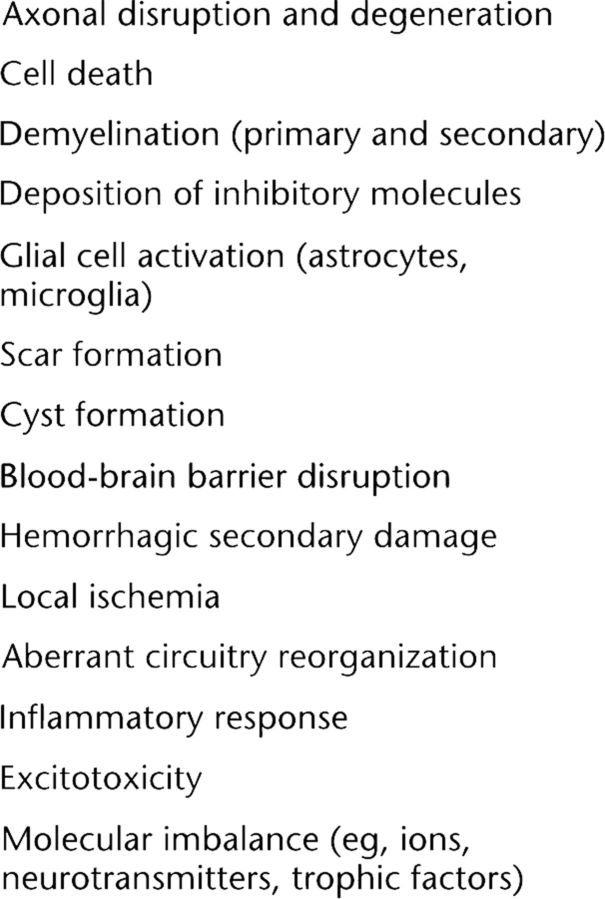
Each year, an estimated 50 million people sustain CNS damage, including stroke, traumatic brain injury, SCI, demyelinating diseases, and neurodegenerative disorders.34 Although CNS pathologies are variable in mechanism and presentation, the uniform and resultant regenerative failure is the principal rationale for pursuing regenerative medicine–based repair—including stem cell transplantation. The primary regenerative goals are the genesis of new neurons and supporting glial cells, as well as long-distance regrowth of injured nerve fibers. The injured CNS is characterized by a complex cascade of biochemical events that contribute to secondary tissue loss beyond the initial insult21,35 (summarized in Fig. 2). In this hostile host environment, donor or experimentally introduced cells are susceptible to conditions that can negatively affect cell engraftment (as noted below). Thus, donor cell populations shown to be capable of long-term survival in all neural cell types in pretransplant culture systems27,28,36 demonstrate significant reductions in proliferation and differentiation following transplantation into the injured CNS environment. This fact dramatically limits transplantation efficiency and underlies the theory that additional cotherapies such as rehabilitation may be required to optimize cell therapy outcomes.
Figure 2.
Overview of damage following central nervous system injury.
The selection process for therapeutic stem cells is a topic fueled by both ethical and logistical considerations. Stem cell sources from early developmental time points (eg, embryonic stem cells) are attractive given their high rate of proliferation. However, cell division must be harnessed in order to avoid tumor formation—a major risk factor in using embryonic stem cells. Conversely, cells obtained from a later developmental time point (eg, fetal or postnatal stem cells) are less likely to form tumors. Yet, this option could pose difficulties in generating ample transplant material due to the age-related decline in stem cell robustness with increasing age of the donor source. Feasibility also remains an important consideration for obtaining stem cell populations. Accessing embryonic and fetal material is both controversial and heavily regulated in the United States. On the other hand, harvesting of autologous stem cells from the postnatal or adult brain germinal zones is difficult to envision at this time. Another selection factor is using a donor cell that makes the mature cell type required. “Cell fate” is the identity of a mature cell (ie, neuron, astrocyte, oligodendrocyte) obtained following lineage progression from a stem cell to a final progeny cell. The importance of the potential fates of engrafted cells is dependent on the therapeutic goals. The general consensus is that, for robust tissue-specific cell replacement, postnatal donor material must come from the tissue of origin.†,37 Reports to the contrary for neural cell replacement using stem cells from other tissues are controversial, but should not be ignored.38–40 Additionally, donor cells from other tissue types (eg, mesenchymal stem cells for neural injury) offer the potential for non-cell replacement-related support, such as nutrition and counteraction of immune responses.41,42
CNS Stem Cell Transplantation Studies
Because of the nature and properties inherent to stem cells, and in light of the limitations presented by a lesion to an essentially nonregenerative CNS, stem cell transplantation is first theorized to replace neural cells lost due to injury through cell replacement.43 The notion that stem cell grafts could additionally provide trophic or nutritive support44 to healing tissue is potentially just as powerful. In the case of SCI, transplantation of many cell types (eg, fetal material, embryonic and adult neural stem cells, marrow stromal stem cells, olfactory ensheathing cells, Schwann cells) from various donor sources has been tested in different experimental SCI models.43,45–47 Collectively, these reports have demonstrated safety with rare exception,48 and donor cell survival is seen weeks to months following transplantation. However, optimal cell delivery protocols (eg, cell types versus times of delivery versus means of delivery)—for human applications especially—remain to be defined.49 Furthermore, a “transplant effect” is often assigned to behavioral improvements in experimental animals, but the underlying mechanism of a graft-mediated effect is often insufficiently demonstrated. Overall, experimental recovery is quite variable, and there is little information that correlates recovery with metrics of functional engraftment. For example, data demonstrating host-graft synaptic connectivity are sparse.
