Abstract
Rosacea is a common chronic inflammatory skin disease of the central facial skin and is of unknown origin. Currently, two classifications of rosacea exist that are based on either “preformed” clinical subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) or patient-tailored analysis of the presented rosacea phenotype. Rosacea etiology and pathophysiology are poorly understood. However, recent findings indicate that genetic and environmental components can trigger rosacea initiation and aggravation by dysregulation of the innate and adaptive immune system. Trigger factors also lead to the release of various mediators such as keratinocytes (for example, cathelicidin, vascular endothelial growth factor, and endothelin-1), endothelial cells (nitric oxide), mast cells (cathelicidin and matrix metalloproteinases), macrophages (interferon-gamma, tumor necrosis factor, matrix metalloproteinases, and interleukin-26), and T helper type 1 (T H1) and T H17 cells. Additionally, trigger factors can directly communicate to the cutaneous nervous system and, by neurovascular and neuro-immune active neuropeptides, lead to the manifestation of rosacea lesions. Here, we aim to summarize the recent advances that preceded the new rosacea classification and address a symptom-based approach in the management of patients with rosacea.
Keywords: Rosacea, Classification, Pathophysiology, Inflammation, Cathelicidin, Immunity, Therapy
From old to new rosacea classification
The first classification of rosacea was published in 2002, recognizing rosacea as a syndrome that is comprehensively depicted by four distinct clinical subtypes defined as erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea 1. The classification was the first to systemize rosacea diagnosis in the daily medical routine and gave resilient criteria at hand to efficiently evaluate therapeutic success in each subtype. The implementation of this new tool in the medical daily routine prompted a marked improvement in the medical care of rosacea patients worldwide. In general, the classification suggests a progression from one subtype to another over time and thereby supports a tendency to disregard deviating rosacea manifestations and overlaps between the subtypes. The progress in scientific insight that was accumulated since the introduction of the original rosacea classification in combination with our detailed clinical experience favored a modernized view of rosacea’s pathophysiology as a product of multivariate disease processes that underlie the patient-specific clinical rosacea presentations 2, 3. An updated rosacea classification was published in 2016, emphasizing a more patient-centric phenotype approach 4, 5. This review aims to summarize the recent developments in our understanding of rosacea’s pathophysiology that preceded the release of the modified rosacea classification and to illustrate the therapeutic management of individual rosacea patients on the basis of their symptoms.
Epidemiology and diagnosis of rosacea
In the US alone, more than 16 million patients are affected by rosacea, and worldwide incidences peak as high as 18%, particularly in populations with a predominant “Celtic” heritage, such as is observed in Ireland 6. Worldwide, the prevalence is estimated to reach over 5%. Females and males are affected equally 7. However, the prevalence of rosacea in many countries, including large countries like China and Australia, is still poorly explored, and the prevalence, especially of erythematous rosacea, demands careful differentiation from that of other erythematous diseases and origins of flushing, such as neuro-endocrine tumors.
Rosacea typically arises symmetrically in the central face with gender- and age-specific preferences with regard to lesion qualities. For instance, rhinophyma nearly exclusively presents in the male gender, flushing and erythema often are the first disease signs in younger ages, and telangiectasias make up first rosacea lesions in older ages. The overall rosacea manifestations are flushing, transient or persistent erythema, telangiectasia, papules, pustules, phymata, and (micro)edema ( Table 1) 1, 8. Additionally, patients often report stinging or burning pain and very rarely pruritic sensations. Despite the typical centro-facial localization of rosacea, a causative association with the unique centro-facial skin composition that is characterized by a dense presence of sebaceous glands, dense nerval and vascular networks, and Demodex mites cannot be drawn conclusively as of yet. However, Demodex infestation is increased in some patients with rosacea, and eradication seems to alleviate rosacea symptoms probably by preventing the formation of pro-inflammatory cytokines 9, 10.
Table 1. Novel classification of rosacea on the basis of diagnostic, major, and secondary features of rosacea.
| Diagnostic features | Major features | Secondary features |
|---|---|---|
| Persistent centro-facial erythema associated
with aggravation by trigger factors |
Flushing/transient erythema | Burning sensation |
| Phymatous changes | Inflammatory papules and pustules | Stinging sensation |
| Telangiectasia | Edema | |
| Ocular manifestations
Lid margin telangiectasia Blepharitis, keratitis, conjunctivitis, and sclerokeratitis |
Dry sensation of the skin |
The patient’s awareness about rosacea’s centro-facial localization often promotes severe psychosocial symptoms, including impaired self-esteem, problems in socializing, and changes in the way the patient thinks, feels, or copes. Recent epidemiological studies confirm these clinical observations and report significant psychological disease burden and decreased quality of life in patients with rosacea 11– 16.
Rosacea can be initiated or aggravated by a variety of endogenous and exogenous trigger factors, including heat, noxious cold, ultraviolet (UV) irradiation, and food and beverages. Activation pathways to some of the rosacea triggers have been delineated recently ( Table 2) and might point to future therapeutic targets. The identification of patient-specific trigger factors represents the main and fundamental pillar of rosacea therapy and enables selective and therapeutic trigger avoidance. This strategy in particular is helpful to prevent or alleviate rosacea manifestations that respond dynamically to a trigger such as flushing and transient erythema. In general, the individual clinical manifestation of rosacea appears to be influenced by the special trigger constellation and susceptibilities of a patient. For instance, a bald patient with an enhanced susceptibility toward UV irradiation is imperiled to develop frontoparietal papulopustular lesions, whereas a patient devoid of UV susceptibility is less likely to develop similar rosacea lesions.
