Abstract
Background
The importance of assessing proprioceptive function for rehabilitation after neurological or orthopedic injury has long been recognized. Yet, neither the validity nor the accuracy of the available tests is firmly established. Testing typically involves repeated matching of a given joint position with the same or opposite limb where the difference between the 2 positions indicates proprioceptive acuity.
Objectives
The aim of this study was to compare position sense acuity between ipsilateral and contralateral matching methods against a psychophysical threshold method to establish the accuracy and relationships between these models.
Design
A repeated-measures design was used.
Method
Assessment of forearm position sense for a 10-degree reference position in 27 young adults who were healthy.
Results
Psychophysical thresholds were revealed to be the most precise and least variable acuity measure. The mean (±SD) threshold (1.05°±0.47°) was significantly lower than mean position errors obtained by both joint position matching tasks (ipsilateral: 1.51°±0.64°; contralateral: 1.84°±0.73°)—a 44% to 75% difference in measurement accuracy. Individual participant position errors correlated poorly with respective thresholds, indicating a lack of concurrent validity. Position errors for both matching methods correlated only mildly with each other.
Limitations
The data represent performance of a healthy, young adult cohort. Differences between methods will likely be more pronounced in aging and clinical populations.
Conclusions
Threshold testing and joint position matching methods examine different physiological aspects of proprioceptive function. Because threshold testing is based on passive motion, it most closely reflects afferent sensory feedback processing (ie, proprioception). Matching methods require active motion and are consequently influenced by additional sensorimotor processes. Factors such as working memory and transmission between brain hemispheres also influence joint matching task outcomes.
Mechanoreceptors of the joints, muscles, tendons, and skin provide information about muscle length, contractile speed, muscle tension, and joint position. They form the basis for proprioception, giving rise to the passive motion, active motion, and limb position senses and the sense of heaviness.1
Numerous neurological and orthopedic conditions, such as Parkinson disease,2–4 focal dystonia,5,6 peripheral sensory neuropathies,7,8 or injuries to ligaments, joint capsules, and muscles, are associated with proprioceptive impairment.9–11 Furthermore, proprioceptive feedback is considered an important training signal for the neural reorganization underlying the recovery of behavioral function.12–14 Although the importance of proprioception for motor control and motor rehabilitation is widely recognized, there is no universally accepted method that allows for an objective and precise evaluation of proprioceptive function—namely, the assessment of proprioceptive sensitivity (ie, the intensity of the smallest detectable stimulus) and acuity (ie, the smallest perceived difference between 2 detectable stimuli).
In clinical practice, proprioceptive evaluation most often involves some form of limb position sense testing, requiring the patient to match the joint position of homologous limbs without vision. The examiner passively moves the patient's limb to a target position, which the patient either matches with the contralateral limb or, after the examiner moves the ipsilateral limb back to its starting position, actively moves the ipsilateral limb to the remembered position. The visually observed mismatch in position serves as a measure of proprioceptive precision. These clinical examinations constitute a coarse measure of proprioceptive function and are ill-suited to provide the necessary level of accuracy needed to evaluate rehabilitation protocols. Thus, researchers have designed specialized equipment for testing joint position sense in a more controlled and precise manner. Typically, this equipment allows for bilateral movement of the upper or lower limbs and utilizes a joint position matching paradigm where position sense is assessed by measuring positional error between the reference and the matching movement.11,15,16
Alternatively, psychophysical threshold hunting methods have been established that yield 2 types of thresholds: the detection threshold, which is the smallest perceivable change in position, and the discrimination threshold, which is the just-noticeable difference between 2 perceived positions.17 The detection threshold is considered a measure of sensitivity, and the discrimination threshold represents a measure of acuity. In contrast to joint matching methods that rely on active motion of the test person, threshold hunting paradigms often use specialized equipment that passively moves a person's limb in a highly controlled manner.18–22
A number of studies investigated the reliability and accuracy of joint position matching as well as passive motion paradigms. From these studies emerged that test-retest reliability for position matching methods is highly variable, with one study demonstrating excellent reliability (r=.88–.92) for wrist position sense testing,23 whereas others showed moderate to poor reliability (r=.18–.64) for shoulder and knee joint position sense evaluation.24,25 In contrast, procedures relying on measuring position sense during passive motion were shown to be valid and very reliable for the ankle, hip, and elbow joints.18,19,26,27
In summary, although joint position matching paradigms are routinely used in clinical examinations and are widely applied in many studies to obtain a measure of proprioceptive function, there is a paucity of data on the accuracy and validity of these measures. In addition, studies that reported proprioceptive thresholds often focused on detection rather than discrimination, whereas the joint position matching tasks usually reflect an error in discrimination. That is, these measures are not directly comparable. Finally, many studies18,19,24–27 relied on averaging over relatively few trials (<12) to arrive at a detection threshold, a notion that does influence accuracy, and compared movements with different amplitudes. This study sought to expand on this previous research by: (1) determining the accuracy of joint position matching tests using a defined movement amplitude as reference, (2) comparing these results with an appropriate psychophysical discrimination threshold to understand the differences induced by passive versus active motion, and (3) establishing a measure of validity by analyzing how well joint position matching methods relate to a psychophysical test of proprioceptive acuity.
