Abstract
Objective . Dry eye is a multi-factorial disorder that manifests with painful ocular symptoms and visual disturbances, which can only be partly attributed to tear dysfunction. This disorder may also involve neuroplasticity in response to neuronal injury. This review will emphasize the key characteristics of dry eye pain and its pathologic mechanisms, making the argument that a subset of dry eye represents a neuropathic pain disorder of the eye, more appropriately called “burning eye syndrome.”
Methods . A literature review was conducted using a PubMed search focusing on dry eye, corneal nociception, and neuropathic pain. Articles were reviewed and those discussing clinical course, pathophysiology, and neuronal regulation of chronic ocular pain as related to dry eye were summarized.
Results . We found that there is a discordance between ocular pain and dryness on the ocular surface. Although tear dysfunction may be one of the initial insults, its persistence may be associated with repeated ocular sensory nerve injury leading to an acute-to-chronic pain transition associated with neuropathologic changes (peripheral and central sensitization), neuronal dysfunction, and spontaneous ocular pain.
Conclusion . Dry eye is becoming a major health concern due to its increasing incidence, significant morbidity, and economic burden. Recent evidence suggests that a subset of dry eye may be better represented as a chronic neuropathic pain disorder due to its features of dysesthesia, spontaneous pain, allodynia, and hyperalgesia. Future therapies targeted at the underlying neuroplasticity may yield improved efficacy for patients with this subset of dry eye, which we term “burning eye syndrome.”
Keywords: Dry Eye, Cornea, Neuropathic Pain, Neuronal Dysfunction, Hyperalgesia, Sensitization
Introduction
Dry eye is a common cause of chronic ocular pain and a growing epidemic [ 1 ]. It is a multi-factorial disorder that consists of painful ocular symptoms and visual disturbances that can only partly be attributed to tear dysfunction; other postulated mechanisms include inflammation and sensory neuronal dysregulation [ 2 ]. Dry eye is a leading cause for visits to the eye clinic with patients often complaining of a variety of dysesthesias including “dryness,” “burning,” and “foreign body sensation,” as well as photophobia and blurry vision [ 3 ]. However, effective therapies are lacking as most treatment strategies traditionally focus on tear dysfunction. This likely reflects the discordance between ocular pain and dryness on the ocular surface. An alternative explanation is that pathology in the corneal somatosensory pathway may underlie ocular pain in a subset of dry eye patients, and this subset may be better represented as a chronic neuropathic pain disorder, or “burning eye” (in a concept analogous to that of “burning mouth”) [ 4 ]. We will be referring to this subset of dry eye with neuropathic pain features as burning eye syndrome (BES). This review article is intended to address the idea of BES as a chronic neuropathic pain disorder associated with dry eye, focusing on its clinical presentation, pain mechanisms, and future directions in research and treatment.
Methods
The references for this review were obtained using a PubMed search with the following terms (in order of relevance): dry eye, cornea, neuropathic pain, chronic pain, nociception, neurogenic inflammation, corneal innervation, substance P (SP), calcitonin-gene related peptide (CGRP), nerve growth factor (NGF), transient receptor potential cation channels subfamily M member 8 (TRPM8), transient receptor potential vanilloid 1 (TRPV1), glutamate, N-methyl-D-aspartate (NMDA), gamma amino butyric acid (GABA), topical opioids, topical non-steroidal anti-inflammatory drugs (NSAIDs), and gabapentinoids. All searches were limited to the English language and conducted back to 1970. Articles were reviewed and those discussing clinical course, pathophysiology, and neuronal regulation of chronic ocular pain as related to dry eye were summarized. One textbook was used as a reference for further review of pain mechanisms of the eye including Encyclopedia of Pain , Second Edition, G.F. Gebhart, R.F. Schmidt (Eds.), 2013.
Burning Eye Syndrome Through the Eyes of an Ophthalmologist
Prevalence and Burden of Dry Eye
Despite controversy over disease definition, most ophthalmologists agree that dry eye is a highly prevalent condition. Symptoms of dry eye have been estimated to affect up to 30% of the population aged 50 years and older [ 1 ]. Surveys using the Impact of Dry Eye on Everyday Life (IDEEL) questionnaire have emphasized the negative effect of dry eye symptoms on tasks of daily living and its repercussions on psychological health and quality of life [ 5 , 6 ]. Utility scores for severe dry eye symptoms parallel those for patients suffering from angina and receiving dialysis [ 7 , 8 ]. The economic burden of dry eye is significant with an estimated indirect cost of $55.4 billion dollars annually, based on an average annual indirect cost to society of $11,302 per patient [ 9 ].
Clinical Presentation of Dry Eye
A multitude of terms are used to describe ocular pain associated with dry eye including “dryness,” “burning,” “itching,” and “foreign body sensation,” all of which can be episodic or continuous and usually wax and wane in severity [ 6 ]. Clinical findings in dry eye are similarly variable and include a spectrum of features that may reflect tear dysfunction [ 10 ]. Further, meibomian gland dysfunction and surrounding anatomic abnormalities, such as eyelid laxity and redundant conjunctivae (conjunctivochalasis), affect both the signs and symptoms of dry eye [ 10 ].
Diagnosis of Dry Eye
The fundamental definition of dry eye is not standardized and consequently, there is no gold standard for diagnosis. Studies have used symptoms alone, signs, or a combination of both as diagnostic criteria. Various tests are employed to grade dry eye severity, including measuring osmolarity of the inferior tear meniscus (TearLab, San Diego, CA), using fluorescein to assess tear break up time and corneal staining, placing strips of paper in the eyes to measure tearing over a 5 minute period (Schirmer test), assessing vascularity of the eyelid margin, and squeezing the eyelids to express and grade meibum [ 11 ]. Interestingly, none of the test results explain the variability in dry eye symptoms. The longstanding explanation for this discrepancy is that clinicians are not adequately capturing the temporal variation of the tear film and of the disease process itself [ 10 , 11 ]. Alternatively, BES may constitute a significant subset of dry eye patients. In this scenario, the underlying mechanism driving disease pathogenesis is a primary abnormality in the ocular sensory-nociceptive apparatus [ 12 ]. Unfortunately, none of the established ocular tests assess the function of the ocular sensory-nociceptive apparatus and this represents a major challenge in the characterization of BES. In light of this, the clinical diagnosis of dry eye frequently predicates on the use of validated questionnaires such as the 5-item Dry Eye Questionnaire (DEQ-5) and Ocular Surface Disability Index (OSDI), which focus on patient’s symptomology [ 10 , 13 ].