Table 2. Common rosacea triggers and their activation pathways and current therapy regimen.
| Pathway of activation | Trigger | Therapeutic regimen |
|---|---|---|
| Inflammasome (NALP3) | Sun exposure, wind, heavy exercise, alcohol consumption, emotional stress, skin
care products and cosmetics (formaldehyde), medication, and microorganisms |
Avoidance, anti-inflammatory therapy,
and antibiotics |
| TLR-2 | Sun exposure, emotional stress, alcohol, exercise, microorganisms/gut
microbiome, topicals, and medication |
Avoidance, 30+ SPF sunscreen, and
brimonidine |
| TRPV1 | Emotional stress, heat/hot weather/hot steam, exercise, alcohol, and spicy food
(capsaicin) |
Avoidance and brimonidine |
| TRPV2 | Heat | Avoidance |
| TRPV4 | Sun exposure/ultraviolet irradiation, humidity, and osmotic changes | Avoidance and 30+ SPF sunscreen |
| TRPA1 | Cold weather, garlic/mustard oil (pungency), and skin care products and
cosmetics (formaldehyde) |
Avoidance and brimonidine |
| PAR 2 | Proteinases and microorganisms | Anti-inflammatory therapy and
antibiotics |
NALP3, NACHT, LRR, and PYD domain-containing protein 3; PAR 2, proteinase-activated receptor 2; SPF, sun protection factor; TLR-2, Toll-like receptor 2; TRPA1, transient receptor potential ankyrin 1; TRPV, transient receptor potential vanilloid.
The newly introduced classification of rosacea emphasizes the importance of each rosacea manifestation and distinguishes diagnostic features (signs) from major and secondary features (symptoms) ( Table 1) 17. Briefly, phymatous changes and persistent centro-facial erythema are considered the only diagnostic features (signs) of rosacea, whereas flushing, telangiectasia, and inflammatory papules/pustules are considered major symptoms and only in combination can suggest the diagnosis of rosacea. Stinging or burning pain, edema, and dry sensation are defined as secondary features of rosacea ( Table 1).
Novelties in the pathophysiology of rosacea
Rosacea skin is characterized by dysregulated inflammatory (perivascular or pilosebaceous infiltrate), vascular (dilation), lymphatic (dilation), glandular (hyperplasia), and fibrotic processes, a composition that reflects the multivariate process of the skin disease. Simultaneously, this heterogeneous histological picture hints at rosacea’s unclear pathophysiologic event of onset. Does rosacea originate from an initial dysregulation in inflammatory processes? Is rosacea a disease of the vasculature or rather of the lymphatic system? Does it result from glandular tissue? Or does rosacea represent a skin disease that ultimately arises from combined dysfunctional systems that could involve the gut?
Immunity
The adaptive immune system along with the innate immune system might take a central part in rosacea’s pathophysiology. Both the early stage perivascular and later-stage pilosebaceous infiltrates are strongly composed of T helper type 1 (T H1) and T H17 cells and show marked expression of innate immune cells such as additional macrophages and mast cells in papules and erythema and additional neutrophils in pustules and plasma cells in phymata 18, 19. CD4 + T H cells dominate the immune cell infiltrate, but overall rosacea, like its differential diagnosis acne vulgaris, displays a T H1/T H17 polarization pattern 20. These immuno-histochemical findings have been confirmed by transcriptome analysis, where markedly elevated expressions of T H1 signature genes—interferon-gamma and tumor necrosis factor-alpha (TNF-α)—and upregulated expression of T H17-associated genes coding for IL17A, IL22, IL6, IL20, and CCL20 were found. Neither T H2-associated genes nor signature markers for regulatory T cells (FOXP3, IL10, CCR4, and CCR8) were found to be altered in the expression in rosacea when compared with healthy skin 19. In acne vulgaris, Propionibacterium acnes capably drives the polarization of the immune cell infiltrate to a T H1/T H17 type 21, 22. This mechanism too could be of importance in rosacea, since some studies could associate patient colonization with Demodex spp., Bacillus oleronius, Staphylococcus epidermidis, Helicobacter pylori, and Bartonella quintana with the development of rosacea 19, 23– 25. Rosacea’s association with facial Demodex spp. colonization was described quite some time ago, but its consequence for rosacea pathophysiology is still not understood and is even disputed by some. These concerns were substantiated by multiple clinical trials that achieved a reduction or eradication of Demodex colonization but often did not observe a marked amelioration of the clinical presentation in patients 26. However, in case microbes initiate the shift of the infiltrate observed in rosacea toward a stable T H1/T H17 polarization, eradication of an “early” rosacea signal might have a smaller therapeutic effect than anticipated in later disease stages. The causative role of especially Demodex spp. and Demodex-dependent and -independent microbes for the T H1/T H17 polarization observed in rosacea will need to be investigated, and the importance of the T-cell pattern for the initiation and perpetuation of rosacea is in need of detailed clarification.
Another pathogen that has been suggested to be involved in the pathophysiology of rosacea is H. pylori 27, 28. However, a recent meta-analysis found only a weak association between H. pylori infection and rosacea and between successful eradication of H. pylori and improvement of rosacea manifestations 29.