Method
Participants
Twenty-seven young adults (mean age±SD=25.7±4.1 years; 26 right-handed, 1 left-handed) who had no known injuries or pathologies involving the upper limb participated in the study. Handedness of all participants was assessed using the Edinburgh Handedness Inventory.28
Instruments
A passive motion apparatus was used to test the position sense acuity in the psychophysical evaluation method (Fig. 1A). Participants placed their forearm on an aluminum splint that was moved passively by a DC 5-phase stepping motor (precision: 5,466 steps per 1°=0.00018°/step; Nyden Inc, San Jose, California). The splint was padded with 16-cm-thick foam to attenuate possible vibration effects of the DC motor. Control of the apparatus was realized through customized software routines coded in MATLAB technical programming language (The MathWorks, Natick, Massachusetts).
Figure 1.
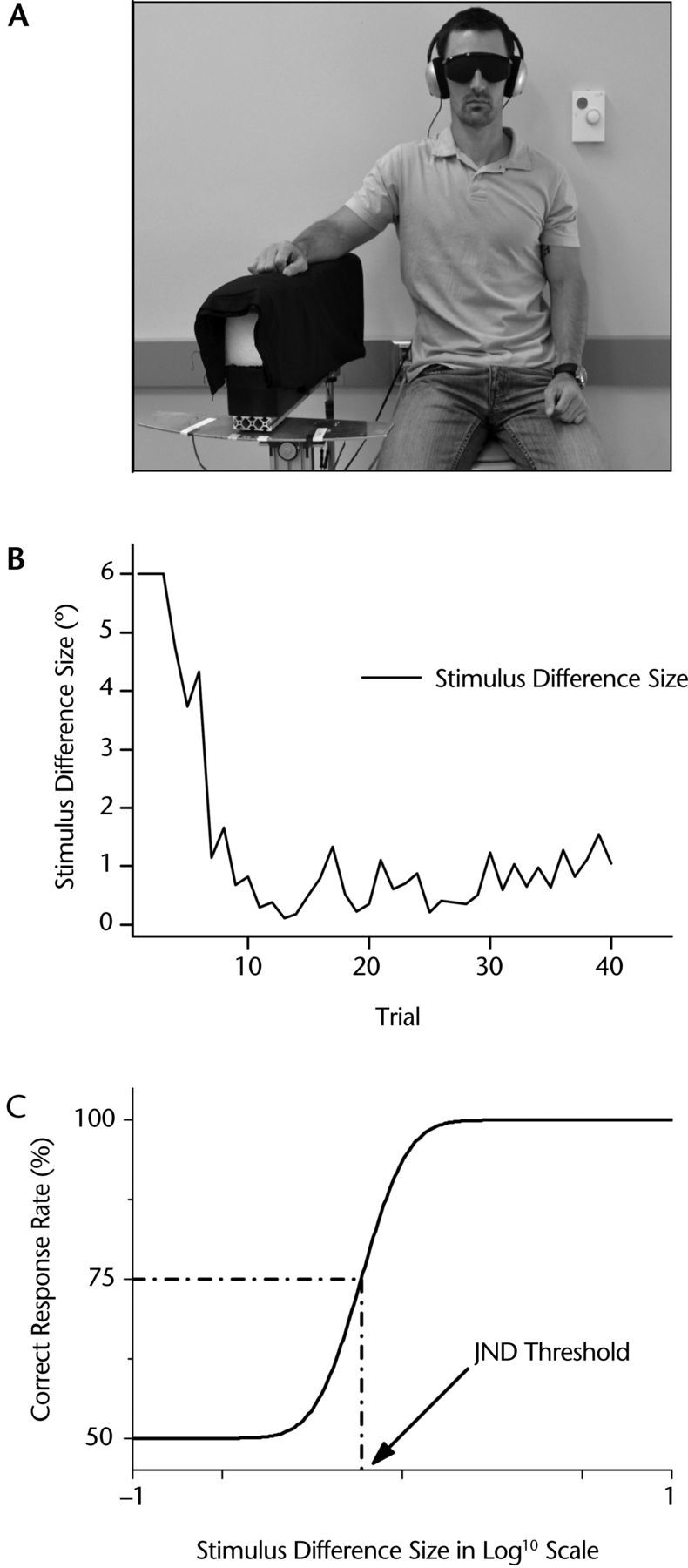
(A) Setup for psychophysical threshold method. The passive motion apparatus flexed or extended the forearm in the horizontal plane at a constant speed of 2°/s. (B) Exemplar data of one participant showing difference between the standard and comparison movement amplitude. Note how the stimulus difference size converged toward the participant's threshold. (C) Based on the response data, a cumulative Gaussian function was fitted. The difference value at the 75% correct response level marked the just-noticeable difference (JND) threshold.
A bimanual manipulandum consisting of 2 horizontal levers acting as movable armrests was used for the ipsilateral and contralateral limb position matching methods. Participants placed each forearm on one of the armrests and aligned the joint axis of their elbow to the armrest's axis of rotation (Fig. 2A). The angular position of the armrests was monitored by embedded potentiometers attached to their respective axles (spatial resolution=0.008°). The 2 armrests could be moved relative to each other. The distance between armrests was adjusted to shoulder width for each participant in such a way that shoulder abduction angle was comfortable (<20°) and very similar between shoulders and across participants.
