Abstract
Introduction:
There are mixed reports on nicotine’s effects on alcohol-induced impairment in cognitive performance and behavior in humans. The main objective of this study was to characterize the interactive effects of acute intravenous (IV) alcohol and nicotine administration on behavior and cognition in healthy nonsmokers.
Methods:
Healthy subjects aged 21–44 years participated in 3 test days. On each test day, they received in a double-blind randomized manner one of three IV alcohol infusion conditions using a “clamp”: placebo, targeted breathalyzer of 40 mg%, or targeted breathalyzer of 80 mg%. Alcohol infusion was delivered over 20 min and lasted for 120 min. They also received both placebo and active nicotine in a fixed order delivered intravenously. Placebo nicotine was delivered first over 10 min at the timepoint when the breath alcohol was “clamped”; active nicotine (1.0 mcg/kg/min) was delivered for 10 min, 70 min after the alcohol infusion was clamped. Subjective effects of alcohol were measured using the Biphasic Alcohol Effects Scale and the Number of Drinks Scale. Cognitive inhibition and attention were measured by the Continuous Performance Task—Identical Pairs and working memory by the Rey Auditory Verbal Learning Task (RAVLT).
Results:
Nicotine significantly reversed subjective intoxication and sedation of alcohol at the low dose. Alcohol impaired performance on the RAVLT, and nicotine further impaired verbal learning and recall at both doses of alcohol.
Conclusions:
The data showed that nicotine had an effect on subjective alcohol effects but did not reverse and actually worsened alcohol-induced deficits in memory.
Introduction
Alcohol consumption and tobacco use are highly correlated in the general population (Dawson, 2000; Grant, Hasin, Chou, Stinson, & Dawson, 2004; McKee, Falba, O’Malley, Sindelar, & O’Connor, 2007), in clinical samples (Ait-Daoud et al., 2005; John, Meyer, Rumpf, & Hapke, 2003), and in young adults (Dierker et al., 2006; Jackson, Sher, Cooper, & Wood, 2002; Jackson, Sher, & Park, 2005). A number of studies, including findings from the National Epidemiological Survey on Alcohol and Related Conditions (NESARC), suggest higher rates of tobacco use among people who use, misuse, or are dependent on alcohol (Bobo & Huston, 2000; Dawson, 2000; Falk, Yi, & Hiller-Sturmhofel, 2006). Studies of young adults show that 59% of college students who drink also smoke, and this rate is higher than in the nondrinking college student population (Harrison, Desai, & McKee, 2008; Weitzman & Chen, 2005). Longitudinal studies such as the National Longitudinal Study of Adolescent Health and the Adolescent Risk Study suggest alcohol and tobacco use escalate in adolescence and that drinking is associated with greater risk for tobacco use (Jackson et al., 2002). A large proportion of smokers start smoking in high school and develop their pattern of daily smoking by early adulthood (Chassin, Presson, Pitts, & Sherman, 2000); this coincides with the timeframe when rates of drinking increase (Dawson, Grant, Stinson, & Chou, 2004). These and other data have shown that alcohol use and tobacco use are clearly linked.
The epidemiological data of the association between drinking and smoking are supported and expanded by laboratory studies showing that administration of alcohol increases the rewarding effects of nicotine (Rose et al., 2004), increases the urge to smoke in a dose-dependent manner (Burton & Tiffany, 1997; Epstein, Sher, Young, & King, 2007; A. C. King & Epstein, 2005), and increases voluntary smoking in both past (Hughes, Rose, & Callas, 2000) and present alcoholics (Griffiths, Bigelow, & Liebson, 1976; Henningfield, Chait, & Griffiths, 1983) as well as social drinkers (Henningfield, Chait, & Griffiths, 1984; Mello, Mendelson, & Palmieri, 1987) and light smokers (A. King, McNamara, Conrad, & Cao, 2009). Administration of nicotine increases voluntary alcohol consumption in male social drinkers (Perkins, Fonte, & Grobe, 2000) and occasional male smokers (Barrett et al., 2006), enhances the alcohol-induced euphoria and relaxation (Kouri, McCarthy, Faust, & Lukas, 2004), and reverses the sedative effects of alcohol (Rose et al., 2004).
While the link between alcohol and nicotine has been well established, some questions about this relationship remain unclear. For example, it is possible that alcohol increases smoking behavior (or vice versa) because of the reinforcing effects of nicotine on alcohol (or vice versa). It is also possible that alcohol increases smoking behavior because of specific external features related to smoking such as the handling of cigarettes or the smoke itself. Alternatively, nicotine may attenuate or reverse the negative subjective effects of alcohol or may reverse the locomotor impairment associated with alcohol. The majority of laboratory studies that have examined the interaction between alcohol and nicotine have been done in heavy or regular smokers. Little is known about the interaction of alcohol and nicotine in nondaily smokers who are also social drinkers. This group may experience the alcohol–nicotine interaction in a laboratory differently since their responses are not confounded by possible withdrawal effects from either substance (Shiffman, 1989; Shiffman, Paty, Gnys, Kassel, & Elash, 1995). Also they may report greater sensitivity to the effects of alcohol on nicotine since their smoking occurs most often in the context of drinking (Harrison & McKee, 2008; McKee, Harrison, & Shi, 2010; Shiffman & Paty, 2006).
