Abstract
Background
Radiotherapy and lymphadenectomy have been associated with improved survival in population-based studies of endometrial cancer, which is in contrast with findings from randomized trials and meta-analyses. The primary study aim was to estimate the cause-specific effects of adjuvant radiotherapy and lymphadenectomy on competing causes of mortality.
Methods
We analyzed Surveillance, Epidemiology, and End Results (SEER) data from 1988 to 2006. The sample comprised 58172 patients with stage I and II endometrial adenocarcinoma. Patients were risk stratified by stage, grade, and age. Cumulative incidences and cause-specific hazards of competing causes of mortality were estimated according to treatment. All statistical tests were two-sided.
Results
Pelvic radiotherapy was associated with statistically significantly increased endometrial cancer mortality (hazard ratio [HR] = 1.66; 95% confidence interval [CI] = 1.52 to 1.82) in all stage I and II patients and decreased noncancer mortality in intermediate and high-risk stage I and II patients (HR = 0.82; 95% CI = 0.77 to 0.89). Lymphadenectomy was associated with increased endometrial cancer mortality in stage I patients (HR = 1.27; 95% CI = 1.16 to 1.39), decreased endometrial cancer mortality in stage II patients (HR = 0.61; 95% CI = 0.52 to 0.72), and decreased noncancer mortality in both stage I and II patients (HR = 0.84; 95% CI = 0.80 to 0.88). Effects of radiotherapy and lymphadenectomy on second cancer mortality varied according to risk strata.
Conclusions
Radiotherapy and lymphadenectomy are associated with statistically significantly reduced noncancer mortality in stage I and II endometrial cancer. The improved overall survival associated with these treatments reported from SEER studies is largely attributable to their selective application in healthier patients rather than their effects on endometrial cancer.
Endometrial cancer is the most common gynecologic cancer in the United States ( 1 ). Population-based studies and retrospective cohort studies have reported associations between improved survival and the use of both adjuvant external beam radiotherapy and lymphadenectomy in early stage endometrial cancer ( 2–5 ). In contrast, randomized trials and meta-analyses of controlled trials have not found evidence that these interventions prolong survival, despite favorable effects on cancer-specific events ( 6–13 ). The effectiveness of these interventions is thus controversial.
Because endometrial cancer is not the principal cause of death in patients with treated early stage endometrial cancer ( 14 ), observed effects of treatment on overall survival may be partially or solely attributable to effects on competing causes of mortality, rather than mortality from endometrial cancer. A previous study in stage I to IV endometrial cancer reported that lymphadenectomy is associated with statistically significant improvement in both cancer-specific and noncancer mortality ( 15 ). The extent to which the observed effects of radiotherapy and lymphadenectomy on overall survival in population-based studies is attributable to the cause-specific effects of these therapies vs favorable selection is unclear. Therefore, we sought to determine the cause-specific effects of both radiotherapy and lymphadenectomy in stage I and II endometrial cancer within Surveillance, Epidemiology, and End Results (SEER) data.
Methods
Sample
Patients were selected from the SEER 17 Registries plus Hurricane Katrina Impacted Louisiana Cases dataset (1973–2008; November 2010 edition). The 17 registries dataset covers approximately 26% of the US cancer population ( 16 ). Data were extracted using SEERstat 7.1.0 on August 22, 2012. We identified 63 595 patients diagnosed with stage I or stage II endometrial adenocarcinoma after total hysterectomy between 1988 and 2006. Data extracted were age, race, tumor node metastasis (TNM) stage (American Joint Committee on Cancer third edition), depth of myometrial invasion, histology, grade, type of radiotherapy delivered (if any), whether lymphadenectomy was administered and number of lymph nodes removed (if any), diagnosis date, and cause of death (if any). Patients with histologies other than adenocarcinoma (clear cell, papillary serous, carcinosarcoma, sarcoma, squamous cell) were excluded. Patients without total hysterectomy as initial treatment (n = 807) or unknown information about radiation therapy (n = 587), lymph node dissection (n = 60), grade (n = 3654), or stage (n = 1353) were also excluded. The proportion with missing grade or stage data did not differ statistically significantly according to radiotherapy use (χ 2P = .13) but was lower for patients who had lymphadenectomy compared with those who did not (3.6% vs 4.6%; P < .01).
Risk Stratification and Treatment
Patients were assigned to one of five risk groups for analysis. Stage I patients were stratified using the 2009 Fédération Internationale de Gynécologie Obstétrique (FIGO) system (IA: <1/2 myometrial invasion; IB: >1/2 myometrial invasion; II: cervical stromal invasion, without extrauterine or lymph node involvement). Grade 1 was defined as well differentiated, grade 2 was defined as moderately differentiated, and grade 3 was defined as poorly differentiated or undifferentiated. Stage I patients were classified into high-, intermediate-, and low-risk strata according to age and grade, as done by Chino et al. ( 3 ). Patients with stage IB and grade 3 disease were classified as high risk. Patients with any of the following conditions were classified as intermediate risk: 1) stage IA and grade 2–3 disease; 2) aged ≥ 70 years and stage IB or grade 1 or 2 disease; 3) aged ≥ 50 years and stage IB and grade 2 disease. Patients not meeting the above criteria were classified as low risk. Because of changes in the staging system, patients with 1988 FIGO stage IIA or stage II not otherwise specified (NOS) that could not be recategorized as FIGO 2009 stage I or stage II were classified together as a separate risk group. Patients were identified as receiving no radiation, whole pelvic radiation (WPRT), vaginal brachytherapy (VB), or a combination of WPRT and VB. The extent of lymphadenectomy was categorized according to the number of lymph nodes sampled (0 vs 1–10 vs >10).
Statistical Analysis
T tests were used to examine differences in age according to treatment. The χ 2 test and analysis of variance were used to analyze differences in race and age according to risk group and treatment. Causes of mortality were classified as due to endometrial cancer, secondary cancers, or not due to cancer. Event times were defined as the time from diagnosis to death from the respective cause, with censoring at the time of last follow-up for patients without an event or at death for causes other than the event of interest. Multivariable analyses were performed using the Cox proportional hazards model ( 17 , 18 ), controlling for age (continuous), black race (vs white/other), marital status (yes vs no), year of cancer diagnosis (1988–1992 vs 1993–1997 vs 1998–2002 vs 2003–2006), socioeconomic status (higher vs lower; higher socioeconomic status [SES] was defined by earnings above the mean of the median household income for the sample), radiotherapy use (WPRT ± VB vs VB alone vs none), and lymphadenectomy (>10 nodes vs 1–10 nodes vs 0 nodes). Results were similar when controlling for age as a categorical variable (by decade), or an age-squared term, or stratifying by 5-year age groups. Each of the five risk strata was analyzed separately. Stage and grade were omitted from models analyzed within separate strata but were controlled for (stage I vs stage IIA/II/NOS; grade 1 vs grade 2 vs grade 3) in models with pooled risk strata. The proportional hazards assumption was assessed using the Grambsch–Therneau method ( 19 ). Primary covariable and treatment interaction terms were tested, with statistically significant ( P < .05) interactions retained in the final regression models. Cumulative probabilities of cancer-specific and noncancer-specific mortality were calculated using nonparametric cumulative incidence functions ( 20 ). Gray’s test ( 21 ) was used to test for differences in cause-specific cumulative incidences. Data were analyzed using R version 2.15.1 ( www.r-project.org ). All statistical tests were two-sided, with P values less than .05 considered statistically significant.