Translation of clinical stem cell transplantation to the clinic is an ongoing effort that is characterized by both promise and limitation. Clinical fetal cell transplantation studies initiated more than 3 decades ago provided an early road map for translating the basic science of neural tissue replacement to the clinical arena, with safety and feasibility being the endpoints of primary interest.50–53 Translational studies initially focused on treating people with Parkinson disease with dopamine-producing embryonic CNS donor tissue, which has since been recognized to be a rich source of neural stem cells.50,51 Preclinical results obtained from animal models of Parkinson disease and SCI led to human trials, which demonstrated that human neural progenitors could be safely grafted into human recipients.50–53 Subsequently, clinical trials have been initiated in the United States and overseas using stem cells to treat people with stroke, amyotrophic lateral sclerosis, and SCI43,54–56 (Table). An in-depth review of that work is beyond the scope of this article, but here we focus on relevant general comments. In addition to being an active and exciting area of translational research around the world, current and future clinical trials can broaden our knowledge through thoughtful consideration of experimental variability in areas such as nonuniform recruitment base, nonstandardized study designs, variability in procedural control, cell source definition, and peer-reviewed documentation. Limitations to preclinical central nervous system transplant literature include: heterogeneity of starting cell material, differences in cell isolation/preparation between laboratories, timing of transplant postinjury, mode of cell delivery, and lack of positive finding in a chronic injury model. Considerable caution must be exercised when drawing conclusions about the safety of different cell sources, efficacy of engraftment, or reliability of clinical benefits.43
Table.
Summary of Current Stem Cell Transplantation Trials (Available Reports)
| Agency or Individual (Country) | Cell Type | Reported Transplant (n) | Safety Reported? | Functional Gains Reported? |
|---|---|---|---|---|
| Geron (USA) terminated | Human embryonic stem–derived cells (oligodendrocyte precursors) | 2 | Yes | No |
| Barros (Brazil) | Bone marrow–derived stem cells | 39 | Yes | Yes |
| Moviglia (Argentina) | Bone marrow–derived stem cells | 10 | Yes | Yes |
| Geffner (Ecuador) | Bone marrow–derived stem cells | 52 | Yes | Yes |
| Callera (Brazil) | Bone marrow–derived stem cells | 10 | Yes | No |
| Jacques (Mexico) | Umbilical cord stem cells | 59 | Yes | Yes |
| Medra Inc (Dominican Republic/United States) | Fetal stem cells | Unknown | Unknown | Unknown |
| Sykova (Czech Republic) | Bone marrow–derived stem cells | 36 | Yes | Yes |
| Klienbloesem (Cells4Health: Turkey/the Netherlands); (Xcell-Center: Germany) | Bone marrow–derived stem cells | 180 | Yes | Yes |
| Bussarsky (Bulgaria) | Bone marrow–derived stem cells | 115 | Yes | Yes |
| Trossel (the Netherlands) | Umbilical stem cells | Unknown | Unknown | Unknown |
| Curt/Stem Cells Inc (Switzerland/ United States) | Fetal central nervous system stem cells | Unknown | Unknown | Unknown |
| Neuralstem Inc | Human fetal spinal cord stem cells | Unknown | Unknown | Unknown |
| Bryukhovetskiy (Russia) | Embryonic stem/fetal stem cells (former); adult stem cells, type unknown; olfactory ensheathing cells | ~60 | Yes | Premature |
| Rabinovich (Russia) | Combinations of various cells | 122 | Yes | Yes |
| Chernykh (Russia) | Bone marrow–derived stem cells | 18 | Yes | Yes |
| Asia (greater than 12 investigators) | Multiple sources | Unknown | Varied | Varied |
| Caribbean | Unclear | Unknown | Unknown | Unknown |
These overall results would suggest that, although an incredibly promising venture, CNS transplantation is still an evolving approach that will benefit from more extensive study in preclinical animal models. Current and future studies should seek to ensure that the optimal model is in use so that rehabilitation has the best opportunity to elicit biologically relevant improvements.