Microbes and the cathelicidin axis in rosacea
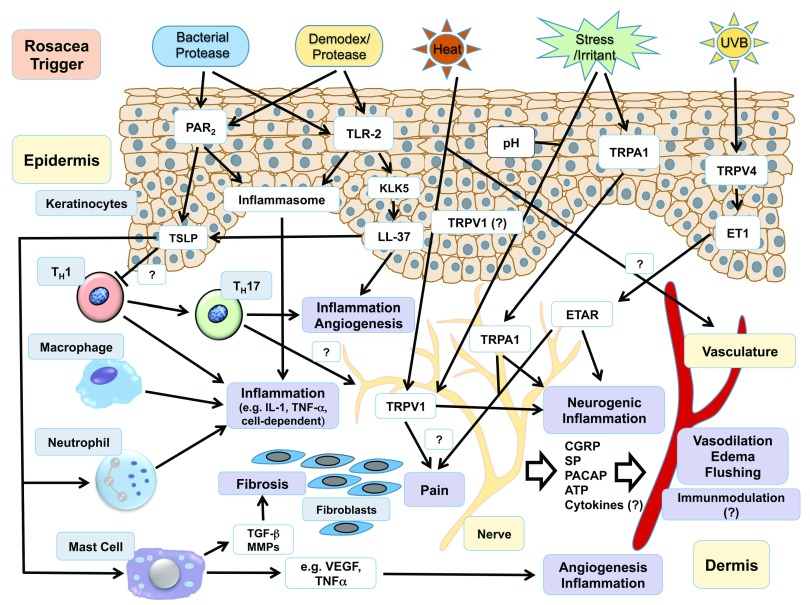
Products of microbes can be recognized by cells of the innate immune system and activate, for example, Toll-like receptors (TLRs) and the G-protein-coupled receptor proteinase-activated receptor 2 (PAR 2) that are expressed by keratinocytes and can nurture inflammatory processes 30. Notably, TLR-2 and probably PAR 2 are upregulated in patients with rosacea, and in vitro activation of both receptors promotes the activation of cathelicidin, an anti-microbial peptide that is also overexpressed in patients with rosacea ( Figure 1) 30, 31. TLR-2 signaling can further activate the NLRP3 inflammasome with subsequent IL-1β- and TNF-mediated inflammation amplification and prostaglandin E 2 synthesis, which support pustule formation, pain sensation, and vascular responses 32. TLR-2 activation additionally promotes the release of pro-inflammatory cytokines, chemokines, proteases, and pro-angiogenic factors, which are mediators associated with rosacea symptoms such as erythema, telangiectasia, or inflammation or a combination of these 10, 33– 35. A direct link among Demodex mites, microbes, and PAR 2 or TLR-2 has not yet been demonstrated in rosacea. Also, functional in vivo data for cathelicidin in humans are still lacking. In mice, TLR-2-induced cathelicidin activation needs functional kallikrein-5 (KLK-5) protease activity for the formation of rosacea-like erythema and angiogenesis 36. However, KLK-5 is increased in patients with rosacea and KLK(s)-5 can activate PAR 2 37. PAR 2 is a known mediator of (neuro)inflammation, pruritus and pain sensation, T-cell and neutrophil recruitment to sites of inflammation, mast cell degranulation and vasodilation, and promotion of release of inflammatory mediators such as IL-1, IL-6, IL-8, TNF, chemokines, matrix metalloproteinases, and prostaglandins 38. PAR 2 is expressed by various skin cell types, including keratinocytes, endothelial cells, and innate and adaptive immune cells, activated by microbe-derived proteases, and interacts with TLRs 39– 43. The upstream signal or signals of enhanced PAR 2, TLR-2, and KLK-5 proteins in rosacea have not been identified yet. However, vitamin D can increase TLR-2 and KLK-5 expression in keratinocytes and is found in excess in some patients with rosacea and might be a candidate ( Figure 1) 44. Further activation of pro-inflammatory receptors (for example, PAR 2) can lead to a secondary skin barrier deficit which may lead to more inflammation 45, 46.
Figure 1. Current understanding of the pathomechanisms in rosacea.
Rosacea triggers lead to the activation of downstream effectors (white boxes) in various cell types (gray boxes) probably by the activation of a few specific receptors and channels (white boxes), which in cooperation nurture processes of (neurogenic) inflammation, including edema and vasodilation, fibrosis, pain, and angiogenesis (lilac boxes). For instance, epidermal and probably immune cell-expressed proteinase-activated receptor-2 (PAR 2) and Toll-like receptor-2 (TLR-2) are activated by rosacea-associated bacterial and Demodex-derived proteases, leading to the induction of the inflammasome and subsequent release of pro-inflammatory agents such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) as well as enhanced expression of the innate immune peptide LL-37. ATP, adenosine triphosphate; CGRP, calcitonin gene-related peptide; ET1, endothelin-1; ETAR, endothelin A receptor; KLK-5, kallikrein-5; LL-37, cathelicidin; MMP, matrix metalloproteinase; NALP3, NACHT, LRR, and PYD domain-containing protein 3; PACAP, pituitary adenylate cyclase-activating peptide; SP, substance P; TGF-β, transforming growth factor-beta; TRP, transient receptor potential; TSLP, thymic stromal lymphopoietin; VEGF, vascular endothelial growth factor.
The importance of microbes and microbe-associated products and their respective target receptor(s) for rosacea pathophysiology is further supported by the recent observation that rosacea patients with SIBO (small intestinal bacterial overgrowth) benefit from treatment with the T-cell modulator rifaximin and present with improved rosacea symptoms 47– 50.
