Abstract
Background:
Survivors of Hodgkin’s lymphoma (HL) treated as adults are at risk for late effects of therapy. However, the burden of late morbidity and mortality among adults treated for HL remains incompletely characterized.
Methods:
Vital status and, for deceased, cause of death were determined for 746 adults treated on a first-line trial at a single center from 1975 to 2000. Survivors completed a detailed survey describing their physical and mental health. A severity score (grades 1–4, ranging from mild to life-threatening or disabling) was assigned to self-reported conditions.
Results:
At a median follow-up of 22 years, 227 of patients (30.4%) had died, 107 (47.1%) from HL, 120 (52.9%) from other causes, including second primary malignancies (SPMs) (n = 52) and cardiovascular disease (n = 27). Across the duration of follow-up, all-cause and SPM-specific risk of death remained higher than predicted by normative data. Among survivors, late morbidity survey data are available for 238 patients (45.9%). Ninety-four-point-one percent of respondents reported at least one morbidity, and 47.5% reported at least one grade 3 or 4 morbidity; 20.2% reported two or more grade 3 morbidities. Commonly reported morbidities included cardiovascular (54.6%), endocrine (68.5%), pulmonary disease (21.4%), and nonfatal second malignancy (23.1%). Anxiety, depression, and fear of recurrence were frequently reported.
Conclusions:
Among a large cohort of patients treated for HL with extensive follow-up, risk of late mortality from causes other than HL and prevalence of late medical morbidity are high. Guidelines for prevention, screening, and management of late effects in adult survivors of HL are needed.
The outcome for adults with Hodgkin’s lymphoma (HL) has dramatically improved because of progressive improvements in chemotherapy, radiation, and combined modality therapy (CMT). Cure rates are now 85% to 90% for early stage disease (1,2) and 65% to 85% for advanced stage disease (3,4). As more cured patients survive longer, the impact of late complications of treatment is increasingly clear. Among children, by fifteen years post-treatment, the risks of death from causes other than HL surpass those from HL itself.(5) The most common causes of mortality in survivors of HL include second primary malignancy (SPM) and cardiovascular, pulmonary, and infectious diseases (6–8).
However, mortality alone does not describe the burden of illness experienced by survivors. A more comprehensive understanding of the impact of treatment for HL on long-term health requires a broad assessment of late morbidity. While late mortality from SPM (9–12) and cardiovascular disease (13–15) has been well described, late morbidity in adult HL survivors is less well characterized. Studies have often evaluated individual late effects, such as thyroid dysfunction (16), infertility (17,18), or fatigue (19–22). The Childhood Cancer Survivor Study (CCSS) describes the breadth and depth of late morbidity experienced by survivors of childhood cancers (23–25), and others have evaluated survivors of pediatric HL (26–30). Because children and adults differ in both biology and therapeutic exposures, and given marked differences in comorbid conditions, environmental exposures, and health behaviors, extrapolating this understanding to adults may not be appropriate. Given limitations in understanding of late treatment effects among survivors of adult HL, we assessed the late morbidity and mortality of adults treated at our institution over a 25-year period.
Methods
Sample
To characterize morbidity and mortality in adult HL patients, we identified all patients from six consecutive clinical trials of first-line therapy for HL at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1975 and 2000. All six studies (Table 1) were single-center phase II trials of CMT for HL except for one arm of one study, which was chemotherapy alone. Details of these studies have been published previously (31–35). Complete treatment records were available, including chemotherapy doses and radiation therapy fields and dosages. Informed consent was obtained from respondents prior to completion of the study survey. The institutional review board of MSKCC approved this nontherapeutic protocol, and it was registered with clinicaltrials.gov (http://clinicaltrials.gov/ct2/show/NCT00598728).
Table 1.
Baseline demographic characteristics at time of survey response among participants
| Characteristic | No. (%) |
|---|---|
| Median age, y | 49 |
| Range, y | 25–88 |
| Median age at treatment, y | 29 |
| Range, y | 14–66 |
| Median duration of follow-up, y | 21 |
| Range, y | 5.5–32 |
| Sex | |
| Male | 115 (48.3) |
| Female | 123 (51.7) |
| Race | |
| White | 229 (96.6) |
| African American | 4 (1.7) |
| Other | 4 (1.7) |
| Missing | 1 (n/a) |
| Ethnicity | |
| Hispanic | 10 (4.2) |
| Non-Hispanic | 226 (95.8) |
| Missing | 2 (n/a) |
| Marital status | |
| Married/living with partner | 192 (80.7) |
| Single | 24 (10.1) |
| Divorced/separated | 18 (7.6) |
| Widowed | 4 (1.7) |
| Educational achievement | |
| Less than high school | 1 (0.4) |
| Partial high school | 2 (0.8) |
| High school graduate | 35 (14.8) |
| Partial college or vocational training | 52 (22.0) |
| College degree | 78 (33.1) |
| Graduate degree or professional training | 68 (28.8) |
| Missing | 2 (n/a) |
| Family income level | |
| Less than $10 000 | 4 (1.8) |
| $10 000 - $29 999 | 9 (4.0) |
| $30 000 - $49 999 | 22 (9.9) |
| $50 000 - $69 999 | 24 (10.8) |
| $70 000 - $89 999 | 35 (15.7) |
| More than $90 000 | 129 (57.8) |
| Missing | 15 (n/a) |
Outcomess
Mortality
We determined vital status through hospital records and the National Death Index (NDI); for deceased patients, NDI and death certificate data determined cause of death. Patients not identified by hospital records or NDI as deceased were presumed alive. Cause-specific mortality was categorized as HL, SPM, cardiovascular, other, and unknown.
Morbidity
Survivors were invited to participate in a cross-sectional survey study. First contact was by mail, and subsequently for nonresponders by mail, telephone, and e-mail. Participants completed an 18-page questionnaire detailing demographic characteristics, physical health, and mental health. Physical domains, adapted from the CCSS annual cohort study, assessed included cardiovascular, pulmonary, gastrointestinal, urinary, endocrine, musculoskeletal, neurologic, dental, ophthalmologic, and dermatologic health (24). Mental health was evaluated with Functional Assessment of Chronical Illiness Therapy–fatigue, Fears of Recurrence Questionnaire (FRQ), Hospital Anxiety and Depression Scale (HADS), and relevant questions within the SF-12 (36–39). Current medications and supplements were ascertained. Self-reported occurrences of SPM were confirmed through chart review. To characterize factors associated with morbidity, comparisons were performed by sex, age at treatment (≤25, 26 to 35, and ≥35 years), radiotherapy dose (none, <35 Gy, ≥35 Gy), and time from treatment (<14, 15 to 23, and ≥23 years); determination of cutpoints for comparisons were informed by the distribution of patients’ clinical characteristics. Severity of reported conditions was scored according to a modification of the Common Terminology Criteria for Adverse Events (23). This system grades self-reported health conditions as grade 1 (mild), grade 2 (moderate), grade 3 (severe), or grade 4 (life-threatening or disabling). Severity scores were reviewed by three coauthors (MM, KO, DS), and discrepant scoring was resolved by consensus.