Building Evidence for Rehabilitation to Augment CNS Stem Cell Therapies
Stem cell transplantation offers the potential benefit of introducing an additional and sustained source of neuroplasticity. Neuroplasticity in this context refers to changes in the structure or function of a neural system in response to a stimulus. Therefore, following injury, stem cells would contribute structurally or functionally to what is essentially a novel neural substrate, within which lies an opportunity for the application of rehabilitation approaches to maximize the efficacy of current transplantation approaches. Activation of the nervous system through injury or sensory or motor stimuli provides a rationale to pursue combined therapies.
Stem cells in the CNS are responsive to injury and, therefore, could contribute to the recovery process. For example, following SCI in rodents, ependymal spinal cord stem cells rapidly proliferate, primarily producing glial cells.57–59 In animal models of stroke, endogenous, subventricular, zone-derived progenitors proliferate and migrate toward the lesion.33,60,61 Even more striking, these cells can differentiate into mature neurons62 and appear to integrate with host tissue.63 In these studies, the time frame of donor cell integration occurs over a period of several months, an interval that, perhaps only coincidentally, corresponds to a typical poststroke recovery. In support of a possible endogenous neural stem cell contribution to recovery, chemical reduction of the host stem cell population results in a worse functional outcome following stroke compared with naive animals that generate a normal stem cell response to injury.64 These data suggest that CNS stem cells are mobilized following injury and could contribute to recovery.65 What clinical outcomes affirm, however, is that this endogenous stem cell response to injury is inadequate to mount a complete recovery of function following injury without further intervention.
Stem cells also respond to externally applied sensory and motor stimuli, which underlies the potential for rehabilitation to augment innate stem cell-mediated recovery. This finding was established in studies on the effects of sensory input and motor activity on the birth of new neurons (neurogenesis) in the hippocampus (ie, through proliferation or survival of stem cells and their progeny cells).66,67 Animals housed in an enriched environment (ie, increased sensory input and social interaction) exhibited increased survival of newborn hippocampal neurons over time. In addition, voluntary exercise on a running wheel resulted in a temporally significant increase in the birth of new hippocampal neurons and improved their overall survival.67 In aged animals, an average of 3.9 km per day (SD=0.1) on a running wheel results in a 50% reversal of the natural decline in hippocampal neurogenesis.68 Thus, appropriately timed and dosed stimuli may influence endogenous stem cell responses. Furthermore, the same stimuli could complement transplantation by enhancing donor cell survival, fate, and integration, thereby leading to more consistent and biologically significant functional outcomes.
The mechanisms underlying stem cell responses to general exercise and environmental enrichment are slowly emerging.69 Teng and colleagues65 have proposed that activity stimulates endogenous or transplanted stem cells to secrete neurotrophins (molecules that promote neuronal health and plasticity). This proposal is supported by correlative data on the effects of activity at the anatomical and physiological levels. At the anatomical level, exercise can affect dendritic morphology and increase vascularity.70 At the physiological level, exercise influences expression of synaptic and receptor proteins.70 Such plasticity can directly influence neural stem cell pools.67,68 On the other hand, activity or physiological modalities could counteract negative factors within the injury environment.
Physical rehabilitation approaches that focus on activation of the neuromuscular system could have an impact at the cellular level, with the aim of augmenting a behavioral change and improving outcomes.71 In this light, rehabilitation is a promising partner to stem cell technologies. A recent review emphasized the convergent themes of CNS regenerative medicine and neurorehabilitation approaches.21 The authors discussed that functional gains seen using these approaches in isolation, to date, are modest. They posited that the introduction of complementary approaches (eg, motor stimulation combined with small molecule therapy) will be needed to achieve improved outcomes. Applied to the current topic, patterned input through modalities and activity could address current limitations to cell replacement therapies by conditioning transplanted cells and the injured environment for survival and function over long periods.