Burning Eye Syndrome Through the Eyes of a Pain Specialist
Pain specialists rarely, if ever, encounter and treat patients with ocular pain, and in the same context, are unfamiliar with the diagnostic evaluation and therapeutic considerations with dry eye. In contrast, patients with chronic, spontaneous burning pain of the face are likely diagnosed with neuropathic pain after exclusion of other primary painful conditions. Why should it be any different with chronic burning pain of the eye? Based on recent publications, BES may be classified under the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain (NeuPSIG) definition of neuropathic pain (NP), as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system [ 14 ].” While physiologic pain is a critical protective mechanism that attempts to prevent further injury, a minority of patients with acute nerve injury transition to chronic NP, which has no protective role [ 15 ]. Neuropathic pain appears to be a pathological manifestation of nerve injury leading to maladaptive plasticity of both the peripheral and central nervous system components of the pain pathway [ 14 ]. This review will emphasize the key features of BES, which characterize it as a neuropathic ocular pain disorder.
Burning Eye Syndrome as a Chronic Neuropathic Pain Disorder
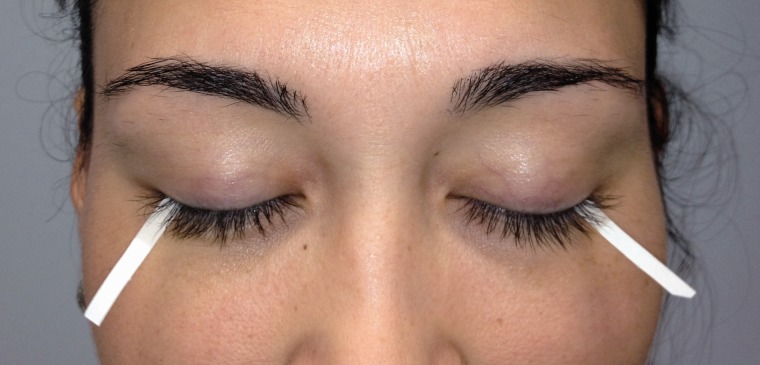
The manifestations of BES are analogous to those reported by patients suffering from chronic neuropathic pain disorders. The most important features of neuropathic pain include spontaneous pain, burning sensation, dysesthesias, allodynia, hyperalgesia, and difficulty with medical management [ 16 ]. A similar panoply of descriptors have been used by BES patients including “dryness,” “burning,” “aching,” “grittiness,” “jabbing,” and “foreign body sensation [ 12 , 17 ].” These dysesthesias may occur spontaneously, but can also be triggered by otherwise, trivial stimuli, such as air conditioning, wind, low humidity, and even light [ 1 ]. A frequent example of allodynia in BES patients is the overwhelming pain response to the Schirmer test, a relatively benign test, in which a paper strip is placed onto the eye to measure tear production ( Figure 1 ) [ 17 ]. Patients can also have painful or unpleasant manifestations in tissues surrounding the injury, in a fashion suggestive of the phenomenon, secondary hyperalgesia. These include headaches, blepharospasm, or generalized discomfort around the orbit, face, and jaw [ 12 , 18 ]. All of these features suggest a pathophysiological connection between BES and other neuropathic pain conditions.
Figure 1.
Schirmer strips are used to quantify ocular surface wetness by measuring the amount of moisture (in mm) on a strip after 5 minutes in the eye.
The Role of the Corneal Somatosensory Pathway in BES
Corneal nociception is exquisitely sensitive with an estimated density of nociceptors 300 to 600 times that of the dermis [ 17 , 19 ]. These nociceptors are largely unmyelinated and are located superficially between the epithelial cells just inferior to the thin tear film layer [ 19 ]. The different types of corneal sensory receptors include cold-sensing thermoreceptors, mechanoreceptors, and polymodal receptors [ 20 ]. Cold-sensing thermoreceptors make up 10% of this population; they likely use the TRPM8 as its cold sensor and have a homeostatic role in regulating basal tear production [ 21 ]. Mechanoreceptors account for roughly 20% of corneal sensory fibers and respond to noxious mechanical forces, but their molecular pathways are still unknown. They are likely responsible for the acute, sharp pain triggered by tactile contact with the ocular surface [ 20 ]. The remainder of the corneal sensory fibers (70%) are polymodal nociceptors, which are stimulated by endogenous inflammatory mediators, exogenous chemical irritants, noxious mechanical forces, heating, and cooling [ 21 , 22 ]. The channel ion predominantly associated with these nociceptors is the TRPV1 [ 21 ]. These three classes of nociceptors constitute the primary defense of the ocular surface against environmental insults.
