Abstract
Objective. We aimed to examine whether the current users of specific NSAIDs have an increased risk of venous thromboembolism (VTE) among knee OA patients.
Methods. We conducted a population-based case-control study using The Health Improvement Network, a database of patient records from general practices in the UK. For every VTE case, we identified five controls matched on age, sex and calendar year of study enrolment. We used conditional logistic regression to assess the association between current use of specific NSAIDs and risk of VTE relative to remote NSAID users.
Results. Among knee OA patients with at least one NSAID prescription, we identified 4020 incident cases of VTE and 20 059 matched controls. Adjusted odd ratios (ORs) relative to the remote users were 1.38 (95% CI: 1.32, 1.44) for recent users and 1.43 (95% CI: 1.36, 1.49) for current users. Among the current NSAID users, the risk of VTE was increased with diclofenac [OR 1.63 (95% CI: 1.53, 1.74)], ibuprofen [OR = 1.49 (95% CI: 1.38, 1.62)], meloxicam [OR = 1.29 (95% CI: 1.11, 1.50)] and coxibs [celecoxib, OR = 1.30 (95% CI: 1.11, 1.51); rofecoxib, OR = 1.44 (95% CI: 1.18, 1.76)]; naproxen did not increase VTE risk [OR = 1.00 (95% CI: 0.89, 1.12)].
Conclusion. Compared with the remote users of NSAIDs, the risk of VTE increased for current users of diclofenac, ibuprofen, meloxicam, and coxibs, but not for naproxen, in the knee OA population. Clinicians should consider the risk profile for specific NSAIDs when recommending their use.
Keywords: NSAIDs, VTE, knee osteoarthritis
Rheumatology key messages
In knee OA, venous thromboembolism risk was increased in users of diclofenac, ibuprofen, meloxicam and coxibs.
Venous thromboembolism risk with diclofenac and ibuprofen use appeared higher than risk in coxib use in knee OA patients.
Among knee OA patients, venous thromboembolism risk was not increased among current users of naproxen.
Introduction
NSAIDs are one of the most frequently prescribed classes of medicine for treating arthritis or pain [1]. Despite their long history of use, previously unrecognized adverse effects are still being reported [2–10]. Recent findings of NSAIDs’ effect on venous thromboembolism (VTE) have raised questions on how NSAIDs affect the risk of VTE, though the results are still controversial [6, 11, 12].
VTE, which includes deep venous thrombosis (DVT) and pulmonary embolism, has an incidence of >0.1% of people per year in the USA and is often fatal [6, 9]. Several studies [6–10] were conducted to assess the relation between NSAIDs and VTE, but none of these controlled for the disease indication for NSAIDs. VTE risk could be associated with the indication for NSAIDs rather the effect of NSAIDs by itself. For example, pain from knee OA may limit the mobility of the patients, and may put them in an increased risk of VTE [13–15]. Additionally, leg pain ultimately caused by a DVT could trigger using NSAIDs to lessen the pain, creating a spurious association of NSAID with DVT (protopathic bias).
A recent meta-analysis also showed that, compared with non-users of NSAIDs, both non-selective NSAIDs (nsNSAIDs) users and coxib users had an increased risk of VTE [5]. However, in this study, the VTE risk among NSAID users in the general population was compared with that of NSAID non-users, which raises an issue of a potential confounding by indication [16]. It has been pointed out that the differences in the definitions of NSAIDs exposure and concerns about the heterogeneity of the populations in the included studies limits the generalizability of results [5, 12]. Furthermore, this study attempts to apply results to all NSAIDs as a class, which could be misleading in interpreting the effects of individual NSAIDs.
Cumulative findings of NSAIDs’ different effects on thrombosis suggest that the degree of VTE risk might vary depending on the individual NSAIDs. How NSAIDs are associated with arterial thrombosis has been studied through multiple randomized controlled trials [4, 17] and observational studies [2, 18–20, D. Dalal et al., submitted for publication], yet the mechanism of how NSAIDs affect coagulation has not been clearly explained. Considering that arterial and venous thrombosis have common pathophysiological mechanisms, and that each one is often a risk factor for one another [21, 22], attention on the individual NSAIDs on VTE risk may lead to practical clinical insights. To assess the VTE risk of NSAIDs, we conducted a nested case-control study of individual NSAIDs by restricting the study population to knee OA patients.