Experimental evidence is needed to determine whether rehabilitation, with the appropriate timing and dosage of administration, can augment stem cell therapies aimed at CNS repair. Such evidence is emerging across the neuromuscular spectrum. For example, in the musculoskeletal system, exercise augments stem cell transplantation following muscular injury in a mouse model.13 In the CNS, locomotor training alone has elicited gains in hind-limb functional recovery in a number of neurological populations.72–74 Yet, recovery is enhanced when locomotor training is paired with regenerative modalities, such as electrical stimulation5 or pharmacological agents.75 Another recent report demonstrated that improvements in rodent motor function following cervical SCI were absent following gene therapy unless combined with rehabilitation.76
A final example of particular interest is intermittent hypoxia, a systemic and physical therapy–administered physiologic stimulus. Relevant to stem cell therapies, recent in vitro findings outline that CNS neural stem cells harvested following intermittent hypoxia are more proliferative and yield a greater percentage of neurons than those harvested from control animals.77,78 This is a compelling finding in that individuals with chronic incomplete SCI demonstrate greater plantar flexion force and ambulatory speed and endurance following acute mild intermittent hypoxia protocols.12,79 Similar to the aforementioned findings in conjunction with locomotor training, the combination of ambulatory training with mild intermittent hypoxia results in an additive benefit to gait endurance.79 Together, these findings suggest that systemic intermittent hypoxia is sufficient to elicit changes in neural stem cell biology that support study of their combined use to offset some limitations to CNS stem cell therapies (specifically, reduced proliferation or differentiation of transplanted cells with preconditioning and conditioning of a toxic injury environment).
Thus, rehabilitation modalities, in theory, gain ground as a component of multipronged approaches75 to potentially optimize stem cell transplantation. This notion is in keeping with the concept of rehabilitating the patient on the cellular level13,80 and, if carefully optimized for timing and dose of administration, may prove to be effective beyond the capabilities of the injured system alone.
Professional Opportunities to Forward Regenerative Rehabilitation
The potential for physical therapy to augment advanced technologies is supported by initiatives already set forth to address progressive therapeutics. In 2006, the American Physical Therapy Association (APTA) House of Delegates' Vision 20209 mandated innovative practice models as part of the profession's mission. Subsequently, emphasis has been placed on the effective translation of advanced technologies into clinical practice to meet this mission. In 2010, the Physical Therapy and Society Summit (PASS) produced recommendations for professional growth to meet scientific advances.81,82 These recommendations called for effective collaborations in testing new technologies, progression of technology-based intervention to health care delivery, establishment of mechanisms to promote clinical competencies with new technologies, and the promotion of multidisciplinary efforts in science and technology developments. Finally, during the 2011 National Institute of Child Health and Human Development Scientific Vision Workshop on Plasticity, basic and clinical scientists identified emerging developments regarding plasticity-related research. As outlined in the white paper report,83 relevant questions raised included:
Do different modes of plasticity converge to promote healing?
How do therapeutic interventions tap into plasticity, and are multiple interventions needed to optimize access to multiple forms of biological plasticity?
What new tools and approaches are needed to study plasticity?
How do we evaluate the effects of combination therapies on plasticity?
Do we have the most appropriate preclinical models?
Therefore, there is a significant drive for physical therapists to engage in advanced technologies research, such as those technologies encompassed by regenerative medicine.
In the spirit of acting on these initiatives, several recent sessions have focused on the growth of regenerative rehabilitation. Regenerative rehabilitation has been the focus of an educational session (2011) and the Eugene Michels Researchers Forum (2012) at the APTA Combined Sections Meetings. Building on this educational platform, Ambrosio and colleagues have initiated a series of symposia on regenerative rehabilitation that overview current research efforts and progress toward combined therapies, with other similar symposia now occurring internationally. Regenerative rehabilitation was the subject of the 2012 APTA Section on Research retreat and has been highlighted in print84 and via podcast in PT in Motion.85 Finally, with the inception of the Alliance for Regenerative Rehabilitation Research and Training (AR3T), major research institutions are joining together to fund research and to educate and disseminate current research to the broadest audience possible.86
Clearly, more physical therapists are becoming involved in regenerative medicine research. For example, a survey of the top 27 programs listed in the US News and World Report revealed that 43% actively participate in some form of regenerative medicine–oriented research (unpublished observation). This observation supports the growing interface between physical therapy and regenerative medicine research teams. Building partnerships between physical therapy researchers and clinicians will become a critical next step, where each can play a significant role in the design and application of emerging regenerative rehabilitation protocols. Early collaboration between the 2 disciplines is the root of a fusion model proposed by Ambrosio and Russell,15 which contends that rehabilitation and regenerative medicine interactions should be assessed concurrently during research development, rather than in a separated and linear fashion. The rationale is to assess rehabilitation approaches in the context of developing advanced technologies and as a component of preclinical trials to ensure that dosing parameters and clinical outcomes are optimized.