The Transition from Acute Nerve Injury to Neuropathic Ocular Pain
Acute Changes in the Peripheral Corneal Somatosensory Pathway
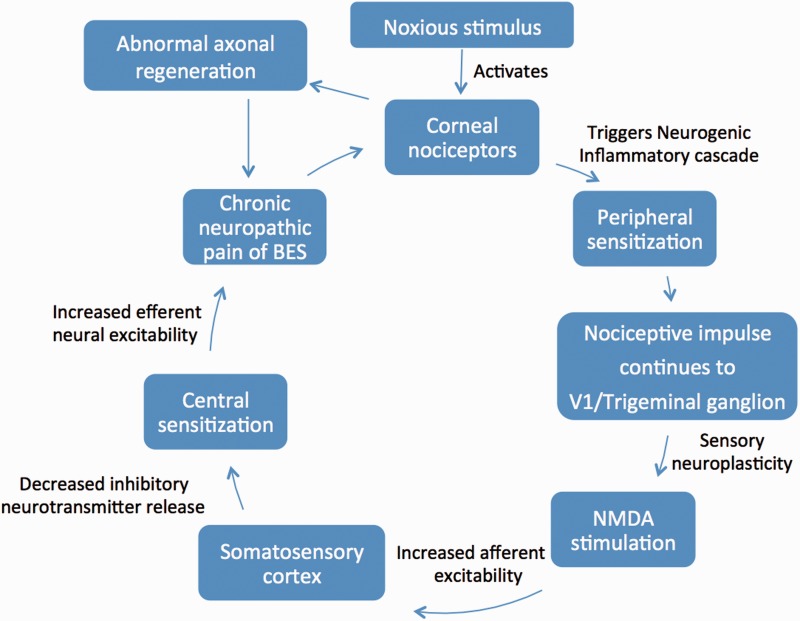
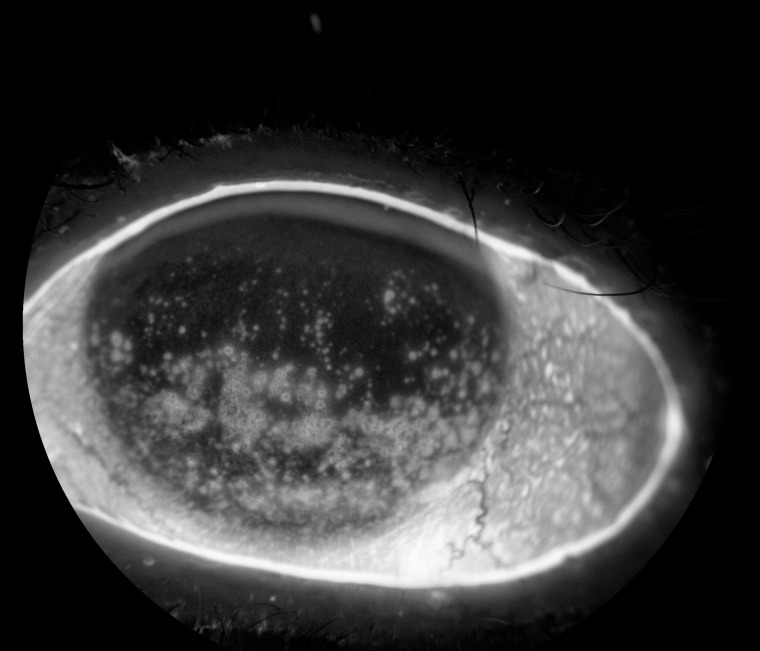
The rich density and superficial location of the corneal nociceptors render them effective at detecting noxious stimuli, but also increases their vulnerability to damage and injury ( Figures 2 and 3 ) [ 23 ]. Once axonal injury occurs, neuronal and glial cells trigger an inflammatory cascade, which leads to the release of mediators often detected in tears including cytokines, interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α) [ 24 , 25 ].
Figure 2.
Proposed pathogenesis of Burning Eye Syndrome.
Figure 3.
Diffuse corneal staining (white areas on cornea) due to epithelial cell disruption with subsequent exposure and activation of corneal nerves.
The cytokine response is accompanied by neurogenic inflammation with peripheral terminal release of multiple neuropeptides, most importantly SP and CGRP [ 24 , 26 ]. These peptides cause vasodilation, edema, and further release of inflammatory mediators and polymorphonuclear leukocytes (PMNs) [ 19 , 27 ]. The inflammatory mediators lead to the release of NGF, which has a key role in the functional modification of nociceptors [ 20 ]. Through activation of tyrosine kinase (trkA) receptors located in the peripheral terminals of nociceptors, NGF up-regulates existing genes and induces expression of new genes [ 17 , 19 ]. This culminates in peripheral sensitization, which renders corneal nociceptors hypersensitive to an activating stimulus and may contribute to the heightened pain sensation accompanying BES [ 15 , 28 ]. Studies supporting this have found increased levels of NGF in the tears of dry eye patients [ 29 , 30 ]. The role of NGF specifically within the ocular nociceptive pathway was explored by De Castro et al. who showed that mutant (-/-) trkA knockout mice (unresponsive to NGF due to the loss of the trkA receptor) had a significant reduction in corneal nerve terminals and a very reduced blinking response to noxious stimuli when compared with the wild types and heterozygous (+/-) trkA animals [ 28 ]. Taken together, these findings suggest that NGF appears especially germane to nociception within the ocular surface. Although the primary role of NGF is to promote nerve healing and the initial hypersensitivity response is intended to protect the injured area, prolonged activity can clearly be detrimental [ 15 , 31 ].
Chronic Changes in the Peripheral Corneal Somatosensory Pathway
Pathological neuropathic pain develops in the setting of traumatic insult or repeated ongoing damage to nerves, resulting in prolonged hypersensitivity or altered neuronal healing. This probably contributes to the abnormal painful sensations, including allodynia and hyperalgesia, seen in BES patients. The initial insult that precipitates BES has often resolved by the time the neuropathic pain develops. A prime example of this is laser in situ keratomileusis (LASIK)-induced dry eye [ 2 ]. Chao et al. demonstrated that decreases in corneal sensitivity and tear dysfunction resulting from LASIK-induced corneal nerve damage returned to preoperative levels by the time 20–40% of post-LASIK patients reported dry eye symptoms [ 32 ]. This dichotomy between BES patient’s subjective complaints and lack of clinical signs is another commonly seen feature of typical chronic neuropathic pain conditions.
With severe or repeated corneal axonal injury, there can be a phenotypic switch within the nociceptor’s functional state from signal transmission to nerve regeneration [ 12 , 17 ]. The regenerating axon can form nerve sprouts at the damaged fiber ends and microneuromas that spontaneously discharge leading to pain without overt activation by a noxious stimulus; they can also become hyper-responsive to stimuli [ 17 , 33 ]. Recent studies with in vivo confocal imaging of the sub-basal corneal nerves demonstrate this abnormal morphology in some dry eye patients including nerve sprouts, increased nerve thickness, beadlike/microneuroma formations, and tortuosity [ 34–41 ]. Further, confocal microscopy findings in patients with dry eye have been non-uniform with separate studies demonstrating either decreased, similar, or even an increased density of corneal sub-basal nerves compared with controls [ 34–38 , 42–43 ]. Correlations of corneal nerve density to corneal sensation (as assessed by Belmonte and Cochet-Bonnet aesthesiometry) have been similarly conflicting [ 34 , 37 , 42–43 ]. This observed heterogeneity suggests that confocal microscopy may be a useful tool to identify BES endotypes based on corneal nerve morphology. We hypothesize that BES patients are the sub-population of dry eye more likely to have nerve abnormalities on confocal microscopy due to abnormal healing of damaged corneal axons. Clinically, this may manifest as the spontaneous pain of BES [ 44 , 45 ].