Figure 2.
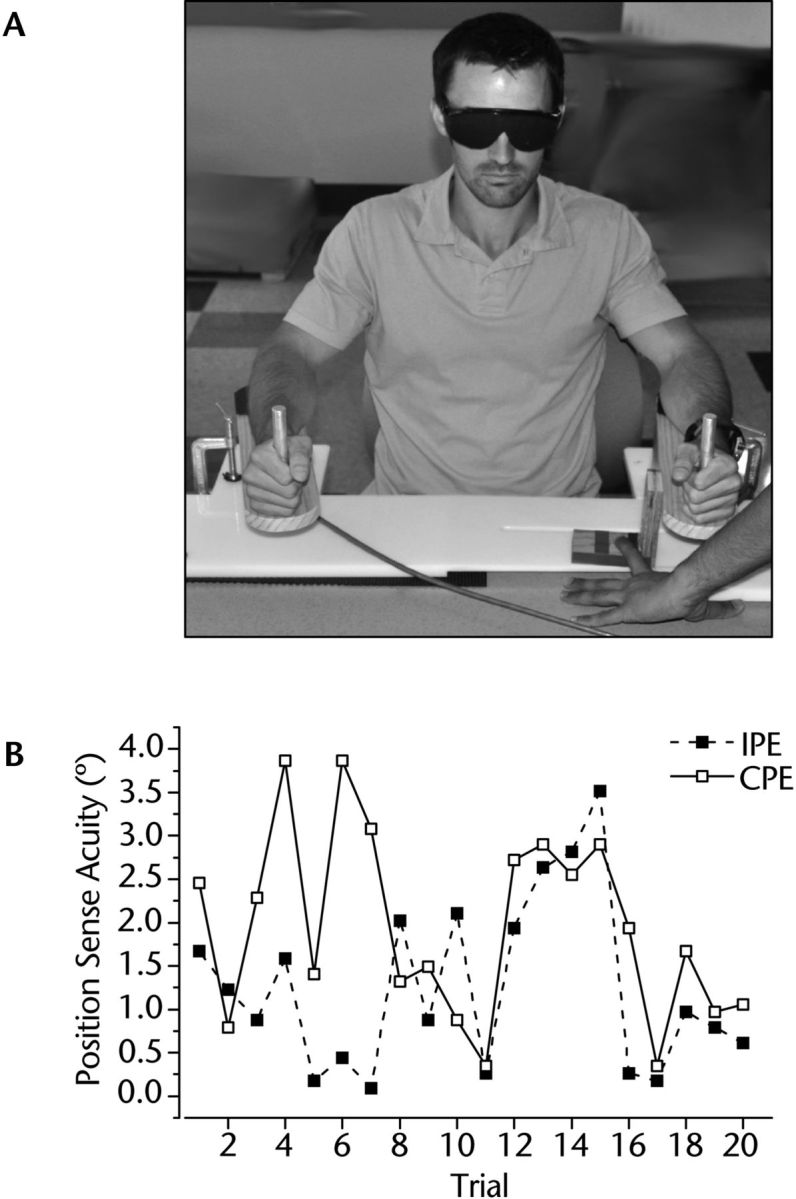
(A) The bimanual manipulandum used ipsilateral and contralateral matching of forearm position. High precision potentiometers (resolution: 8/1,000°) encoded the position of each arm. (B) Exemplar data of one participant showing the recorded position errors for each trial of ipsilateral and contralateral joint position matching tasks. IPE=ipsilateral position error, CPE=contralateral position error.
Procedure
In all participants, position sense acuity was measured by 3 methods (psychophysical threshold, contralateral and ipsilateral matching). The order of the tasks was counterbalanced across participants using a Latin square design. To control for the effects of hand dominance and hemispheric lateralization on proprioceptive acuity, the dominant arm was tested for all participants. During testing, the participants' vision was blocked with a pair of opaque glasses.
Psychophysical threshold method
During the psychophysical evaluation, participants sat in an upright position and placed their arm on the splint of the passive motion apparatus. Participants wore headphones that played pink noise to mask any possible auditory cues. Starting position was an arm position in which the elbow was at approximately 90 degrees of flexion. The passive motion apparatus then flexed the forearm in the horizontal plane at a constant velocity of 2°/s. The rationale for choosing 2°/s was to move the arm slowly and deliberately so that the participant had ample time to begin to concentrate on the final displacement. At the same time, it was fast enough to reach the target amplitude at a sufficiently short time period (maximum movement time <5 seconds), avoiding memory issues (ie, the amplitude comparison within a trial becomes compromised because the participant already forgot the amplitude of the first movement). Each trial involved sensing 2 forearm positions: a standard position (80° flexion), resulting in a 10-degree movement amplitude from the start, and a comparison position <10 degrees from the start. After each trial, participants were asked, “Which of these 2 positions was closer to the body?” They had to indicate the first movement or the second movement (forced choice). Based on these responses, an adaptive QUEST algorithm29 selected the comparison stimulus position for the subsequent trial. The comparison position varied between 4 and 10 degrees (86°–80° flexion) across trials. The adaptive algorithm ensured that the difference between the standard and the comparison stimuli converged almost monotonically to the threshold.