What does this conceptualization mean for the experienced clinician and for interested clinical doctor of physical therapy (DPT) students? Experienced clinical insight will be critical to the successful and wide implementation of regenerative rehabilitation. Advanced knowledge of patient responses to regenerative approaches will be important to the accurate assessment of treatment efficacy. The development of appropriate clinical outcomes that capture nuanced aspects of recovery will also be important.87 A preliminary step in establishing prerequisite clinician involvement could be the creation of specialized clinical trial sites that will interface novel rehabilitation approaches with specific patient groups receiving novel regenerative approaches. As an example, the mission of the Christopher and Dana Reeve Foundation NeuroRecovery Network88 is to fast-track the translation of scientific evidence for activity-based therapies into clinical practice in concert with ongoing program evaluation. Essential to the NeuroRecovery Network is the use of: (1) standardized therapy protocols for specific patient populations, (2) standardized outcome measures, and (3) standardized training of staff across the NeuroRecovery Network. Parameters and dosage of locomotor training (current activity-based therapy), patient benefit (eg, physical performance, health, quality of life), population benefit, and rate of recovery are among the many aspects of clinical data collected from the patients. Thus, program evaluation provides data with which to track the progress of developing technologies, problem-solve within the clinical team (eg, physician, therapist, administrator, manager, scientist), and ultimately inform and guide clinical practice.
Although perhaps premature at this stage, the practice of physical therapy, specifically neurorehabilitation, will change as advances continue in brain and spinal cord repair. This change will require new material within academic programs and advanced continuing education to prepare physical therapists for safe and effective implementation of evidence-based rehabilitation protocols in patients having undergone treatments targeting neuroregeneration. Finally, clinicians will need to acknowledge the current state of practice, as patients are exposed to considerable unfiltered information regarding treatment options, benefits, and controversies. A well-informed clinician must have resources to best help their patients to sift through the “hype versus the hope.” This concern is important for all therapists dedicated to best informing their patients, whether or not engaged in regenerative rehabilitation research.
Conclusions
Physical therapy is poised to play a significant role in regenerative medicine through the development and implementation of regenerative rehabilitation approaches. We have used the example of stem cell therapies following CNS injury to highlight the potential for rehabilitation to augment regenerative processes. We hope we also have continued a discourse that outlines the important role experienced clinicians can play to effect meaningful translation of advanced technologies and to emphasize that the physical therapy profession must prepare for its role as a key partner in the evolution of regenerative rehabilitation.
Just as advances in electronic communication have enlightened the world's populace to the immediacy of information, such technologies also have spawned the recognition that discovery should become synonymous with instant gratification. These discoveries will inevitably include the recognition that health is becoming intimately entwined with molecules and that translating this information into improved health will become an expectation. Examples of this expectation already abound in recognition of achievements such as the mapping of the human genome or the interface between the delineation of genetic polymorphisms and implications for new treatment approaches. Discoveries demonstrating that human tissue can regenerate beckons the call for consumer expectation. There is every reason to anticipate that as these electronic communications inform us about the association between regenerative rehabilitation and quality or longevity of our lives, the implications for physical therapist practice will become obvious to even the most casual of observers. Our collective spirit of compassion and creativity demands our diligence and preparation in becoming proactive participants in the discovery and implementation processes.
From an academic perspective, it may be argued that the incorporation of translational regenerative rehabilitation concepts (from molecules to man) into DPT curricula will be sine qua non for training the next generation of physical therapists, and terminology and principles commonly implemented in the field of regenerative medicine should be a part of our rehabilitation vernacular. Accordingly, given the rapid pace by which medical advances are occurring, educators should continually strive toward a culture of lifelong learning such that today's students (and tomorrow's clinicians) may ever be up-to-date with the latest breakthroughs, well beyond the time of graduation and throughout clinical practice.
To learn more about regenerative rehabilitation, visit the APTA website89 for a list of resources on the topic, including references, podcasts, audio courses and upcoming events.
Footnotes
With regard to nomenclature, “stem cell populations” generally consist of true stem cells, lineage-restricted progenitors, and postmitotic progeny cells maturing into CNS cell types (Fig. 1). Because of their heterogeneous nature, it is most accurate to refer to stem cell populations as neural stem progenitors or neural progenitors. In the remainder of this article, we will continue with the terminology of stem cell populations.
The exception to this rule appears to be induced pluripotent stem cells, in which skin fibroblasts are reprogrammed using gene transfer or small molecules, or both, to make stem cells that are then induced to make mature cell types. This technology has been used to create multiple tissue-specific adult cell types, including neural cells.