Anatomy and Function of the Ocular Central Pain Pathway
Another aspect of the molecular pathogenesis of BES involves neuronal dysregulation within the second and higher order neurons of the ascending excitatory pain pathway (central sensitization). Under physiologic conditions, once corneal nociceptors discharge an action potential in response to a noxious challenge, it travels along small-diameter primary afferent unmyelinated C and lightly myelinated A-delta fibers of the nasociliary branch of the trigeminal nerve (V1) and through the somata in the trigeminal ganglion [ 12 ]. This action triggers the release of a host of neurotransmitters including glutamate [ 45 ]. Glutamate binds to multiple types of receptors of the second order neurons of the trigeminal subnucleus caudalis (TSNC), but most importantly to AMPA (α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA receptors [ 46 ]. Binding to AMPA receptors results in continued transmission of the noxious signal to higher order neurons; this action is responsible for the “fast” nociceptive impulses seen with acute pain [ 45 , 46 ]. The NMDA receptor is also activated by glutamate but requires much stronger, repetitive noxious signals and is considered to be responsible for central sensitization [ 46 ]. Following activation of second-order neurons of the TSNC, the impulse is eventually relayed to the thalamus, and following potential modification by subcortical/limbic structures, it terminates in the somatosensory cortex and other supraspinal centers. There, the original noxious signal is perceived as pain [ 12 , 23 ].
Neuronal Dysregulation Within the Central Pain Pathways
Within the corneal somatosensory pathway, peripheral sensitization and ectopic activity can lead to neuroplasticity of the central nociceptive pathway [ 45 ]. Persistent activity of nociceptors is often the source for central sensitization and it can result in NMDA receptor activation and increased synaptic efficacy of second order neurons of the TSNC even with sub-threshold noxious stimuli [ 14 ]. This is also commonly referred to as the “wind-up” phenomenon and manifests as allodynia and hyperalgesia in many chronic pain states [ 47 ]. In vitro evidence supporting this concept within the eye has revealed cross-talk between corneal epithelial cells and TSNC mediated by NMDA receptors [ 48 ]. In addition, Bereiter et al. showed in a rat model that NMDA receptors relay signals to the corneal central pain pathway [ 49 ]. Further studies are needed to elucidate the exact interactions of the NMDA receptor within the ocular pain pathway.
Dysregulation of the Descending Inhibitory Nociceptive Pathway
The ascending excitatory nociceptive pathway is modulated by the descending inhibitory pathway, and an imbalance between the two may facilitate the development of neuropathic pain in BES. Normally, interneurons within the central pain pathway release inhibitory neurotransmitters of nociceptive signaling, including GABA and glycine [ 50 ]. After a noxious insult, the ensuing inflammatory cascade reduces GABA’s inhibitory influence on the ascending pathway [ 45 ]. In a rat study, Hirata et al. showed that GABA does have a role in inhibiting corneal input by acting on the TSNC [ 51 ]. With neuronal dysregulation, a persistent loss of the inhibitory GABA-mediated chloride current allows for unopposed activation of the ascending pain pathway, contributing to a chronic neuropathic pain state [ 12 , 17 , 52 ].
Neuronal Dysfunction Can Contribute to Ocular Surface Dryness of BES
While BES patients primarily present with burning pain and hyperalgesia, some do have signs of ocular surface dryness from tear dysfunction. This ocular dryness can be attributable to neuronal dysregulation, specifically of the lacrimal functional unit (LFU). The LFU is composed of the lacrimal gland (primarily responsible for tear secretion), ocular surface, and lids, which are all integrated by afferent and efferent nerves [ 2 ]. The moisture of the ocular surface requires continuous tear secretion, which is primarily regulated by TRPM8 of corneal receptors. TRPM8 is activated by the cooler temperature associated with evaporative tear loss and sends signals through the trigeminal (V1) nerve to maintain basal tearing [ 53 ]. TRPM8 is very sensitive to minute temperature variations within the ocular surface and is thus relevant in maintaining the tear film layer during blinking or exposure to dry environments [ 12 ]. Neuronal damage or dysfunction in any component of the LFU can lead to tear dysfunction and generate a positive feedback loop of further inflammation and neuroplasticity [ 2 , 54 ]. With corneal injury, the subsequent release of inflammatory mediators, specifically IL-1β and TNF-α, has been shown to directly inhibit neural activity [ 55 ]. Further, in a study by Zoukhri et al., exogenous addition of IL-1β and TNF-α inhibited neurotransmitter release, which led to decreased tear section by the lacrimal gland [ 56 ]. These studies suggest that the corneal inflammatory cascade can cause neuronal dysfunction of the LFU culminating in ocular surface dryness.
Burning Eye Syndrome Treatment Through the Eyes of an Ophthalmologist
Dry eye therapy is tailored to the severity of the patient’s symptoms [ 57 ]. A major challenge in identifying effective treatment is the lack of universal diagnostic criteria for dry eye. Further, in a recent systematic review, Alves et al. highlighted the limitations in evaluation of dry eye treatment due to the heterogeneity of outcome endpoints [ 58 ]. As stated previously, most therapies aim at improving tear film function. Mild symptoms are managed with environmental interventions such as avoiding air conditioning, low humidity, and air pollution [ 59 , 60 ]. Nutritional modification such as omega-3 fatty acid supplementation has been found to improve tear dysfunction and reduce ocular surface inflammation [ 61 , 62 ]. Artificial tear substitutes, gels/ointments are used as needed for symptomatic relief [ 59 ]. For moderate symptoms, topical anti-inflammatory agents are often used to target ocular surface inflammation. More severe cases are often treated with punctal occlusion, a procedure in which the inferior punctae (conduits for tear drainage) are blocked, thereby increasing the surface time of tears [ 63 ]. Autologous serum tears (tears constituted from patient’s serum) have been employed in refractory cases [ 64 ]. Dry eye symptoms recalcitrant to medical management may require contact lens placement (such as fitting of a PROSE lens (prosthetic replacement of the ocular surface ecosystem) and less commonly surgery [ 59 , 65 ]. Interestingly, one theory behind the efficacy of the PROSE lens in dampening dry eye symptoms is decreased nociceptor firing due to the constant fluid that bathes and protects the end terminals of the corneal nociceptors [ 65 ].