Results
The sample comprised 58 172 patients meeting inclusion criteria ( Table 1 ). At last follow-up, 44 549 patients were alive; 89 were lost to follow-up. There were 2589 deaths from endometrial cancer, 3019 deaths from secondary malignancies, and 8015 deaths from other causes. Median follow-up time was 81 months for surviving patients and 77 months overall (range = 0–251). The median times to death from endometrial cancer, noncancer causes, and secondary malignancy were 31, 78, and 57 months, respectively. Overall, 77.1% of endometrial cancer cases were stage IA, 13.6% were stage IB, 2.7% were stage II, and 6.6% were FIGO stage IIA or stage II NOS. Among low-risk stage I patients, 3.7% were stage IB. Among intermediate-risk stage I patients, 42.1% were stage IB. Mean age was statistically significantly higher in the adjuvant radiotherapy group compared with the no radiotherapy group (64.6 vs 62.0 years; P < .001). These differences were consistently observed across all risk strata. No differences in age were observed according to lymphadenectomy (62.5 vs 62.6 years; P = .63). Compared with white patients, black patients were more likely to receive both radiotherapy (21.7% vs 26.0%; P < .001) and lymphadenectomy (46.8% vs 51.8%; P < .001). These differences were statistically significant within the low- and intermediate-risk strata.
Table 1.
Sample characteristics*
| Characteristic | Stage I, low risk | Stage I, intermediate risk | Stage I, high fisk | Stage IIA† /stage II NOS | Stage II | All |
|---|---|---|---|---|---|---|
| Number of patients | 40 121 | 10747 | 1897 | 3857 | 1550 | 58172 |
| Mean age, years (SD) | 58.9 (10.9) | 74.8 (7.5) | 68.9 (11.3) | 63.8 (13.2) | 62.7 (13.1) | 62.6 (12.3) |
| Race, No. (%) | ||||||
| White | 34 972 (87.2) | 9911 (92.2) | 1723 (90.8) | 3310 (85.8) | 1302 (84.0) | 51 218 (88.0) |
| Black | 1798 (4.5) | 392 (3.7) | 75 (4.0) | 253 (6.6) | 122 (7.9) | 2640 (4.6) |
| Other | 3351 (8.3) | 444 (4.1) | 99 (5.2) | 294 (7.6) | 126 (8.1) | 4314 (7.4) |
| 2009 FIGO stage I, No. (%) | ||||||
| IA | 38 637 (96.3) | 6223 (57.9) | — | — | — | 44 851 (77.1) |
| IB | 1484 (3.7) | 4524 (42.1) | 1897(100) | — | — | 7911 (13.6) |
| Grade, No. (%) | ||||||
| 1 | 26 320 (65.6) | 1200 (11.2) | — | 1227 (31.8) | 439 (28.3) | 29 186 (50.2) |
| 2 | 10 617 (26.5) | 7642 (71.1) | — | 1737 (45.0) | 646 (41.7) | 20 642 (35.5) |
| 3 | 3184 (7.9) | 1905 (17.7) | 1897 | 893 (23.2) | 465 (30.0) | 8344 (14.3) |
| LND, No. (%) | ||||||
| No LND | 23 062 (57.5) | 4853 (45.2) | 517 (27.2) | 1663 (43.2) | 451 (29.1) | 30 546 (52.8) |
| LND, 1–10 nodes | 7478 (18.6) | 2640 (24.5) | 536 (28.3) | 916 (23.7) | 419 (27.0) | 11 989 (20.6) |
| LND, >10 nodes | 9581 (23.9) | 3254 (30.3) | 844 (44.5) | 1278 (33.1) | 680 (43.9) | 15 637 (26.9) |
| Any LND | 17 059 (42.5) | 5894 (54.8) | 1380 (72.8) | 2194 (56.8) | 1099 (70.9) | 27 626 (47.5) |
| Adjuvant RT, No. (%) | ||||||
| No RT | 35 549 (88.6) | 7212 (67.1) | 680 (35.8) | 1737 (45.0) | 497 (32.1) | 45 675 (78.5) |
| VB | 1487 (3.7) | 693 (6.4) | 135 (7.1) | 383 (9.9) | 156 (10.0) | 2854 (4.9) |
| WPRT, no VB | 2123 (5.3) | 2052 (19.1) | 747 (39.4) | 936 (24.3) | 446 (28.8) | 6304 (10.8) |
| WPRT+VB | 962 (2.4) | 790 (7.4) | 335 (17.7) | 801 (20.8) | 451 (29.1) | 3339 (5.8) |
| Higher SES, No. (%) | 19 613 (48.9) | 5025 (46.8) | 893 (47.1) | 1829 (47.4) | 701 (45.2) | 28 061 (48.2) |
| Married, No. (%) | 23 910 (59.6) | 4666 (43.4) | 881 (46.4) | 1926 (49.9) | 752 (48.5) | 32135 (55.2) |
* FIGO = Fédération Internationale de Gynécologie Obstétrique; LND = lymphadenectomy; NOS = not otherwise specified; RT = radiation therapy; SD = standard deviation; SES = socioeconomic status; VB = vaginal brachytherapy; WPRT = whole pelvic radiation therapy; — = data unavailable/not applicable.
† 1988 FIGO staging system.
Overall, the cumulative incidence of endometrial cancer mortality was statistically significantly higher in patients undergoing WPRT than patients receiving no radiotherapy ( P < .001) ( Table 2 ; Figure 1 ). When controlling for demographic, disease, and treatment characteristics, the association between WPRT and increased endometrial cancer mortality was confirmed within all stage I risk strata ( Table 3 ). The adjusted hazard ratio (HR) for the effect of WPRT in all stage I and II patients was 1.66 (95% confidence interval (CI) = 1.52 to 1.82). In general, significant interactions were not observed, except between WPRT and age (HR = 0.99 per year; P < .001) in all stage I and II patients and between WPRT and high SES (HR = 1.71; P = .02) in the high-risk stage I patients. When stratified by age decile, the effect of WPRT was highest in patients aged 31 to 40 years (HR = 3.84; P = .01) and lowest in patients aged 81 to 90 years (HR = 1.21; P = .12). The effect of WPRT was also greater in high SES patients (HR = 1.40; P = .06) compared with low SES patients (HR = 0.96; P = .82). Both VB alone ( Table 2 ; Figure 1 ) and lymphadenectomy ( Table 3 ; Figure 2 ) were associated with statistically significantly higher endometrial cancer mortality in low-risk stage I patients but lower endometrial cancer mortality in stage II patients. The adjusted effect of any lymphadenectomy in low-risk stage I patients was a hazard ratio of 1.27 (95% CI = 1.16 to 1.39); in all stage I patients, the hazard ratio was 0.93 (95% CI = 0.85 to1.02), and in stage II patients, the hazard ratio was 0.61 (95% CI = 0.52 to 0.72).