References
- 1. Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3:1–5. [DOI] [PubMed] [Google Scholar]
- 2. Byrne BJ, Falk DJ, Pacak CA, et al. Pompe disease gene therapy. Hum Mol Genet. 2011;20:R61–R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jäderstad J, Jäderstad LM, Li J, et al. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc Natl Acad Sci USA. 2010;107:5184–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. [DOI] [PubMed] [Google Scholar]
- 5. Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahmoudi B, Sanchez JC. A symbiotic brain-machine interface through value-based decision making. PLoS One. 2011;6:e14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Badylak SF, Tullius R, Kokini K, et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995;29:977–985. [DOI] [PubMed] [Google Scholar]
- 8. Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. [DOI] [PubMed] [Google Scholar]
- 9. Interagency Federal Working Group on Regenerative Medicine. 2020: A New Vision. A Future for Regenerative Medicine. Washington, DC: US Department of Health and Human Services; 2005. [Google Scholar]
- 10. O'Brien C. California Proposition 71 (2004). Embryo Project Encyclopedia (2014-04-03). Available at: https://embryo.asu.edu/pages/california-proposition-71-2004.
- 11. Lee J, Garcia AM, Lee WP, Munin MC. Inpatient rehabilitation challenges in a quadrimembral amputee after bilateral hand transplantation. Am J Phys Med Rehabil. 2011;90:688–693. [DOI] [PubMed] [Google Scholar]
- 12. Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. [DOI] [PubMed] [Google Scholar]
- 13. Ambrosio F, Ferrari RJ, Distefano G, et al. The synergistic effect of treadmill running on stem-cell transplantation to heal injured skeletal muscle. Tissue Eng Part A. 2010;16:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Distefano G, Ferrari RJ, Weiss C, et al. Neuromuscular electrical stimulation as a method to maximize the beneficial effects of muscle stem cells transplanted into dystrophic skeletal muscle. PLoS One. 2013;8:e54922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ambrosio F, Russell A. Regenerative rehabilitation: a call to action. J Rehabil Res Dev. 2010;47:xi–xv. [DOI] [PubMed] [Google Scholar]
- 16. Ambrosio F, Wolf SL, Delitto A, et al. The emerging relationship between regenerative medicine and physical therapeutics. Phys Ther. 2010;90:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ambrosio F, Boninger ML, Brubaker CE, et al. Guest editorial: emergent themes from second annual symposium on regenerative rehabilitation, Pittsburgh, Pennsylvania. J Rehabil Res Dev. 2013;50:vii–xiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reier PJ, Lane MA. Degeneration, regeneration and plasticity in the nervous system. In: Conn PM, ed. Neuroscience in Medicine. 3rd ed Totowa, NJ: Humana Press; 2008:691. [Google Scholar]
- 19. Treherne JM, Woodward SK, Varga ZM, et al. Restoration of conduction and growth of axons through injured spinal cord of neonatal opossum in culture. Proc Natl Acad Sci USA. 1992;89:431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicholls J, Saunders N. Regeneration of immature mammalian spinal cord after injury. Trends Neurosci. 1996;19:229–234. [DOI] [PubMed] [Google Scholar]
- 21. Aravamudhan S, Bellamkonda RV. Toward a convergence of regenerative medicine, rehabilitation, and neuroprosthetics. J Neurotrauma. 2011;28:2329–2347. [DOI] [PubMed] [Google Scholar]
- 22. Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. [DOI] [PubMed] [Google Scholar]
- 23. Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–591. [DOI] [PubMed] [Google Scholar]
- 24. Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. [DOI] [PubMed] [Google Scholar]
- 25. Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124;319–335. [DOI] [PubMed] [Google Scholar]
- 26. Altman J. Autoradiographic and histological studies of postnatal neurogenesis, IV: cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. [DOI] [PubMed] [Google Scholar]
- 27. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. [DOI] [PubMed] [Google Scholar]
- 28. Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. [DOI] [PubMed] [Google Scholar]
- 29. Kukekov VG, Laywell ED, Suslov O, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. [DOI] [PubMed] [Google Scholar]
- 30. Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14;186–191. [DOI] [PubMed] [Google Scholar]
- 31. Redmond L, Hockfield S, Morabito MA. The divergent homeobox gene PBX1 is expressed in the postnatal subventricular zone and interneurons of the olfactory bulb. J Neurosci. 1996;16:2972–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. [DOI] [PubMed] [Google Scholar]
- 34. National Institute of Neurological Disorders and Stroke. Overview page. Available at: http://www.ninds.nih.gov/about_ninds/ninds_overview.htm. Accessed December 5, 2015.