The order of the standard and comparison positions switched randomly between trials. The armrest moved at a constant angular velocity of 2°/s for both the standard and comparison movements of all the trials for all participants. The task involved a minimum of 40 trials. If convergence was not clearly seen, we added 20 more trials. This procedure ensured that the threshold data were not compromised by a lack of data (ie, the participant had a lower physiological threshold, but we failed to measure it because we had stopped testing too early). The participants were given rest periods of 1 to 2 minutes for every 12 to 15 trials to ensure an active focus on the task.
Ipsilateral matching method
Participants rested their dominant forearm on the movable splint of a bimanual manipulandum while being seated in an upright position (Fig. 2A). They were instructed to hold the handgrip in a relaxed manner. The armrest had a stopper to ensure the same starting position for all the trials (90° flexion; ie, the same as for the psychophysical method). In each trial, the examiner moved the splint on each participant's dominant side by 10 degrees from the starting position toward the body. The experimenter verbally informed the participants when the desired target position was reached. Their forearm was then passively moved back to the starting position. Next, the participants actively moved their forearm to the remembered target position. They were instructed to stop their movement and provide verbal notification when they felt they had reached the same forearm position as the reference target. The participants held the position for about 2 seconds to allow for collecting a solid sample of angular data through the potentiometer. A total of 20 trials were administered. The participants were allowed practice trials before the start of the task to understand the procedure. During the whole task, all participants wore a pair of opaque glasses that blocked the vision of the arm position.
Contralateral matching method
In the contralateral matching method, both forearms of a participant rested on the movable splints of a bimanual manipulandum. Starting position and number of trials were the same as during ipsilateral testing (90° of elbow flexion; 20 trials). The closeness of each arm to the trunk was controlled. The bimanual manipulandum allowed us to “body scale” the distance of the 2 arm splints relative to the trunk by moving them horizontally to each other. This procedure effectively meant that the abduction angle for both arms was similar and constant within and across participants. In each trial, the examiner moved the participant's nondominant arm to the target position of 10 degrees from the start position in the horizontal plane toward the body. While holding the position of the nondominant arm, the participant then actively moved the dominant arm to match the target position of the nondominant arm. The angular displacements on both sides were recorded. As in the ipsilateral matching method, participants were allowed a few practice trials until they were comfortable with the procedure.
Measurements
Psychophysical threshold
Figure 1B shows exemplar data of one participant, indicating how the angular difference between the 2 stimuli converged during psychophysical testing. Based on the participants' verbal responses for every trial, the percentage of correct responses was computed. These response rates were then fitted as a function of stimulus intensity using a cumulative Gaussian function (Fig. 1C). Based on the fitted function, the stimulus difference value at the 75% correct response rate was defined as the participant's discrimination or just-noticeable difference (JND) threshold.
Joint position error during matching
For both matching methods, an arm position error was computed for each trial. The recorded angular positions recorded for both the positions (target and matched) were used to compute the absolute angular difference between these 2 values, indicating a joint position error for either the ipsilateral or contralateral matching.
Data Analysis
The position error values obtained through the ipsilateral and contralateral matching procedure and the JND threshold were analyzed using custom-written scripts based on R statistical programming language (R Foundation for Statistical Computing, Vienna, Austria).30 The density distribution function of each measure and the respective means and standard deviations were computed. A univariate, type III, repeated-measures analysis of variance (ANOVA) of ipsilateral position error, contralateral position error, and threshold was performed to determine mean differences among methods. Subsequent correlation and linear regression analyses were performed to determine the strength of relationship among these 3 measures of position sense.
Results
Outlier Analysis
Three participants had mean data that were 1.5 times outside the interquartile range and above 2 standard deviations of the respective means in one of the joint position matching tests. Following Frigge and colleagues,31 these participants were classified as outliers, and all of their data were excluded from further analysis.