Table 2.
Ten-year cumulative incidences of mortality (95% confidence interval) by cause of death, treatment, and risk group*
| Category | Stage I, low risk | Stage I, intermediate risk | Stage I, high risk | Stage IIA†/stage II NOS | Stage II | All |
|---|---|---|---|---|---|---|
| Endometrial cancer mortality | ||||||
| No RT | 2.01 (1.83 to 2.18) | 7.30 (6.64 to 7.96) | 19.1 (15.8 to 22.3) | 12.0 (10.3 to 13.7) | 16.5 (12.8 to 20.2) | 3.65 (3.45 to 3.84) |
| WPRT +/- VB | 7.15 (6.16 to 8.14) | 9.90 (8.72 to 11.1) | 23.2 (20.4 to 25.9) | 15.8 (14.0 to 17.7) | 14.1 (11.3 to 16.9) | 11.9 (11.2 to 12.6) |
| VB | 3.79 (2.64 to 4.95) | 7.21 (4.82 to 9.61) | 15.9 (8.27 to 23.6) | 9.08 (5.68 to 12.5) | 8.90 (0.54 to 17.3) | 6.00 (4.96 to 7.05) |
| No LND | 2.25 (2.03 to 2.46) | 8.08 (7.27 to 8.89) | 23.6 (19.7 to 27.5) | 15.7 (13.9 to 17.6) | 19.7 (15.6 to 23.8) | 4.57 (4.31 to 4.83) |
| LND, 1–10 nodes | 3.00 (2.52 to 3.47) | 7.21 (6.10 to 8.32) | 22.9 (18.9 to 26.8) | 12.1 (9.86 to 14.3) | 14.9 (10.8 to 19.0) | 5.96 (5.48 to 6.45) |
| LND, >10 nodes | 2.74 (2.34 to 3.15) | 8.70 (7.55 to 9.84) | 18.6 (15.5 to 21.6) | 11.5 (9.53 to 13.5) | 9.98 (7.00 to 13.0) | 5.94 (5.49 to 6.38) |
| Noncancer mortality | ||||||
| No RT | 10.4 (9.97 to 10.8) | 33.1 (31.7 to 34.4) | 32.9 (28.1 to 37.7) | 22.2 (19.7 to 24.7) | 20.9 (16.0 to 25.8) | 15.1 (14.7 to 15.6) |
| WPRT +/- VB | 8.43 (7.24 to 9.62) | 24.4 (22.5 to 26.3) | 16.5 (13.6 to 19.4) | 14.7 (12.8 to 16.6) | 10.7 (7.19 to 14.2) | 15.7 (14.8 to 16.6) |
| VB | 7.81 (5.91 to 9.72) | 23.3 (18.1 to 28.5) | 16.0 (4.14 to 27.8) | 13.3 (8.71 to 17.9) | 20.6 (3.47 to 37.8) | 12.5 (10.7 to 14.3) |
| No LND | 11.5 (10.9 to 12.0) | 34.1 (32.5 to 35.6) | 29.4 (24.8 to 34.0) | 21.4 (19.2 to 23.7) | 22.1 (15.9 to 28.3) | 16.5 (16.0 to 17.0) |
| LND, 1–10 nodes | 8.85 (7.92 to 9.78) | 27.4 (25.2 to 29.7) | 19.7 (15.3 to 24.2) | 15.0 (12.2 to 17.8) | 10.9 (7.28 to 14.6) | 14.4 (13.6 to 15.3) |
| LND, >10 nodes | 7.05 (6.29 to 7.82) | 24.8 (22.7 to 26.9) | 18.1 (14.3 to 21.8) | 14.8 (12.2 to 17.4) | 11.8 (6.98 to 16.6) | 12.6 (11.8 to 13.3) |
| Second cancer mortality | ||||||
| No RT | 4.59 (4.31 to 4.87) | 9.53 (8.73 to 10.3) | 11.1 (8.26 to 14.0) | 8.10 (6.60 to 9.60) | 10.4 (5.99 to 14.8) | 5.69 (5.43 to 5.96) |
| WPRT +/- VB | 5.13 (4.20 to 6.06) | 7.54 (6.41 to 8.68) | 9.19 (7.10 to 11.3) | 7.28 (5.92 to 8.63) | 10.3 (5.40 to 15.3) | 6.93 (6.33 to 7.54) |
| VB | 5.00 (3.48 to 6.53) | 6.44 (3.85 to 9.03) | 13.5 (4.46 to 22.5) | 4.52 (2.15 to 6.89) | 1.29 (-0.49 to 3.06) | 5.56 (4.40 to 6.72) |
| No LND | 4.82 (4.48 to 5.16) | 9.13 (8.23 to 10.0) | 10.2 (7.32 to 13.1) | 8.92 (7.41 to 10.4) | 13.6 (4.81 to 22.4) | 5.94 (5.62 to 6.25) |
| LND, 1–10 nodes | 5.03 (4.38 to 5.69) | 8.94 (7.56 to 10.3) | 11.0 (7.80 to 14.3) | 7.64 (5.66 to 9.61) | 7.45 (3.19 to 11.7) | 6.52 (5.95 to 7.09) |
| LND, >10 nodes | 3.76 (3.23 to 4.28) | 8.52 (7.24 to 9.79) | 9.41 (6.77 to 12.1) | 5.24 (3.73 to 6.74) | 8.80 (4.68 to 12.9) | 5.41 (4.93 to 5.90) |
* LND = lymphadenectomy; NOS = not otherwise specified; RT = radiation therapy; VB = vaginal brachytherapy; WPRT = whole pelvic radiation therapy.
† 1988 Fédération Internationale de Gynécologie Obstétrique staging system.
Figure 1.