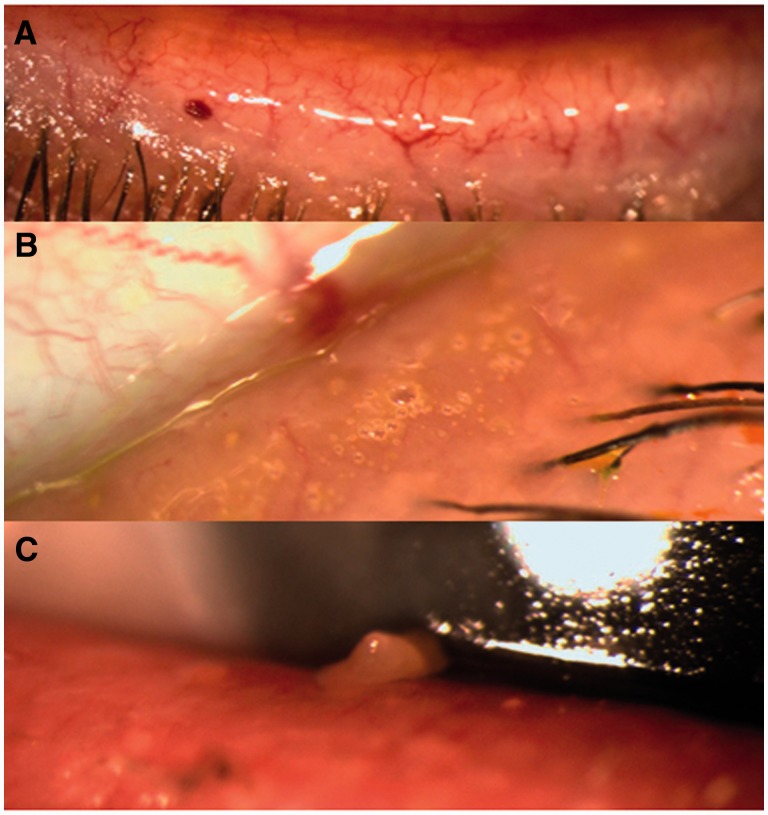
A large subset of patients with dry eye also has meibomian gland dysfunction, which affects tear film stability (evaporative dry eye) ( Figure 4 ). Treatment for these cases include eyelid cleaning, short-term topical corticosteroids, and topical or oral antibiotics [ 66 ]. Newer treatments for this sub-type of dry eye include eyelid heating and massage with the LipiFlow ® Thermal Pulsation system (Tear Science, Morrisville, NC), intraductal probing of the meibomian glands, and intense pulsed light therapy [ 67–69 ]. Limited information is available on their efficacy and outcomes due to lack of long term follow up.
Figure 4.
Various clinical examples of meibomian gland dysfunction. ( A ) Telangiectasias on the eyelid margin. ( B ) Foaminess on the eyelid margin indicating abnormal meibum quality. ( C ) Abnormal meibum quality detected by manual expression (meibum is thickened and opaque).
It is important to note that none of these therapies directly address the function of the corneal somatosensory pathway. Given this reality, an important first-step in individualizing management is to identify endotypes that may influence prognosis and alter treatment modalities. This paradigm is already being applied to dry eye in regards to the presence of aqueous deficiency or evaporative dry eye; however, it has not been specifically applied to patients’ symptoms [ 10 , 13 ]. This is likely contributory to the sub-optimal patient satisfaction with current dry eye therapy. As our understanding of these neuronal mechanisms evolve, new therapeutic strategies will hopefully emerge to target the BES sub-type of dry eye.
Burning Eye Syndrome Treatment Through the Eyes of a Pain Specialist
Primary Prevention
While patients may improve with treatments aimed at ameliorating tear film dysfunction, most continue to have symptoms and some continue to have debilitating disease. Extrapolation of data from other pain disorders suggests that protection of the ocular surface from damage may be helpful in the initial stages of this disorder. Once central sensitization occurs, however, BES pain may be spontaneous and unrelated to tear film dysfunction. Consequently, a primary goal should be to prevent acute ocular pain altogether (primary prevention) [ 70 ]. For general surgical procedures, analgesic strategies that attenuate the impact of the peripheral nociceptive barrage have been shown to prevent peripheral and central sensitization and reduce postoperative pain intensity and analgesic requirements [ 71 ]. Similar strategies may be applied to patients undergoing ocular surgery (refractive, cataract extraction), where a significant percentage may develop BES secondary to trauma [ 32 ]. Topical ocular opioids may ameliorate the acute ocular pain associated with these procedures. While topical opioids are not currently used for ocular surgery, µ-receptors are localized in the cornea [ 72 ]. Moreover, in a murine pain model, Wenk et al. found that topical application of morphine decreased chemical-induced blinking suggesting an analgesic effect [ 73 ]. The development of topical opioids may be worth exploring for the prevention of surgical pain.
Secondary Prevention and Multi-Modal Therapy
Secondary prevention consists of early recognition and aggressive treatment of acute pain, thereby preventing transition to chronicity [ 70 ]. One of the main pathologic components of BES is an initial prolonged inflammatory cascade; thus, anti-inflammatory agents (such as NSAIDS) may be particularly helpful in attenuating this response and the subsequent peripheral sensitization. Some success has been reported with the use of ophthalmic diclofenac for acute postoperative pain following refractive surgery [ 74–76 ]. Furthermore, Szucs et al. demonstrated a significant analgesic effect of ophthalmic diclofenac for acute corneal abrasions when compared with placebo [ 77 ]. Diclofenac, in particular, may confer an additional analgesic effect above other NSAIDs by decreasing neuronal excitability via opening of neuronal ATP-sensitive potassium channels [ 78 ]. Deficient opening of these channels is known to mediate neuropathic pain after traumatic nerve injury; therefore, restoration of their activation may mitigate its development [ 79 ]. Thus, the use of topical NSAIDs in the initial stages of ocular inflammation may lessen peripheral and central neuroplasticity.
The clinical manifestations of BES can result from pathology at multiple levels within the corneal somatosensory pathway. Multi-modal therapy targeting different sites of the pain pathway may therefore be the best strategy [ 70 ]. Chronic neuropathic pain is often managed with gabapentinoids and antidepressants due to their effects on central pain pathways. In particular, gabapentinoids have been shown to be effective in the treatment of diabetic peripheral neuropathy and post-herpetic neuralgia. They block N-type voltage-gated calcium channels in the primary afferent presynaptic terminals, with consequent decrease in the release of multiple excitatory neurotransmitters [ 70 ]. Gabapentinoids may prove similarly beneficial for the treatment of BES. In a prospective, randomized, double-blind, placebo-controlled study, Lichtinger et al. showed that gabapentin significantly reduced post-operative pain after photorefractive keratectomy [ 80 ]. The anti-depressants typically used to treat chronic neuropathic pain are tricyclic antidepressants (TCA) and serotonin-norepinephrine reuptake inhibitors (SNRI), which both act on the descending pain pathway to reduce pain [ 70 ]. These medications may also have a role in the treatment of BES; however, their potential use in BES may be limited by their prominent anti-histamine and anti-muscarinic effects, which can exacerbate ocular dryness. Further studies are needed to evaluate the efficacy of these centrally acting neuromodulators in the BES population.
