Abstract
Background: Insecticide treated nets (ITNs) protect humans against bites from the Anopheles mosquito vectors that transmit malaria, thereby reducing malaria morbidity and mortality. It has been noted that ITN use leads to a switch from indoor to outdoor feeding among these vectors. It might be expected that outdoor feeding would undermine the effectiveness of ITNs that target indoors vectors, but data are limited. Methods: We linked homestead level geospatial data to clinical surveillance data at a primary healthcare facility in Kilifi County in order to map geographical heterogeneity in ITN effectiveness and observed vector feeding behaviour using landing catches and CDC light traps in seven selected areas of high and low ITN effectiveness. Results: We observed 33% and 39% visits associated with positive malaria slides among ITN users and non-ITN-users, respectively; ITN use was associated with 22% protection from malaria (crude OR = 0.78, 95% CI: 0.72, 0.84). We obtained significant modification of ITN effectiveness by geographical area (p=0.022), and identified significant hotspots using the spatial scan statistic. Most biting occurred outdoors (62%) and was by An. funestus (76%), and appeared to be more frequent in low ITN effectiveness areas compared with high ITN effectiveness areas (69% vs. 26%, p<0.001), but this was due to a single outlying area. After excluding this outlying area, outdoor biting was similar in low vs. high ITN effectiveness area (69% vs. 75%, p=0.76). Conclusion: Our data therefore do not support the hypothesis that outdoor biting undermines the effectiveness of ITNs in our study area.
Keywords: ITNs, outdoor, Anopheles mosquito, effectiveness, Kilifi, Kenya, KHDSS
Introduction
Despite the recent scale-up effort to achieve control, malaria continues to cause morbidity and mortality, especially in sub-Saharan Africa. There are uncertainties in global estimates 1– 3; however in 2015, the World Health Organization estimated global deaths due to malaria to be 438,000 (range: 236,000–635,000) and the burden of febrile illness at 214 million cases (range: 149–303 million) 4. Modelling studies suggest that approximately 1.4 billion of the world’s population live at risk of stable malaria and ~1.1 billion at risk of unstable malaria 5.
The frontline tools for malaria control in sub-Saharan Africa, insecticide treated nets (ITNs) and indoor residual spray, are optimally effective if baseline transmission occurs indoors 6. The major vectors of human malaria mostly feed indoors, and transmission can therefore be substantially reduced by these tools 6. The proportion of the at risk population who have access to ITNs was modeled to have increased from 4% to 67% between 2004 and 2015 7. ITNs operate in three ways: deterrence, excito-repellence and killing, thereby reducing the density, feeding frequency, feeding success, and survival of Anopheles mosquito vectors 6. By reducing vector densities and vector survival, ITNs not only directly protect the individual ITN user, but also reduce the overall transmission intensity and protect the whole community when a particular threshold of bed net coverage is reached 8– 10. The evidence base supports ITN use over a range of transmission intensities 11 and protective efficacy has been demonstrated against infection, clinical disease and mortality 12– 16. However, residual malaria transmission is well described even after optimal ITN use, which could be caused by changes in the behaviour of the mosquito vector that allows them to evade fatal contact with these frontline tools of intervention 17, 18. The most obvious behavioural change is the mosquito vector exhibiting exophagic tendencies – i.e. the vector feeds outdoors on humans.
Among malaria vectors in Africa, the two principal species complexes are: Anopheles gambiae sensu lato (s.l.) and Anopheles funestus group. Both species complexes feed primarily indoors; however both have exhibited behavioral shifts to outdoor biting or feeding in the early part of the evening following prolonged use of ITNs in some areas 6, 19, 20. This behavioral change might have resulted from one of three processes: (i) selection, either for species that more readily engages in outdoor feeding, for instance in favour of An. arabiensis rather than An. gambiae sensu strictu (s.s.); (ii) by selecting for evolutionary change within a species; or (iii) a response to inability to feed during the night in the absence of genetic variation 21. In Western Kenya and South-eastern Tanzania there have been reports of a reduction in indoor feeding by An. gambiae sensu stricto ( s.s.) and an increase in the relative abundance of An. arabiensis. The latter has a broader range of feeding times and biting behavior, including outdoor feeding 8, 21– 23. In northern Tanzania, where ITNs have been used for several years, the mosquitoes are biting more frequently during the hours of the early evening and early morning when people are more likely to be awake and vulnerable outside of their nets 6, 24. The potential for ITNs to result in species switches was appreciated in earlier controlled trials 21, 22, 25, and is now reported more widely as ITN use is scaled up in Western Kenya and on the East African coast 21, 22.