The effect of the combination of nicotine and alcohol on cognition is even murkier. It has been well documented that alcohol causes cognitive impairment (T. Weissenborn & Duka, 2003). A recent review designed to summarize the findings in the literature since 1994 concluded that the most robust finding was that nicotine enhanced attention and memory (Heishman, Kleykamp, & Singleton, 2010). Fewer studies have examined the combined effects of nicotine and alcohol on cognition and the results are mixed. In animal studies, treatment with nicotinic receptor agonists or cholinesterase inhibitors reversed alcohol-induced learning deficits (Hodges et al., 1991), and pretreatment with nicotine reduced the effects of alcohol on both reference and working memory (Tracy, Wayner, & Armstrong, 1999). However, while nicotine attenuated the impairment induced by alcohol on motor reaction time (RT) and accuracy, the combination caused impairment of short-term memory processing beyond alcohol alone or placebo (Kerr, Sherwood, & Hindmarch, 1991). Others have also found working memory impairment with co-administration of alcohol and nicotine at doses that had no effect when given alone (Rezvani & Levin, 2002). In humans, nicotine attenuated the impairment induced by alcohol on rapid visual information processing in young female smokers compared with nonsmokers (Michel & Battig, 1989). Nicotine improved alcohol-induced impairment on finger-tapping speed and on rapid visual information-processing tasks in male and female moderate smokers (Glautier, Clements, White, Taylor, & Stolerman, 1996).
The main purpose of this study was to characterize the interactive effects of acute intravenous (IV) alcohol and nicotine administration on alcohol-induced subjective effects and cognition in healthy nonsmokers who use alcohol recreationally. This was an IV alcohol and nicotine administration study to: (a) allow for direct comparisons of the behavioral and cognitive effects of specific alcohol doses and (b) maintain stable alcohol levels without the confounding factors of variable alcohol absorption and peak blood alcohol levels. In the case of nicotine to: (a) allow precise dosage administration, (b) imitate the rapid delivery of nicotine when smoking a cigarette, and (c) avoid the confounding of other gaseous compounds in cigarette smoke.
Methods
The study was approved by the institutional review boards of the VA Connecticut Healthcare System (West Haven, CT) and the Yale University School of Medicine (New Haven, CT). The administration of alcohol to human subjects was in compliance with the Guidelines on Ethyl Alcohol Administration in Human Experimentation developed by the United States National Institute on Alcohol Abuse and Alcoholism (NIAAA) of the National Institutes of Health (NIAAA, 2005). Subjects aged 21–44 years were recruited via public advertisements and were paid for their study participation. Written informed consent was obtained from all subjects. None of the participants were nicotine naive. Nonsmokers were defined as those who did not smoke regularly (nondaily smokers) but all subjects had sampled cigarettes at some point in the past (smoked less than 100 cigarettes in their lifetime). All participants were social drinkers and had at least four drinks on at least two occasions over the past year—this minimal alcohol limit was defined a priori to ensure that potential participants had experience with the amount of alcohol that was given in this study. No maximum level of alcohol consumption was defined a priori but individuals who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for alcohol abuse and dependence were excluded from the study. Subjects were medically healthy by physical examination, history, electrocardiography, laboratory testing, and had no current substance abuse or dependence—assessed using the Structured Clinical Interview for DSM-IV (SCID) and verified by urine toxicology and breath alcohol levels at screening and on each test day. Subjects were excluded if they had any current or past Axis I DSM-IV psychiatric diagnosis, including lifetime substance abuse or dependence—assessed using the SCID and confirmed by negative urine toxicology at screening and on each test day—or were alcohol and nicotine naive.
Study Design
This was a double-blind, placebo-controlled, randomized, within-subject design study of IV administration of three doses of alcohol using an IV “clamp” method. The alcohol clamp procedure is a method designed to achieve and maintain a target breath alcohol level for a predetermined and extended period of time. The procedure is tailored for each subject in order to achieve the same alcohol exposure. The testing consisted of three separate test days scheduled at least 3 days apart. Test days included a placebo day (saline solution), low alcohol condition (alcohol clamped to targeted breathalyzer = 40 mg%), and high alcohol condition (alcohol clamped to targeted breathalyzer = 80 mg%). On each test day, participants also received two infusions consisting of placebo nicotine and active nicotine (1.0 mcg/kg/min) in a fixed order (raters were blinded to condition). All testing was done on the Biological Unit at the VA Connecticut Healthcare System, West Haven campus. Table 1 outlines the study schedule in detail. Alcohol and nicotine were administered through an infusion pump that was programmed to titrate the dose of alcohol or nicotine to a desired level.
Table 1.