Statistical Analyses
All statistical tests were two-sided and a P value of less than .05 was considered statistically significant.
Survival Analysis
Overall survival (OS) of the entire cohort was calculated using the Kaplan-Meier method. Cumulative incidence curves estimating cause-specific mortality were also calculated (40).
Risk ratios were calculated to compare mortality from SPM in the cohort to cancer mortality in the general population, retrieved from the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) (41). Risk of death from SPM was estimated by compiling person-years of follow-up (PY) by age (in five-year intervals), sex, and calendar year from protocol registration date until death from SPM or last follow-up. The expected number of deaths from cancer was estimated by multiplying the SEER cancer mortality rates specific for each age group, sex, and calendar year by the accumulated PY at risk. Observed and expected numbers of deaths were summed, and relative risks expressed as the ratio of observed-to-expected (O/E) cases. Statistical tests and confidence intervals were based on the assumption that observed deaths from SPM were distributed as a Poisson variable. Risk ratios comparing all-cause mortality in the cohort to mortality in the general population were calculated similarly, using data from the National Center for Heath Statistics (NCHS, www.cdc.gov/nchs).
Morbidity Analysis
Associations between patient characteristics or therapeutic exposures and late morbidity were examined using Fisher’s exact test or the Mantel-Haenszel test for trend. Associations with psychiatric morbidity were examined using the Wilcoxon Rank Sum Test or Kruskal-Wallis test.
Risk ratios were calculated to compare the incidence of SPM among survey respondents to the incidence of first cancer in the general population, derived from the SEER Program. PYs were aggregated by age, sex, and calendar year from protocol registration date until date of diagnosis of second cancer, death, or last follow-up. The expected number of cancers was estimated using SEER cancer incidence rates, and the risk ratios for cancer incidence were calculated as described above. Date of diagnosis of SPM was not universally available among respondents, so risk ratios were calculated assuming three different time points for date of SPM: five years post-treatment, midway between treatment and survey response, and one year before response. Risk ratios did not appreciably vary under these three scenarios. Using one year before response as the date of SPM yielded the most conservative risk ratio estimates; these results are presented.
Results
Patient Characteristics
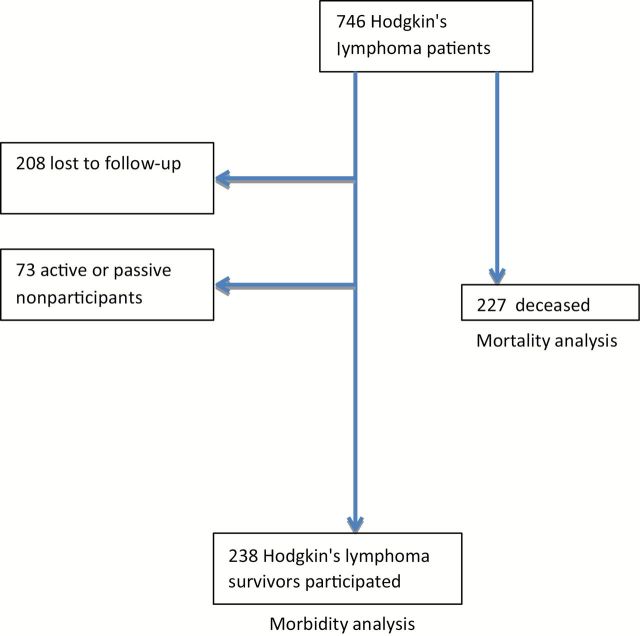
From 1975 to 2000, 746 patients were treated on one of six sequential first-line protocols at our center. At time of protocol initiation, of these 746 patients, 226 (30.3%) had died and 520 were alive (Figure 1). Of the 520 survivors, 213 (41.0%) were lost to follow-up. Of the remaining 306, 238 (45.8% of survivors) consented to participation and completed the baseline questionnaire, with 20 active and 53 passive nonparticipants. Demographic characteristics of respondents are shown in Table 1, and treatment programs are detailed in Table 2. Comparison of demographic characteristics of respondents to nonrespondents (both those lost to follow-up and active or passive nonrespondents) demonstrated an imbalance of patients treated on the more recent protocols (41.2% of respondents vs 27.3% of nonrespondents, P = .004) and to have been slightly older at treatment (median age 29 years old vs 27 years old, P = .006), but did not differ by gender, race, ethnicity, treatment with doxorubicin, or dose of radiotherapy.
Figure 1.
Consolidated Standards of Reporting Trials diagram of protocol-treated patients eligible for mortality and/or morbidity analysis.
Table 2.
Sequential therapeutic protocols for newly diagnosed Hodgkin’s lymphoma, 1975–2008
| Protocol | Eligibility | Chemotherapy | Cycles | Radiation dose |
Radiation field |
Number enrolled |
|---|---|---|---|---|---|---|
| 75–103 | Stage I, II | MOPP | 6 (sandwich) | 3500 cGy | IFRT | 82 |
| 75–104 | Stage IIB, III, IV | MOPP/ABVD | 6 | 2000–3000 cGy* | IFRT | 69 |
| 79-017 | Stage IIB, III, IV | MOPP/ABV/CAD | 9 (sandwich RT) | 2000–3000 cGy* | EFRT† | 116 |
| MOPP/ABVD | 9 (sandwich RT) | 2000–3000 cGy* | EFRT† | 111 | ||
| 81–103 | Stage I, II, IIIA | MOPP | 4 (sandwich RT) | 3600 cGy | EFRT† | 89 |
| TBV | 4 (sandwich RT) | 3600 cGy | EFRT† | 80 | ||
| 90-044 | Stage I, II, IIIA | ABVD | 6 | none | n/a | 76 |
| 3600 cGy | EFRT†or IFRT‡ | 77 | ||||
| 91-069 | Stage IIB, III, IV | ABVD | 6 | 3600 cGy | IFRT | 23 |
| 3600 cGy | EFRT† | 23 | ||||
* All patients received 2000 cGy to extended fields. Patients with bulk at diagnosis received 1000 cGy boosts to site(s) of bulk. ABV = doxorubicin, bleomycin, vinblastine; ABVD = doxorubicin, bleomycin, vinblastine, dacarbazine; CAD = lomustine, doxorubicin, vindesine; EFRT = extended field radiotherapy; IFRT = involved field radiotherapy; MOPP = mechlorethamine, vincristine, procarbazine, prednisone; RT = radiotherapy; TBV = thiotepa, bleomycin, vinblastine.