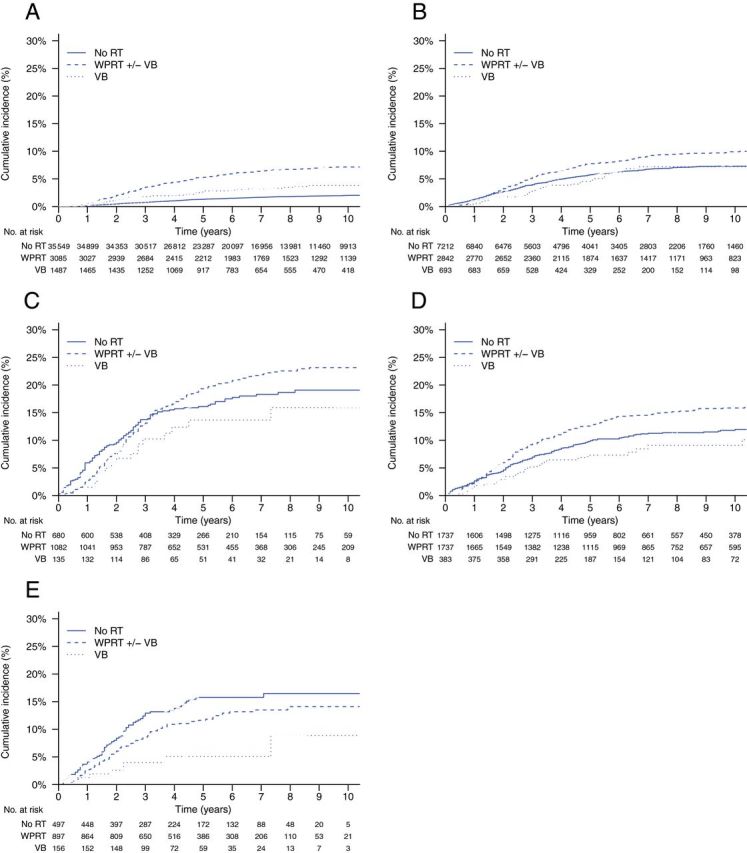
Cumulative incidences of endometrial cancer mortality by type of radiotherapy treatment and risk group. Gray’s test P values are for overall, pelvic radiotherapy vs no radiotherapy, and vaginal brachytherapy alone vs no radiotherapy, respectively. A ) Low-risk, stage I ( P < .001, P < .001, P < .001). B ) Intermediate-risk, stage I ( P < .001, P < .001, P = .49). C ) High-risk, stage I ( P = .15, P = .21, P = .27). D ) Stage IIA* (1988 Fédération Internationale de Gynécologie Obstétrique staging system)/stage II not otherwise specified ( P < .001, P < .001, P = .23). E ) Stage II ( P < .05, P = .10, P < .01). RT = radiotherapy; VB = vaginal brachytherapy; WPRT = whole pelvic radiotherapy.
Table 3.
Multivariable analysis of factors associated with endometrial cancer mortality*
| Characteristic | Stage I, low risk | Stage I, intermediate risk | Stage I, high risk | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age, per year | 1.03 (1.03 to 1.04) | <.001 | 1.03 (1.02 to 1.04) | <.001 | 1.03 (1.02 to 1.04) | <.001 |
| Race | ||||||
| Other | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Black | 2.61 (2.09 to 3.27) | <.001 | 1.48 (1.06 to 2.06) | .02 | 1.91 (1.22 to 2.99) | .004 |
| RT | ||||||
| None | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| VB Alone | 1.93 (1.43 to 2.62) | <.001 | 0.88 (0.62 to 1.24) | .50 | 0.78 (0.47 to 1.31) | .35 |
| WPRT +/- VB | 3.59 (3.04 to 4.23) | <.001 | 1.47 1.26 to 1.72) | <.001 | 1.13 (0.89 to 1.43) | .32 |
| LND | ||||||
| 0 nodes | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| 1–10 nodes | 1.16 (0.97 to 1.39) | .10 | 0.89 (0.74 to 1.07) | .21 | 1.04 (0.79 to 1.36) | .79 |
| >10 nodes | 1.22 (1.03—1.45) | .02 | 1.05 (0.89—1.25) | .56 | 0.86 (0.66 to 1.12) | .27 |
| SES | ||||||
| Lower | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Higher | 0.90 (0.79 to 1.04) | .15 | 0.99 (0.86 to 1.14) | .91 | 0.98 (0.79 to 1.21) | .87 |
| Marital status | ||||||
| Unmarried | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Married | 0.83 (0.72 to 0.95) | .009 | 0.81 (0.69 to 0.94) | .005 | 1.06 (0.85 to 1.32) | .59 |
| Year of diagnosis | ||||||
| 1988–1992 | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| 1993–1997 | 0.90 (0.73 to 1.11) | .31 | 1.19 (0.95 to 1.48) | .13 | 0.66 (0.47 to 0.94) | .02 |
| 1998–2002 | 0.88 (0.72 to 1.07) | .20 | 1.11 (0.89 to 1.38) | .35 | 0.69 (0.51 to 0.93) | .02 |
| 2003–2006 | 0.88 (0.70 to 1.11) | .28 | 1.18 (0.93 to 1.50) | .18 | 0.76 (0.56 to 1.05) | .10 |
| Stage IIA†/stage II NOS | Stage II | All | ||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age, per year | 1.05 (1.04 to 1.05) | <.001 | 1.04 (1.03 to 1.06) | <.001 | 1.04 (1.04 to 1.05) | <.001 |
| Race | ||||||
| Other | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Black | 1.78 (1.30 to 2.44) | <.001 | 1.90 (1.25 to 2.90) | .003 | 1.65 (1.43 to 1.90) | <.001 |
| RT | ||||||
| None | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| VB Alone | 0.85 (0.57 to 1.25) | .41 | 0.42 (0.20 to 0.89) | .02 | 1.03 (0.87 to 1.24) | .71 |
| WPRT +/- VB | 1.32 (1.09 to 1.60) | .005 | 0.82 (0.60 to 1.12) | .21 | 1.66 (1.52 to 1.82) | <.001 |
| LND | ||||||
| 0 nodes | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| 1–10 nodes | 0.78 (0.62 to 0.99) | .04 | 0.74 (0.52 to 1.05) | .09 | 0.86 (0.78 to 0.95) | .002 |
| >10 nodes | 0.77 (0.61 to 0.96) | .02 | 0.54 (0.38 to 0.77) | .001 | 0.82 (0.74 to 0.90) | <.001 |
| SES | ||||||
| Lower | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Higher | 0.90 (0.75 to 1.09) | .29 | 0.82 (0.61 to 1.11) | .21 | 0.97 (0.89 to 1.05) | .39 |
| Marital status | ||||||
| Unmarried | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Married | 0.95 (0.79 to 1.15) | .60 | 0.84 (0.61 to 1.14) | .26 | 0.86 (0.80 to 0.94) | <.001 |
| Year of diagnosis | ||||||
| 1988–1992 | 1.00 (referent) | Referent | — — | — | 1.00 (referent) | Referent |
| 1993–1997 | 0.96 (0.74 to 1.25) | .77 | — — | — | 0.97 (0.86 to 1.10) | .67 |
| 1998–2002 | 0.99 (0.76 to 1.29) | .95 | 1.00 (referent) | Referent | 0.97 (0.87 to 1.09) | .64 |
| 2003–2006 | 0.98 (0.73 to 1.31) | .90 | 1.30 (0.96 to 1.76) | .10 | 1.06 (0.94 to 1.20) | .35 |
* CI = confidence interval; HR = hazard ratio; LND = lymphadenectomy; NOS = not otherwise specified; RT = radiotherapy; SES = socioeconomic status; VB = vaginal brachytherapy; WPRT = whole pelvic radiation therapy; — = data unavailable / not applicable
† 1988 Fédération Internationale de Gynécologie Obstétrique staging system.