Neurovascular processes and neurogenic inflammation in rosacea
Patients with rosacea react to a vast panel of trigger factors such as temperature changes, heat, cold, exercise, UV radiation, and spicy food and alcoholic beverages with deterioration of rosacea lesions 51. The precise mediating receptors and messengers for each trigger factor are in most cases not identified, but recent transcriptomic analysis and immunohistochemical findings indicate that the transient receptor potential family—in particular, members of the ankyrin subfamily (TRPA1) and the vanilloid subfamily (TRPV1 and TRPV4)—might convey cellular responses to several of the rosacea-specific trigger factors 52, 53. TRPV1 and TRPA1 are well-described targets for various pungent compounds such as capsaicin (TRPV1) and mustard oil (TRPA1 and TRPV1) 54 and could render rosacea stimuli such as heat (TRPV1) 55, possibly cold temperatures (TRPA1) 56, UVB irradiation (TRPV4) 57, and toxins and cosmetics ingredients (for example, TRPA1) 58 into clinical rosacea manifestations. In particular, neuronally expressed TRP channels could be responsible for the activation of the cutaneous vasculature leading to flushing, one hallmark feature of rosacea, and erythema by a neurovascular mechanism involving neurogenic inflammation mediators (see Figure 1) 59– 61. Rosacea “trigger factor activated TRP channels” in fact lead to the release of vasoactive neuropeptides such as substance P (SP), pituitary adenylate cyclase-activating peptide (PACAP), and the migraine-associated calcitonin gene-related peptide (CGRP) 61– 63. Sensory nerves also express the neuro-inflammatory TLR-2 and PAR 2 which might perpetuate the neurovascular dysregulation observed in rosacea. Because TRP channels (particularly TRPA1 and TRPV1) and PAR 2 can crosstalk with neuropeptide receptors or at least trigger neuropeptide release, these interactions could help to sustain the neurovascular loop and neurogenic inflammation in rosacea 52, 53, 64.
Along sensory nerves, dysregulation of the autonomic nervous system (ANS) can produce facial flushing. However, a distinct role for this mechanism in rosacea-typical flushing is not confirmed at this stage but could be promoted by ANS-expressed PACAP or stress-induced increase of skin sympathetic nerve activity 65– 67. In summary, the neurovascular circuits that appear to be involved in rosacea’s pathophysiology might explain the patient-specific trigger profiles and differences in the clinical presentation of rosacea. Altered downstream signaling and target structures might account for the phenotypic variability observed in rosacea.
Genetics of rosacea
The existence of genetic traits that underlie rosacea is expressed by a positive family history for rosacea. Only recently, a handful of genome-wide association studies have been conducted that propose genetic risk loci for rosacea. An American study included individuals of European descent (all customers of the genetic company 23andme, Mountain View, CA, USA) identified single-nucleotide polymorphisms in the butyrophilin-like 2 (BTNL2) and the human leukocyte antigen–DRA genes. Both genes are associated with the major histocompatibility complex of the acquired immune system, indicating a central role for dysregulations of the immune system in rosacea’s pathogenesis. Another study found a null mutation polymorphism in the glutathione S-transferase (GST) gene that encodes for an enzyme involved in cellular oxidative stress 68, 69. In a case study of a patient with granulomatous rosacea, a polymorphism in the NOD2/CARD15 gene was observed 70. NOD2/CARD15 protein functions as a caspase recruitment protein and is associated with the function of innate immune system receptors such as TLR-2 and subsequent inflammatory processes. Interestingly, a recent population-based case control study revealed that rosacea shares genetic risk loci with various autoimmune diseases such as multiple sclerosis, type 1 diabetes mellitus, celiac disease, and rheumatoid arthritis. This observation underlines the importance of a thorough risk assessment for the individual patient with rosacea at risk of developing an autoimmune disorder so that multidisciplinary medical care can be organized for affected patients 71.
Therapeutic management of rosacea
General skin care
Consequent adherence to skin care advice and consequent application of adequate non-irritating skin care can significantly prevent events of rosacea aggravation and improve the patient’s quality of life. Skin care advice consists primarily of avoidance of trigger factors (including stress management), usage of sunscreen with 30+ sun protection factor, application of moisturizers for dry skin and drying applications for oily skin, and gentle cleansing of the whole face 72.
Symptom-based treatment
Because rosacea symptoms derive from distinct pathophysiologies, the therapeutic regimen will in most cases consist of combinations of topicals with systemics or physical therapy or both 73, 74.
Flushing and erythema
According to recent guidelines, two approved topicals can be used to treat persistent erythema in adults with rosacea: brimonidine 75– 77 (a beta2-adrenergic agonist) and oxymetazoline hydrochloride 1% cream 78 (an alpha1A-adrenoreceptor agonist that also activates alpha2-receptors at higher concentrations) 4, 17, 72, 73.
Certain laser therapies can be used, but they should be avoided in pain-sensitized patients 79– 81. Off-label usage of beta-blockers such as carvedilol or adrenergic receptor modulators (for example, brimonidine) may alleviate these symptoms 80. In case of pain association, an analgesic therapy with, for instance, lidocaine gel (4%) or polidocanol cream in mild cases and antiphlogistics (for example, ibuprofen), anti-depressants (for example, amitriptyline), or anticonvulsants (for example, gabapentin and pregabalin) in more severe cases may be helpful 82.
Telangiectasia
Only a few options exist for the treatment of telangiectasia, among which physical laser therapy and intravascular aethoxysklerol (0.5%–1%) injections are most commonly used.
Papules and pustules
Patients with mild to moderate papules and pustules benefit from topical treatment with ivermectin (1%) 83, metronidazole (1%) 84– 86, azelaic acid (15%) 87, or sodium sulfacetamide sulfur 88. Off-label therapy with topical erythromycin (2%), isotretinoin, clindamycin, permethrin, doxycycline, minocycline, and oral erythromycin has been reported with good results. Combination therapy often helps to prolong symptom-free periods. In severe or therapy-refractory cases, systemic treatment with metronidazole, clarithromycin, and azithromycin can be conducted. In cases of Demodex infestation, permethrin (off-label) or ivermectin cream and oral ivermectin can improve the therapeutic result 72, 89.