Methods
Study population
We drew our study population from The Health Improvement Network (THIN), a database of patient records from general practices (GPs) in the UK from 1986 to 2013. THIN contains health related records including information on diagnoses (using Read codes), symptoms, referrals to secondary care, medication prescriptions and other physical characteristics such as BMI, blood pressure and smoking status. Subjects with a diagnosis of knee OA were eligible to enter the study if they were aged 18–90 years, had at least one prescription record for NSAIDs, and were enrolled with a GP participating in THIN for at least 1 year during the study period of 1 January 1995 through 31 September 2013. This study was approved by the UK National Health Service Research Ethics Committee (Protocol 14-020).
Case ascertainment
We identified incident VTE cases as persons with a Read code for either pulmonary embolism or DVT and a subsequent prescription record of anticoagulation (see supplementary data available at Rheumatology Online). We also included cases with a VTE Read code without following prescription data for anticoagulant if the subject died within 30 days [15]. Subjects who had a VTE event prior to the knee OA diagnosis were excluded from the study, and the date of the first diagnosis of VTE was taken as the index date for the cases.
Control selection
For each VTE case, we identified a maximum of five controls with knee OA, matched on the year of birth, sex and calendar year of index date. We used risk-set sampling to randomly select matched control subjects from knee OA patients without VTE. Controls were assigned the same index date as their matched case.
Exposure to NSAIDS
Information on NSAID prescriptions was extracted by using Anatomical Therapeutic Chemical codes (see supplementary data available at Rheumatology Online). Using the date of the most recent NSAID prescription, we categorized subjects into three groups: current, recent and remote users. Current users were defined by those with an NSAID prescription dated within 60 days prior to the index date [2]. Current users were further classified into eight categories [23]: diclofenac, ibuprofen, naproxen, meloxicam, celecoxib, rofecoxib, other coxibs, and users of any other NSAID. Recent and remote users were defined by those with a prescription of any NSAID within 60–365 or >365 days, respectively, before the index date. Judging that the remote users were likely to have the least exposure to NSAIDs, we set this group as the reference group for analyses. Remote NSAID users serve better as a reference group rather than non-users because it assures both the exposures of interest and the referent group had an indication for NSAID use. We excluded subjects whose NSAID prescriptions were before the knee OA diagnosis and those who were prescribed more than one NSAID within the 60-day period preceding the index date because whether the VTE is correlated to one NSAID or another would be ambiguous. Knee OA patients without an NSAID prescription were excluded to reduce the potential for confounding by indication.
Covariates
To allow adjustment for potential confounding, we obtained information on the covariates during the period 12–24 months prior to the index date. The covariates included major risk factors for VTE (surgery, trauma and cancer), smoking status, alcohol consumption, comorbidities (stroke, ischaemic heart disease, chronic kidney disease, liver disease, hypertension, diabetes, hyperlipidaemia, inflammatory rheumatic conditions), concomitant drugs (glucocorticoids, aspirin, HRT) and utilization of medical care (specialist referrals, number of GP visits and hospitalizations).
Statistical analysis
To assess whether the risk of VTE increased in relation to NSAID usage, we compared current with remote users. The crude association between NSAID usage and VTE risk was assessed by using a conditional logistic regression model only adjusted for the matching factors: age, sex and calendar year. A conditional logistic regression model was used to assess the effect of NSAIDs on VTE risk with adjustment for the baseline covariates. In addition to the major VTE risk factors (surgery, any trauma and cancer) and lifestyle related factors (smoking status and BMI), we added additional covariates to the multivariate model individually and assessed if the effect estimate changed by > 10%. Our primary analysis used imputed missing values for covariates (BMI, smoking status and alcohol use), and combined estimates from five different imputed datasets. All of the analyses were performed with SAS software (version 9.3, SAS Institute, Cary, NC, USA). IVEware (version 0.2) [24] was used for imputation.
Results
Characteristics of the study population
The baseline characteristics of the study population are summarized in Table 1. A total of 24 079 knee OA patients were included in the analysis: 4020 cases of VTE and 20 059 matched controls. More than half of subjects were female and aged 70 years or older. Cases and controls were similar with respect to the distribution of most major VTE risk factors and comorbidities, although obesity, cancer, glucocorticoid use and specialist referral were more prevalent in the cases than the control group.
Table 1.