In Kilifi, Kenya, a switch in the most common vector, from An. gambiae sensu stricto (s.s.) to An. arabienses, occurred during the period of ITN scale-up 19. The increased ability of An. arabiensis to feed outdoors might be expected to result in a decrease in ITN effectiveness. However, there is little data to support this contention, and some data and models that are available suggest that ITNs continue to be effective despite outdoor feeding 26, 27. The objectives of this study were (i) to examine whether there has been a shift in vector biting patterns and/or vector behaviour, during the period of intense ITN use along the Kenyan coast; (ii) to test for geographical heterogeneity in ITN effectiveness within the surveillance area of a primary healthcare facility in Kilifi County; and (iii) to assess whether outdoor vector biting is a potential explanation for the variation in ITN effectiveness.
Methods
Study area
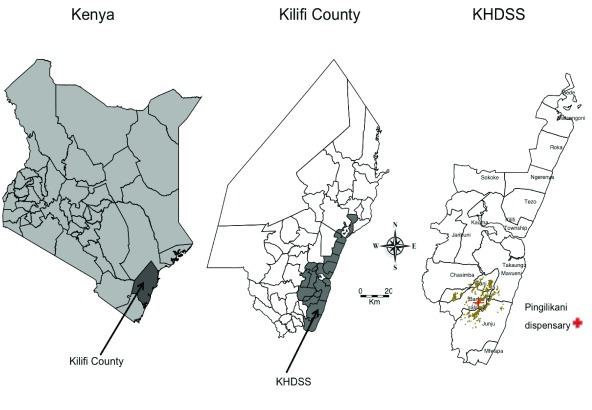
The clinical surveillance study was conducted between January 2009 and December 2014 within a 6km radius of Pingilikani dispensary in Kilifi County on the Kenyan Coast ( Figure 1): within the Kilifi Health and Demographic Surveillance System (KHDSS). All children under 13 years presenting for medical assessment to Pingilikani dispensary (except those with trauma as their only concern) were assessed by research staff and had finger-prick blood samples examined for malaria parasites. Thick and thin blood smears were stained with 10% Giemsa and examined at 1000X magnification for asexual Plasmodium falciparum parasites. Before slides could be considered negative, 100 fields were examined. Children with malaria positive slides were treated with co-artemether.
Figure 1. Situation of Kilifi County in Kenya and the map of Kilifi County showing the boundaries of the KHDSS.
The map of KHDSS shows the locations and the situation of homesteads and Pingilikani dispensary where the study was conducted. The brown plotted point on the KHDSS map represents homesteads.
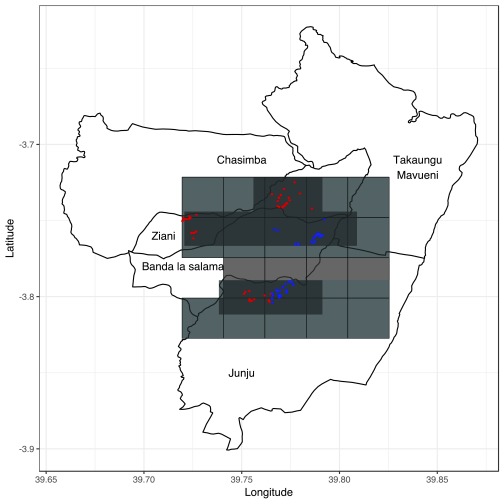
Transmission of malaria peaks after the long rains from April to June and the short rains from October to November each year, although transmission has been declining 28– 31. The surveillance area was divided into 2.5x2.5 km regular polygons resulting in 21 geographical areas ( Figure 2). As part of KHDSS, four-monthly enumeration rounds were conducted to identify births, deaths and migration events. Each inhabitant was described by their family relationships and their homestead of residence, with geospatial coordinates, and assigned a unique personal identifier 32. These details were used to link children visiting Pingilikani dispensary to geospatial coordinates for the homestead of residence. Data on ITN use was collected once yearly during cross-sectional surveys integrated into the regular KHDSS enumeration since 2008. Questionnaires were used to collect household data on ITN ownership and use on the night prior to enumeration. Seven geographical areas were selected for mosquito sampling out of 21 areas for which clinical effectiveness estimates were determined ( Figure 2). The basis of selecting the seven areas was (i) geographical areas with >60 homesteads available for randomization; (ii) areas representative of highest and lowest ITN effectiveness.
Figure 2. Map of the 2.5X2.5 km geographical areas (grids in light gray), the geographical areas where mosquito sampling was conducted (grids in dark gray) and the homesteads where mosquito sampling was done.
Each plotted point represents an individual homestead, where color shading indicates ITN effectiveness, with red shading indicating low effectiveness and blue shading indicating high effectiveness.