† Extended fields consisted of mantle for supradiaphragmatic disease, inverted-Y plus spleen or splenic pedicle for infradiaphragmatic disease, and total lymphoid irradiation.
‡ Radiation fields were changed during study to align with guidelines favoring involved field radiotherapy. Eleven patients received IFRT, the remainder EFRT.
Mortality
The median OS of the entire cohort was 32 years (Figure 2A). Two hundred and twenty-six patients had died at time of protocol initiation, and one patient died subsequent to accrual to protocol and is included in mortality assessment. Of the 227 deaths, 107 (47.1%) were from HL, and 120 (52.9%) from causes other than HL, 52 from SPM, 27 cardiovascular disease, 21 other illnesses, and 20 unknown. The cumulative incidence of death from HL rose quickly during the first five years and then plateaued after 10 years (10-year estimate: 12.8%, 95% confidence interval [CI] = 10.6% to 15.4%), while the cumulative incidence of death from causes other than HL rose steadily to 4.9% (95% CI = 3.6% to 6.7%) by 10 years and to 12.0% (95% CI = 9.7% to 14.8%) by 20 years (Figure 2B). The cumulative incidence of death from causes other than HL surpassed that from HL at 22 years post-treatment. Death from SPM and cardiovascular disease increased continually over time, following an initial latency of five years for SPM and 12 years for cardiovascular disease (Figure 2C). We compared the mortality rates in our cohort, both SPM cause-specific mortality and all-cause mortality (Table 3), to population-based normative comparators. Compared to SEER incidence–based mortality data, cause-specific mortality risk in our cohort was elevated across follow-up time periods. For patients with 14 or fewer years of follow-up post-treatment, the risk ratio (RR) for SPM cause-specific survival was 3.34 (95% CI = 2.29 to 4.72); for 14 to 23 years of follow-up, the risk ratio was 1.89 (95% CI = 0.98 to 3.31); and for 23 ore more years of follow-up, the risk ratio was 3.54 (95% CI = 1.53 to 6.98). Using NCHS data, we again observed a persistent elevation in all-cause mortality risk across follow-up strata. Patients 14 or fewer years after treatment had a risk ratio of 5.48 (95% CI = 4.68 to 6.37); for those with 14 to 23 years of follow-up, the risk ratio was 2.54 (95% CI = 1.86 to 3.39); and for those with 23 or more years of follow-up, the risk ratio was 2.05 (1.09 to 3.50). We analyzed data by era of therapy to characterize differences in risks of overall and cause-specific death (data not shown); sensitivity analyses showed no discernible differences with regards to overall or cause-specific mortality.However, conclusions regarding differential late mortality by regimen are limited by statistically significantly shorter median follow-up from more recent trials and by the paucity of deaths among patients from these trials.
Figure 2.
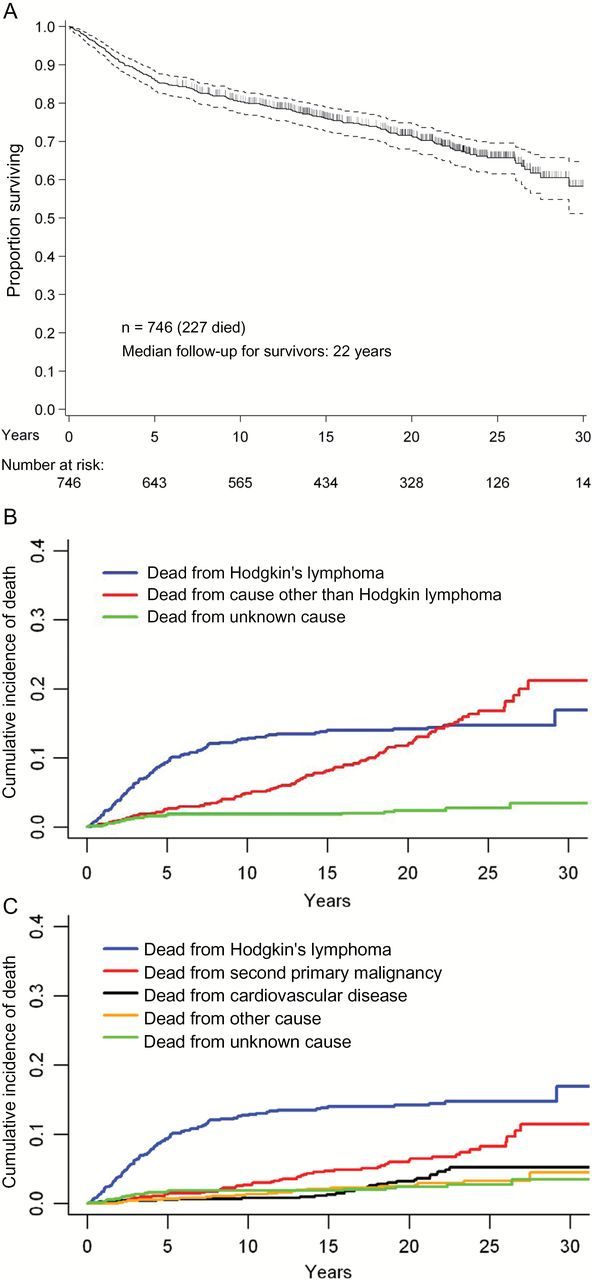
Overall survival, Hodgkin’s lymphoma–related mortality, and cause-specific mortality among participants in six consecutive first-line clinical trials, 1975–2008. A) Overall survival. B) Cause-specific mortality from Hodgkin’s lymphoma and causes other than Hodgkin’s lymphoma. C) Cause-specific mortality among 746 treated patients.
Table 3.