Conclusion
Dry eye is becoming a major health concern due to its significant morbidity and economic burden. The lack of effective therapy is a challenge that needs to be addressed by continued exploration of the intricate pathophysiology behind this disease. Recent evidence suggests that dry eye is a heterogeneous condition and a sub-group of dry eye may be better represented as a chronic neuropathic pain disorder; that is, “burning eye syndrome,” due to its features of dysesthesia, spontaneous pain, allodynia, and hyperalgesia. The wide variability in the BES phenotype is likely related to peripheral and central sensitization that can develop at multiple points within the pain pathway. Interestingly, ophthalmologists and pain specialists think about dry eye and ocular pain in very different ways, and a blend of their expertise is needed to develop more robust assessments of dry eye to further evaluate and target the neuroplasticity of the corneal somatosensory pathway. Another important avenue of future study will include genetic predisposition. Genetic polymorphisms are known to influence inter-individual variability in pain sensitivity and further research is needed to better elucidate how these polymorphisms specifically contribute to the susceptibility and severity of neuropathic pain within the dry eye population [ 81 ]. An improved understanding of the corneal somatosensory system in dry eye is needed in order to individualize treatments for the disease, with the goal of decreasing morbidity.
Funding sources: This article was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development’s Career Development Award CDA-2-024-10S (Dr. Galor), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD- Grant#W81XWH-09-1-0675 and Grant#W81XWH-13-1-0048 ONOVA) (institutional), and NIH NIDCR R01 DE022903 (Dr. Levitt).
Conflicts of interest: There are no conflicts of interest to report.
References
- 1. The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop . Ocul Surf 2007. ; 5 : 93 – 107 . [DOI] [PubMed] [Google Scholar]
- 2. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop . Ocul Surf 2007. ; 5 : 75 – 92 . [DOI] [PubMed] [Google Scholar]
- 3. Galor A, Zheng DD, Arheart KL. et al. . Dry eye medication use and expenditures: Data from the medical expenditure panel survery 2001 to 2006 . Cornea 2012. ; 31 : 1403 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarantopoulos C. Burning mouth syndrome: A misunderstood, underinvestigated, and undertreated clinical challenge . Reg Anesth Pain Med 2013. ; 38 : 378 – 9 . [DOI] [PubMed] [Google Scholar]
- 5. Abetz L, Rajagopalan K, Mertzanis P. et al. . Development and validation of the Impact of Dry Eye on Everyday Life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients . Health Qual Life Outcomes 2011. ; 9 : 111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mertzanis P, Abetz L, Rajagopalan K. et al. . The relative burden of dry eye in patients’ lives: Comparisons to a US normative sample . Invest Ophthalmol Vis Sci 2005. ; 46 : 46 – 50 . [DOI] [PubMed] [Google Scholar]
- 7. Schiffman RM, Walt JG, Jacobsen G. et al. . Utility assessment among patients with dry eye disease . Ophthalmology 2003. ; 110 : 1412 – 9 . [DOI] [PubMed] [Google Scholar]
- 8. Buchholz P, Steeds CS, Stern LS. et al. . Utility assessment to measure the impact of dry eye disease . Ocul Surf 2006. ; 4 : 155 – 61 . [DOI] [PubMed] [Google Scholar]
- 9. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: A decision tree analysis . Cornea 2011. ; 30 : 379 – 87 . [DOI] [PubMed] [Google Scholar]
- 10. Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop . Ocul Surf 2007. ; 5 : 108 – 52 . [DOI] [PubMed] [Google Scholar]
- 11. Galor A, Feuer W, Lee DJ. et al. . Ocular surface parameters in older male veterans . Invest Ophthalmol Vis Sci 2013. ; 54 : 1426 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenthal P, Borsook D. The corneal pain system. Part I: The missing piece of the dry eye puzzle . Ocul Surf 2012. ; 10 : 2 – 14 . [DOI] [PubMed] [Google Scholar]
- 13. Chalmers RL, Begley CG, Caffery B. Validation of the 5-item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses . Cont Lens Anterior Eye 2010. ; 33 : 55 – 60 . [DOI] [PubMed] [Google Scholar]
- 14. Ro LS, Chang KH. Neuropathic pain: Mechanisms and treatments . Chang Gung Med J 2005. ; 28 : 597 – 605 . [PubMed] [Google Scholar]
- 15. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage . Annu Rev Neurosci 2009. ; 32 : 1 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Searle RD, Howell SJ, Bennett MI. Diagnosing postoperative neuropathic pain: A Delphi survey . Br J Anaesth 2012. ; 109 : 240 – 4 . [DOI] [PubMed] [Google Scholar]
- 17. Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: Is it real? Ocul Surf 2009. ; 7 : 28 – 40 . [DOI] [PubMed] [Google Scholar]
- 18. Borsook D, Rosenthal P. Chronic (neuropathic) corneal pain and blepharospasm: Five case reports . Pain 2011. ; 152 : 2427 – 31 . [DOI] [PubMed] [Google Scholar]
- 19. Muller LJ, Marfurt CF, Kruse F. et al. . Corneal nerves: Structure, contents and function . Exp Eye Res 76 : 521 – 42 . [DOI] [PubMed] [Google Scholar]
- 20. Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas . Exp Eye Res 2004. ; 78 : 513 – 25 . [DOI] [PubMed] [Google Scholar]
- 21. Belmonte C. Eye dryness sensations after refractive surgery: Impaired tear secretion or “phantom cornea”? J Refract Surg 2007. ; 23 : 598 – 602 . [DOI] [PubMed] [Google Scholar]
- 22. Belmonte C, Viana F. Transduction and encoding of noxious stimuli . In: Schmidt RF, Willis W , eds. Encyclopedia of Pain. vol. 3 . Berlin, Germany: : Springer-Verlag; ; 2007. : 2515 – 28 . [Google Scholar]