Mosquito sampling
Indoor and outdoor biting profiles of An. gambiae s.l. and the An. funestus group were estimated using human landing catches (HLC) and CDC-light traps (CDC-LT) by visiting randomly selected houses (random selection done by stratified sampling) between July and August 2016. For both indoor and outdoor mosquito collection, HLC was conducted by two pairs of trained male volunteers (one pair was located indoors and the other pair outdoors, but at the same homestead), who sat with their legs exposed and caught mosquitoes that attempted to bite them using an aspirator. HLC was conducted between 18:00hours and 06:00hours for 45 minutes each hour, allowing 15 minutes break for rest. The catches for each hourly interval were stored in separate collection cups. CDC-light traps were also set indoor and outdoor between 18:00hours and 06:00hours. The HLC and the CDC-LT collections took place in different houses. In each geographical area, sampling was conducted for at least 3 days in at least 16 houses; 8 houses for HLC and 8 houses for CDC-LT. In total, 26 days of sampling were conducted across 115 houses in the seven selected geographical areas within the surveillance area.
Mosquito processing
The mosquito samples were morphologically separated for sex and identified for species 6, 24. The female Anopheles mosquitoes were tested for falciparum infection using a sandwich circumsporozoite protein (CSP) enzyme linked immunosorbent assay (ELISA) 33 (anti-CSP capture: Pf2A10-28 and conjugate : Pf2A10-CDC antibodies; KPL, Gaithersburg, MD, USA). Individual mosquitoes were stored at -20°C in micro-centrifuge tubes containing a small amount of desiccant (silica gel) separated from the mosquito by a thin layer of cotton prior to ELISA and molecular analysis for sibling species by polymerase chain reaction 6, 34.
Statistical analysis
Statistical analyses were performed using STATA v13.1 (StataCorp, College Station, TX, USA). To assess for geographical heterogeneity, we used the logistic regression model to analyze data on over 20,000 visits from children attending Pingilikani dispensary. The outcome of interest was presence of malaria by microscopy on presentation to the dispensary. The potential risk factors included: ITN use, age of the child, year of presentation to the dispensary and the geographical area, as defined by the 2.5×2.5 km regular polygons. We assessed whether the effect of ITN use on malaria was altered by geographical area by including an interaction term between geographical area and ITN use. We also assessed whether the effect of ITN use was altered by the age of the child and whether geographical areas altered the effect of age. To assess the nonlinear effect of age in the regression models, multiple fractional polynomial transformation was used. Given that the hospital malaria episodes were clustered within patients, we allowed for clustering by using a logistic regression model with robust standard errors. The robust standard errors were used to account for the clustering effect in the estimation of the standard errors. The ratio of malaria in the non-ITN users to that in the ITN users was expressed as an odds ratio (OR) as determined by logistic regression. ITN effectiveness was calculated as (1 – OR) × 100. Model fit was assessed by examining residuals against covariates. Spearman’s rank correlation was used to assess the association between ITN effectiveness and prevalence of malaria. SaTScan software (version 9.4; https://www.satscan.org/), a spatial scan statistic developed by Kulldorf 35, was used to detect potential spatial variations of ITN effectiveness by identifying statistically significant geographical clustering of ITN effectiveness.
In order to compare counts of female Anopheles captured, we determined the relative proportion of each mosquito species in each geographical area and ITN effectiveness levels (ITN effectiveness was divided into 2 levels based on the estimates obtained from the logistic regression above – i.e. high and low ITN effectiveness). Three areas with high ITN effectiveness and four areas with low ITN effectiveness were selected based on the findings of the scan statistic. We compared the proportion of vectors biting outdoors in each geographical area. We estimated the confidence intervals of these proportions using the binomial distributions, and tested for an association between biting preference and ITN effectiveness (at the level of geographical area).
Results
Geographical variations in ITN effectiveness
Between 2009 and 2014, there were 20,827 visits to Pingilikani dispensary made by 4,992 children aged between 3 months to 12 years ( Supplementary Table 1). Of these visits, 7220 (35%) were classified as episodes of malaria, with a median number of 7 (IQR: 4,12) episodes per child during this time period. The number of children, cases of malaria and ITN use in the 21 geographical areas examined is summarized in detail in Supplementary Table 1. ITN use was consistently >50% in all geographical areas and the prevalence of ITN use in non-malaria cases was 74.9% (95% CI: 74.2, 75.6).
Table 1. Proportion of Anopheles mosquitoes collected indoors and outdoors.