Risk ratios of mortality because of second primary malignancy and all-cause mortality, in comparison to Surveillance, Epidemiology, and End Results normative data*
| Length of follow-up | Observed | Expected | RR (95% CI) |
|---|---|---|---|
| Death because of second primary malignancy | |||
| ≤14 y | 32 | 9.6 | 3.34 (2.29 to 4.72) |
| 14–23 y | 12 | 6.3 | 1.89 (0.98 to 3.31) |
| >23 y | 8 | 2.3 | 3.54 (1.53 to 6.98) |
| Total | 52 | 18.2 | 2.86 (2.14 to 3.75) |
| Death because of all causes among men | |||
| ≤14 y | 101 | 21.3 | 4.75 (3.87 to 5.77) |
| 14–23 y | 29 | 9.9 | 2.93 (1.96 to 4.21) |
| >23 y | 6 | 2.9 | 2.05 (0.75 to 4.46) |
| Total | 136 | 34.1 | 3.99 (3.35 to 4.72) |
| Death because of all causes among women | |||
| ≤14 y | 67 | 9.4 | 7.11 (5.51 to 9.03) |
| 14–23 y | 17 | 8.2 | 2.07 (1.21 to 3.32) |
| >23 y | 7 | 3.4 | 2.04 (0.82 to 4.21) |
| Total | 91 | 21.0 | 4.32 (3.48 to 5.31) |
| Death because of all causes among all patients | |||
| ≤14 y | 168 | 30.7 | 5.48 (4.68 to 6.37) |
| 14–23 y | 46 | 18.1 | 2.54 (1.86 to 3.39) |
| >23 y | 13 | 6.4 | 2.05 (1.09 to 3.50) |
| Total | 227 | 55.1 | 4.12 (3.60 to 4.69) |
* CI = confidence interval; RR = risk ratio.
Morbidity
Two hundred and thirty-eight patients completed the survey characterizing late morbidity (Table 4). The median age of respondents was 49, with a median length of follow-up from treatment of 22 years. Among respondents, 94.1% identified one or more morbidity, and 47.5% identified one or more morbidity of grade 3 or 4 severity: 28 (11.8%) reported one or more morbidity with a maximum severity of grade 1, 83 (34.9%) maximum grade 2, 65 (27.3%) maximum grade 3, and 48 (20.2%) maximum grade 4. Patients frequently reported multiple morbidities: 99 (41.6%) reported three or more conditions of grade 2 or greater severity, 48 (20.2%) two or more morbidities of grade 3 or greater, and 15 (6.3%) with three or more morbidities of grade 3 or greater. Frequencies of key self-reported morbidities include nonfatal SPM (23.1%), cardiovascular (54.6%), endocrine (68.5%), musculoskeletal (37.0%), pulmonary (21.4%), and infectious diseases (32.4%) (Table 5).
Table 4.
Summary of frequency and severity of health conditions among respondents
| Health Condition | Frequency (%) |
|---|---|
| Conditions by grade | |
| No condition | 14 (5.8) |
| Grade 1 | 28 (11.8) |
| Grade 2 | 83 (34.9) |
| Grade 3 | 65 (27.3) |
| Grade 4 | 48 (20.2) |
| Any condition | |
| Grades 1–4 | 224 (94.1) |
| Grade 3 or 4 | 113 (47.5) |
| Multiple health conditions | |
| ≥Grade 1 | |
| ≥2 conditions | 199 (83.6) |
| ≥3 conditions | 166 (69.7) |
| ≥Grade 2 | |
| ≥2 conditions | 156 (65.5) |
| ≥3 conditions | 99 (41.6) |
| ≥Grade 3 | |
| ≥2 conditions | 48 (20.2) |
| ≥3 conditions | 15 (6.3) |
Table 5.
Frequency and severity of morbidity by individual health domain among respondents
| Health domain | Morbidity present? | No. (%) | Maximum grade | No. (%) |
|---|---|---|---|---|
| Vision | No | 193 (81.1) | ||
| Yes | 45 (18.9) | 1 | 38 (16.0) | |
| Hearing | No | 209 (87.8) | 2 | 7 (2.9) |
| Yes | 29 (12.2) | 1 | 26 (10.9) | |
| 2 | 3 (1.3) | |||
| Oral | No | 173 (72.7) | ||
| Yes | 65 (27.3) | 2 | 59 (24.8) | |
| 3 | 2 (0.8) | |||
| 4 | 4 (1.7) | |||
| Cardiovascular | No | 108 (45.4) | ||
| Yes | 130 (54.6) | 1 | 35 (14.7) | |
| 2 | 54 (22.7) | |||
| 3 | 28 (11.8) | |||
| 4 | 13 (5.5) | |||
| Pulmonary | No | 187 (78.6) | ||
| Yes | 51 (21.4) | 1 | 42 (17.6) | |
| 2 | 2 (0.8) | |||
| 3 | 5 (2.1) | |||
| 4 | 2 (0.8) | |||
| Gastrointestinal | No | 178 (74.8) | ||
| Yes | 60 (25.2) | 1 | 30 (12.6) | |
| 2 | 23 (9.7) | |||
| 3 | 7 (2.9) | |||
| Genitourinary | No | 234 (98.3) | ||
| Yes | 4 (1.7) | 1 | 4 (1.7) | |
| Musculoskeletal | No | 150 (63.0) | ||
| Yes | 88 (37.0) | 1 | 35 (14.7) | |
| 2 | 38 (16.0) | |||
| 3 | 15 (6.3) | |||
| Neurological | No | 215 (90.3) | ||
| Yes | 23 (9.7) | 1 | 12 (5.0) | |
| 2 | 5 (2.1) | |||
| 3 | 3 (1.3) | |||
| 4 | 3 (1.3) | |||
| Endocrine | No | 75 (31.5) | ||
| Yes | 163 (68.5) | 1 | 27 (11.3) | |
| 2 | 95 (39.9) | |||
| 3 | 41 (17.2) | |||
| Psychiatric | No | 185 (77.7) | ||
| Yes | 53 (22.3) | 1 | 5 (2.1) | |
| 2 | 46 (19.3) | |||
| 3 | 1 (0.4) | |||
| 4 | 1 (0.4) | |||
| Hematological | No | 227 (95.4) | ||
| Yes | 11 (4.6) | 1 | 11 (4.6) | |
| Dermatological | No | 184 (77.3) | ||
| Yes | 54 (22.7) | 1 | 53 (22.3) | |
| Infectious disease | No | 161 (67.6) | 2 | 1 (0.4) |
| Yes | 77 (32.4) | 1 | 67 (28.2) | |
| 3 | 10 (4.2) | |||
| Second malignancy | No | 183 (76.9) | ||
| Yes | 55 (23.1) | 2 | 17 (7.1) | |
| 3 | 4 (1.7) | |||
| 4 | 34 (14.3) | |||
Determinants of Medical Morbidity
Sex, age at treatment, treatment exposures, and time from treatment were associated with specific morbidities (Tables 6 and 7). Endocrine and dermatologic morbidities were more frequently reported by women (77.2% vs 59.1%, 35.8% vs 8.7%; both P ≤ .003). Age at treatment was associated with visual, musculoskeletal, and endocrine morbidity: advanced age with increased frequency of visual and musculoskeletal morbidity, younger age with increased frequency of endocrine morbidity (including infertility and thyroid disease), all P values were less than or equal to .05. Although few respondents received chemotherapy alone (37/238, 15.5%), these patients reported a lower frequency of endocrine dysfunction (P = .008), but no differences in late cardiovascular or neoplastic events in comparison to survivors of CMT were identified. Increased time from treatment was associated with cardiovascular disease, SPM, musculoskeletal disease, endocrine dysfunction, infectious diseases, and impaired oral health (all P ≤ .05). Radiation therapy doses of 3500 cGy or more were associated with more frequent endocrine dysfunction (P < .001) and dermatologic morbidity (P = .009), and possibly pulmonary morbidity and impaired oral health (P = .06 and P = .08, respectively). Patients receiving doses of radiation of less than 3500 cGy (typically, 2000–3000 cGy) did not have lower rates of SPM or cardiovascular disease than did those receiving 3500 or more cGy.