Baseline characteristics of the VTE cases and matched controls
| VTE cases (n = 4020) | Controls (n = 20 059) | |
|---|---|---|
| Female, n (%) | 2431 (60.5) | 12 137 (60.5) |
| Age (years), n (%) | ||
| <60 | 479 (11.9) | 2357 (11.8) |
| 60–69 | 968 (24.1) | 4840 (24.1) |
| ≥70 | 2573 (64.0) | 12 862 (64.1) |
| Age, years, mean (s.d.) | 72.7 (10.1) | 72.8 (10.0) |
| BMI category, kg/m2, n (%) | ||
| <18.5 | 15 (0.4) | 97 (0.5) |
| 18.5–24.9 | 574 (15.9) | 4079 (22.7) |
| 25–29.9 | 1346 (37.2) | 7420 (41.3) |
| ≥30 | 1682 (46.5) | 6373 (35.5) |
| Major risk factors for VTE, n (%) | ||
| Surgery | 338 (8.4) | 1522 (7.6) |
| Any trauma | 274 (6.8) | 1133 (5.6) |
| Cancer | 632 (15.7) | 2276 (11.3) |
| Smoking, n (%) | ||
| None | 2319 (60.4) | 11 408 (59.4) |
| Past | 1114 (29.0) | 5544 (28.8) |
| Current | 408 (10.6) | 2267 (11.8) |
| Alcohol, n (%) | ||
| None | 877 (24.3) | 4113 (22.8) |
| Past | 97 (2.7) | 426 (2.4) |
| Current | 2634 (73.0) | 13 489 (74.8) |
| Other comorbidities, n (%) | ||
| Stroke | 375 (9.3) | 1768 (8.8) |
| Ischaemic heart disease | 788 (19.6) | 3525 (17.6) |
| Chronic kidney disease | 468 (11.6) | 2062 (10.3) |
| Liver disease | 98 (2.4) | 476 (2.4) |
| Hypertension | 2012 (50.0) | 9980 (49.8) |
| Diabetes | 484 (12.0) | 2365 (11.8) |
| Hyperlipidaemia | 1298 (32.3) | 6654 (33.2) |
| Inflammatory conditionsa | 594 (14.8) | 2516 (12.5) |
| RA | 110 (2.7) | 477 (2.4) |
| NSAID use, n (%) | ||
| Remote user (any NSAID) | 2114 (52.6) | 12 208 (60.9) |
| Recent user (any NSAID) | 950 (23.6) | 3991 (19.9) |
| Current diclofenac user | 396 (9.9) | 1433 (7.1) |
| Current ibuprofen | 218 (5.4) | 861 (4.3) |
| Current naproxen user | 96 (2.4) | 550 (2.7) |
| Current meloxicam user | 57 (1.4) | 250 (1.2) |
| Current celecoxib user | 59 (1.5) | 248 (1.2) |
| Current rofecoxib user | 34 (0.8) | 129 (0.6) |
| Current other coxib user | 25 (0.6) | 100 (0.5) |
| Current other NSAIDs user | 71 (1.8) | 289 (1.4) |
| Other medication use, n (%) | ||
| Glucocorticoids | 445 (11.1) | 1459 (7.3) |
| Aspirin | 1147 (28.5) | 5319 (26.5) |
| HRT | 141 (3.5) | 689 (3.4) |
| Medical service utilization | ||
| Specialist referral, n (%) | 1547 (38.5) | 6670 (33.3) |
| GP visits, mean (s.d.) | 5.6 (4.4) | 4.8 (3.9) |
| Hospitalizations, mean (s.d.) | 0.37 (1.0) | 0.28 (0.8) |
aSeronegative spondyloarthropathy/psoriasis, CTD, vasculitides and crystal arthropathies. VTE: venous thromboembolism.
NSAIDs usage and VTE risks
Compared with the remote users, the risk of VTE was increased in both current and recent NSAID users. After adjustment for all potential confounders, VTE risk was increased by 38% [OR = 1.38 (95% CI: 1.32, 1.44)] among recent users, and 43% [OR = 1.43 (95% CI: 1.36, 1.49)] among current users (Table 2). Among current users, risk of VTE was increased in the diclofenac users [OR =1.63 (95% CI: 1.53, 1.74)], ibuprofen users [OR = 1.49 (95% CI: 1.38, 1.62)], meloxicam users [OR = 1.29 (95% CI: 1.11, 1.50)] and users of both celecoxib [OR = 1.30 (95% CI: 1.11, 1.51)] and rofecoxib [OR = 1.44 (95% CI: 1.18, 1.76)]. However, such an association was not observed among current naproxen users [OR = 1.00 (95% CI: 0.89, 1.12)] (Fig. 1).