Phymata
Patients affected by mild phymata benefit from tetracycline therapy. Standard therapies for progressed phymata are ablative (destructive) laser and dermato-surgery. Low-dose isotretinoin appears to reduce phymata by its anti-inflammatory capacity and by lessening the number of sebaceous glands and inhibiting their proliferation 90– 92. Systemic immunomodulatory therapy such as dapsone was used in some cases with mixed results.
Facial (lymph)edema
No US Food and Drug Administration-approved therapy exists. Immunomodulators such as isotretinoin and dapsone or combination therapies with doxycycline/prednisolone have been tried, but the therapeutic value is unclear 93.
Ocular rosacea
The appropriate treatment of ocular rosacea requires a multidisciplinary effort from ophthalmologists and dermatologists. Basic lid hygiene routines such as warm compresses and lubricating drops can be conducted by the patient. Artificial tear substitutes help ocular dryness and accompanying burning and stinging. Successful therapy with topical ivermectin was recently reported 94. In more severe cases, cyclosporine eye drops and systemic tetracycline can be prescribed 73.
Summary and future directions
Basic, translational, and clinical research has significantly increased our understanding of a common skin disease, rosacea, leading to novel anti-inflammatory and anti-erythematous treatments. Combination therapies, similar to those for acne and atopic dermatitis, are a key for the successful therapy of this poly-symptomatic disease 95.
Several questions remain to be answered: which genes are involved in rosacea? What is the prevalence of rosacea globally? Which are the key mediators and receptors of rosacea in the various clinical symptoms and signs? Which comorbidities are associated with rosacea? How can we optimize the diagnosis and treatment of rosacea? Translational research is demanded to better understand and treat this commonly neglected skin disease.
Acknowledgments
We thank Hasna Amal Boumenar for proofreading the manuscript and assisting in the preparation of Figure 1.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Jacob Pontoppidan Thyssen, Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, DK-2900, Denmark
Adam Reich, Department of Dermatology, University of Rzeszów, Rzeszów, Poland
Steven R Feldman, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, NC, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Wilkin J, Dahl M, Detmar M, et al. : Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46(4):584–7. 10.1067/mjd.2002.120625 [DOI] [PubMed] [Google Scholar]
- 2. Gallo RL, Granstein RD, Kang S, et al. : Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–55. 10.1016/j.jaad.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 3. Tan J, Steinhoff M, Berg M, et al. : Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176(1):197–9. 10.1111/bjd.14819 [DOI] [PubMed] [Google Scholar]
- 4. Gallo RL, Granstein RD, Kang S, et al. : Rosacea comorbidities and future research: The 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):167–70. 10.1016/j.jaad.2017.06.150 [DOI] [PubMed] [Google Scholar]
- 5. Tan J, Berg M, Gallo RL, et al. : Applying the phenotype approach for rosacea to practice and research. Br J Dermatol. 2018;179(3):741–6. 10.1111/bjd.16815 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Tan J, Berg M: Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 Suppl 1):S27–35. 10.1016/j.jaad.2013.04.043 [DOI] [PubMed] [Google Scholar]
- 7. Gether L, Overgaard LK, Egeberg A, et al. : Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–9. 10.1111/bjd.16481 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Powell FC: Clinical practice. Rosacea. N Engl J Med. 2005;352(8):793–803. 10.1056/NEJMcp042829 [DOI] [PubMed] [Google Scholar]
- 9. Sattler EC, Hoffmann VS, Ruzicka T, et al. : Reflectance confocal microscopy for monitoring the density of Demodex mites in patients with rosacea before and after treatment. Br J Dermatol. 2015;173(1):69–75. 10.1111/bjd.13783 [DOI] [PubMed] [Google Scholar]
- 10. Casas C, Paul C, Lahfa M, et al. : Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol. 2012;21(12):906–10. 10.1111/exd.12030 [DOI] [PubMed] [Google Scholar]
- 11. Egeberg A, Hansen PR, Gislason GH, et al. : Patients with Rosacea Have Increased Risk of Depression and Anxiety Disorders: A Danish Nationwide Cohort Study. Dermatology. 2016;232(2):208–13. 10.1159/000444082 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Bewley A, Fowler J, Schöfer H, et al. : Erythema of Rosacea Impairs Health-Related Quality of Life: Results of a Meta-analysis. Dermatol Ther (Heidelb). 2016;6(2):237–47. 10.1007/s13555-016-0106-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Halioua B, Cribier B, Frey M, et al. : Feelings of stigmatization in patients with rosacea. J Eur Acad Dermatol Venereol. 2017;31(1):163–8. 10.1111/jdv.13748 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Haber R, El Gemayel M: Comorbidities in rosacea: A systematic review and update. J Am Acad Dermatol. 2018;78(4):786–792.e8. 10.1016/j.jaad.2017.09.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Egeberg A, Hansen PR, Gislason GH, et al. : Patients with rosacea have increased risk of dementia. Ann Neurol. 2016;79(6):921–8. 10.1002/ana.24645 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Holmes AD, Spoendlin J, Chien AL, et al. : Evidence-based update on rosacea comorbidities and their common physiologic pathways. J Am Acad Dermatol. 2018;78(1):156–66. 10.1016/j.jaad.2017.07.055 [DOI] [PubMed] [Google Scholar]