Table 6.
Relationship between gender and age at first treatment with frequency of morbidity by individual health domain among respondents
| Health domain | Sex | Age at first treatment, y | |||||
|---|---|---|---|---|---|---|---|
| M (n = 115) No. (%) | F (n = 123) No. (%) | P* | ≤25 (n = 85) No. (%) | 26–35 (n = 89) No. (%) | >35 (n = 64) No. (%) | P† | |
| Vision | 18 (15.6) | 27 (22.0) | .25 | 11 (12.9) | 17 (19.1) | 17 (26.6) | .04 |
| Hearing | 13 (11.3) | 16 (13.0) | .70 | 7 (8.2) | 13 (14.1) | 9 (14.1) | .25 |
| Speech | 26 (22.6) | 39 (31.7) | .15 | 23 (27.1) | 27 (30.3) | 15 (23.4) | .68 |
| Cardiovascular | 64 (55.6) | 66 (53.7) | .80 | 42 (49.4) | 48 (53.9) | 40 (62.5) | .12 |
| Pulmonary | 20 (17.4) | 31 (25.2) | .16 | 15 (17.7) | 18 (20.2) | 18 (28.1) | .13 |
| Gastrointestinal | 24 (20.9) | 36 (29.3) | .19 | 22 (25.9) | 25 (28.1) | 13 (20.3) | .48 |
| Genitourinary | 1 (0.9) | 3 (2.4) | .62 | 2 (2.4) | 1 (1.1) | 1 (1.6) | .68 |
| Musculoskeletal | 38 (33.0) | 50 (40.7) | .23 | 24 (28.2) | 34 (38.2) | 30 (46.9) | .02 |
| Neurological | 13 (11.3) | 10 (8.1) | .51 | 8 (9.4) | 8 (9.0) | 7 (10.9) | .77 |
| Endocrine | 68 (59.1) | 95 (77.2) | .003 | 63 (74.1) | 68 (76.4) | 32 (50.0) | .003 |
| Psychiatric | 14 (12.2) | 39 (31.7) | <.001 | 25 (29.4) | 19 (21.4) | 9 (14.1) | .03 |
| Hematologic | 2 (1.7) | 9 (7.3) | .06 | 7 (8.2) | 1 (1.1) | 3 (4.7) | .24 |
| Dermatologic | 10 (8.7) | 44 (35.8) | <.001 | 21 (24.7) | 20 (22.5) | 13 (20.3) | .53 |
| Infectious disease | 31 (27.0) | 46 (37.4) | .10 | 27 (31.8) | 33 (37.1) | 17 (26.6) | .57 |
| Cancer | 20 (17.4) | 35 (28.5) | .05 | 20 (23.5) | 15 (16.9) | 20 (31.2) | .34 |
* Fisher’s exact test, two-sided.
† Mantel-Haenszel test for trend, two-sided.
Table 7.
Relationship between radiotherapy dose and time from treatment with frequency of morbidity by individual health domain among respondents
| Health domain | Radiotherapy dose, cGy | Time from treatment to questionnaire, y | ||||||
|---|---|---|---|---|---|---|---|---|
| No RT (n = 37) No. (%) | <3500 (n = 65) No. (%) | ≥3500 (n = 135) No. (%) | P* | ≤14 (n = 70) No. (%) | 15–23 (n = 85) No. (%) | >23 (n = 83) No. (%) | P* | |
| Vision | 5 (13.5) | 14 (21.5) | 26 (19.3) | .60 | 10 (14.3) | 14 (16.5) | 21 (25.3) | .08 |
| Hearing | 4 (10.8) | 8 (12.3) | 17 (12.6) | .79 | 4 (5.7) | 12 (14.1) | 13 (15.7) | .07 |
| Speech | 5 (13.5) | 19 (29.2) | 41 (30.4) | .08 | 13 (18.6) | 22 (25.9) | 30 (36.1) | .01 |
| Cardiovascular | 16 (43.2) | 38 (58.5) | 76 (56.3) | .27 | 28 (40.0) | 47 (55.3) | 55 (66.3) | .001 |
| Pulmonary | 5 (13.5) | 11 (16.9) | 35 (25.9) | .06 | 14 (20.0) | 18 (21.2) | 19 (22.9) | .66 |
| GI | 8 (21.6) | 17 (26.2) | 35 (25.9) | .66 | 12 (17.1) | 24 (28.2) | 24 (28.9) | .10 |
| GU | 0 (0.0) | 1 (1.5) | 3 (2.2) | .36 | 2 (2.9) | 0 (0.0) | 2 (2.4) | .89 |
| Musculoskeletal | 14 (37.8) | 29 (44.6) | 45 (33.3) | .33 | 15 (21.4) | 35 (41.2) | 38 (45.8) | .002 |
| Neurological | 4 (10.8) | 7 (10.7) | 12 (8.9) | .66 | 7 (10.0) | 7 (8.2) | 9 (10.8) | .84 |
| Endocrine | 12 (32.4) | 49 (75.4) | 101 (74.8) | <.001 | 35 (50.0) | 63 (74.1) | 65 (78.3) | <.001 |
| Psychiatric | 4 (10.8) | 11 (16.9) | 38 (28.2) | .01 | 14 (20.0) | 17 (20.0) | 22 (26.5) | .32 |
| Hematologic | 1 (2.7) | 4 (6.2) | 6 (4.4) | .85 | 1 (1.4) | 5 (5.9) | 5 (6.0) | .19 |
| Dermatologic | 5 (13.5) | 9 (13.9) | 40 (29.6) | .009 | 18 (25.7) | 17 (20.0) | 19 (22.9) | .71 |
| Infectious disease | 8 (21.6) | 23 (35.4) | 46 (34.1) | .25 | 18 (25.7) | 23 (27.1) | 36 (43.4) | .02 |
| Cancer | 7 (18.9) | 15 (23.1) | 33 (24.4) | .50 | 10 (14.3) | 20 (23.5) | 25 (30.1) | .02 |
* Mantel-Haenszel test for trend, two-sided.