| Number
collected |
% Indoor (CI) | % Outdoor (CI) | |
|---|---|---|---|
| All | 411 | 38 [33, 43] | 62 [57, 67] |
| Species | |||
| An. gambiae s.l. | 76 | 17 [9, 27] | 83 [73, 91] |
| An. funestus | 314 | 46 [40, 52] | 54 [48, 60] |
| Other anopheles | 21 | 0 | 100 [84, 100 *] |
| Geographical area | |||
| 5 | 171 | 46 [38, 53] | 54 [47, 62] |
| 6 | 59 | 83 [71, 92] | 17 [8, 29] |
| 13 | 9 | 44 [14, 79] | 56 [21, 86] |
| 15 | 6 | 33 [4, 78] | 67 [22, 96] |
| 16 | 6 | 17 [0.4, 64] | 83 [36, 99] |
| 19 | 113 | 12 [7, 20] | 88 [80, 93] |
| 20 | 47 | 19 [9, 33] | 81 [67, 91] |
*CI: Confidence Interval, %: Proportion per 100
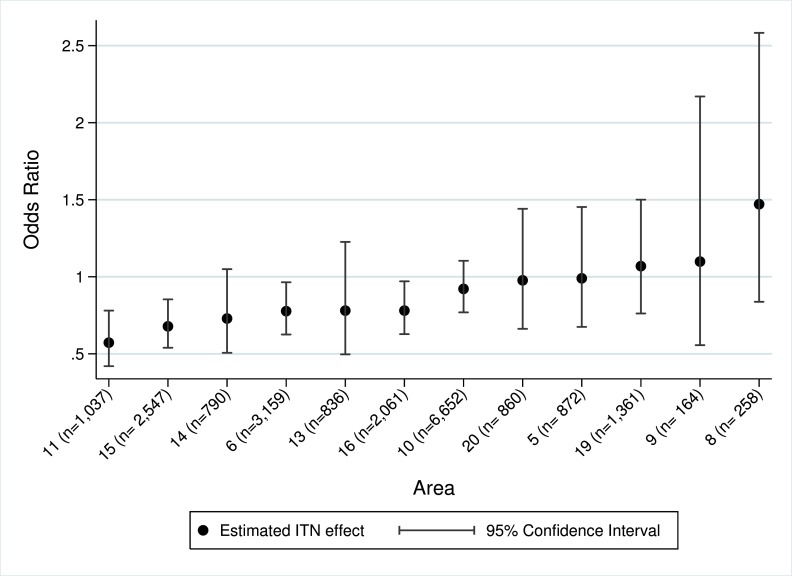
Among children who were ITN users, 33% (5045/15234) of the visits were associated with positive malaria slides, whereas among non-ITN-users 39% (2175/5593) of the visits were associated with positive malaria slides. ITN use was associated with a 22% protection from malaria; crude OR = 0.78, 95% CI: 0.72, 0.84 (p<0.001). When geographical area was added to the model as an interaction term with ITN use, we obtained a statistically significant variation in ITN effectiveness between the geographical areas (p=0.014). Geographical variation in ITN effectiveness remained robust (p=0.022) even after adjusting for the year of visitation to the dispensary and plausible interactions (i.e. interactions between ITN use and nonlinear age, and between geographical area and nonlinear age). The stratum specific adjusted OR for the association of ITN use on malaria in the geographical areas was calculated and shown in the order of decreasing effectiveness ( Figure 3 & Supplementary Table 1). Previous data have shown that ITN effectiveness is lower in areas of high malaria transmission 11, 36. This did not appear to be the explanation for variation in effectiveness in this data ( Supplementary Figure 1); the Spearman rho coefficient value for the association of ITN effectiveness and prevalence of malaria was 0.308, p=0.331.
Figure 3. Scatter plot of stratum specific adjusted Odds Ratio and 95% confidence intervals of 12 geographical areas in order of decreasing effectiveness.
Hotspots
Using the logistic regression model, we estimated ITN effectiveness for each individual homestead where there was sufficient data to calculate a point estimate (i.e. >30 observations from homestead aggregated at a 2.5 km smoothing). Using SaTScan software, we identified 6 significant hotspots of low ITN effectiveness: p=0.001 for 4 hotspots, p=0.002 and p=0.014 for a 5th and 6th hotspot ( Figure 4). We concluded that spatial variation in ITN effectiveness was not due to random noise based on the 95% confidence intervals obtained from the logistic regression analysis for geographical areas and the existence of significant hotspots by SaTScan, and selected seven geographical areas for further entomological studies to represent a range of ITN effectiveness estimates.
Figure 4. Scatter plot of estimated insecticide treated net (ITN) effectiveness for individual homesteads aggregated at a 2.5km smoothing.
Each plotted point represents an individual homestead, where color shading indicates ITN effectiveness, with red shading indicating low effectiveness and blue shading indicating high effectiveness. The large black circles indicate the significant hotspots (analyzed without smoothing).
Vector abundance
Over 26 nights, 411 female Anopheles mosquitoes were collected by both methods (i.e. 259 by HLC and 152 by CDC-LT), representing a mean of 15.8 mosquitoes per night. 63% of mosquitoes were collected using HLC. Of the 411 mosquitoes, 314 (76%, 95% CIs 72%, 80%) were An. funestus group, which was significantly greater than An. gambiae s.l (p<0.001). The proportion of A nopheles mosquitoes caught outdoors (62%; 95% CI: 57,67) was significantly greater than the proportion caught indoors (p<0.001). There were more Anopheles mosquitoes collected outdoors in all geographical areas except area 6, where most of the mosquitoes were collected indoors ( Table 1). The frequencies of species collected in each geographical area are summarized in Supplementary Table 2. An. funestus group was the most prevalent species in all areas.