- 17. Tan J, Almeida LM, Bewley A, et al. : Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):431–8. 10.1111/bjd.15122 [DOI] [PubMed] [Google Scholar]
- 18. Schwab VD, Sulk M, Seeliger S, et al. : Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):53–62. 10.1038/jidsymp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buhl T, Sulk M, Nowak P, et al. : Molecular and Morphological Characterization of Inflammatory Infiltrate in Rosacea Reveals Activation of Th1/Th17 Pathways. J Invest Dermatol. 2015;135(9):2198–208. 10.1038/jid.2015.141 [DOI] [PubMed] [Google Scholar]
- 20. Kelhälä HL, Palatsi R, Fyhrquist N, et al. : IL-17/Th17 pathway is activated in acne lesions. PLoS One. 2014;9(8):e105238. 10.1371/journal.pone.0105238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kistowska M, Meier B, Proust T, et al. : Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135(1):110–8. 10.1038/jid.2014.290 [DOI] [PubMed] [Google Scholar]
- 22. Agak GW, Qin M, Nobe J, et al. : Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J Invest Dermatol. 2014;134(2):366–73. 10.1038/jid.2013.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holmes AD: Potential role of microorganisms in the pathogenesis of rosacea. J Am Acad Dermatol. 2013;69(6):1025–32. 10.1016/j.jaad.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 24. Murillo N, Aubert J, Raoult D: Microbiota of Demodex mites from rosacea patients and controls. Microb Pathog. 2014;71–72:37–40. 10.1016/j.micpath.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 25. Murillo N, Mediannikov O, Aubert J, et al. : Bartonella quintana detection in Demodex from erythematotelangiectatic rosacea patients. Int J Infect Dis. 2014;29:176–7. 10.1016/j.ijid.2014.07.021 [DOI] [PubMed] [Google Scholar]
- 26. Koçak M, Yağli S, Vahapoğlu G, et al. : Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. A randomized double-blind placebo-controlled study. Dermatology. 2002;205(3):265–70. 10.1159/000065849 [DOI] [PubMed] [Google Scholar]
- 27. Rebora A, Drago F, Picciotto A: Helicobacter pylori in patients with rosacea. Am J Gastroenterol. 1994;89(9):1603–4. [PubMed] [Google Scholar]
- 28. Kolibásová K, Tóthová I, Baumgartner J, et al. : Eradication of Helicobacter pylori as the only successful treatment in rosacea. Arch Dermatol. 1996;132(11):1393. 10.1001/archderm.1996.03890350137032 [DOI] [PubMed] [Google Scholar]
- 29. Jørgensen AR, Egeberg A, Gideonsson R, et al. : Rosacea is associated with Helicobacter pylori: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2017;31(12):2010–5. 10.1111/jdv.14352 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Yamasaki K, Kanada K, Macleod DT, et al. : TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131(3):688–97. 10.1038/jid.2010.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim JY, Kim YJ, Lim BJ, et al. : Increased expression of cathelicidin by direct activation of protease-activated receptor 2: possible implications on the pathogenesis of rosacea. Yonsei Med J. 2014;55(6):1648–55. 10.3349/ymj.2014.55.6.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Segovia J, Sabbah A, Mgbemena V, et al. : TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One. 2012;7(1):e29695. 10.1371/journal.pone.0029695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meyer-Hoffert U, Schröder JM: Epidermal proteases in the pathogenesis of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):16–23. 10.1038/jidsymp.2011.2 [DOI] [PubMed] [Google Scholar]
- 34. Gerber PA, Buhren BA, Steinhoff M, et al. : Rosacea: The cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15(1):40–7. 10.1038/jidsymp.2011.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steinhoff M, Schauber J, Leyden JJ: New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 Suppl 1):S15–26. 10.1016/j.jaad.2013.04.045 [DOI] [PubMed] [Google Scholar]
- 36. Yamasaki K, Di Nardo A, Bardan A, et al. : Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975–80. 10.1038/nm1616 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Oikonomopoulou K, Hansen KK, Saifeddine M, et al. : Kallikrein-mediated cell signalling: targeting proteinase-activated receptors (PARs). Biol Chem. 2006;387(6):817–24. 10.1515/BC.2006.104 [DOI] [PubMed] [Google Scholar]
- 38. Steinhoff M, Buddenkotte J, Shpacovitch V, et al. : Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26(1):1–43. 10.1210/er.2003-0025 [DOI] [PubMed] [Google Scholar]
- 39. Steinhoff M, Vergnolle N, Young SH, et al. : Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6(2):151–8. 10.1038/72247 [DOI] [PubMed] [Google Scholar]
- 40. Vergnolle N, Bunnett NW, Sharkey KA, et al. : Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7(7):821–6. 10.1038/89945 [DOI] [PubMed] [Google Scholar]
- 41. Shpacovitch VM, Brzoska T, Buddenkotte J, et al. : Agonists of proteinase-activated receptor 2 induce cytokine release and activation of nuclear transcription factor kappaB in human dermal microvascular endothelial cells. J Invest Dermatol. 2002;118(2):380–5. 10.1046/j.0022-202x.2001.01658.x [DOI] [PubMed] [Google Scholar]
- 42. Buddenkotte J, Stroh C, Engels IH, et al. : Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J Invest Dermatol. 2005;124(1):38–45. 10.1111/j.0022-202X.2004.23539.x [DOI] [PubMed] [Google Scholar]
- 43. Moormann C, Artuc M, Pohl E, et al. : Functional characterization and expression analysis of the proteinase-activated receptor-2 in human cutaneous mast cells. J Invest Dermatol. 2006;126(4):746–55. 10.1038/sj.jid.5700169 [DOI] [PubMed] [Google Scholar]