Contrasting the Position Sense Acuity Measures
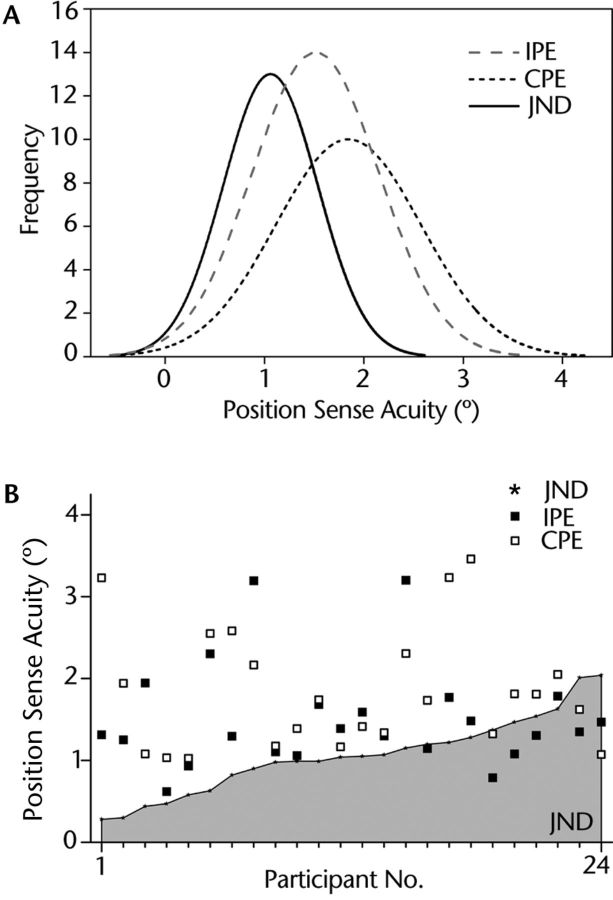
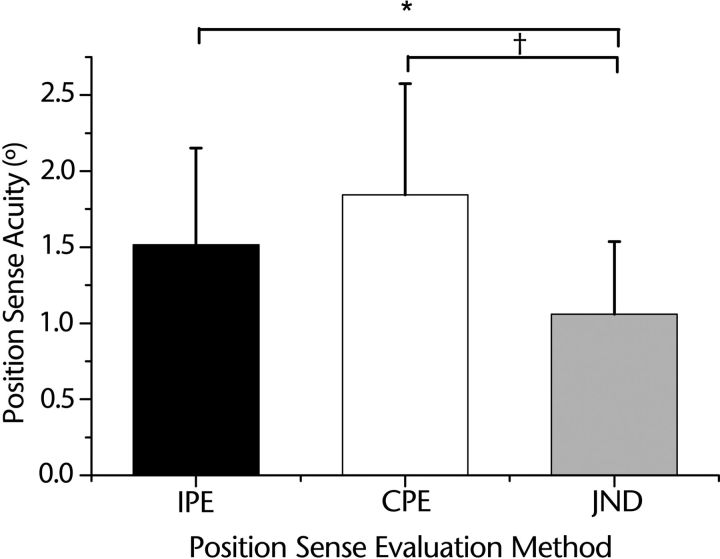
Based on our sample data, the probability density functions of each of the 3 position sense measures were derived (Fig. 3A). The threshold data had a tall and narrow distribution shifted to the left compared with the position errors obtained through the joint position matching tests, indicating a lower mean and variability of the threshold data. The differences among position sense test methods were confirmed by a repeated-measures one-way ANOVA, which yielded a highly significant effect for test method (F2,46=10.8; P<.001). The results of a Mauchly test for sphericity validated the use of the ANOVA, indicating that the assumption of equality of variances had not been violated (P=.66). Subsequent Tukey honestly significant difference post hoc tests revealed that the mean (±SD) JND threshold (1.05°±0.47°) was significantly different from the mean ipsilateral position error (1.51°±0.64°; P=.01) and contralateral position error (1.84°±0.73°; P<.001). The position error means were not significantly different from each other (P=.57) (Fig. 4).
Figure 3.
(A) Probability density distributions for all 3 position sense acuity measures. Note how the distribution of the threshold data is shifted to the left of the 2 curves representing the matching error distributions. (B) Acuity measures of the 3 methods by individual participants. Data are sorted in ascending order with respect to their just-noticeable difference (JND) threshold values. Note that JND threshold was consistently lower for the majority of participants. IPE=ipsilateral position error, CPE=contralateral position error.
Figure 4.
Mean positional error for each matching method in comparison with the mean threshold. Error bars indicate standard deviation. Mean ipsilateral position error (IPE) was 44% larger and mean contralateral position error (CPE) was 75% larger than the mean threshold. JND=just noticeable difference. * P<.01; †P<.001.
To obtain a better understanding of these differences, we analyzed the individual participant performance for each test. This analysis did not show a consistent trend that 1 of the 2 matching methods yielded lower threshold values. However, it revealed that 75% of all participants exhibited position errors in either test that were above their respective threshold (Fig. 3B), indicating that threshold testing was associated with lower position sense measurements.
Relationships Between Position Error and JND Threshold
In order to examine the relationships between position errors and JND thresholds, we performed respective correlation analyses. Pearson product moment correlations between the JND thresholds and position errors were poor (ipsilateral: r=−.004; contralateral: r=−.13) and failed to reach statistical significance. The corresponding linear regression analysis confirmed that the position error values of both matching tests are poor predictors of the respective JND threshold (ipsilateral-JND: r2=.00002, P=.98; contralateral-JND: r2=.007, P=.7). The correlation between the ipsilateral and contralateral position error values was mild (r=.35, P=.09).
Discussion
Clinical researchers have long advocated the need for precise assessment tools for proprioceptive function in patients that overcome the limitations of current testing protocols.11,16,24 This study investigated the accuracy of 3 methods for testing joint position sense acuity. Its main findings were as follows. First, the psychophysical threshold method yielded the most precise and least variable measure of position sense acuity. Mean position errors obtained by the 3 joint position matching tasks were substantially larger (Fig. 4). Second, individual participant position errors correlated poorly with respective thresholds, indicating a lack of concurrent validity of these measures. Third, error values of both matching methods correlated only mildly with each other, indicating that both methods are not interchangeable but may measure different aspects of proprioceptive function.