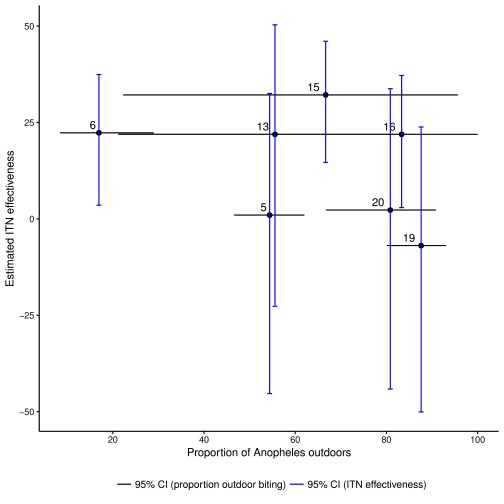
The species and proportion of mosquitoes collected in areas of high vs. low ITN effectiveness are summarised in Table 2 and Supplementary Figure 2. Overall, the proportion of outdoor biting was higher in the low ITN effectiveness areas (69% vs. 27%, p <0.001), but this apparent significance was due to a single area (labelled area 6), which was an outlier for indoor biting ( Figure 5). When we excluded area 6, the proportion of outdoor biting in the low vs. high ITN effectiveness areas was non-significant (69% vs. 75%, p=0.76). Moreover, when analysed by individual geographical area there was not a visually obvious trend associating increasing outdoor biting with decreasing ITN effectiveness in the seven geographical areas ( Figure 5). The Spearman rho coefficient value for the association of ITN effectiveness and proportion of mosquitoes collected outdoors was -0.464, p=0.302.
Table 2. Composition of the mosquito species in areas of high and low ITN effectiveness.
| Trap type | Species | Low ITN effectiveness areas | High ITN effectiveness area | ||||
|---|---|---|---|---|---|---|---|
| Total
(N) |
Outdoor
(n) |
Outdoor
(%) |
Total
(N) |
Outdoor
(n) |
Outdoor
(%) |
||
| HLC | An. gambiae | 57 | 45 | 78.9 | 5 | 4 | 80.0 |
| An. funestus | 152 | 69 | 45.4 | 24 | 10 | 41.7 | |
| Other Anopheles | 21 | 21 | 100 | 0 | 0 | 0 | |
| Total | 230 | 135 | 58.7 | 29 | 14 | 48.3 | |
| CDC-LT | An. gambiae | 14 | 14 | 100 | 0 | 0 | 0 |
| An. funestus | 96 | 86 | 89.6 | 42 | 5 | 11.9 | |
| Other Anopheles | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 110 | 100 | 90.9 | 42 | 5 | 11.9 | |
*HLC: Human landing catches, CDC-LT: CDC light trap, %: Proportion per 100, N & n: number of mosquitoes collected.
Figure 5. Scatter plot of estimated insecticide treated net (ITN) effectiveness and the proportion of Anopheles mosquitoes collected outdoors and 95% confidence intervals of these estimates.
Discussion
Malaria is an important public health problem in sub-Saharan Africa, and many countries, including Kenya, have attempted to reduce this burden by increasing ITN ownership and usage 37, 38. However, previous reports have shown that prolonged ITN use leads to behavioral shifts in the mosquito vector from indoor to outdoor biting or feeding in the early part of the evening 6, 19, 24. This shift in mosquito feeding behavior might be expected to result in a decrease in ITN effectiveness. We identified statistically significant geographical variation in the effectiveness of ITN and identified areas where ITN effectiveness was found to be consistent with the 50% estimate reported in the literature 11, 39, 40, and other areas where ITNs were less effective ( Figure 3). This variation could conceivably have arisen as a result of variations in quality of ITNs, patterns of use, host resistance, insecticide resistance or other factors, including random variation. We investigated whether variations in outdoor vector biting was a potential explanation.
We found that An. funestus was more prevalent than An. gambiae s.l. species complex, consistent with a previous report 19. We observed small-scale spatial variability in vector abundance ( Table 1), which is consistent with previous reports on the Kenyan Coast 20, 41. We also observed a higher proportion of mosquito vectors collected outdoors than indoors, in areas of both high and low ITN effectiveness ( Figure 5). On first principles one would expect that outdoor biting would lead to ITNs becoming ineffective. However, despite seeing consistent outdoor biting throughout the study area this did not appear to be associated with an overall reduction in ITN effectiveness. We may have observed an apparently statistically significant increase in the prevalence of outdoor biting in areas of low ITN effectiveness. However, this was due to a single outlying geographical area and there was no variation in prevalence of outdoor biting after this area was excluded. This suggests the statistical significance of the initial comparison may have been due to ecological confounding, where a geographical area with high ITN effectiveness happened to have more indoor mosquitoes, but this relationship was not confirmed in other areas ( Figure 5).