Table 2.
Risk of VTE with usage of specific NSAIDs compared with remote usage of any NSAID
| Cases, n | Controls, n | Crude ORa (95% CI) | Adjusted ORb (95% CI) | |
|---|---|---|---|---|
| Remote use (any NSAID) | 2114 | 12 208 | 1 (reference) | 1 (reference) |
| Recent use (any NSAID) | 950 | 3991 | 1.40 (1.34, 1.47) | 1.38 (1.32, 1.44) |
| Current use | 956 | 3860 | 1.47 (1.40, 1.53) | 1.43 (1.36, 1.49) |
| Diclofenac | 396 | 1433 | 1.66 (1.56, 1.77) | 1.63 (1.53, 1.74) |
| Ibuprofen | 218 | 861 | 1.49 (1.37, 1.61) | 1.49 (1.38, 1.62) |
| Naproxen | 96 | 550 | 1.02 (0.91, 1.14) | 1.00 (0.89, 1.12) |
| Meloxicam | 57 | 250 | 1.34 (1.16, 1.56) | 1.29 (1.11, 1.50) |
| Celecoxib | 59 | 248 | 1.43 (1.23, 1.66) | 1.30 (1.11, 1.51) |
| Rofecoxib | 34 | 129 | 1.60 (1.32, 1.95) | 1.44 (1.18, 1.76) |
| Other coxibs | 25 | 100 | 1.46 (1.17, 1.83) | 1.45 (1.15, 1.83) |
| Other NSAIDs | 71 | 289 | 1.46 (1.27, 1.67) | 1.34 (1.17, 1.54) |
aAdjusted for age, sex and calendar year. bAdjusted for age, sex, calendar year, surgery, trauma, cancer, BMI, smoking, ischaemic heart disease, hypertension, diabetes, hyperlipidaemia, inflammatory conditions, glucocorticoid use, number of GP visits, specialist referral and hospitalizations. VTE: venous thromboembolism.
Fig. 1.
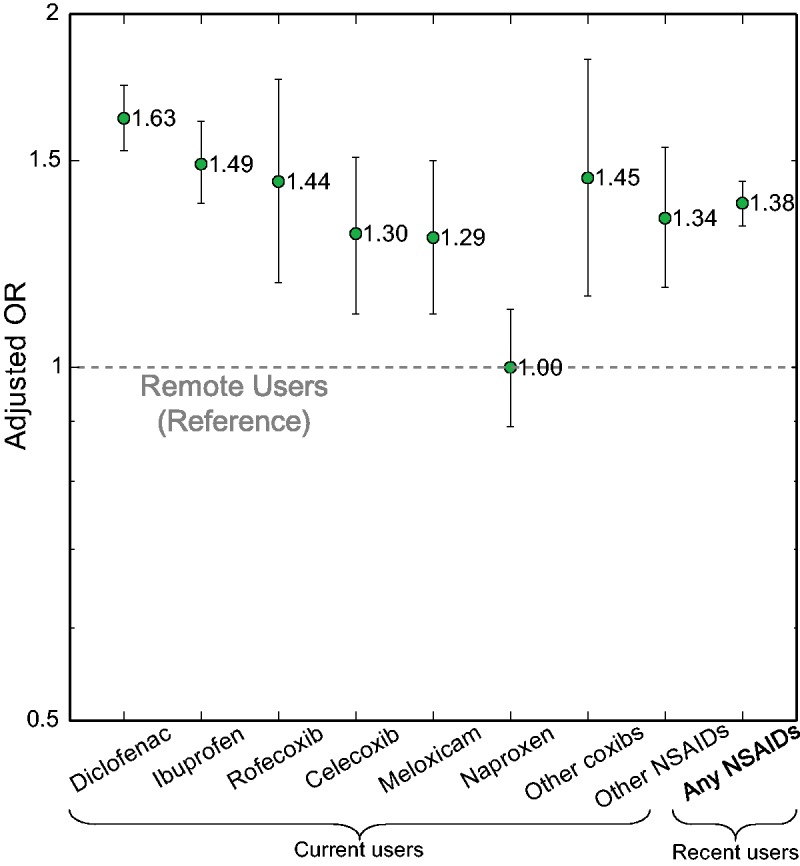
Risk of VTE with usage of specific NSAIDs after adjustment
VTE: venous thromboembolism.