When trying to understand the differences among these 3 measures of position sense acuity and the implications of these results for the clinical assessment of proprioceptive function for diagnosis and rehabilitation, one needs to consider the physiological and methodological factors that may have influenced the outcome measures.
Influence of Active Versus Passive Movement on Position Sense Testing
One defining difference between the threshold method and the joint position matching methods is that threshold hunting relies on passive motion, whereas the matching methods both involved active motion when the test person matched a given joint position. The threshold method mainly reflects the processing of external feedback (ie, the peripheral transmission of proprioceptive signals and a cortical activation of regions in the contralateral somatosensory cortex).32 In contrast, matching methods reflect the processing of external sensory feedback plus an array of underlying sensorimotor processes (ie, because the active motion required to match a target position produces a second sensory source, called internal or predicted sensory feedback).33,34 This information is derived from a copy of the motor commands that give rise to the voluntary movement (the so-called efference copy or motor command corollary discharge). Both internal and external feedback are then combined in a process of sensorimotor integration involving the activation of the somatosensory cortices, the primary motor and premotor cortical regions, and the subcortical regions (such as the ipsilateral cerebellum and contralateral putamen).35
In addition, active motion is necessarily subject to movement variability. Consequently, the limits of movement precision impose limits on measurable sensory precision. In summary, the observed differences between the joint position matching task and the threshold method may partly have been due to the fact that the underlying joint displacements were achieved by different types of motion (Table). The superior accuracy obtained under passive motion implies that researchers or clinicians who desire an exact measure of proprioception should rely on tests using passive motion because sensing joint position after active motion is necessarily influenced or confounded by processes of sensorimotor integration and motor control. This explanation does not imply that joint position testing under active movement conditions is meaningless. It implies that additional aspects of proprioceptive processing closer to motor control also will be tested.
Table.
Overview of the Characteristics of the Evaluated Joint Position Sense Testing Methods
| Method | Type of Motion | Working Memory Required? | Interhemispheric Transfer/Integration Required? | Can Test for: |
|---|---|---|---|---|
| Contralateral matching | Active | No | Yes | Acuity |
| Ipsilateral matching | Active | Yes | No | Acuity |
| Psychophysical threshold | Passive | Yes | No | Acuity, sensitivity |
Involvement of 1 or 2 Brain Hemispheres
Surprisingly, not even the 2 joint position matching methods relying on active motion showed close correspondence. At first glance, this finding does not reflect well on the validity of these methods. Again, one needs to consider whether the 2 methods actually test different aspects of position sense. Using the ipsilateral limb or the contralateral limb to assess position sense acuity implies the utilization of different brain hemispheres to accomplish the task. Although ipsilateral testing mostly reflects the activation of the contralateral brain hemisphere,36 contralateral testing is a bimanual task requiring bilateral activation of frontoparietal cortical networks32,35 and the exchange of proprioceptive information across the cerebral hemispheres. Previous studies have shown that transcallosal projections, especially those crossing the posterior portions of the corpus callosum,37 convey corollary motor signals and somatosensory feedback signals across the hemispheres in bimanual tasks.38,39
If the callosal projections are compromised due to either injury or agenesis, the spatiotemporal parameters of bimanual movements become impaired, and localization of the contralateral limb degrades.40–42 Given that the contralateral matching task requires the sensing of limb position at one side and reproduction of this position at the other side, and knowing that the transfer of the underlying proprioceptive and tactile information relies on intact transcallosal projections, it becomes plausible that injury of these projections will lead to position and localization errors.38,42 Thus, a comparison of ipsilateral and contralateral position error may constitute an indirect measure of the integrity of the interhemispheric connections that warrants closer examination.
Different Demands on Working Memory
The psychophysical method and the ipsilateral joint position matching method present 2 joint positions at 2 different points in time. In order to compare the 2 positions, the tested person needs to keep the first position in working memory. In contrast, the contralateral matching method requires the concurrent evaluation of 2 joint positions. Thus, the results of the 2 methods—ipsilateral joint position matching method and psychophysical method—are potentially influenced by differences in working memory or memory-based feedback43 (Table). In our sample of young individuals who were healthy, the additional demand on memory-based proprioceptive feedback had an impact on acuity measures. The threshold and ipsilateral matching method revealed lower acuity values compared with the contralateral method. However, it has been shown that ipsilateral joint position matching is affected by lower working memory capabilities in older adults.44 When testing patients with disease states known to impair working memory and processing and the utilization of somatosensory information such as stroke, the contralateral matching method may be quite appropriate,23 although no data on a direct comparison of joint acuity measures of ipsilateral and contralateral methods involving patients with stroke are available.
Differences in Device Technology
Could differences in the spatial resolution of the 2 testing devices account for the differences in the measured accuracy? This result is highly unlikely given that the resolution of the encoders in the manipulandum was 0.008 degrees and the spatial resolution of the passive motion apparatus was 0.00018 degrees. Thus, the reported mean differences are not explained by a lack of precision of the instrumentation where the proprioceptive precision difference fell within the measurement error of the systems.