- 23. Guthoff RF, Wienss H, Hahnel C. et al. . Epithelial innervation of human cornea . Cornea 2005. ; 24 : 608 – 13 . [DOI] [PubMed] [Google Scholar]
- 24. Mantelli F, Massaro-Giordano M, Macchi I. et al. . The cellular mechanisms of dry eye: From pathogenesis to treatment . J Cell Physiol 2013. ; 228 : 2253 – 6 . [DOI] [PubMed] [Google Scholar]
- 25. Massingale ML, Li X, Vallabhajosyulla M. et al. . Analysis of inflammatory cytokines in the tears of dry eye patients . Cornea 2009. ; 28 : 1023 – 7 . [DOI] [PubMed] [Google Scholar]
- 26. Beuerman RW, Stern ME. Neurogenic inflammation: A first line of defense for the ocular surface . Ocul Surf 2005. ; 3 : S203 – 6 . [DOI] [PubMed] [Google Scholar]
- 27. Benemei S, Nicoletti P, Capone JG. et al. . CGRP receptors in the control of pain and inflammation . Curr Opin Pharmacol 2009. ; 9 : 9 – 14 . [DOI] [PubMed] [Google Scholar]
- 28. De Castro F, Silos-Santiago I, Lopez de Armentia M. et al. . Corneal innervation and sensitivity to noxious stimuli in trkA knockout mice . Eur J Neurosci 1998. ; 10 : 146 – 52 . [DOI] [PubMed] [Google Scholar]
- 29. Lambiase A, Micera A, Sacchetti M. et al. . Alterations of tear neuromediators in dry eye disease . Arch Ophthalmol 2011. ; 129 : 981 – 6 . [DOI] [PubMed] [Google Scholar]
- 30. Liu Q, McDermott AM, Miller WL. Elevated nerve growth factor in dry eye associated with established contact lens wear . Eye Contact Lens 2009. ; 35 : 232 – 7 . [DOI] [PubMed] [Google Scholar]
- 31. Aloe L, Tirassa P, Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers . Pharmacol Res 2008. ; 57 : 253 – 8 . [DOI] [PubMed] [Google Scholar]
- 32. Chao C, Golebiowsi B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye . Ocul Surf 2014. ; 12 : 32 – 45 . [DOI] [PubMed] [Google Scholar]
- 33. Neumann S, Doubell TP, Leslie T. et al. . Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons . Nature 1996. ; 384 : 360 – 4 . [DOI] [PubMed] [Google Scholar]
- 34. Benitez-Del-Castille JM, Acosta MC, Wassfi MA. et al. . Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye . Invest Ophthalmol Vis Sci 2007. ; 48 : 173 – 81 . [DOI] [PubMed] [Google Scholar]
- 35. Villani E, Galimberti D, Viola F. et al. . The cornea in Sjogren’s syndrome: An in vivo confocal study . Invest Ophthalmol Vis Sci 2007. ; 48 : 2017 – 22 . [DOI] [PubMed] [Google Scholar]
- 36. Tuominen IS, Konttinen YT, Vesaluoma MH. et al. . Corneal innervation and morphology in primary Sjogren’s syndrome . Invest Ophthalmol Vis Sci 2003. ; 44 : 2545 – 9 . [DOI] [PubMed] [Google Scholar]
- 37. Tuisku IS, Konttinen YT, Konttinen LM. et al. . Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren’s syndrome . Exp Eye Res 2008. ; 86 : 879 – 85 . [DOI] [PubMed] [Google Scholar]
- 38. Zhang M, Chen J, Luo L. et al. . Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal micropscopy . Cornea 2005. ; 24 : 818 – 24 . [DOI] [PubMed] [Google Scholar]
- 39. Benitez del Castillo JM, Wasfy MA, Fernandez C. et al. . An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye . Invest Ophthalmol Vis Sci 2004. ; 45 : 3030 – 5 . [DOI] [PubMed] [Google Scholar]
- 40. Erdelyi B, Kraak R, Zhivov A. et al. . In vivo confocal laser scanning microscopy of the cornea in dry eye . Graefe’s Arch Clin Exp Ophthalmol 2007. ; 245 : 39 – 44 . [DOI] [PubMed] [Google Scholar]
- 41. Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions . Semin Ophthalmol 2012. ; 27 : 138 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Labbe A, Alalwani H, Van Went C. et al. . The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease . Invest Ophthalmol Vis Sci 2012. ; 53 : 4926 – 31 . [DOI] [PubMed] [Google Scholar]
- 43. Labbe A, Liang Q, Wang Z. et al. . Corneal nerve structure and function in patients with non-sjogren dry eye: Clinical correlations . Invest Ophthalmol Vis Sci 2013. ; 54 : 5144 – 50 . [DOI] [PubMed] [Google Scholar]
- 44. Belmonte C, Aracil A, Acosta C. et al. . Nerves and sensations from the eye surface . Ocul Surf 2004. ; 2 : 248 – 53 . [DOI] [PubMed] [Google Scholar]
- 45. Von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms . Neuron 2012. ; 73 : 638 – 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hudspith MJ. Glutamate: A role in normal brain function, anaesthesia, analgesia, and CNS injury . Br J Anasth 1997. ; 78 : 731 – 47 . [DOI] [PubMed] [Google Scholar]
- 47. Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective . Eur J Pain 2000. ; 4 : 5 – 17 . [DOI] [PubMed] [Google Scholar]
- 48. Oswald DJ, Lee A, Trinidad M. et al. . Communication between corneal epithelial cells and trigeminal neurons is facilitated by purinergic (P2) glutamatergic receptors . PLoS One 2012. ; 7 : 1 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bereiter DA, Bereiter DF. NMethyl-D-Aspartate and non-N-Methyl-D-Aspartate receptor antagonism reduces Fos-like immunoreactivity in central trigeminal neurons after corneal stimulation in the rat . Neuroscience 1996. ; 73 : 249 – 58 . [DOI] [PubMed] [Google Scholar]
- 50. Barr MS, Farzan F, Davis KD. et al. . Measuring GABAergic inhibitory activity with TMS-EEG and its potential clinical application for chronic pain . J Neuroimmune Pharmacol 2013. ; 8 : 535 – 46 . [DOI] [PubMed] [Google Scholar]
- 51. Hirata H, Okamoto K, Bereiter DA. GABAA receptor activation modulates corneal unit activity in rostral and caudal portions of trigeminal subnucleus caudalis . J Neurophysiol 2003. ; 90 : 2837 – 49 . [DOI] [PubMed] [Google Scholar]
- 52. Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells, and glia . Nature Neuroscience 2007. ; 10 : 1361 – 8 . [DOI] [PubMed] [Google Scholar]