How should we interpret the finding that outdoor feeding does not consistently lead to a reduction in ITN effectiveness? It is possible that the higher proportion of mosquitoes biting outdoors represents a behavioral response to unsuccessful feeding attempts made indoors during the night, and therefore it may simply be a marker of successful ITN use. This avoidance behavior may exert a cost on the vector, and so ITNs may in fact still be protective in areas where outdoor biting is observed, as has been suggested previously 27.
Spatial heterogeneity in malaria exposure has been described at micro-epidemiological level at varying transmission settings 42 and is responsible for variations in disease risk within small geographical areas and is evidenced by local clustering of malaria infections. Within the 2.5 km squared geographical areas, ITN effectiveness appears to have been spatially heterogeneous ( Figure 4); however, we were unable to demonstrate a significant association between ITN effectiveness and outdoor biting at the level of seven small geographical areas. The observed geographical variation in ITN effectiveness therefore remains unexplained. Possibilities include insecticide resistance, or geographical variations in human behaviour in terms of ITN use.
Our study has some limitations. Data on ITN use may have been incorrectly reported, as we did not require each resident to be present to respond to the ITN ownership and use questions. We attempted to minimize this by instructing data collecting teams to interview only residents of the same homestead regarding ITN ownership and usage. There may have been some misclassification as we did not ascertain ITN use during hospital visitation but instead used the yearly ITN data collected by the KHDSS. The results may also be biased and confounded by other unmeasured factors (e.g., variation in the quality and type of ITN, urbanization, socio-economic status and mother’s education). Therefore, the estimates obtained could be an overestimation or underestimation of the true effectiveness. It is likely that we underestimated the protection afforded by the use of high-quality ITN because we included all ITNs, regardless of quality. The vast majority of ITNs in the area are long-lasting insecticidal nets, hence we do not expect substantial variation in insecticidal efficacy. The accuracy of the human landing catches may be affected by the inter-individual differences in attracting mosquitoes. The size of our study limits power: with a sample size of 411, and the proportion of mosquitoes biting outdoors at 69% in low ITN effectiveness areas we therefore had >90% power to detect a reduction to 27% or lower in high ITN effectiveness areas. Our study was therefore powered to detect only a large difference in the proportion of vectors caught outdoors. However, we reasoned that reductions of ITN effectiveness to less than half of the previously documented efficacy of 50% would require a doubling of the proportion of mosquitoes feeding outdoors. Hence our study was powered to detect large variations in the frequency of outdoor biting. Furthermore, since the proportion of vectors collected outdoors was high throughout the study area despite preserved ITN effectiveness in many areas, we conclude that the pattern of outdoor feeding identified in our site does not undermine ITN effectiveness.
In summary, we found no evidence that the currently observed switch from indoor to outdoor biting leads to reduced ITN effectiveness. The outdoor biting observed may therefore have been the result of high levels of ITN use leading to unsuccessful attempts at indoor feeding. It remains possible that selection pressures might lead to the emergence of populations of mosquitoes that are better adapted to outdoor feeding. Outdoor feeding is becoming more common in parts of Africa 43 and may represent evolutionary change in some areas, with a potential to undermine ITN effectiveness. Therefore, malaria control programs require monitoring to assess the impact of ITNs on vector populations and vector behavioral change as well as monitoring ITN effectiveness as vectors evolve 6, 21, 22, 24, 25. Detailed studies of vector bionomics, continuous monitoring and malaria transmission dynamics are essential for predicting disease outbreaks and vector control in the region.
Ethical approval
This study was approved by the Kenya Medical Research Institute Scientific Ethics Review Unit (KEMRI/SERU/CGMR-C/024/3148). Written informed consent was obtained from the parents/guardians of the children attending the dispensary.
Data availability
Data that support the findings of this study (hospital surveillance, ITN community surveys and mosquito collection) are available from the KEMRI Institutional Data Access/Ethics Committee, for researchers who meet the criteria for access to confidential data. Details of the criteria can be found in the KEMRI-Wellcome data sharing guidelines. The data includes homestead level coordinates as an essential component and these are personally identifiable data. Access to data is provided via the KEMRI-Wellcome Data Governance Committee: Data_Governance_Committee@kemriwellcome.org; Tel, +254708 587 210; Contact person, Marianne Munene (Secretary; Tel, +254709 983 436).
Acknowledgements
We are grateful to the entomology field workers, Festus Yaa, Gabriel Nzae and David Shida, who helped with the fieldwork implementation of the study.
This paper is published with the permission of the Director of KEMRI.