- 35. Reier PJ, Lane MA, Behrman AL, Howland DR. Spinal cord: repair and rehabilitation. Encyclopedia of Life Sciences. 2009;1–7. 10.1002/9780470015902.a0021403. [Google Scholar]
- 36. Ross HH, Levkoff LH, Marshall GP Jr, et al. Bromodeoxyuridine induces senescence in neural stem and progenitor cells. Stem Cells. 2008;26:3218–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 38. Chen KA, Laywell ED, Marshall G, et al. Fusion of neural stem cells in culture. Exp Neurol. 2006;198:129–135. [DOI] [PubMed] [Google Scholar]
- 39. Franco Lambert AP, Fraga Zandonai A, Bonatto D, et al. Differentiation of human adipose-derived adult stem cells into neuronal tissue: does it work? Differentiation. 2009;77:221–228. [DOI] [PubMed] [Google Scholar]
- 40. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 41. Onda T, Honmou O, Harada K, et al. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen J, Li Y, Wang L, Lu M, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. [DOI] [PubMed] [Google Scholar]
- 43. Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yan J, Welsh AM, Bora SH, et al. Differentiation and tropic/trophic effects of exogenous neural precursors in the adult spinal cord. J Comp Neurol. 2004;480:101–114. [DOI] [PubMed] [Google Scholar]
- 45. Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus. 2008;24:E19. [DOI] [PubMed] [Google Scholar]
- 46. Reier PJ. Neural tissue grafts and repair of the injured spinal cord. Neuropathol Appl Neurobiol. 1985;11:81–104. [DOI] [PubMed] [Google Scholar]
- 47. Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hofstetter CP, Holmström MA, Lilja JA, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nature Neurosci. 2005;8:346–353. [DOI] [PubMed] [Google Scholar]
- 49. Guest J, Benavides F, Padgett K, et al. Technical aspects of spinal cord injections for cell transplantation: clinical and translational considerations. Brain Res Bull. 2011;84:267–279. [DOI] [PubMed] [Google Scholar]
- 50. Freed CR, Breeze RE, Rosenberg NL, et al. Therapeutic effects of human fetal dopamine cells transplanted in a patient with Parkinson's disease. Prog Brain Res. 1990;82:715–721. [DOI] [PubMed] [Google Scholar]
- 51. Freed CR, Breeze RE, Rosenberg NL, et al. Transplantation of human fetal dopamine cells for Parkinson's disease: results at 1 year. Arch Neurol. 1990;47:505–512. [DOI] [PubMed] [Google Scholar]
- 52. Thompson FJ, Reier PJ, Uthman B, et al. Neurophysiological assessment of the feasibility and safety of neural tissue transplantation in patients with syringomyelia. J Neurotrauma. 2001;18:931–945. [DOI] [PubMed] [Google Scholar]
- 53. Wirth ED III, Reier PJ, Fessler RG, et al. Feasibility and safety of neural tissue transplantation in patients with syringomyelia. J Neurotrauma. 2001;18:911–929. [DOI] [PubMed] [Google Scholar]
- 54. Svendsen CN, Ebert AD, eds. Encyclopedia of Stem Cell Research. Vol. 2 Thousand Oaks, CA; Sage Publications Inc: 2008. [Google Scholar]
- 55. Tsukamoto A, Uchida N, Capela A, et al. Clinical translation of human neural stem cells. Stem Cell Res Ther. 2013;4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Neuralstem Inc. Neuralstem cell therapy for spinal cord injury. Available at: http://www.neuralstem.com/cell-therapy-for-sci. Accessed March 8, 2016.