The increased risk of VTE in the current diclofenac, ibuprofen, meloxicam and coxibs usage categories persisted across the strata of sex, age (<60, 60–69, ⩾70) and calendar period (1995–99, 2000–04, 2005–13) (supplementary Tables S1–S3, available at Rheumatology Online). However, the effects of diclofenac, ibuprofen, meloxicam and celecoxib appeared to be greater in the subjects <70 years of age (supplementary Table S2, available at Rheumatology Online).
Discussion
This large population-based study of knee OA patients demonstrates that all current users of nsNSAIDs and coxibs had an increased risk of VTE except for users of naproxen. The findings from our study largely agree with the results from the general population [7–9]. On the other hand, a subgroup analysis from Huerta et al. [6] found no increase in the VTE risk among the OA patients taking NSAIDs. However, including patients with OA at any site may incompletely describe the baseline VTE risk because OA at specific sites may confer greater risk than others. For instance, knee OA may have a greater association with impaired mobility [13, 14] and obesity [25, 26] than hand OA. The effect estimates observed in our study are lower than those of the previous studies from the general population [7–9], which may be due to a greater baseline risk for VTE in knee OA patients, or because we reduced the potential for confounding by indication by establishing remote NSAID users as the referent group.
The definition of current users and selection of specific NSAIDs of interest in this study was based on the findings from a previous study on the usage of NSAIDs in the UK using THIN [23]. According to this study, the most common duration of an NSAID prescription is ⩽60 days; therefore our definition of current users is consistent with the practice of NSAID prescriptions in the UK. Considering the rare occurrence of VTE in the general population, we selected the most frequently used NSAIDs to ensure adequate power for the analysis while including both coxibs and nsNSAIDs.
We found that, among nsNSAIDs, the degree of VTE risk was dependent on the specific NSAID. As compared with the 63% increase in the risk among current diclofenac users and 49% increase in current ibuprofen users, the risk was not increased at all among current naproxen users. Therefore, it may be misleading to suggest that all NSAIDs significantly increase the risk of VTE [5].
The trend of increased VTE risk among individual NSAIDs is parallel to that for the risk of other vascular events including stroke, myocardial infarction and vascular death, as was found from meta-analyses [27–29]; the increased risk of both arterial and venous thrombotic events was present in the descending order of diclofenac, ibuprofen and coxibs (celecoxib and rofecoxib); naproxen had no increase in risk.
While there is no consensus on how NSAIDs influence thrombotic events, one common hypothesis [5, 7, 30] is that NSAIDs cause an imbalance between prostacyclin and thromboxane, triggering an unopposed expression of thromboxane due to the selective inhibition of COX-2. This increase in thromboxane is thought to lead to a prothrombotic state and increased risk of thrombotic diseases. However, in our study, the VTE risk of individual NSAIDs was not correlated with the selectivity on the COX-2. The lack of clinical evidence to generally associate COX-2 selectivity with cardiovascular risks was extensively reviewed by Joshi et al. [31]. Furthermore, according to Kirkby et al. [32], the production of prostacyclin, which has been assumed to be controlled by the COX-2 enzyme under the imbalance hypothesis, seems to be driven by COX-1 in the cardiovascular system. In this case, it is questionable to consider COX-2 selectivity as a cause of increased thrombotic events.
Another perspective explaining the association between NSAIDs and VTE is the increased production of reactive oxygen species (ROS) by NSAIDs [31]. Increased production of ROS can contribute to endothelial dysfunction such as activation of proinflammatory signalling pathways and inhibition of nitric oxide synthase [33]. It has been shown from studies on oxidative stress that individual NSAIDs could differ in the degree of inducing endothelial dysfunction; diclofenac produced the most oxidative stress [33, 35]. This mechanism may explain the association of diclofenac and VTE found in our study. However, naproxen, which showed least association with VTE risk in our case, was also found with high oxidative stress [34, 35].
Alternatively, the pharmacokinetics of naproxen could be considered to help understand the differential effects compared with other NSAIDs on thrombotic risk. Compared with diclofenac or ibuprofen whose half-life is 1–2 h, the long half-life (about 14 h) of naproxen would make it capable of sustained COX-1 inhibition, which resembles the antiplatelet effect of low-dose aspirin [27, 36]. Therefore, COX-1/COX-2 inhibition, and drug-specific ROS production and pharmacokinetics may all be competing mechanisms within a complex pathway leading to thromboembolic events.