Outline of Study Procedure for Each Test Day
| Time (min) | Procedure |
| −60 | IV lines placed, VS, breathalyzer, urine toxicology, urine pregnancy test (women), alcohol, and nicotine intake questions (Day 1 only), BAES, NDS |
| Alcohol infusion, adjusted until target BrAc is achieved (approximately 20 min), clamped for 120 min | |
| 0 | Placebo nicotine infusion over 10 min |
| +5 | RAVLT |
| +10 | BAES, NDS |
| +15 | CPT-IP, RAVLT (delayed) |
| +45 | BAES, NDS |
| +70 | Active nicotine infusion (1.0 μg/kg/min over 10 min) |
| +75 | RAVLT |
| +80 | CPT-IP, RAVLT (delayed) |
| +115 | BAES, NDS |
Note. VS = vital signs; BrAc = breath alcohol level; BAES = Biphasic Alcohol Effects Scale; NDS = Number of Drinks Scale; RAVLT = Rey Auditory Verbal Learning Test; CPT-IP = Continuous Performance Task—Identical Pairs.
Alcohol and Nicotine Infusion
The alcohol infusate was prepared by the pharmacy and the concentration of alcohol was 6% by volume, approximately two oz. ethanol. Alcohol was administered through an IV from two, 1-l bags of the infusate based on a preset computerized schedule that considered subject’s weight and height. The infusion of alcohol was over 20 min until the desired breath alcohol (BrAc) was achieved (low-dose–targeted breathalyzer = 40 mg% and high-dose–targeted breathalyzer = 80 mg%). Frequent BrAc measurements (every 2 min until target and every 8 min until infusion was complete) using a breathalyzer (Alcotest 7410Plus) assured that the infusion was maintained within the specified range. After the target BrAc was achieved, it was maintained for 120 min (Ramchandani, Bolane, Li, & O’Connor, 1999; Subramanian et al., 2002).
On each test day, subjects also received in fixed order, placebo and nicotine in a single-blind fashion. Placebo nicotine was delivered intravenously, over 10 min as soon as the desired BrAc was achieved and alcohol infusion was clamped. Active nicotine was delivered intravenously at a dose of 1.0 mcg/kg/min for 10 min (a range of 0.5–1.5 mg), 70 min after the alcohol infusion was clamped. According to a report by the Federal Trade Commission (2003) an average of 0.88 mg of nicotine is delivered to a smoker from each cigarette. Therefore, the dose of nicotine delivered in this study (0.5–1.5mg) was within the range of 1 cigarette (0.88 mg).
Measures
Main Outcome Measures
Subjective measures of alcohol effects.
The stimulant and sedative effects of alcohol were assessed with the Biphasic Alcohol Effects Scale (BAES; Martin, Earleywine, Musty, Perrine, & Swift, 1993). The stimulant subscale includes items that measure feeling: elated, energized, excited, stimulated, talkative, up, and vigorous while the sedative subscale includes items that measure feeling: down, inactive, sedated, sluggish, having difficulty concentrating, having a heavy head, or having slow thoughts. A single item Number of Drinks Scale (NDS) was also used to rate the number of standard ethanol drinks participants believe that they have been administered. As all subjects will have consumed the level of alcohol administered in this study, we instructed subjects to use their prior experience with ethanol as a reference in relation to “standard drinks” (10 g of ethanol). For this item, subjects were asked to subjectively report on the number of drinks they felt they had consumed using the NDS scale. This item was administered four times: at baseline (−60), after start of alcohol infusion (time = +10) and during clamp (time = +45 and time = +115). The NDS scale has been used in a number of previous challenge studies conducted by our group (Dickerson et al., 2010; Krystal et al., 1998).
Cognitive measures.
Attention was measured by the Continuous Performance Task—Identical Pairs (CPT-IP; DelBello et al., 2004). The CPT-IP is a measure of sustained visual attention with a working memory component (Cornblatt & Keilp, 1994; Cornblatt, Risch, Faris, Friedman, & Erlenmeyer-Kimling, 1988). Digits (two digits, three digits, or four digits) are presented on a computer monitor one at a time, in random sequence. Subjects are told to click the mouse as quickly as possible whenever the same number sequences appear twice in a row. A brief training trial precedes the actual test. The CPT program generates separate scores for the number of hits, misses, false alarms, and mean hit RTs. Discriminability scores (d’) are also computer generated based on hits and false alarms for two-digit, three-digit, and four-digit pairs. As others have suggested, discriminability (d’) was selected as a better measure of decline in sensitivity or attention capacity than number of correct responses (Cornblatt et al., 1988). An average d’ measure was used as a single measure of attention and was calculated averaging the two-digit, three-digit, and four-digit d’ scores. Working memory was assessed using the Rey Auditory Verbal Learning Test (RAVLT; Mungas, 1983). The RAVLT is a 15-word list learning task of verbal memory and hippocampal function (Rosenberg, Ryan, & Prifitera, 1984; Ryan, Geisser, Randall, & Georgemiller, 1986) that measures immediate free recall, delayed free recall, and recognition recall. One of the five different word lists of the RAVLT—validated separately (Crawford, Stewart, & Moore, 1989; Lezak, 1983; Rey, 1964; Shapiro & Harrison, 1990)—was administered on each test day and randomized across subjects.