Summary and Recommendations
There is great interest in and need for a reliable, valid, and easy-to-administer test of proprioceptive function for diagnosis and rehabilitation.15,16 Unfortunately, there is little consensus about the best method to use; the results from numerous methodological studies are mixed and provide little guidance.15,18,19,24–27,45 This lack of clarity is partly due to the fact that measures of proprioceptive sensitivity are compared with measures of acuity derived by another method, or motion sense is compared with position sense. That is, the measures are not directly comparable but are expressing different aspects of proprioceptive function. In order to control for such issues, this study examined only measures of position sense acuity derived by 3 methods. The results show that even under those controlled conditions, the methods may yield varying measures of precision and show little correlation to each other. We contend that these differences in acuity are not necessarily a sign of a methodological weakness of a particular method but are due to several physiological factors inherent to each method that affect the outcome measure (Table).
Researchers in rehabilitation, neurology, and orthopedics have advocated the need for new measurement protocols for proprioception that overcome the limitations of current testing protocols.16 Our findings imply that researchers and clinicians interested in assessing proprioceptive function by measuring position sense acuity need to be cognizant about the benefits and limitations of each method. If the main emphasis is to obtain the “purest” possible measure of proprioceptive function, a method using passively induced joint motion will be superior. If, in addition, the intactness of sensorimotor integration processes is of interest, methods requiring active motion are appropriate. The difference between active and passive motion acuity may provide an indirect measure of those sensorimotor contributions. Similarly, comparing ipsilateral and contralateral acuity measures may be informative about how well somatosensory information can be exchanged across brain hemispheres.
Footnotes
Study participation was approved by the Institutional Review Board of the University of Minnesota.
References
- 1. Goldscheider A. Physiologie des Muskelsinnes. Leipzig, Germany: Johann Ambrosius Barth; 1898. [Google Scholar]
- 2. Khudados E, Cody F, O'Boyle D. Proprioceptive regulation of voluntary ankle movements, demonstrated using muscle vibration, is impaired by Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mongeon D, Blanchet P, Messier J. Impact of Parkinson's disease and dopaminergic medication on proprioceptive processing. Neuroscience. 2009;158:426–440. [DOI] [PubMed] [Google Scholar]
- 4. Rickards C, Cody F. Proprioceptive control of wrist movements in Parkinson's disease: reduced muscle vibration-induced errors. Brain. 1997;120(pt 6):977–1067. [DOI] [PubMed] [Google Scholar]
- 5. Putzki N, Stude P, Konczak J, et al. Kinesthesia is impaired in focal dystonia. Mov Disord. 2006;21:754–760. [DOI] [PubMed] [Google Scholar]
- 6. Rosenkranz K, Altenmüller E, Siggelkow S, Dengler R. Alteration of sensorimotor integration in musician's cramp: impaired focusing of proprioception. Clin Neurophysiol. 2000;111:2040–2045. [DOI] [PubMed] [Google Scholar]
- 7. Ghez C, Gordon J, Ghilardi MF, et al. Roles of proprioceptive input in the programming of arm trajectories. Cold Spring Harb Symp Quant Biol. 1990;55:837–847. [DOI] [PubMed] [Google Scholar]
- 8. Rothwell J, Traub M, Day B, et al. Manual motor performance in a deafferented man. Brain. 1982;105(pt 3):515–557. [DOI] [PubMed] [Google Scholar]
- 9. Barrack R, Skinner H, Buckley S. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17:1–7. [DOI] [PubMed] [Google Scholar]
- 10. Fridén T, Roberts D, Zätterström R, et al. Proprioception after an acute knee ligament injury: a longitudinal study on 16 consecutive patients. J Orthop Res. 1997;15:637–644. [DOI] [PubMed] [Google Scholar]
- 11. Lephart SM, Warner JJP, Borsa PA, Fu FH. Proprioception of the shoulder joint in healthy, unstable, and surgically repaired shoulders. J Shoulder Elbow Surg. 1994;3:371–380. [DOI] [PubMed] [Google Scholar]
- 12. Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38(2 suppl):840–845. [DOI] [PubMed] [Google Scholar]
- 13. Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol. 1998;79:2119–2148. [DOI] [PubMed] [Google Scholar]
- 14. Sabbah P, de SS, Leveque C, et al. Sensorimotor cortical activity in patients with complete spinal cord injury: a functional magnetic resonance imaging study. J Neurotrauma. 2002;19:53–60. [DOI] [PubMed] [Google Scholar]
- 15. Goble D. Proprioceptive acuity assessment via joint position matching: from basic science to general practice. Phys Ther. 2010;90:1176–1260. [DOI] [PubMed] [Google Scholar]
- 16. Knoop J, Steultjens MP, van der Leeden M, et al. Proprioception in knee osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2011;19:381–388. [DOI] [PubMed] [Google Scholar]