- 53. Parra A, Madrid R, Echevarria D. et al. . Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea . Nat Med 2010. ; 16 : 1396 – 9 . [DOI] [PubMed] [Google Scholar]
- 54. Acosta MC, Peral A, Luna C. et al. . Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers . Invest Ophthalmol Vis Sci 2004. ; 45 : 2333 – 6 . [DOI] [PubMed] [Google Scholar]
- 55. Collins SM, Hurst SM, Main C. et al. . Effect of inflammation of enteric nerves. Cytokine-induced changes in neurotransmitter content and release . Ann Ny Acad Sci 1992. ; 664 : 415 – 24 . [DOI] [PubMed] [Google Scholar]
- 56. Zoukhri D. Effect of inflammation on lacrimal gland function . Exp Eye Res 2006. ; 82 : 885 – 98 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Management and therapy of dry eye disease: Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop . Ocul Surf 2007. ; 5 : 163 – 178 . [DOI] [PubMed] [Google Scholar]
- 58. Alves M, Fonseca EC, Alves MF. et al. . Dry eye disease treatment: A systematic review of published trials and a critical apprasial of therapeutic strategies . Ocul Surf 2013. ; 11 : 181 – 92 . [DOI] [PubMed] [Google Scholar]
- 59. Sahai A, Malik P. Dry eye: Prevalence and attributable risk factors in a hospital-based population . Indian J Ophthalmol 2005. ; 53 : 87 – 91 . [DOI] [PubMed] [Google Scholar]
- 60. Versura P, Profazio V, Cellini M. et al. . Eye discomfort and air pollution . Ophthalmologica 1999. ; 213 : 103 – 9 . [DOI] [PubMed] [Google Scholar]
- 61. Bhargava R, Kumar P, Kumar M. et al. . A randomized controlled trial of omega-3 fatty acids in dry eye syndrome . Int J Ophthalmol 2013. ; 6 : 811 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brignole-Baudouin F, Baudouin C, Aragona P. et al. . A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients . Acta Ophthalmol 2011. ; 89 : e591 – 7 . [DOI] [PubMed] [Google Scholar]
- 63. Alfawaz AM, Algehedan S, Jastaneiah SS. et al. . Efficacy of punctal occlusion in management of dry eyes after laser in situ keratomileusis for myopia . Curr Eye Res 2014. ; 39 : 257 – 62 . [DOI] [PubMed] [Google Scholar]
- 64. Pan Q, Angelina A, Zambrano A. et al. . Autologous serum eye drops for dry eye . Cochrane Database Syst Rev 2013. ; 8 : CD009327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dimit R, Gire A, Pflugfelder SC. et al. . Patient ocular conditions and clinical outcomes using a PROSE scleral device . Cont Lens Anterior Eye 2013. ; 36 : 159 – 63 . [DOI] [PubMed] [Google Scholar]
- 66. Fiscella RG. Understanding dry eye disease: A managed care perspective . Am J Manag Care 2011. ; 17 : S432 – 9 . [PubMed] [Google Scholar]
- 67. Finis D, Hayaineh J, Konig C. et al. . Evaluation of an automated thermodynamic treatment (LipiFlow) system for meibomian gland dysfunction: A prospective, randomized, observer-masked trial . Ocul Surf 2014. ; 12 : 146 – 54 . [DOI] [PubMed] [Google Scholar]
- 68. Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction . Cornea 2010. ; 29 : 1145 – 52 . [DOI] [PubMed] [Google Scholar]
- 69. Fahmy AM, Hardten DR. Treating ocular surface disease: New agents in development . Clin Ophthalmol 2011. ; 5 : 465 – 72 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McGreevy K, Bottros MM, Raja SN. Preventing chronic pain following acute pain: Risk factors, preventive strategies, and their efficacy . Eur J Pain Suppl 2011. ; 5 : 365 – 72 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Katz J, Clarke H, Seltzer Z. Preventive analgesia:quo vadimus? Anesth Analg 2011. ; 113 : 1242 – 53 . [DOI] [PubMed] [Google Scholar]
- 72. Zollner C, Mousa S, Klinger A. et al. . Topical fentanyl in a randomized, double-blind study in patients with corneal damage . Clin J Pain 2008. ; 24 : 690 – 6 . [DOI] [PubMed] [Google Scholar]
- 73. Wenk HN, Nannenga MN, Honda CN. Effect of morphine sulphate eye drops on hyperalgesia in the rat cornea . Pain 2003. ; 105 : 455 – 65 . [DOI] [PubMed] [Google Scholar]
- 74. Zaidman GW. Diclofenac and its effect on corneal sensation . Arch Ophthalmol 1995. ; 113 : 262 . [DOI] [PubMed] [Google Scholar]
- 75. Fry LL. Efficacy of diclofenac sodium solution in reducing discomfort after cataract surgery . J Cataract Refract Surg 1995. ; 21 : 187 – 90 . [DOI] [PubMed] [Google Scholar]
- 76. Mohammadpour M, Jabbarvand M, Nikdel M. et al. . Effect of preemptive topical diclofenac on postoperative pain relief after photorefractive keratectomy . Cataract Refract Surg 2011. ; 37 : 633 – 7 . [DOI] [PubMed] [Google Scholar]
- 77. Szucs PA, Nashed AH, Allegra JR. et al. . Safety and efficacy of diclofenac ophthalmic solution in the treatment of corneal abrasions . Ann Emerg Med 2000. ; 35 : 131 – 7 . [DOI] [PubMed] [Google Scholar]
- 78. Ortiz MI, Castañeda-Hernández G, Izquierdo-Vega JA. et al. . Role of ATP-sensitive K+ channels in the antinociception induced by non-steroidal anti-inflammatory drugs in streptozotocin-diabetic and non-diabetic rats . Pharmacol Biochem Behav 2012. ; 102 : 163 – 9 . [DOI] [PubMed] [Google Scholar]
- 79. Kawano T, Zoga V, Gemes G. et al. . Suppressed Ca2+/CaM/CaMKII-dependent KATP channel activity in primary afferent neurons mediates hyperalgesia after axotomy . Proc Natl Acad Sci USA 2009. ; 106 : 8725 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lichtinger A, Purcell TL, Schanzlin DJ. et al. . Gabapentin for postoperative pain after photorefractive keratectomy: A prospective, randomized, double-blind, placebo-controlled trial . J Refract Surg 2011. ; 27 : 613 – 7 . [DOI] [PubMed] [Google Scholar]
- 81. Moller AT, Jensen TS. Pain and genes: Genetic contribution to pain variability, chronic pain and analgesic responses . Eur J Pain Suppl 2010. ; 4 : 197 – 201 . [Google Scholar]