Data Analysis
Descriptive statistics were used to summarize the data on all randomized subjects. All continuous variables were examined for adherence to the normal distribution using normal probability plots and Kolmogorov–Smirnov tests. Only subjects that completed all test days were included in the analysis. The outcome variables included: (a) subjective stimulant (BAES stimulant) effects of alcohol, subjective sedative (BAES sedative) effects of alcohol, and NDS scale and (b) cognitive measures of attention (CPT-IP), memory recognition (calculated from the RAVLT), and memory recall (calculated from the RAVLT). All analyses were performed using the 17.0 version of SPSS. All statistical testing was at a two-tailed alpha level of 0.05. Repeated measures analysis of variance was used to assess changes in behavior and cognition as a result of alcohol and nicotine infusion. For behavioral variables, alcohol dose (placebo, BrAc = 40 mg%, and BrAc = 80 mg%) and time (four timepoints including before and after nicotine infusion) were used as within-subject factors. After a significant dose × time interaction post-hoc tests were performed to examine the effects of active nicotine on behavioral variables. A change score was calculated for each behavioral measure (BAES stimulant, BAES sedative, and NDS scores) using the scores before active nicotine infusion (time +45) minus the score after active nicotine infusion (time +115). This change score was analyzed using alcohol dose as a within-subject factor. Alcohol dose and nicotine (placebo vs. active nicotine) were used as within-subject factors for cognitive variables.
Results
Demographic Characteristics
Eighteen subjects participated in the study and fifteen completed all three test days. Of the three subjects who did not complete all three test days, two completed one test day (did not return repeated phone calls), and one completed two test days (moved out of state). The study procedures were well tolerated with no serious adverse events. There was one nonserious adverse event (eyelid puffiness and difficulty swallowing that occurred more than 3 hr after the last nicotine infusion and more than 2.5 hr after the alcohol infusion was completed) that resolved completely by the end of the test day. CPT-IP data for three subjects were lost due to error in setting up the electronic files for these assessments, and for those measures, analyses were performed on 12 subjects.
As shown in Table 2, the participants were young adults (mean age = 25.33, SD = 5.7) with at least some college education (years of education = 15.87, SD = 1.68), and with above-average intelligence (mean IQ = 120.93, SD = 14.64). The majority were females (n = 8) and Caucasian (n = 11).
Table 2.
Demographic and Clinical Characteristics of Healthy, Nonsmokers
| Variables | N = 15 |
| Age, mean (SD) | 25.33 (5.7) |
| Gender, n (%) | |
| Male | 7 (46.7) |
| Female | 8 (53.3) |
| Ethnicity, n (%) | |
| Asian | 3 (20) |
| African American | 1 (6.7) |
| Caucasian | 11 (73.3) |
| Education (years), mean (SD) | 15.87 (1.68) |
| IQ (WASI), mean (SD) | 120.93 (14.64) |
| Largest Number of Drinks (past year), mean (SD) | 5.40 (1.40) |
| How Often Drinking per Week (past year), mean (SD) | 4.40 (1.68) |
| Age at First Drink, mean (SD) | 16.93 (2.12) |
| Age Started Drinking Regularly, mean (SD) | 18.64 (1.86) |
Note. WASI = Wechsler Abbreviated Scale of Intelligence.
Subjective Alcohol effects
BAES (Stimulant and Sedative Subscales)
There was no significant main effect for alcohol dose on the “stimulant” scale of the BAES (F2,13 = 1.35, p = .293) and no significant differences over time (F3,12 = 2.18, p = .143), but there was a significant interaction between alcohol dose × time (F6,19 = 4.004, p = .031). There was no significant change on the BAES stimulant scores before and after nicotine infusion (F2,1.57 = 0.469, p = .58) indicating that nicotine had no significant effect on the stimulant effects of alcohol (see Figure 1). There was a significant main effect for alcohol dose on the sedative scale of the BAES (F2,13 = 6.107, p = .013), a significant main effect for time (F3,12 = 5.92, p = .01), and there was a significant alcohol dose × time interaction (F6,9 = 3.21, p = .05) (see Figure 2). The sedative effects of alcohol were dose dependent with high dose of alcohol producing most sedation. Also, there was a significant change on the BAES sedative scores before and after nicotine infusion (F2,1.8 = 5.2, p = .014). Sedative scores were significantly lower after the nicotine infusion on the low dose of alcohol but not the high dose of alcohol.
Figure 1.
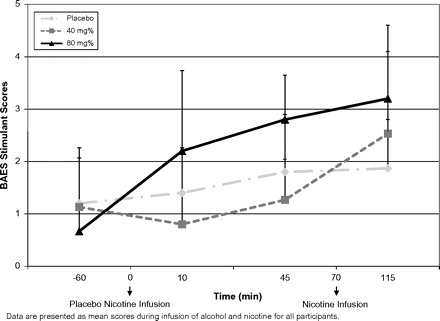
Self-rated stimulant alcohol effects as reflected by the Biphasic Alcohol Effects Scale.