Figure 2.
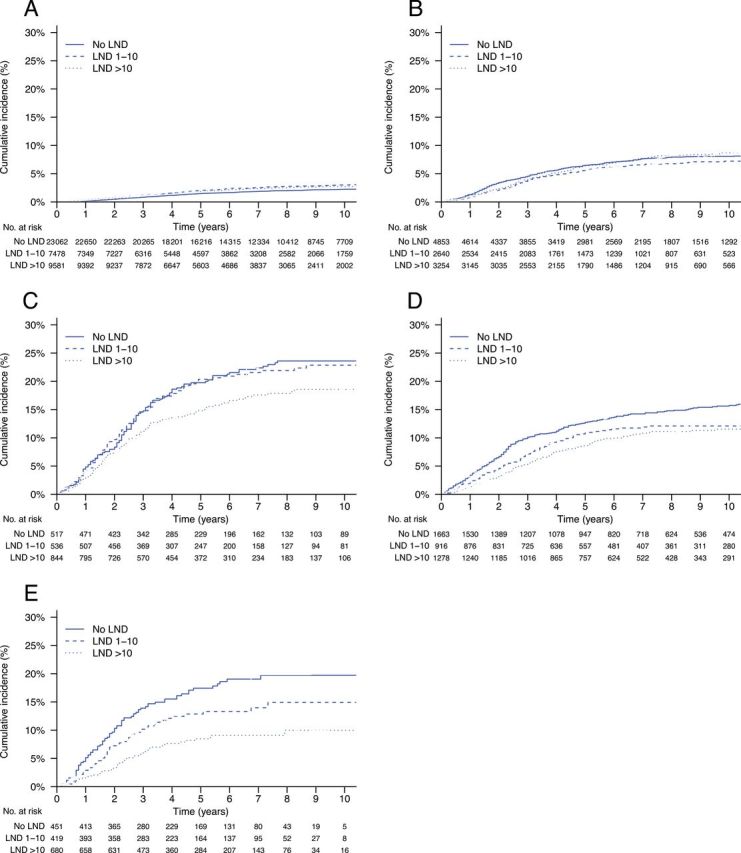
Cumulative incidences of endometrial cancer mortality by lymphadenectomy (LND) and risk group. Gray’s test P values are for overall, LND 1–10 nodes vs no LND, and LND >10 nodes vs no LND, respectively. A ) Low-risk, stage I ( P < .01, P < .01, P < .01). B ) Intermediate-risk, stage I ( P = .29, P = .16, P = .81). C ) High-risk, stage I ( P < .05, P = .81, P < .05). D ) Stage IIA* (1988 Fédération Internationale de Gynécologie Obstétrique staging system)/stage II not otherwise specified ( P < .01, P < .05, P < .001). E ) Stage II ( P < .001, P = .06, P < .001).
The overall cumulative incidence of noncancer mortality was statistically significantly higher in patients undergoing WPRT compared with no radiotherapy ( P = .03) and was statistically significantly lower in patients undergoing VB alone vs those undergoing no radiotherapy ( P = .003) and lymphadenectomy vs those undergoing no lymphadenectomy ( P < .001) ( Table 2 ). Within all risk strata, the cumulative incidence of noncancer mortality was statistically significantly lower in patients receiving WPRT compared with no radiotherapy ( P < .05 in all five strata), in patients receiving VB alone compared with no radiotherapy ( P < .05 in all five strata), and in patients receiving lymphadenectomy compared with no lymphadenectomy ( P < .05 in all five strata) ( Table 2 ; Figures 3 and 4 ). When controlling for demographic, disease, and treatment characteristics, the associations between decreased noncancer mortality and use of WPRT (HR = 0.90; 95% CI = 0.85 to 0.96), VB alone (HR = 0.82; 95% CI = 0.73 to 0.93), and lymphadenectomy (HR = 0.88; 95% CI = 0.84 to 0.92) were all statistically significant ( Table 4 ). This effect was confined to intermediate- and high-risk stage I and stage II patients for WPRT and VB alone, whereas for lymphadenectomy it was generally observed across all risk strata. WPRT was particularly associated with decreased noncancer mortality in intermediate- and high-risk stage I and stage II patients (pooled HR = 0.82; 95% CI = 0.77 to 0.89), whereas any lymphadenectomy was associated with decreased noncancer mortality in all stage I and II patients (pooled HR = 0.84; 95% CI = 0.80 to 0.88). Adjusted effects of treatment on both endometrial and noncancer mortality were similar when fully stratified according to 5-year age groups ( Supplementary Table 1 , available online).
Figure 3.