Funding Statement
This work was supported by the Wellcome Trust [104015].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 4 approved with reservations]
Supplementary material
Supplementary Table 1: Description of insecticide treated net (ITN) use, cases of malaria and ITN effectiveness in the 2.5x2.5 km geographical areas. Data includes the number of children observed, number visits made to Pingilikani hospital by the children, the number and proportion of malaria among ITN use or non-ITN-users in the 21 geographical areas, the stratum specific adjusted Odds Ratio (aOR) and the Confidence Interval (95% CI); ‡ areas with fewer than 35 observations were excluded from the logistic regression due to perfect prediction and/or collinearity.
Supplementary Table 2: Composition of the mosquito species in seven geographical areas. Data includes number of Anopheles mosquitoes collected by human landing catches (HLC) and CDC light trap (CDC-LT) indoor or outdoor in the seven geographical areas, and the overall proportion.
References
- 1. Snow RW, Guerra CA, Noor AM, et al. : The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–217. 10.1038/nature03342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nkumama IN, O'Meara WP, Osier FH: Changes in Malaria Epidemiology in Africa and New Challenges for Elimination. Trends Parasitol. 2017;33(2):128–140. 10.1016/j.pt.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cibulskis RE, Aregawi M, Williams R, et al. : Worldwide incidence of malaria in 2009: estimates, time trends, and a critique of methods. PLoS Med. 2011;8(12):e1001142. 10.1371/journal.pmed.1001142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO: World malaria report. World Health Organization. 2015. Reference Source [Google Scholar]
- 5. Gething PW, Patil AP, Smith DL, et al. : A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. 10.1186/1475-2875-10-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell TL, Govella NJ, Azizi S, et al. : Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. 10.1186/1475-2875-10-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker PG, Griffin JT, Ferguson NM, et al. : Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Glob Health. 2016;4(7):e474–484. 10.1016/S2214-109X(16)30073-0 [DOI] [PubMed] [Google Scholar]
- 8. Gimnig JE, Vulule JM, Lo TQ, et al. : Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. Am J Trop Med Hyg. 2003;68(4 Suppl):16–22. [PubMed] [Google Scholar]
- 9. Howard SC, Omumbo J, Nevill C, et al. : Evidence for a mass community effect of insecticide-treated bednets on the incidence of malaria on the Kenyan coast. Trans R Soc Trop Med Hyg. 2000;94(4):357–360. 10.1016/S0035-9203(00)90103-2 [DOI] [PubMed] [Google Scholar]
- 10. Lindblade KA, Eisele TP, Gimnig JE, et al. : Sustainability of reductions in malaria transmission and infant mortality in western Kenya with use of insecticide-treated bednets: 4 to 6 years of follow-up. JAMA. 2004;291(21):2571–2580. 10.1001/jama.291.21.2571 [DOI] [PubMed] [Google Scholar]
- 11. Lengeler C: Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004; (2):CD000363. 10.1002/14651858.CD000363.pub2 [DOI] [PubMed] [Google Scholar]
- 12. Binka FN, Hodgson A, Adjuik M, et al. : Mortality in a seven-and-a-half-year follow-up of a trial of insecticide-treated mosquito nets in Ghana. Trans R Soc Trop Med Hyg. 2002;96(6):597–599. 10.1016/S0035-9203(02)90321-4 [DOI] [PubMed] [Google Scholar]
- 13. ter Kuile FO, Terlouw DJ, Phillips-Howard PA, et al. : Impact of permethrin-treated bed nets on malaria and all-cause morbidity in young children in an area of intense perennial malaria transmission in western Kenya: cross-sectional survey. Am J Trop Med Hyg. 2003;68(4 Suppl):100–107. [PubMed] [Google Scholar]
- 14. Phillips-Howard PA, Nahlen BL, Kolczak MS, et al. : Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68(4 Suppl):23–29. [PubMed] [Google Scholar]
- 15. Noor AM, Moloney G, Borle M, et al. : The use of mosquito nets and the prevalence of Plasmodium falciparum infection in rural South Central Somalia. PLoS One. 2008;3(5):e2081. 10.1371/journal.pone.0002081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Snow RW, Rowan KM, Greenwood BM: A trial of permethrin-treated bed nets in the prevention of malaria in Gambian children. Trans R Soc Trop Med Hyg. 1987;81(4):563–567. 10.1016/0035-9203(87)90408-1 [DOI] [PubMed] [Google Scholar]
- 17. Killeen GF, Govella NJ, Lwetoijera DW, et al. : Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J. 2016;15:225. 10.1186/s12936-016-1280-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Govella NJ, Chaki PP, Killeen GF: Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar J. 2013;12:124. 10.1186/1475-2875-12-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mwangangi JM, Mbogo CM, Orindi BO, et al. : Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13. 10.1186/1475-2875-12-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mbogo CN, Baya NM, Ofulla AV, et al. : The impact of permethrin-impregnated bednets on malaria vectors of the Kenyan coast. Med Vet Entomol. 1996;10(3):251–259. 10.1111/j.1365-2915.1996.tb00739.x [DOI] [PubMed] [Google Scholar]