- 44. Morizane S, Yamasaki K, Kabigting FD, et al. : Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D 3, and retinoic acid. J Invest Dermatol. 2010;130(5):1297–306. 10.1038/jid.2009.435 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Demerjian M, Hachem JP, Tschachler E, et al. : Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008;172(1):86–97. 10.2353/ajpath.2008.070161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elias PM: Skin barrier function. Curr Allergy Asthma Rep. 2008;8(4):299–305. 10.1007/s11882-008-0048-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Drago F, De Col E, Agnoletti AF, et al. : The role of small intestinal bacterial overgrowth in rosacea: A 3-year follow-up. J Am Acad Dermatol. 2016;75(3):e113–e115. 10.1016/j.jaad.2016.01.059 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Parodi A, Paolino S, Greco A, et al. : Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6(7):759–64. 10.1016/j.cgh.2008.02.054 [DOI] [PubMed] [Google Scholar]
- 49. Drago F, Ciccarese G, Parodi A: Effects of the treatment for small intestine bacterial overgrowth on rosacea. J Dermatol. 2017;44(12):e321. 10.1111/1346-8138.13985 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Weinstock LB, Steinhoff M: Rosacea and small intestinal bacterial overgrowth: Prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68(5):875–6. 10.1016/j.jaad.2012.11.038 [DOI] [PubMed] [Google Scholar]
- 51. Jarmuda S, O'Reilly N, Zaba R, et al. : Potential role of Demodex mites and bacteria in the induction of rosacea. J Med Microbiol. 2012;61(Pt 11):1504–10. 10.1099/jmm.0.048090-0 [DOI] [PubMed] [Google Scholar]
- 52. Steinhoff M, Buddenkotte J, Aubert J, et al. : Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):2–11. 10.1038/jidsymp.2011.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sulk M, Seeliger S, Aubert J, et al. : Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012;132(4):1253–62. 10.1038/jid.2011.424 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Everaerts W, Gees M, Alpizar YA, et al. : The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21(4):316–21. 10.1016/j.cub.2011.01.031 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Caterina MJ, Schumacher MA, Tominaga M, et al. : The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 56. Story GM, Peier AM, Reeve AJ, et al. : ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–29. 10.1016/S0092-8674(03)00158-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Moore C, Cevikbas F, Pasolli HA, et al. : UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci U S A. 2013;110(34):E3225–34. 10.1073/pnas.1312933110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Mihara S, Shibamoto T: The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin Immunol. 2015;11(1):11. 10.1186/s13223-015-0074-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Drummond PD, Su D: Endothelial and axon reflex vasodilatation to acetylcholine in rosacea-affected skin. Arch Dermatol Res. 2012;304(2):133–7. 10.1007/s00403-011-1177-1 [DOI] [PubMed] [Google Scholar]
- 60. Steinhoff M, Ständer S, Seeliger S, et al. : Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139(11):1479–88. 10.1001/archderm.139.11.1479 [DOI] [PubMed] [Google Scholar]
- 61. Aubdool AA, Brain SD: Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc. 2011;15(1):33–9. 10.1038/jidsymp.2011.8 [DOI] [PubMed] [Google Scholar]
- 62. Baylie RL, Brayden JE: TRPV channels and vascular function. Acta Physiol (Oxf). 2011;203(1):99–116. 10.1111/j.1748-1716.2010.02217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Madva EN, Granstein RD: Nerve-derived transmitters including peptides influence cutaneous immunology. Brain Behav Immun. 2013;34:1–10. 10.1016/j.bbi.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hajna Z, Sághy É, Payrits M, et al. : Capsaicin-Sensitive Sensory Nerves Mediate the Cellular and Microvascular Effects of H 2S via TRPA1 Receptor Activation and Neuropeptide Release. J Mol Neurosci. 2016;60(2):157–70. 10.1007/s12031-016-0802-z [DOI] [PubMed] [Google Scholar]
- 65. Steinhoff M, Schmelz M, Schauber J: Facial Erythema of Rosacea - Aetiology, Different Pathophysiologies and Treatment Options. Acta Derm Venereol. 2016;96(5):579–86. 10.2340/00015555-2335 [DOI] [PubMed] [Google Scholar]
- 66. Metzler-Wilson K, Toma K, Sammons DL, et al. : Augmented supraorbital skin sympathetic nerve activity responses to symptom trigger events in rosacea patients. J Neurophysiol. 2015;114(3):1530–7. 10.1152/jn.00458.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Del Rosso JQ: Management of facial erythema of rosacea: what is the role of topical α-adrenergic receptor agonist therapy? J Am Acad Dermatol. 2013;69(6 Suppl 1):S44–56. 10.1016/j.jaad.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 68. Chang ALS, Raber I, Xu J, et al. : Assessment of the genetic basis of rosacea by genome-wide association study. J Invest Dermatol. 2015;135(6):1548–55. 10.1038/jid.2015.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Woo YR, Lim JH, Cho DH, et al. : Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition. Int J Mol Sci. 2016;17(9): pii: E1562. 10.3390/ijms17091562 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. van Steensel MA, Badeloe S, Winnepenninckx V, et al. : Granulomatous rosacea and Crohn's disease in a patient homozygous for the Crohn-associated NOD2/CARD15 polymorphism R702W. Exp Dermatol. 2008;17(12):1057–8. 10.1111/j.1600-0625.2008.00753.x [DOI] [PubMed] [Google Scholar]