Figure 2.
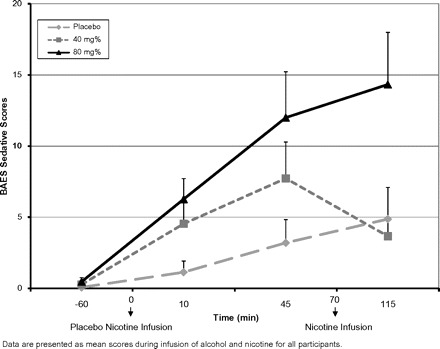
Self-rated sedative alcohol effects as reflected by the Biphasic Alcohol Effects Scale.
Number of Drinks Scale
Overall, subjects reported that the low dose of alcohol felt similar to consuming 1.1 drinks (SE = 0.122) and the high dose felt similar to 1.7 drinks (SE = 0.259). There was a significant main effect for alcohol dose on the NDS scale (F2,13 = 27.36, p = .0001; there was a significant main effect for time (F3,12 = 19.02, p = .0001) and a significant alcohol dose × time interaction (F6,9 = 11.546, p = .001). In addition, there was a significant change on the NDS scores before and after nicotine infusion (F2,1.4 = 3.7, p = .05). NDS scores were significantly lower after the nicotine infusion (on high and lose dose of alcohol vs. placebo) with the most significant change on the low dose of alcohol (see Figure 3).
Figure 3.
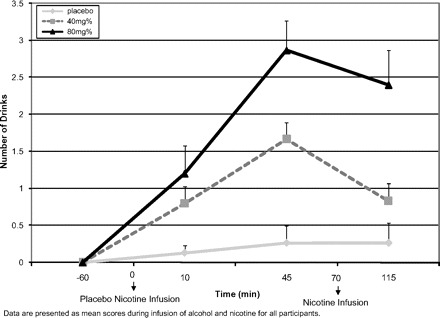
Self-rated alcohol intoxication measured by the “Number of Drinks” Scale.
Cognitive Effects
Attention
Neither alcohol nor nicotine or the interaction had a significant effect on attention. There were no statistically significant differences in attention based on different doses of alcohol (F2,10 = 1.18, p = .348), no differences in attention after administration of placebo and nicotine (F1,11 = 0.048, p = .831), and no significant interaction between alcohol and nicotine administration (F2,10 = 2.63, p = .121; see Table 3).
Table 3.
Means and SDs for Attention and Memory Measured by the Continuous Performance Task—Identical Pairs and the Rey Auditory Verbal Learning Test under All Three Alcohol Conditions
| Variables | Alcohol conditions | Before infusion of active nicotine, mean (SD) | After infusion of active nicotine mean (SD) |
| CPT-IP | Placebo | 3.185 (0.55) | 3.348 (0.48) |
| 40 mg% | 3.199 (0.54) | 3.182 (0.46) | |
| 80 mg% | 3.208 (0.59) | 3.017 (0.58) | |
| RAVLT: Recognition | Placebo | 13.80 (1.37) | 13.00 (2.42) |
| 40 mg% | 13.53 (1.99) | 12.73 (2.49) | |
| 80 mg% | 12.20 (2.70) | 10.80 (2.42) | |
| RAVLT: Delayed Recall | Placebo | 11.80 (2.73) | 10.53 (4.45) |
| 40 mg% | 10.00 (3.45) | 8.29 (4.09) | |
| 80 mg% | 8.35 (3.90) | 5.82 (4.50) | |
| RAVLT: Immediate Recall | Placebo | 56.65 (10.45) | 53.67 (9.45) |
| 40 mg% | 51.85 (9.08) | 56.65 (10.45) |
Note. CPT-IP = Continuous Performance Task—Identical Pairs; RAVLT = Rey Auditory Verbal Learning Test.
Memory Recall and Recognition
Alcohol impaired immediate recall (F2,13 = 6.11, p = .013), delayed recall (F2,13 = 15.75, p = .0001), and recognition (F2,13 = 7.85, p = .006) measured by the RVALT; high dose of alcohol produced greater impairment in immediate recall, delayed recall, and recognition than low dose of alcohol or placebo (see Table 3). Nicotine further impaired immediate recall (F1,14 = 11.53, p = .004), delayed recall (F1,14 = 9.82, p = .007), and recognition (F1,14 = 5.08, p = .04). There were no significant interactions between alcohol and nicotine administration on immediate recall (F2,13 = 0.029, p = .971), delayed recall (F2,13 = 0.905, p = .428), or recognition (F2,13 = 0.386, p = .687).