- 21. Bayoh MN, Mathias DK, Odiere MR, et al. : Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. 10.1186/1475-2875-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mutuku FM, King CH, Mungai P, et al. : Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J. 2011;10:356. 10.1186/1475-2875-10-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindblade KA, Gimnig JE, Kamau L, et al. : Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. J Med Entomol. 2006;43(2):428–432. [DOI] [PubMed] [Google Scholar]
- 24. Killeen GF, Kihonda J, Lyimo E, et al. : Quantifying behavioural interactions between humans and mosquitoes: evaluating the protective efficacy of insecticidal nets against malaria transmission in rural Tanzania. BMC Infect Dis. 2006;6:161. 10.1186/1471-2334-6-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gimnig JE, Kolczak MS, Hightower AW, et al. : Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in western Kenya. Am J Trop Med Hyg. 2003;68(4 Suppl):115–120. [PubMed] [Google Scholar]
- 26. Charlwood JD, Alcantara J, Pinto J, et al. : Do bednets reduce malaria transmission by exophagic mosquitoes? Trans R Soc Trop Med Hyg. 2005;99(12):901–904. 10.1016/j.trstmh.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 27. Govella NJ, Okumu FO, Killeen GF: Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg. 2010;82(3):415–419. 10.4269/ajtmh.2010.09-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Meara WP, Bejon P, Mwangi TW, et al. : Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372(9649):1555–1562. 10.1016/S0140-6736(08)61655-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okiro EA, Alegana VA, Noor AM, et al. : Malaria paediatric hospitalization between 1999 and 2008 across Kenya. BMC Med. 2009;7:75. 10.1186/1741-7015-7-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mogeni P, Williams TN, Fegan G, et al. : Age, Spatial, and Temporal Variations in Hospital Admissions with Malaria in Kilifi County, Kenya: A 25-Year Longitudinal Observational Study. PLoS Med. 2016;13(6):e1002047. 10.1371/journal.pmed.1002047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Snow RW, Kibuchi E, Karuri SW, et al. : Changing Malaria Prevalence on the Kenyan Coast since 1974: Climate, Drugs and Vector Control. PLoS One. 2015;10(6):e0128792. 10.1371/journal.pone.0128792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott JA, Bauni E, Moisi JC, et al. : Profile: The Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol. 2012;41(3):650–657. 10.1093/ije/dys062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wirtz RA, Zavala F, Charoenvit Y, et al. : Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65(1):39–45. [PMC free article] [PubMed] [Google Scholar]
- 34. Scott JA, Brogdon WG, Collins FH: Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49(4):520–529. [DOI] [PubMed] [Google Scholar]
- 35. Kulldorff M: A spatial scan statistic. Commun Stat Theory. 1997;26(6):1481–1496. 10.1080/03610929708831995 [DOI] [Google Scholar]
- 36. Bermejo A, Veeken H: Insecticide-impregnated bed nets for malaria control: a review of the field trials. Bull World Health Organ. 1992;70(3):293–296. [PMC free article] [PubMed] [Google Scholar]
- 37. Noor AM, Mutheu JJ, Tatem AJ, et al. : Insecticide-treated net coverage in Africa: mapping progress in 2000–07. Lancet. 2009;373(9657):58–67. 10.1016/S0140-6736(08)61596-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fegan GW, Noor AM, Akhwale WS, et al. : Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: a longitudinal study. Lancet. 2007;370(9592):1035–1039. 10.1016/S0140-6736(07)61477-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nevill CG, Some ES, Mung'ala VO, et al. : Insecticide-treated bednets reduce mortality and severe morbidity from malaria among children on the Kenyan coast. Trop Med Int Health. 1996;1(2):139–146. 10.1111/j.1365-3156.1996.tb00019.x [DOI] [PubMed] [Google Scholar]
- 40. Phillips-Howard PA, Nahlen BL, Alaii JA, et al. : The efficacy of permethrin-treated bed nets on child mortality and morbidity in western Kenya I. Development of infrastructure and description of study site. Am J Trop Med Hyg. 2003;68(4 Suppl):3–9. [PubMed] [Google Scholar]
- 41. Mbogo CM, Mwangangi JM, Nzovu J, et al. : Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg. 2003;68(6):734–742. [PubMed] [Google Scholar]
- 42. Kreuels B, Kobbe R, Adjei S, et al. : Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J Infect Dis. 2008;197(1):85–93. 10.1086/524066 [DOI] [PubMed] [Google Scholar]
- 43. Githeko AK, Adungo NI, Karanja DM, et al. : Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82(3):306–315. [DOI] [PubMed] [Google Scholar]