Survivors reported an excess risk of (nonfatal) SPM, with an observed/expected (O/E) risk ratio of 3.41 (95% CI = 2.57 to 4.44). Radiation dose was not clearly associated with risk of SPM (Table 7): For patients receiving less than 35 Gy, the estimated risk ratio was 2.79 (95% CI = 1.56 to 4.60), and for those receiving 35 or more Gy the estimated risk ratio was 3.82 (95% CI = 2.63 to 5.36).
Psychosocial Function
Of respondents, 80.7% were married or had a life partner at time of survey and 7.6% were divorced; 61.9% had completed collegiate or graduate education, 22.0% had completed some college or vocational training, and 14.8% had graduated high school. Regarding lifestyle behaviors, 40.7% reported having smoked at least 100 cigarettes during their lifetime, and 16.3% were current smokers. Problem drinking or illicit drug use following treatment for HL was reported by 5.6% (data not shown).
Anxiety and depression were frequently reported by respondents. High levels of anxiety, as indicated by a HADS subscale score of 11 or higher, were reported by 14.0%, and an additional 11.4% had a borderline score of 8 to 10. Further, 5.9% had a subscale score of 11 or higher for depression, and an additional 5.5% had a borderline score of 8 to 10. Borderline and major anxiety were both more common in women (P = .002) and in patients who were younger at times of treatment and survey (each, P = .01). No differences in depression were noted by age, gender, time from treatment, or treatment exposure. Subjective depression, anxiety, other psychiatric illness, or multiple psychiatric morbidities requiring psychotherapy or medical therapy were reported by 22.3% of respondents. Higher radiation therapy doses were associated with increased rates of self-reported psychiatric morbidity (no radiation, 10.8%; radiation dose ≤35 Gy, 16.9%; radiation dose >35 Gy, 28.1%, P = .01), as was younger age at treatment (P = .03). Fear of recurrence or second malignancy was common in this remotely treated cohort, with mean scores by the FRQ in women of 70 (SD = 17) and men of 66 (SD = 17); associated factors included younger age at time of survey (P = .03) and higher doses of radiation (P = .009).
Discussion
Because of improved outcomes in the treatment of adult HL, there now exists a large, and growing, population of survivors, but delivery of optimal care is hindered by a paucity of data. Investigators have identified increased risks of SPM (10-12,42), including breast cancer among younger women receiving radiation to breast tissue (42–44), lung cancer among smokers receiving thoracic radiotherapy (45), and thyroid cancer among patients with exposure of the thyroid to radiotherapy (46,47). Cardiovascular mortality is increased among survivors of HL, particularly because of atherosclerosis among patients receiving mediastinal radiation, although risks of congestive heart failure, valvular heart disease, and stroke are increased among HL survivors having received mediastinal radiotherapy and anthracycline-based chemotherapy (13,14,48). Despite excellent cure rates for our patients, mortality from causes other than HL and late medical morbidity were common. Risk of death from causes other than HL surpassed that from HL itself by 22 years after diagnosis; among causes of death other than HL, SPM and cardiovascular disease (myocardial infarction, congestive heart failure, and cerebrovascular accident) predominated. Relative risks of SPM in our cohort were markedly increased, with 7.0% of treated patients having died from SPM, and at least another 23.1% having survived a second malignancy. Of note, lower radiation dose was not associated with decreased risk of SPM, although more modern techniques of limiting normal tissue exposure such as intensity modulation or image-guided radiotherapy—techniques capable of reducing oncogenic radiation exposure to normal tissues—were not used. Our results affirm prior investigators’ identification of SPM as a critical cause of late mortality in patients treated with combined modality. In the long-term results of the German Hodgkin Study Group HD10 study, 5% of patients treated with two or four cycles of ABVD and radiation had experienced an SPM at a brief median follow-up (7.5 years); indeed, by that time point, deaths from cardiovascular disease or SPM had already exceeded that from HL (49). Recently, Meyer and colleagues reported mature findings from the HD.6 trial, comparing four to six cycles of ABVD to subtotal nodal radiotherapy alone or with two cycles of ABVD (1). The primary endpoint of the study was 12-year OS, and at median follow-up of 11 years survival was inferior for the radiotherapy arms, with a hazard ratio of death of two from increased nonrelapse mortality, largely SPM and cardiovascular disease. While radiation therapy techniques are now dramatically different, whether modifications of field and dose will fully abrogate late effects remains uncertain. Modeling studies predict a reduced risk of SPM with more modern doses and fields (50,51). Fewer data exist projecting changes to cardiac risk, although among pediatric survivors more modern treatment appears associated with reduced cardiac risks (52). However, others found that among patients receiving 15 to 25.5 Gy of involved field radiation (and optional 10 Gy boosts to bulky sites) the cumulative incidence of SPM was 17% at 20 years, with an SIR of 23 (53). These modest doses and fields are similar to those used in our lower-dose radiation protocols, 75 to 104 and 79 to 107 (Table 1), casting doubt upon whether reductions in field and dose necessarily abrogate risks of SPM. It is possible that modern treatment plans may still leave us above an oncogenic threshold, resulting in delayed onset of second malignancy rather than truly mitigated lifetime risk.
Beyond late mortality, these results demonstrate the striking prevalence of medical and psychiatric illnesses among survivors of HL treated as adults. The prevalence of major morbidity among our respondents, all treated during adulthood, is similar to—and in some cases greater than—that reported among survivors of childhood HL. Among 1927 survivors of childhood HL participating in the CCSS, 70% of respondents reported at least one morbidity; 28% reported three or more; and 27% reported at least one of grade 3 or 4 severity (25). While the median age of our respondents was 49 years, compared with 38 years among CCSS respondents, perhaps partially explaining these differences, our results highlight the fact that survivors of adult HL, even more than survivors of childhood HL, suffer from a wide range of often severe illnesses. The relationships between patient characteristics and late effects highlight both the challenge and the opportunity of such work. Whereas the relationships between age and late effects (eg, musculoskeletal and vision complaints) are most likely because of normal aging, the observation that those treated with lower doses of radiotherapy were no less likely to report late cardiovascular morbidity, nor to have been diagnosed with a second primary malignancy, is provocative.