Discussion
This laboratory study was designed to elucidate the interactive effects of alcohol and nicotine on behavior and cognitive performance in healthy, social drinkers, and nonsmokers. First, we replicated earlier findings by others that nicotine may reverse some of the subjective alcohol effects in a laboratory setting (Perkins et al., 1995; Rose et al., 2004); the BAES sedative and NDS scores were significantly lower after infusion of nicotine particularly for the low dose of alcohol. However, nicotine had no effect on the stimulant effects of alcohol. Second, we found that recall and recognition were impaired by alcohol in a dose-related manner with higher doses of alcohol producing more impairment. Nicotine did not reverse alcohol-induced deficits in attention and memory, but in fact, actually further impaired memory recall and recognition.
Our results show that alcohol produced, for most measures, the expected behavioral effects after infusion of low and high dose of alcohol. The exception to this was the unexpected finding that there were no dose-related changes in the stimulant effects of alcohol based on the BAES; alcohol did affect the other measure of subjective intoxication, the NDS. It is not immediately obvious why the sedative effects were strong and consistent with other reports (including those from our group (Perrino et al., 2008) while stimulant effects were somewhat weaker. The use of the IV paradigm rather than the use of oral alcohol might explain the lack of effects. During the IV paradigm, the visual and sensory cues as well as other aspects related to alcohol expectancy are missing, and this may be affecting the stimulant or “high” effects more than the sedative effects. Other groups have also commented on the role of expectancy in laboratory studies and the “inability of participants” to differentiate the pharmacological effects of alcohol from the psychological effects of knowing that one is drunk (Gundersen, Specht, Gruner, Ersland, & Hugdahl, 2008).
The findings indicate that the addition of nicotine after alcohol administration produced less sedation and less intoxication (measured by number of drinks scale) than alcohol alone. These findings seem to support the assertion raised by others that nicotine may reverse subjective alcohol effects (Rose et al., 2004). Surprisingly, we found that nicotine had no effect on the stimulant effects of alcohol (measured by the BAES stimulant scale). One reason for this finding may be that the stimulant effects of alcohol were not strong enough in this study to allow for a significant change after nicotine infusion. Nicotine in this study was administered 70 min after the infusion of alcohol. If, as others have reported (Kouri et al., 2004), the effects of nicotine are strongest while plasma levels of alcohol are rising (early during alcohol infusion) while the effects are stimulatory, nicotine in this study was infused too late to enhance the stimulant effects of alcohol. Why the nicotine effects were more pronounced at the low dose of alcohol is not entirely clear. However, this finding is consistent with other reports (Kouri et al., 2004) showing that the ethanol effects were more rapidly detected at low levels of alcohol when compared with high levels of alcohol due to faster absorption rates. Another possibility is that the dose of nicotine was not strong enough to have an effect on sedation at the high dose of alcohol. Alternatively, the nicotine effects could be a result of acute tolerance to alcohol (Hiltunen, 1997b; Holdstock, King, & de Wit, 2000; Ramchandani et al., 2002). Acute tolerance (reduced effect of alcohol during a descending phase of intoxication) has been studied using an alcohol clamp design (Ramchandani et al., 2002) as the one employed in this study. Since nicotine was administered 70 min after the BrAc was clamped, reduced sedation and decreased subjective intoxication could be a result of acute tolerance rather than nicotine effects. Although acute tolerance can be a rival explanation for the nicotine effects reported on the behavioral measures, our findings on the cognitive functioning do not support acute alcohol tolerance (Hiltunen, 1997a) as an explanation for the cognitive findings in this report. Finally, differences in alcohol delivery (IV vs. alcohol drink), nicotine delivery (IV vs. nasal spray, cigarette, or transdermal patch), and the “speed” of nicotine administration (10 min vs. 60 s which according to some reports [Sofuoglu, Babb, & Hatsukami, 2003; Sofuoglu, Mouratidis, Yoo, Culligan, Kosten, & 2005; Sofuoglu, Poling, Mouratidis, & Kosten, 2006] produces robust physiological and subjective responses) in this study may explain some of the differences in findings.
The results from this study are consistent with the literature showing that alcohol impairs memory performance (R. Weissenborn & Duka, 2000) and that this impairment is dose related, with higher doses of alcohol producing greater impairment in memory (Hindmarch, Kerr, & Sherwood, 1991). The findings that the administration of nicotine did not reverse but further impaired memory performance is consistent with some, but not all research showing that the combination of nicotine and alcohol causes impairment of short-term memory processing beyond alcohol alone or placebo (Kerr et al., 1991) and impairment in working memory at doses that had no effect when given alone (Rezvani & Levin, 2002).