The strength of our study results from the baseline VTE risk adjustment, in that we restricted our study population to knee OA patients with at least one prescription record for NSAIDs. By requiring all subjects to have at least this indication for usage of NSAIDs, we reduced the potential for confounding by indication. In addition, the inclusion of patients from a primary care database, and a long period of study (1995–2013) make the results likely to be generalizable to the other knee OA populations and probably to the general population. One of the limitations in the present study is our use of prescription NSAID records and inability to account for over-the-counter NSAID use, raising concerns of exposure misclassification; however we expect this misclassification to have only biased results toward the null. Additionally, due to small numbers, we were unable to assess for differential effects based on dosage, which has been shown to occur in some NSAIDs (e.g. celecoxib for cardiovascular events) [37]. Lastly, we were unable to compare the bleeding risk of individual NSAIDs, as it was beyond the scope of this study. We note that naproxen could have a higher risk for upper gastrointestinal bleeding compared with celecoxib [38].
In conclusion, we found that the magnitude of VTE risk in knee OA patients varies by current usage of specific NSAIDs: highest with diclofenac, followed by ibuprofen, meloxicam and then coxibs, and there was no increase in VTE risk among naproxen users. In patients with VTE risk factors, diclofenac, ibuprofen, celecoxib and meloxicam should be used cautiously.
Supplementary Material
Acknowledgements
Taeyeon Lee would like to thank Stephen D. Kang from California Institute of Technology for technical support. M.D. was supported by grants from Arthritis Foundation Clinical to Research Transition Award.
Funding: This work was supported by grants from the National Institute of Arthritis, Musculoskeletal and Skin Diseases [AR047785].
Disclosure statement: T.L. is currently an employee of AbbVie. Her appointment started after submission, during the revision process of the manuscript. All other authors have declared no conflicts of interest.
References
- 1. Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001;120:594–606. [DOI] [PubMed] [Google Scholar]
- 2. Graham DJ, Campen D, Hui R. et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 2005;365:475–81. [DOI] [PubMed] [Google Scholar]
- 3. Hochberg MC, Altman RD, April KT. et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465–74. [DOI] [PubMed] [Google Scholar]
- 4. Silverstein FE, Faich G, Goldstein JL. et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284:1247–55. [DOI] [PubMed] [Google Scholar]
- 5. Ungprasert P, Srivali N, Wijarnpreecha K, Charoenpong P, Knight EL. Non-steroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology 2015;54:736–42. [DOI] [PubMed] [Google Scholar]
- 6. Huerta C, Johansson S, Wallander M-A, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med 2007;167:935–43. [DOI] [PubMed] [Google Scholar]
- 7. Bergendal A, Adami J, Bahmanyar S. et al. Non-steroidal anti-inflammatory drugs and venous thromboembolism in women. Pharmacoepidemiol Drug Saf 2013;22:658–66. [DOI] [PubMed] [Google Scholar]
- 8. Biere-Rafi S, Di Nisio M, Gerdes V. et al. Non-steroidal anti-inflammatory drugs and risk of pulmonary embolism. Pharmacoepidemiol Drug Saf 2011;20:635–42. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt M, Christiansen CF, Horváth-Puhó E. et al. Non-steroidal anti-inflammatory drug use and risk of venous thromboembolism. J Thromb Haemost 2011;9:1326–33. [DOI] [PubMed] [Google Scholar]
- 10. Lacut K, van der Maaten J, Le Gal G. et al. Antiplatelet drugs and risk of venous thromboembolism: results from the EDITH case-control study. Haematologica 2008;93:1117–8. [DOI] [PubMed] [Google Scholar]
- 11. Tsai AW, Cushman M, Rosamond WD. et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002;162:1182–9. [DOI] [PubMed] [Google Scholar]
- 12. Alcorn N, Madhok R. Non-steroidal anti-inflammatory drugs and venous thromboembolism. Rheumatology 2015;570–1. [DOI] [PubMed] [Google Scholar]
- 13. Wilkie R, Peat G, Thomas E, Croft P. Factors associated with restricted mobility outside the home in community-dwelling adults ages fifty years and older with knee pain: an example of use of the International Classification of Functioning to investigate participation restriction. Arthritis Rheum 2007;57:1381–9. [DOI] [PubMed] [Google Scholar]