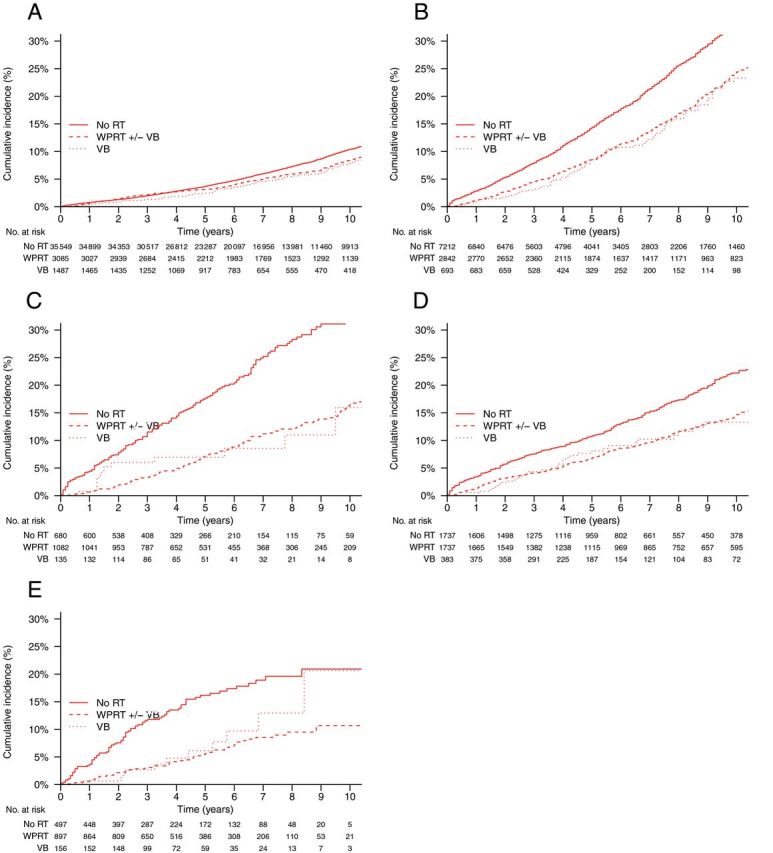
Cumulative incidences of non-cancer mortality by type of radiotherapy treatment and risk group. Gray’s test P values are for overall, pelvic radiotherapy vs no radiotherapy, and vaginal brachytherapy alone vs no radiotherapy, respectively. A ) Low-risk, stage I ( P < .01, P < .05, P < .05). B ) Intermediate-risk, stage I ( P < .001, P < .001, P < .001). C ) High-risk, stage I ( P < .001, P < .001, P < .01). D ) Stage IIA (1988 Fédération Internationale de Gynécologie Obstétrique staging system)/stage II not otherwise specified ( P < .001, P < .001, P < .01). E ) Stage II ( P < .001, P < .001, P < .05). RT = radiotherapy; VB = vaginal brachytherapy; WPRT = whole pelvic radiotherapy.
Figure 4.
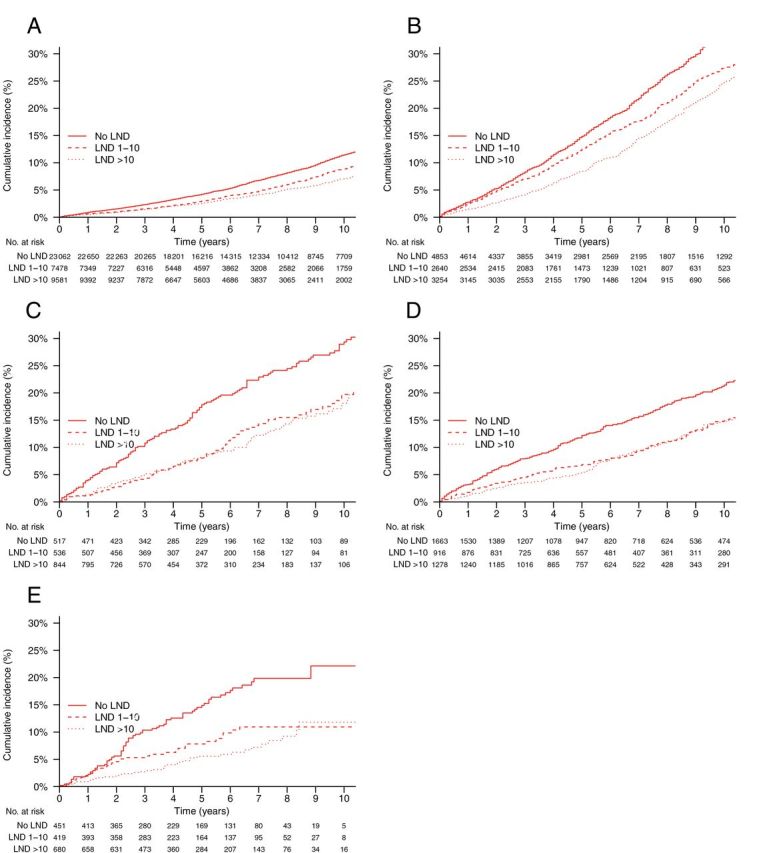
Cumulative incidences of noncancer mortality (NCM) by lymphadenectomy (LND) and risk group. Gray’s test P values are for overall, LND 1–10 nodes vs no LND, and LND >10 nodes vs no LND, respectively. A ) Low-risk, stage I ( P < .001, P < .001, P < .001). B ) Intermediate-risk, stage I ( P < .001, P < .001, P < .001). C ) High-risk, stage I ( P < .001, P < .001, P < .001). D ) Stage IIA (1988 Fédération Internationale de Gynécologie Obstétrique staging system)/ stage II not otherwise specified ( P < .001, P < .05, P < .001). E ) Stage II ( P < .001, P < .01, P < .001).
Table 4.
Multivariable analysis of factors associated with noncancer mortality*
| Characteristic | Stage I, low risk | Stage I, intermediate risk | Stage I, high risk | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age, per year | 1.09 (1.09 to 1.10) | <.001 | 1.10 (1.09 to 1.11) | <.001 | 1.09 (1.08 to 1.10) | <.001 |
| Race | ||||||
| Other | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Black | 2.61 (2.09 to 3.27) | <.001 | 1.48 (1.06 to 2.06) | .02 | 1.91 (1.22 to 2.99) | .004 |
| RT | ||||||
| None | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| VB alone | 0.98 (0.82 to 1.18) | .86 | 0.70 (0.57 to 0.85) | <.001 | 0.53 (0.30 to 0.91) | .02 |
| WPRT +/- VB | 1.03 (0.92 to 1.15) | .62 | 0.89 (0.82 to 0.97) | .007 | 0.55 (0.44 to 0.69) | <.001 |
| LND | ||||||
| 0 nodes | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| 1–10 nodes | 0.90 (0.82 to 0.98) | .02 | 0.95 (0.87 to 1.04) | .26 | 0.82 (0.63 to 1.06) | .13 |
| >10 nodes | 0.81 (0.73 to 0.89) | <.001 | 0.78 (0.71 to 0.85) | <.001 | 0.66 (0.51 to 0.85) | .002 |
| SES | ||||||
| Lower | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Higher | 0.86 (0.81 to 0.92) | <.001 | 0.99 (0.92 to 1.07) | .80 | 1.13 (0.91 to 1.40) | .26 |
| Marital status | ||||||
| Unmarried | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Married | 0.67 (0.63 to 0.72) | <.001 | 0.77 (0.71 to 0.83) | <.001 | 0.82 (0.65 to 1.04) | .10 |
| Year of diagnosis | ||||||
| 1988–1992 | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| 1993–1997 | 0.93 (0.85 to 1.01) | .08 | 1.02 (0.93 to 1.13) | .63 | 1.18 (0.88 to 1.58) | .27 |
| 1998–2002 | 0.93 (0.84 to 1.02) | .13 | 1.02 (0.91 to 1.14) | .74 | 0.92 (0.67 to 1.27) | .63 |
| 2003–2006 | 0.88 (0.77 to 1.02) | .08 | 0.96 (0.82 to 1.11) | .55 | 0.72 (0.47 to 1.09) | .12 |
| Stage IIA†/stage II NOS | Stage II | All | ||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age, per year | 1.10 (1.09 to 1.11) | <.001 | 1.09 (1.07 to 1.11) | <.001 | 1.10 (1.10 to 1.10) | <.001 |
| Race | ||||||
| Other | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Black | 1.25 0.90 to 1.74) | .19 | 1.80 (1.09 to 2.98) | .02 | 1.45 (1.30 to 1.61) | .001 |
| RT | ||||||
| None | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| VB alone | 0.80 (0.58 to 1.10) | .17 | 0.60 (0.32 to 1.15) | .12 | 0.82 (0.73 to 0.92) | .004 |
| WPRT +/- VB | 0.80 (0.68 to 0.93) | .004 | 0.48 (0.34 to 0.69) | <.001 | 0.90 (0.85 to 0.96) | .001 |
| LND | ||||||
| 0 nodes | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| 1–10 nodes | 0.91 (0.76 to 1.10) | .35 | 0.57 (0.38 to 0.86) | .007 | 0.92 (0.87 to 0.97) | <.001 |
| >10 nodes | 0.76 (0.63 to 0.92) | .004 | 0.48 (0.32 to 0.71) | <.001 | 0.78 (0.74 to 0.83) | .001 |
| SES | ||||||
| Lower | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Higher | 0.90 (0.77 to 1.04) | .16 | 0.99 (0.71 to 1.38) | .96 | 0.93 (0.89 to 0.97) | <.001 |
| Marital status | ||||||
| Unmarried | 1.00 (referent) | Referent | 1.00 (referent) | Referent | 1.00 (referent) | Referent |
| Married | 0.82 (0.70 to 0.96) | .02 | 0.69 (0.47 to 1.01) | .06 | 0.72 (0.69 to 0.75) | .001 |
| Year of diagnosis | ||||||
| 1988–1992 | 1.00 (referent) | Referent | — — | — | 1.00 (referent) | Referent |
| 1993–1997 | 0.96 (0.79 to 1.17) | .67 | — — | — | 1.41 (1.31 to 1.52) | .38 |
| 1998–2002 | 1.04 (0.83 to 1.32) | .73 | 1.00 (referent) | Referent | 0.97 (0.92 to 1.03) | .48 |
| 2003–2006 | 1.23 (0.92 to 1.64) | .16 | 1.09 (0.75 to 1.57) | .66 | 0.98 (0.91 to 1.04) | .25 |