One model most frequently cited to explain the interaction of alcohol and nicotine assumes a central role of the nicotinic acetylcholine (nACh) receptors. The major behavioral effects of nicotine, including its discriminative properties, are mediated through central nACh receptors (Korkosz et al., 2005). Further, there is evidence that some of the behavioral effects of “alcohol” are also mediated through the α and β subtypes of nACh receptors such as α7 and α4β2 (Cardoso et al., 1999; Hu, Bai, Tizabi, & Southerland, 2009; Jerlhag, Grotli, Luthman, Svensson, & Engel, 2006). The functional interaction between alcohol and nicotine is complex and not very well understood but could be characterized as antagonistic where one drug attenuates or eliminates the effects of the other (Korkosz et al., 2006b; Tizabi, Manaye, & Taylor, 2005) or agonistic where one drug enhances the effects of the other (Korkosz et al., 2006b; Yang, Criswell, & Breese, 1999). Support for the antagonistic interaction of alcohol and nicotine comes from evidence that indicates α7 and α4β2 agonists reverse the effects of alcohol (Taslim, Al-Rejaie, & Dar, 2008; Taslim & Dar, 2011). Also, α7-selective full agonists and α4β2-selective partial agonists such as varenicline decrease alcohol consumption (Steensland, Simms, Holgate, Richards, & Bartlett, 2007), reverse alcohol-induced effects (Gulick & Gould, 2008), and have been approved for use in smoking cessation. Proponents of the agonistic interaction between alcohol and nicotine indicate that there is considerable similarity in the behavioral effects of both nicotine and alcohol (e.g., relaxation, reward, analgesia), leading them to speculate that these effects may be additive when the two drugs are combined (Korkosz et al., 2006a; Prendergast, Podus, & Change, 2002; Yang et al., 1999). The additive effects can explain the further decline in memory performance when nicotine is added to alcohol. Findings from animal studies designed to test the combined effects of alcohol and nicotine on cognitive functioning (Rezvani & Levin, 2002) have found that the dose as well as the timing of alcohol and nicotine administration was related to whether nicotine enhanced or impaired cognitive performance. The combination of alcohol and high doses of nicotine significantly impaired cognitive performance while the same doses of nicotine or alcohol alone had no effect on cognitive performance. Low doses of nicotine were associated with some improvement in cognitive performance that was blocked when alcohol was administered first. It is therefore possible to argue that in our study the ethanol dose, when administered first was able to block critical mechanisms by which nicotine improves memory (Rezvani & Levin, 2002). One relatively consistent finding in the literature is that nicotine enhances attention and memory (Heishman et al., 2010). The results from this study are not consistent with this finding since there was no improvement in attention and memory under the placebo condition. In light of the failure to observe an interaction in cognitive performance under alcohol and placebo conditions, our results on the effects of nicotine on cognitive performance should be interpreted with caution.
Some other limitations of our study include a relatively small sample size and the focus on nonsmokers, which limits the generalizability of our findings. The fixed order of nicotine and placebo made it difficult to tease apart effects of time and nicotine and may have resulted in learning effects during cognitive testing. It is also likely that more robust cognitive and behavioral effects would have been detected if we used higher doses of alcohol and nicotine or administered the nicotine more rapidly—others have found that rapid nicotine administration over 60 s produces robust physiological and subjective responses (Sofuoglu et al., 2003, 2005, 2006). The strengths of our study include the use of the IV clamp method of administration of alcohol, which allows for direct comparisons of the behavioral, cognitive, and motor effects of specific alcohol doses without the confounding factors of variable alcohol absorption and peak blood alcohol levels, and the IV administration of nicotine, which allows precise dosage administration and avoidance of other gaseous compounds in cigarette smoke. The IV alcohol and nicotine administration, however, does limit the generalizability to smoking and drinking in the natural environment.
In summary, this study examined the combined effects of alcohol and nicotine on cognition and behavior in healthy social drinkers who identified themselves as nonsmokers. Nicotine reversed the sedative and intoxication effects of alcohol particularly at the low dose of alcohol. Nicotine did not reverse alcohol-induced deficits in attention and memory but rather further impaired recall and recognition.
Funding
Alcoholic Beverage Medical Research Foundation; VA Schizophrenia Biological Research Center; VA Alcohol Research Center; the Center for Translational Neuroscience of Alcoholism
Declaration of Interests
None of the funding agencies had any role in study design, in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Acknowledgments
The authors wish to thank Angelina Genovese, R.N.C., M.B.A.; Elizabeth O’Donnell, R.N.; Brenda Breault, R.N., B.S.N.; Sonah Yoo, R.Ph.; Robert Sturwold, R.Ph., BCPP; Rachel Galván, Pharm.D.; and Willie Ford of the Neurobiological Diagnostic Studies Unit at the VA Connecticut Healthcare System, West Haven Campus, for their central contributions to the success of this project.