- 14. Jordan JM, Luta G, Renner JB. et al. Self-reported functional status in osteoarthritis of the knee in a rural southern community: the role of sociodemographic factors, obesity, and knee pain. Arthritis Care Res 1996;9:273–8. [DOI] [PubMed] [Google Scholar]
- 15. Choi HK, Rho Y-H, Zhu Y. et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis 2013;72:1182–7. [DOI] [PubMed] [Google Scholar]
- 16. Corrao G, Ghirardi A, Segafredo G. et al. User-only design to assess drug effectiveness in clinical practice: application to bisphosphonates and secondary prevention of fractures. Pharmacoepidemiol Drug Saf 2014;23:859–67. [DOI] [PubMed] [Google Scholar]
- 17. Bombardier C, Laine L, Reicin A. et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. New Engl J Med 2000;343:1520–8. [DOI] [PubMed] [Google Scholar]
- 18. Ray WA, Stein CM, Daugherty JR. et al. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet 2002;360:1071–3. [DOI] [PubMed] [Google Scholar]
- 19. Mamdani M, Rochon P, Juurlink DN. et al. Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med 2003;163:481–6. [DOI] [PubMed] [Google Scholar]
- 20. Solomon DH, Schneeweiss S, Glynn RJ. et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation 2004;109:2068–73. [DOI] [PubMed] [Google Scholar]
- 21. Franchini M, Mannucci PM. Venous and arterial thrombosis: different sides of the same coin? Eur J Intern Med 2008;19:476–81. [DOI] [PubMed] [Google Scholar]
- 22. Prandoni P. Links between arterial and venous disease. J Intern Med 2007;262:341–50. [DOI] [PubMed] [Google Scholar]
- 23. Arellano FM, Yood MU, Wentworth CE. et al. Use of cyclo-oxygenase 2 inhibitors (COX-2) and prescription non-steroidal anti-inflammatory drugs (NSAIDS) in UK and USA populations. Implications for COX-2 cardiovascular profile. Pharmacoepidemiol Drug Saf 2006;15:861–72. [DOI] [PubMed] [Google Scholar]
- 24. Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 2001;27:85–95. [Google Scholar]
- 25. Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology 1999;10:161–6. [PubMed] [Google Scholar]
- 26. Yusuf E, Nelissen RG, Ioan-Facsinay A. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010;69:761–5. [DOI] [PubMed] [Google Scholar]
- 27. Kearney PM, Baigent C, Godwin J. et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006;332:1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trelle S, Reichenbach S, Wandel S. et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baigent C, Bhala N, Emberson J. et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med 2004;351:1709–11. [DOI] [PubMed] [Google Scholar]
- 31. Joshi GP, Gertler R, Fricker R. Cardiovascular thromboembolic adverse effects associated with cyclooxygenase-2 selective inhibitors and nonselective antiinflammatory drugs. Anesth Analg 2007;105:1793–804. [DOI] [PubMed] [Google Scholar]
- 32. Kirkby NS, Lundberg MH, Harrington LS. et al. Correction for Kirkby et al., Cyclooxygenase-1, not cyclooxygenase-2, is responsible for physiological production of prostacyclin in the cardiovascular system. Proc Natl Acad Sci U S A 2013;110:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bovill EG, van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: what is the link? Annu Rev Physiol 2011;73:527–45. [DOI] [PubMed] [Google Scholar]
- 34. Li H, Hortmann M, Daiber A. et al. Cyclooxygenase 2-selective and nonselective nonsteroidal anti-inflammatory drugs induce oxidative stress by up-regulating vascular NADPH oxidases. J Pharmacol Exp Ther 2008;326:745–53. [DOI] [PubMed] [Google Scholar]
- 35. Van Leeuwen JS, Ünlü B, Vermeulen NPE, Vos JC. Differential involvement of mitochondrial dysfunction, cytochrome P450 activity, and active transport in the toxicity of structurally related NSAIDs. Toxicol Vitr 2012;26:197–205. [DOI] [PubMed] [Google Scholar]
- 36. Capone ML, Tacconelli S, Sciulli MG. et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation 2004;109:1468–71. [DOI] [PubMed] [Google Scholar]
- 37. Solomon SD, Pfeffer MA, McMurray JJV. et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation 2006;114:1028–35. [DOI] [PubMed] [Google Scholar]
- 38. Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002;325:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