* CI = confidence interval; HR = hazard ratio; LND = lymphadenectomy; NOS = not otherwise specified; RT = radiotherapy; SES = socioeconomic status; VB = vaginal brachytherapy; WPRT = whole pelvic radiation therapy; — = data unavailable/not applicable.
† 1988 Fédération Internationale de Gynécologie Obstétrique staging system.
Overall, the cumulative incidence of second cancer mortality was higher ( P < .001) in patients undergoing WPRT than patients receiving no radiotherapy, but only within the low-risk stage I stratum ( P = .35) ( Table 2 ; Supplementary Figure 1 , available online). In all other risk strata, the cumulative incidence of second cancer mortality was lower in patients undergoing WPRT compared with no radiotherapy ( P < .05 in the intermediate-risk stage I stratum; P > .05 in all other strata). The cumulative incidence of second cancer mortality was non-statistically significantly lower in patients undergoing VB alone compared with no radiotherapy ( P = .59), with varying differences within risk strata ( P < .05 in the stage IIA/NOS stratum; P > .05 in all other strata) ( Table 2 ; Supplementary Figure 1 , available online). The cumulative incidence of second cancer mortality was not statistically significantly different in patients undergoing lymphadenectomy compared with no lymphadenectomy ( P = .96), with varying differences within risk strata ( P < .05 in the stage IIA/NOS stratum; P > .05 in all other strata) ( Table 2 ; Supplementary Figure 2 , available online). When controlling for demographic, disease, and treatment characteristics, only the association between use of VB alone nd decreased second cancer mortality was statistically significant (HR = 0.82; 95% CI = 0.68 to 0.99), an effect observed most strongly in stage II patients ( Supplementary Table 2 , available online).
Discussion
Previous studies have reported associations between both radiotherapy and lymphadenectomy and improved overall survival using SEER data ( 2 , 3 ). In addition, a meta-analysis predominantly comprised of observational studies found that removal of more than 10 lymph nodes was associated with statistically significantly increased survival in intermediate- and high-risk endometrial cancer (HR = 0.76) ( 5 ). A large analysis of SEER data similarly found that external beam radiotherapy was associated with improved survival in high-risk stage I endometrial cancer (HR = 0.72). In contrast, two recent meta-analyses of controlled trials found no statistically significant effect of either lymphadenectomy (HR = 1.07) ( 12 ) or external beam radiotherapy (HR = 0.99) ( 13 ) on survival in this population.
In this study, we observed that both adjuvant radiotherapy and lymphadenectomy were associated with statistically significantly decreased noncancer mortality, particularly among intermediate- and high-risk stage I and stage II patients. Within low-risk stage I patients, we observed statistically significantly increased endometrial cancer–specific mortality associated with both treatments. In particular, we did not find evidence that WPRT was associated with statistically significantly improved endometrial cancer–specific mortality in any risk category. We did observe that VB alone and lymphadenectomy were associated with reduced endometrial cancer mortality in higher-risk patients. However, stage migration could partly explain this finding with respect to lymphadenectomy (because some patients not undergoing lymphadenectomy may have occult nodal disease). On the whole, we interpret these findings as evidence that the associations between WPRT and lymphadenectomy and improved overall survival reported in other SEER studies is largely due to the selection of healthier patients with higher-risk disease for these interventions, rather than effects of the treatments per se.
Studies that associate a treatment with improved survival can be easily misconstrued as evidence supporting use of the treatment. Although both radiotherapy and lymphadenectomy can reduce disease recurrence, which can cause mortality from endometrial cancer, we see no consistent argument to explain why these therapies would have a favorable effect on mortality from causes other than endometrial cancer. Furthermore, it is unlikely that these treatments would actually increase mortality from endometrial cancer, or that VB alone—which is given primarily to reduce risk of vaginal cuff recurrences—would have a large impact on disease-related mortality. Our findings indicate that caution should be used in interpreting effects of treatments on overall survival in population-based studies, especially when the background rate of competing mortality events is high. In such settings, analysis of the effects of treatments on competing causes of mortality is important to establish the mechanism(s) for observed effects on overall survival. This approach has been advocated in reporting results of randomized trials ( 22 , 23 ), and is applicable generally when composite endpoints are used in competing risks settings.