- 57. Yamamoto S, Yamamoto N, Kitamura T, et al. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol. 2001;172:115–127. [DOI] [PubMed] [Google Scholar]
- 58. Matthews MA, St Onge MF, Faciane CL. An electron microscopic analysis of abnormal ependymal cell proliferation and envelopment of sprouting axons following spinal cord transection in the rat. Acta Neuropathol. 1979;45:27–36. [DOI] [PubMed] [Google Scholar]
- 59. Meletis K, Narnabé-Heider F, Carlén M, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. [DOI] [PubMed] [Google Scholar]
- 61. Parent JM, Vexler ZS, Gong C, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. [DOI] [PubMed] [Google Scholar]
- 62. Yamashita T, Ninomiya M, Hernández Acosta P, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hou SW, Wang YW, Xu M, et al. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39:2837–2844. [DOI] [PubMed] [Google Scholar]
- 64. Jin K, Wang X, Xie L, et al. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci USA. 2010;107:799–37998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Teng YD, Liao WL, Choi H, et al. Physical activity-mediated functional recovery after spinal cord injury: potential roles of neural stem cells. Regen Med. 2006;1:763–776. [DOI] [PubMed] [Google Scholar]
- 66. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. [DOI] [PubMed] [Google Scholar]
- 67. van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. [DOI] [PubMed] [Google Scholar]
- 68. van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sandrow-Feinberg HR, Izzi J, Shumsky JS, et al. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J Neurotrauma. 2009;26:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. [DOI] [PubMed] [Google Scholar]
- 71. Behrman AL, Harkema SJ. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys Med Rehabil Clin North Am. 2007;18:183–202, v. [DOI] [PubMed] [Google Scholar]
- 72. Behrman AL, Nair PM, Bowden MG, et al. Locomotor training restores walking in a nonambulatory child with chronic, severe, incomplete cervical spinal cord injury. Phys Ther. 2008:88:580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jayaraman A, Shah P, Gregory C, et al. Locomotor training and muscle function after incomplete spinal cord injury: case series. J Spinal Cord Med. 2008;31:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tefertiller C, Pharo B, Evans N, Winchester P. Efficacy of rehabilitation robotics for walking training in neurological disorders: a review. J Rehabil Res Dev. 2011;48:387–416. [DOI] [PubMed] [Google Scholar]
- 75. Musienko P, Heutschi J, Friedli L, et al. Multi-system neurorehabilitative strategies to restore motor functions following severe spinal cord injury. Exp Neurol. 2012;235:100–109. [DOI] [PubMed] [Google Scholar]
- 76. Weishaupt N, Li S, Di Pardo A, et al. Synergistic effects of BDNF and rehabilitative training on recovery after cervical spinal cord injury. Behav Brain Res. 2013;239:31–42. [DOI] [PubMed] [Google Scholar]
- 77. Ross HH, Sandhu MS, Sher WJ, et al. Acute intermittent hypoxia alters cell fate choice in postnatal neural precursors. FASEB. 2011;25:861.2. [Google Scholar]
- 78. Ross HH, Sandhu MS, Sharififar S, Fuller DD. Delivery of in vivo acute intermittent hypoxia in neonatal rodents to prime subventricular zone-derived neural progenitor cell cultures. J Vis Exp. 2015November210.3791/52527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hayes HB, Jayaraman A, Herrmann M, et al. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82:104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wolf SL. Thirty-Third Mary McMillan Lecture: “Look forward, walk tall”: Exploring our “What if” questions. Phys Ther. 2002;82:1108–1118. [PubMed] [Google Scholar]
- 81. Kigin CM, Rodgers MM, Wolf SL; PASS Steering Committee Members. The Physical Therapy and Society Summit (PASS) Meeting: observations and opportunities. Phys Ther. 2010;90:1555–1567. [DOI] [PubMed] [Google Scholar]
- 82. Dean E, Al-Obaidi S, De Andrade AD, et al. The First Physical Therapy Summit on Global Health: implications and recommendations for the 21st century. Physiother Theory Pract. 2011;27:531–547. [DOI] [PubMed] [Google Scholar]
- 83. Chae J, Duncan PW, Pugh K, Selzer ME. Eunice Kennedy Shriver National Institute of Child Health and Human Development Scientific Vision Workshop on Plasticity; January13–14, 2011; Bethesda, Maryland: Workshop White Paper; Available at: https://www.nichd.nih.gov/vision/vision_themes/plasticity/Documents/Plasticity_White_Paper_032111.pdf. Accessed December 5, 2015. [Google Scholar]
- 84. Regenerative Medicine: A Key to Tomorrow's Rehabilitation. PT in Motion. May2013. Available at: http://www.apta.org/PTinMotion/2013/5/Feature/AGrowingConcern/. Accessed March 8, 2016.
- 85. Video: APTA Roundtable: Regenerative Rehabilitation. Available at: http://www.apta.org/RegenerativeMedicine/Roundtable/. Accessed March 8, 2016.
- 86. Alliance for Regenerative Rehabilitation Research and Training. About AR3T. Available at: http://www.ar3t.pitt.edu/index.html. Accessed March 8, 2016.
- 87. Behrman AL, Ardolino E, Vanhiel LR, et al. Assessment of functional improvement without compensation reduces variability of outcome measures after human spinal cord injury. Arch Phys Med Rehabil. 2012;93:1518–1529. [DOI] [PubMed] [Google Scholar]
- 88. Harkema SJ, Schmidt Read M, Behrman AL, et al. Establishing the NeuroRecovery Network: multisite rehabilitation centers that provide activity-based therapies and assessments for neurologic disorders. Arch Phys Med Rehabil. 2012;93:1498–1507. [DOI] [PubMed] [Google Scholar]
- 89. American Physical Therapy Association. Regenerative Rehabilitation. Available at: http://www.apta.org/RegenerativeRehab/. Accessed March 8, 2016.