- 71. Egeberg A, Hansen PR, Gislason GH, et al. : Clustering of autoimmune diseases in patients with rosacea. J Am Acad Dermatol. 2016;74(4):667–72.e1. 10.1016/j.jaad.2015.11.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Schaller M, Schöfer H, Homey B, et al. : Rosacea Management: Update on general measures and topical treatment options. J Dtsch Dermatol Ges. 2016;14 Suppl 6:17–27. 10.1111/ddg.13143 [DOI] [PubMed] [Google Scholar]
- 73. Schaller M, Almeida LM, Bewley A, et al. : Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):465–71. 10.1111/bjd.15173 [DOI] [PubMed] [Google Scholar]
- 74. Reinholz M, Ruzicka T, Steinhoff M, et al. : Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14 Suppl 6:4–15. 10.1111/ddg.13139 [DOI] [PubMed] [Google Scholar]
- 75. Jackson JM, Fowler J, Moore A, et al. : Improvement in facial erythema within 30 minutes of initial application of brimonidine tartrate in patients with rosacea. J Drugs Dermatol. 2014;13(6):699–704. [PubMed] [Google Scholar]
- 76. Fowler J, Jr, Jackson M, Moore A, et al. : Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12(6):650–6. [PubMed] [Google Scholar]
- 77. Docherty JR, Steinhoff M, Lorton D, et al. : Multidisciplinary Consideration of Potential Pathophysiologic Mechanisms of Paradoxical Erythema with Topical Brimonidine Therapy. Adv Ther. 2016;33(11):1885–95. 10.1007/s12325-016-0404-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Draelos ZD, Gold MH, Weiss RA, et al. : Efficacy and safety of oxymetazoline cream 1.0% for treatment of persistent facial erythema associated with rosacea: Findings from the 52-week open label REVEAL trial. J Am Acad Dermatol. 2018;78(6):1156–63. 10.1016/j.jaad.2018.01.027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Holmes AD, Steinhoff M: Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp Dermatol. 2017;26(8):659–67. 10.1111/exd.13143 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Pietschke K, Schaller M: Long-term management of distinct facial flushing and persistent erythema of rosacea by treatment with carvedilol. J Dermatolog Treat. 2018;29(3):310–3. 10.1080/09546634.2017.1360991 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Hofmann MA, Lehmann P: Physical modalities for the treatment of rosacea. J Dtsch Dermatol Ges. 2016;14 Suppl 6:38–43. 10.1111/ddg.13144 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Scharschmidt TC, Yost JM, Truong SV, et al. : Neurogenic rosacea: a distinct clinical subtype requiring a modified approach to treatment. Arch Dermatol. 2011;147(1):123–6. 10.1001/archdermatol.2010.413 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Stein L, Kircik L, Fowler J, et al. : Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13(3):316–23. [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Breneman DL, Stewart D, Hevia O, et al. : A double-blind, multicenter clinical trial comparing efficacy of once-daily metronidazole 1 percent cream to vehicle in patients with rosacea. Cutis. 1998;61(1):44–7. [PubMed] [Google Scholar]
- 85. Dahl MV, Katz HI, Krueger GG, et al. : Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134(6):679–83. 10.1001/archderm.134.6.679 [DOI] [PubMed] [Google Scholar]
- 86. Jorizzo JL, Lebwohl M, Tobey RE: The efficacy of metronidazole 1% cream once daily compared with metronidazole 1% cream twice daily and their vehicles in rosacea: A double-blind clinical trial. J Am Acad Dermatol. 1998;39(3):502–4. 10.1016/S0190-9622(98)70337-8 [DOI] [PubMed] [Google Scholar]
- 87. Thiboutot D, Thieroff-Ekerdt R, Graupe K: Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: Results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48(6):836–45. 10.1067/mjd.2003.308 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Del Rosso JQ: Medical treatment of rosacea with emphasis on topical therapies. Expert Opin Pharmacother. 2004;5(1):5–13. 10.1517/14656566.5.1.5 [DOI] [PubMed] [Google Scholar]
- 89. Schaller M, Schöfer H, Homey B, et al. : State of the art: Systemic rosacea management. J Dtsch Dermatol Ges. 2016;14 Suppl 6:29–37. 10.1111/ddg.13141 [DOI] [PubMed] [Google Scholar]
- 90. Fink C, Lackey J, Grande DJ: Rhinophyma: A Treatment Review. Dermatol Surg. 2018;44(2):275–82. 10.1097/DSS.0000000000001406 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Uslu M, Şavk E, Karaman G, et al. : Rosacea treatment with intermediate-dose isotretinoin: Follow-up with erythema and sebum measurements. Acta Derm Venereol. 2012;92(1):73–7. 10.2340/00015555-1204 [DOI] [PubMed] [Google Scholar]
- 92. van Zuuren EJ, Fedorowicz Z: Interventions for Rosacea. JAMA. 2015;314(22):2403–4. 10.1001/jama.2015.15287 [DOI] [PubMed] [Google Scholar]
- 93. Ranu H, Lee J, Hee TH: Therapeutic hotline: Successful treatment of Morbihan's disease with oral prednisolone and doxycycline. Dermatol Ther. 2010;23(6):682–5. 10.1111/j.1529-8019.2010.01373.x [DOI] [PubMed] [Google Scholar]
- 94. Schaller M, Pietschke K: Successful therapy of ocular rosacea with topical ivermectin. Br J Dermatol. 2018;179(2):520–1. 10.1111/bjd.16534 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Steinhoff M, Vocanson M, Voegel JJ, et al. : Topical Ivermectin 10 mg/g and Oral Doxycycline 40 mg Modified-Release: Current Evidence on the Complementary Use of Anti-Inflammatory Rosacea Treatments. Adv Ther. 2016;33(9):1481–501. 10.1007/s12325-016-0380-z [DOI] [PMC free article] [PubMed] [Google Scholar]