It is important to reconcile findings from both population-based studies and randomized trials because the latter cannot always be relied on to resolve every controversy. Patients represented on randomized trials are not drawn randomly from the population, so the degree to which the findings from clinical trials strictly represent the population to which their findings are applied may be questionable. Moreover, because of the costs of conducting clinical trials, their power to estimate primary, secondary, and subgroup effects is nearly always constrained. It is often assumed that benefits of more aggressive treatment may be underestimated in observational studies because of selective application of these treatments in patients with higher-risk disease. However, our findings indicate that, conversely, their benefits can be overestimated as well because of selective application in patients at low risk of competing mortality.
Strengths of this study include a large population-based sample with demographic and cause-of-death data, permitting a thorough analysis of cause-specific treatment effects. Although cause of mortality may be difficult to attribute accurately, these data are generally accepted as accurate in the SEER database ( 24 ). Notably, some potentially relevant demographic, disease, and treatment characteristics were unavailable in SEER data. We did perform a multivariable analysis to control for measurable factors that might influence the risk of noncancer mortality, but further studies controlling, for example, for the effects of comorbidity, lymphovascular invasion, and radiotherapy techniques could be helpful. Also, we cannot exclude residual effects of unmeasured confounders that could bias our results. Nonetheless, we do not believe these limitations substantially diminish the overall conclusions from this study, which call into question the mechanism responsible for improvements in survival associated with radiotherapy and lymphadenectomy in population-based studies.
It is difficult to ascertain the effects of cancer therapies on overall survival when the predominant cause of mortality is not cancer. As the probability of competing mortality events increases relative to cancer mortality, the effects of therapies on overall survival become attenuated, necessitating ever larger and more expensive randomized trials to prove their effectiveness. Attention to the effects of treatments on competing events takes on particular importance in such studies to ensure that the arms are balanced with respect to competing event risk and that the hypothesized primary effect estimate is plausible, based on the mechanism of the proposed intervention. Retrospective population-based studies may be helpful to detect small net effects on survival, but such analyses are susceptible to myriad forms of bias, particularly selection bias, making the resulting effect estimates dubious. Establishing effect specificity—and lack of nonspecificity—consistent with the hypothesized mechanism of treatment would lend greater credence to findings from such studies.
Supplementary Material
References
- 1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 . CA Cancer J Clin . 2012. ; 62 ( 4 ): 220 – 241 . [DOI] [PubMed] [Google Scholar]
- 2. Lee CM, Szabo A, Shrieve DC, et al. Frequency and effect of adjuvant radiation therapy among women with stage I endometrial adenocarcinoma . JAMA . 2006. ; 295 ( 4 ): 389 – 397 . [DOI] [PubMed] [Google Scholar]
- 3. Chino JP, Jones E, Berchuck A, et al. The influence of radiation modality and lymph node dissection on survival in early-stage endometrial cancer . Int J Radiat Oncol Biol Phys . 2012. ; 82 ( 5 ): 1872 – 1879 . [DOI] [PubMed] [Google Scholar]
- 4. Todo Y, Kato H, Kaneuchi M, et al. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis . Lancet . 2010. ; 375 ( 9721 ): 1165 – 1172 . [DOI] [PubMed] [Google Scholar]
- 5. Kim HS, Suh DH, Kim MK, et al. Systematic lymphadenectomy for survival in patients with endometrial cancer: a meta-analysis . Jpn J Clin Oncol . 2012. ; 42 ( 5 ): 405 – 412 . [DOI] [PubMed] [Google Scholar]
- 6. Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study . Gynecol Oncol . 2004. ; 92 ( 3 ): 744 – 751 . [DOI] [PubMed] [Google Scholar]
- 7. Creutzberg CL, Nout RA, Lybeert ML, et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma . Int J Radiat Oncol Biol Phys . 2011. ; 81 ( 4 ): e631 – e638 . [DOI] [PubMed] [Google Scholar]
- 8. Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis . Lancet . 2009. ; 373 ( 9658 ): 137 – 146 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ASTEC study group. Kitchener H, Swart AM, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study . Lancet . 2009. ; 373 ( 9658 ): 125 – 136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial . J Natl Cancer Inst . 2008. ; 100 ( 23 ): 1707 – 1716 . [DOI] [PubMed] [Google Scholar]
- 11. Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer . Cochrane Database Syst Rev . 2012. ; 4(4) : CD003916 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. May K, Bryant A, Dickinson HO, et al. Lymphadenectomy for the management of endometrial cancer . Cochrane Database Syst Rev . 2010. ; ( 1 ): CD007585 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis . J Natl Cancer Inst . 2012. ; 104 ( 21 ): 1625 – 1634 . [DOI] [PubMed] [Google Scholar]
- 14. Ward KK, Shah NR, Saenz CC, et al. Cardiovascular disease is the leading cause of death among endometrial cancer patients . Gynecol Oncol . 2012. ; 126 ( 2 ): 176 – 179 . [DOI] [PubMed] [Google Scholar]
- 15. Smith DC, Macdonald OK, Lee CM, et al. Survival impact of lymph node dissection in endometrial adenocarcinoma: a surveillance, epidemiology, and end results analysis . Int J Gynecol Cancer . 2008. ; 18 ( 2 ): 255 – 261 . [DOI] [PubMed] [Google Scholar]
- 16. National Cancer Institute . Surveillance, Epidemiology, and End Results (SEER) Program Public-Use Data (1973–2008) . http://www.seer.cancer.gov . Accessed August 22 , 2012. . [Google Scholar]
- 17. Cox DR . Regression models and life tables . J R Stat Soc Series B Stat Methodol . 1972. ; 34 ( 2 ): 187 – 220 . [Google Scholar]
- 18. Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: Examples from clinical oncology data . J Am Stat Assoc . 1993. ; 88 : 400 – 409. [Google Scholar]
- 19. Grambsch P, Therneau T . Proportional hazards tests and diagnostics based on weighted residuals . Biometrika . 1994. ; 81 : 515 – 526 . [Google Scholar]
- 20. Prentice RL, Kalbfleisch JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks . Biometrics . 1978. ; 34 ( 4 ): 541 – 554 . [PubMed] [Google Scholar]
- 21. Gray RJ . A class of K-sample tests for comparing the cumulative incidence of a competing risk . Ann Stat . 1988. ; 16 ( 3 ): 1141 – 1154 . [Google Scholar]
- 22. Ferreira-González I, Busse JW, Heels-Ansdell D, et al. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials . BMJ . 2007. ; 334 ( 7597 ): 786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mell LK, Lau SK, Rose BS, et al. Reporting of cause-specific treatment effects in cancer clinical trials with competing risks: a systematic review . Contemp Clin Trials 2012. ; 33 ( 5 ): 920 – 924 . [DOI] [PubMed] [Google Scholar]
- 24. Lund JL, Harlan LC, Yabroff KR, et al. Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease . Cancer Invest . 2010. ; 28 ( 7 ): 758 – 764 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