Psychiatric morbidity was common among respondents, although survivors fared well in many respects. Self-reported divorce prevalence (7.6%) compares favorably to the 40% to 50% national norm, although typically lower in better-educated and higher socioeconomic strata (54). A 16.3% current-smoker percentage is slightly lower than the 19.3% national average, and that these smokers are potentially at increased risk of lung cancer given past exposure to chest radiation suggests a need for focused tobacco cessation interventions. Anxiety and depression were commonly reported: 14.0% had HADS scores consistent with major anxiety, and 11.4% more with borderline anxiety, while 5.9% scored consistent with major depression and an additional 5.5% with borderline depression. Among survivors of childhood cancer from the CCSS, rates of depression were similar, with 12.4% reporting depression (mild, moderate, or severe), but anxiety may be less common in survivors of childhood cancer, with only 8.6% of childhood cancer survivors scoring as having extreme anxiety by the Brief Symptom Inventory (BSI) assessment (55).
The interpretation of our findings must take into account the investigation’s multiple limitations. Generalizability of these findings is potentially limited by selection bias in two ways. First, eligibility for assessment was limited to patients treated at a tertiary cancer center on clinical trial; the patient population—well educated, relatively affluent, almost exclusively white, and willing to participate in clinical research—is not representative of the US population, nor of the narrower population of long-term survivors of Hodgkin’s lymphoma. Here, selection bias may lead to underestimation of the true prevalence of late morbidity among survivors. Selection bias may further be introduced by the participation rate of 46%, limiting the generalizability of these findings to the broader population of long-term survivors of Hodgkin’s lymphoma. Although nonrespondents did not differ from respondents by gender, race, ethnicity, or treatment with doxorubicin or radiation, whether they suffer from comparable rates of late morbidity is unknown. The treatments used in the constituent clinical trials are varied, with some regimens no longer clinically relevant for newly diagnosed patients. While the ability to extrapolate our aggregate results to patients treated with modern or emerging therapies is limited, these data can inform the management of HL survivors who received and were cured by these historical regimens. Chart abstraction was incapable of providing complete data regarding baseline morbidities of treated patients, and thus we cannot describe relationships between preexisting morbidities and the subsequent risk of late effects. Further, contextualization of our findings is challenged by a lack of normative comparators. Certain morbidities—eg, osteoarthritis or hypertension—are clearly related to aging, and our findings are incapable of assigning causation for reported conditions. Conclusions regarding cause of death are constrained by the quality of data of the National Death Index and of death certificates; while the NDI remains the gold standard for cause of death analyses for cohorts (56,57) and the inclusion of death certificate data potentially improves the assignation of cause of death (58), accuracy remains imperfect. Nonetheless, the broader picture is one that depicts a population with numerous and severe morbidities, and at a frequency that experience suggests is far beyond the norm, with mortality rates driven by late effects as much as by the original diagnosis of Hodgkin’s lymphoma. The breadth and depth of medical, psychiatric, and psychosocial morbidity in adult survivors, shown here in a global sense, as well as the risk of death from late effects, support the need for multidisciplinary care for HL survivors. The imperative, building upon this experience, is to enhance our understanding of host and exposure factors that contribute to risk, which can inform prevention, screening, and therapeutic efforts. But retaining an understanding of the multitude of morbidities that often coexist and complicate the care for survivors will be essential as we apply this evolving understanding to the care of each survivor as an individual.
Funding
This work was supported by the Mortimer J. Lacher Fellowship of The Lymphoma Foundation, the Adam Spector Fund for Hodgkin’s Research, The Ernest and Jeanette Dicker Charitable Foundation, and the Mr. Daniel Moon and Family philanthropic fund.
The study sponsors had no role in design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
References
- 1. Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366(5):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640–652. [DOI] [PubMed] [Google Scholar]
- 3. Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol. 2003;21(4):607–614. [DOI] [PubMed] [Google Scholar]
- 4. Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–4554. [DOI] [PubMed] [Google Scholar]
- 5. Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21(18):3431–3439. [DOI] [PubMed] [Google Scholar]
- 6. Favier O, Heutte N, Stamatoullas-Bastard A, et al. Survival after Hodgkin lymphoma: causes of death and excess mortality in patients treated in 8 consecutive trials. Cancer. 2009;115(8):1680–1691. [DOI] [PubMed] [Google Scholar]
- 7. Kiserud CE, Loge JH, Fossa A, et al. Mortality is persistently increased in Hodgkin’s lymphoma survivors. Eur J Cancer. 2010;46(9):1632–1639. [DOI] [PubMed] [Google Scholar]
- 8. Brusamolino E, Baio A, Orlandi E, et al. Long-term events in adult patients with clinical stage IA-IIA nonbulky Hodgkin’s lymphoma treated with four cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine and adjuvant radiotherapy: a single-institution 15-year follow-up. Clin Cancer Res. 2006;12(21):6487–6493. [DOI] [PubMed] [Google Scholar]
- 9. Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002;100(6):1989–1996. [DOI] [PubMed] [Google Scholar]
- 10. van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin’s disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18(3):487–497. [DOI] [PubMed] [Google Scholar]
- 11. Swerdlow AJ, Higgins CD, Smith P, et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort study. J Clin Oncol. 2011;29(31):4096–4104. [DOI] [PubMed] [Google Scholar]
- 12. Oeffinger KC, Bhatia S. Second primary cancers in survivors of childhood cancer. Lancet. 2009;374(9700):1484–1485. [DOI] [PubMed] [Google Scholar]
- 13. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–1886. [DOI] [PubMed] [Google Scholar]
- 14. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412–418. [DOI] [PubMed] [Google Scholar]
- 15. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cella L, Conson M, Caterino M, et al. Thyroid V30 Predicts Radiation-Induced Hypothyroidism in Patients Treated With Sequential Chemo-Radiotherapy for Hodgkin’s Lymphoma. Int J Radiat Oncol Biol Phys. 2012;82(5):1802–1808. [DOI] [PubMed] [Google Scholar]