- 17. Gescheider GA. Psychophysics: Method, Theory, and Application. 2nd ed Lawrence, NJ: Erlbaum Associates Inc Publishers; 1985. [Google Scholar]
- 18. Westlake K, Wu Y, Culham E. Velocity discrimination: reliability and construct validity in older adults. Hum Mov Sci. 2007;26:443–456. [DOI] [PubMed] [Google Scholar]
- 19. Deshpande N, Connelly D, Culham E, Costigan P. Reliability and validity of ankle proprioceptive measures. Arch Phys Med Rehabil. 2003;84:883–892. [DOI] [PubMed] [Google Scholar]
- 20. Konczak J, Krawczewski K, Tuite P, Maschke M. The perception of passive motion in Parkinson's disease. J Neurol. 2007;254:655–663. [DOI] [PubMed] [Google Scholar]
- 21. Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126(pt 10):2312–2322. [DOI] [PubMed] [Google Scholar]
- 22. Skinner HB, Barrack RL, Cook SD. Age-related decline in proprioception. Clin Orthop Rel Res. 1984;184:208–211. [PubMed] [Google Scholar]
- 23. Carey L, Oke L, Matyas T. Impaired limb position sense after stroke: a quantitative test for clinical use. Arch Phys Med Rehabil. 1996;77:1271–1279. [DOI] [PubMed] [Google Scholar]
- 24. Lönn J, Crenshaw A, Djupsjöbacka M, Johansson H. Reliability of position sense testing assessed with a fully automated system. Clin Physiol. 2000;20:30–37. [DOI] [PubMed] [Google Scholar]
- 25. Fatoye F, Palmer S, Macmillan F, et al. Repeatability of joint proprioception and muscle torque assessment in healthy children and in children diagnosed with hypermobility syndrome. Musculoskeletal Care. 2008;6:108–123. [DOI] [PubMed] [Google Scholar]
- 26. Benjaminse A, Sell T, Abt J, et al. Reliability and precision of hip proprioception methods in healthy individuals. Clin J Sport Med. 2009;19:457–463. [DOI] [PubMed] [Google Scholar]
- 27. Juul-Kristensen B, Lund H, Hansen K, et al. Test-retest reliability of joint position and kinesthetic sense in the elbow of healthy subjects. Physiother Theory Pract. 2008;24:65–72. [DOI] [PubMed] [Google Scholar]
- 28. Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 29. Watson A, Pelli D. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–133. [DOI] [PubMed] [Google Scholar]
- 30. R: A Language and Environment for Statistical Computing. [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 31. Frigge M, Hoaglin D, Iglewicz B. Some implementations of the boxplot. Am Stat. 1989;43:50–54. [Google Scholar]
- 32. Radovanovic S, Korotkov A, Ljubisavljevic M, et al. Comparison of brain activity during different types of proprioceptive inputs: a positron emission tomography study. Exp Brain Res. 2002;143:276–285. [DOI] [PubMed] [Google Scholar]
- 33. Evarts EV. Central control of movement, V: feedback and corollary discharge—a merging of the concepts. Neurosci Res Program Bull. 1971;9:86–112. [PubMed] [Google Scholar]
- 34. Sciutti A, Squeri V, Gori M, et al. Predicted sensory feedback derived from motor commands does not improve haptic sensitivity. Exp Brain Res. 2010;200:259–267. [DOI] [PubMed] [Google Scholar]
- 35. Ciccarelli O, Toosy AT, Marsden JF, et al. Identifying brain regions for integrative sensorimotor processing with ankle movements. Exp Brain Res. 2005;166:31–42. [DOI] [PubMed] [Google Scholar]
- 36. Chang MC, Ahn SH, Cho YW, et al. The comparison of cortical activation patterns by active exercise, proprioceptive input, and touch stimulation in the human brain: a functional MRI study. NeuroRehabilitation. 2009;25:87–92. [DOI] [PubMed] [Google Scholar]
- 37. Hofer S, Frahm J. Topography of the human corpus callosum revisited: comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. [DOI] [PubMed] [Google Scholar]
- 38. Fabri M, Del Pesce M, Paggi A, et al. Contribution of posterior corpus callosum to the interhemispheric transfer of tactile information. Brain Res Cogn Brain Res. 2005;24:73–80. [DOI] [PubMed] [Google Scholar]
- 39. Geffen G, Jones D, Geffen L. Interhemispheric control of manual motor activity. Behav Brain Res. 1994;64:131–140. [DOI] [PubMed] [Google Scholar]
- 40. Eliassen J, Baynes K, Gazzaniga M. Anterior and posterior callosal contributions to simultaneous bimanual movements of the hands and fingers. Brain. 2000;123(pt 12):2501–2511. [DOI] [PubMed] [Google Scholar]
- 41. Bonzano L, Tacchino A, Roccatagliata L, et al. Callosal contributions to simultaneous bimanual finger movements. J Neurosci. 2008;28:3227–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geffen G, Nilsson J, Quinn K, Teng E. The effect of lesions of the corpus callosum on finger localization. Neuropsychologia. 1985;23:497–514. [DOI] [PubMed] [Google Scholar]
- 43. Goble D, Lewis C, Hurvitz E, Brown S. Development of upper limb proprioceptive accuracy in children and adolescents. Hum Mov Sci. 2005;24:155–170. [DOI] [PubMed] [Google Scholar]
- 44. Goble DJ, Mousigian MA, Brown SH. Compromised encoding of proprioceptively determined joint angles in older adults: the role of working memory and attentional load. Exp Brain Res. 2012;216:35–40. [DOI] [PubMed] [Google Scholar]
- 45. Grob K, Kuster M, Higgins S, et al. Lack of correlation between different measurements of proprioception in the knee. J Bone Joint Surg (Br). 2002;84:614–622. [DOI] [PubMed] [Google Scholar]