- 17. Hobbie WL, Ginsberg JP, Ogle SK, et al. Fertility in males treated for Hodgkins disease with COPP/ABV hybrid. Pediatr Blood Cancer. 2005;44(2):193–196. [DOI] [PubMed] [Google Scholar]
- 18. Hodgson DC, Pintilie M, Gitterman L, et al. Fertility among female hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol. 2007;25(1):11–15. [DOI] [PubMed] [Google Scholar]
- 19. Ganz PA, Bower JE. Cancer related fatigue: a focus on breast cancer and Hodgkin’s disease survivors. Acta Oncol. 2007;46(4):474–479. [DOI] [PubMed] [Google Scholar]
- 20. Hjermstad MJ, Fossa SD, Oldervoll L, et al. Fatigue in long-term Hodgkin’s Disease survivors: a follow-up study. J Clin Oncol. 2005;23(27):6587–6595. [DOI] [PubMed] [Google Scholar]
- 21. Ng AK, Li S, Recklitis C, et al. A comparison between long-term survivors of Hodgkin’s disease and their siblings on fatigue level and factors predicting for increased fatigue. Ann Oncol. 2005;16(12):1949–1955. [DOI] [PubMed] [Google Scholar]
- 22. Ruffer JU, Flechtner H, Tralls P, et al. Fatigue in long-term survivors of Hodgkin’s lymphoma; a report from the German Hodgkin Lymphoma Study Group (GHSG). Eur J Cancer. 2003;39(15):2179–2186. [DOI] [PubMed] [Google Scholar]
- 23. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 24. Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. [DOI] [PubMed] [Google Scholar]
- 25. Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117(6):1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ginsberg JP, Goodman P, Leisenring W, et al. Long-term survivors of childhood Ewing sarcoma: report from the childhood cancer survivor study. J Natl Cancer Inst. 2010;102(16):1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood. 2011;118(5):1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hudson MM, Mulrooney DA, Bowers DC, et al. High-risk populations identified in Childhood Cancer Survivor Study investigations: implications for risk-based surveillance. J Clin Oncol. 2009;27(14):2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janeway KA, Grier HE. Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol. 2010;11(7):670–678. [DOI] [PubMed] [Google Scholar]
- 30. Boman KK, Hoven E, Anclair M, et al. Health and persistent functional late effects in adult survivors of childhood CNS tumours: a population-based cohort study. Eur J Cancer. 2009;45(14):2552–2561. [DOI] [PubMed] [Google Scholar]
- 31. Koziner B, Myers J, Cirrincione C, et al. Treatment of stages I and II hodgkin’s disease with three different therapeutic modalities. Am J Med. 1986;80(6):1067–1078. [DOI] [PubMed] [Google Scholar]
- 32. Straus DJ, Myers J, Lee BJ, et al. Treatment of advanced hodgkin’s disease with chemotherapy and irradiation: Controlled trial of two versus three alternating, potentially non-cross-resistant drug combinations. Am J Med. 1984;76(2):270–278. [DOI] [PubMed] [Google Scholar]
- 33. Straus DJ, Myers J, Passe S, et al. The eight-drug/radiation therapy program (MOPP/ABDV/RT) for advanced Hodgkin’s disease. A follow-up report. Cancer. 1980;46(2):233–240. [DOI] [PubMed] [Google Scholar]
- 34. Straus DJ, Portlock CS, Qin J, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) followed by radiation therapy (RT) vs ABVD alone for stages I, II and IIIA non bulky Hodgkin’s disease. Blood. 2004; In press. [DOI] [PubMed] [Google Scholar]
- 35. Straus DJ, Yahalom J, Gaynor J, et al. Four cycles of chemotherapy and regional radiation therapy for clinical early-stage and intermediate-stage Hodgkin’s disease. Cancer. 1992;69(4):1052–1060. [DOI] [PubMed] [Google Scholar]
- 36. Cella D. Manual of the functional assessment of chronic illness therapy (FACIT) scales, version 4. Evanston, IL: Center on Outcomes Research and Education, Evanston Northwestern Healthcare and Northwestern University; 1997. [Google Scholar]
- 37. Northouse LL. Mastectomy patients and the fear of cancer recurrence. Cancer Nurs. 1981;4(3):213–220. [PubMed] [Google Scholar]
- 38. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 39. Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 40. Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: Wiley; 1980. [Google Scholar]
- 41.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2011 Sub (1973–2010) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission.
- 42. Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334(12):745–751. [DOI] [PubMed] [Google Scholar]
- 43. van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95(13):971–980. [DOI] [PubMed] [Google Scholar]
- 44. Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290(4):465–475. [DOI] [PubMed] [Google Scholar]
- 45. Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94(3):182–192. [DOI] [PubMed] [Google Scholar]
- 46. Taylor AJ, Croft AP, Palace AM, et al. Risk of thyroid cancer in survivors of childhood cancer: results from the British Childhood Cancer Survivor Study. Int J Cancer. 2009;125(10):2400–2405. [DOI] [PubMed] [Google Scholar]
- 47. Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365(9476):2014–2023. [DOI] [PubMed] [Google Scholar]
- 48. Myrehaug S, Pintilie M, Tsang R, et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49(8):1486–1493. [DOI] [PubMed] [Google Scholar]
- 49. Engert A, Pluetschow A, Eich HT, et al. Combined Modality Treatment of Two or Four Cycles of ABVD Followed by Involved Field Radiotherapy in the Treatment of Patients with Early Stage Hodgkin’s Lymphoma: Update Interim Analysis of the Randomised HD10 Study of the German Hodgkin Study Group (GHSG). Blood. 2005;106(11):2673-–2673. [Google Scholar]
- 50. Hodgson DC, Koh ES, Tran TH, et al. Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer. 2007;110(11):2576–2586. [DOI] [PubMed] [Google Scholar]
- 51. Koh ES, Sun A, Tran TH, et al. Clinical dose-volume histogram analysis in predicting radiation pneumonitis in Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2006;66(1):223–228. [DOI] [PubMed] [Google Scholar]
- 52. Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55(6):1145–1152. [DOI] [PubMed] [Google Scholar]
- 53. O’Brien MM, Donaldson SS, Balise RR, et al. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28(7):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Copen CE, Daniels K, Vespa J, et al. First marriages in the United States: data from the 2006–2010 National Survey of Family Growth. Natl Health Stat Report. 2012; (49):1–21. [PubMed] [Google Scholar]
- 55. Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology. 2001;12(2):259–261. [DOI] [PubMed] [Google Scholar]
- 57. Sesso HD, Paffenbarger RS, Lee IM. Comparison of National Death Index and World Wide Web death searches. Am J Epidemiol. 2000;152(2):107–111. [DOI] [PubMed] [Google Scholar]
- 58. German RR, Fink AK, Heron M, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35(2):126–131. [DOI] [PubMed] [Google Scholar]