References
- Ait-Daoud N, Wiesbeck G, Bienkowski P, Li M, Pfutzer R, Singer M, et al. Comorbid alcohol and nicotine dependence: From the biomolecular basis to clinical consequences. Alcoholism: Clinical & Experimental Research. 2005;29:1541–1549. doi: 10.1097/01.alc.0000174692.20933.49. doi: 10.1097/01.alc.0000174692.20933.49. [DOI] [PubMed] [Google Scholar]
- Barrett JE, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug and Alcohol Dependence. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. doi:10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bobo JK, Huston C. Sociocultural influences on smoking and drinking. Alcohol and Drug Research. 2000;24:225–232. Retrieved from http://www.jsad.com/ [PMC free article] [PubMed] [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. doi: 10.1111/j.1360-0443.1997.tb03634.x. [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. The Journal of Pharmacology and Experimental Therapeutics. 1999;289:774–780. Retrieved from http://jpet.aspetjournals.org/ [PubMed] [Google Scholar]
- Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a midwestern community sample: Multiple trajectories and their psychosocial correlates. Health Psychology. 2000;19:223–231. doi: 10.10371/0278-6133.19.3.223. [PubMed] [Google Scholar]
- Federal Trade Commission. Federal Trade Commission Cigarette Report for 2001. 2003. Retrieved April 19, 2011, from http://www.ftc.gov/os/2003/06/2001cigreport.pdf. [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophrenia Bulletin. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. Retrieved from http://schizophreniabulletin.oxfordjournals.org/content/20/1/31.short. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): 1. New findings about sustained attention in normal families. Psychiatry Research. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. doi:10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Crawford J, Stewart L, Moore J. Demonstration of savings on the AVLT and development of a parallel form. Journal of Clinical and Experimental Neuropsychology. 1989;11:975–981. doi: 10.1080/01688638908400950. Retrieved from http://www.abdn.ac.uk/∼psy086/dept/pdfs/JCEN_1989_AVLT_Parallel_Form.pdf. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Alcohol consumption, alcohol dependence, and all-cause mortality. Alcoholism: Clinical & Experimental Research. 2000;24:72–81. doi: 10.1111/j.1530-0277.2000.tb04556.x. [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS. Another look at heavy episodic drinking and alcohol use disorders among college and noncollege youth. Journal of Studies on Alcohol. 2004;65:477–488. doi: 10.15288/jsa.2004.65.477. Retrieved from http://www.jsad.com/ [DOI] [PubMed] [Google Scholar]
- DelBello MP, Adler CM, Amicone J, Mills NP, Shear PK, Warner J, et al. Parametric neurocognitive task design: A pilot study of sustained attention in adolescents with bipolar disorder. Journal of Affective Disorders. 2004;82(Suppl. 1):S79–S88. doi: 10.1016/j.jad.2004.05.014. doi:10.1016/j.jad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Dickerson D, Pittman B, Ralevski E, Perrino A, Limoncelli D, Edgecombe J, et al. Ethanol-like effects of thiopental and ketamine in healthy humans. Journal of Psychopharmacology. 2010;24:203–211. doi: 10.1177/0269881108098612. doi: 10.1177/0269881108098612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, Lloyd-Richardson E, Stolar M, Flay B, Tiffany ST, Collins L, et al. The proximal association between smoking and alcohol use among first year college students. Drug and Alcohol Dependence. 2006;81:1–9. doi: 10.1016/j.drugalcdep.2005.05.012. doi:10.1016/j.drugalcdep.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology. 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. doi 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2006;3:162–171. Retrieved from http://archpsyc.ama-assn.org/ [PMC free article] [PubMed] [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behavioral Pharmacology. 1996;7:144–154. Retrieved from http://journals.lww.com/behaviouralpharm/pages/default.aspx. [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. Retrieved from http://archpsyc.ama-assn.org/current.dtl. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Facilitation of human tobacco self-administration by ethanol: A behavioral analysis. Journal of the Experimental Analysis of Behavior. 1976;25:279–292. doi: 10.1901/jeab.1976.25-279. doi:10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Varenicline ameliorates ethanol-induced deficits in learning in C57BL/6mice. Journal of Neurobiology of Learning and Memory. 2008;90:230–236. doi: 10.1016/j.nlm.2008.03.002. doi:10.1016/j.nlm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Specht K, Gruner R, Ersland L, Hugdahl K. Separating the effects of alcohol and expectancy on brain activation: An fMRI working memory study. NeuroImage. 2008;42:1587–1596. doi: 10.1016/j.neuroimage.2008.05.037. doi:10.1016/j.neuroimage.2008.05.037. [DOI] [PubMed] [Google Scholar]
- Harrison EL, Desai RA, McKee SA. Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: Findings from the NESARC. Alcoholism: Clinical & Experimental Research. 2008;32:2081–2087. doi: 10.1111/j.1530-0277.2008.00796.x. doi: 10.1111/j.1530-0277.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison ELR, McKee SA. Young adult non-daily smokers: Patterns of alcohol and cigarette use. Addictive Behaviors. 2008;33:668–674. doi: 10.1016/j.addbeh.2007.11.012. doi:10.1016/j.addbeh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. doi 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Cigarette smoking and subjective response in alcoholics: Effects of pentobarbital. Clinical Pharmacology & Therapeutics. 1983;33:806–812. doi: 10.1038/clpt.1983.110. doi:10.1038/clpt.1983.110. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Effects of ethanol on cigarette smoking by volunteers without histories of alcoholism. Psychopharmacology. 1984;82:1–5. doi: 10.1007/BF00426371. doi: 10.1007/BF00426371. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ. Acute alcohol tolerance in cognitive and psychomotor performance: Influence of the alcohol dose and prior alcohol experience. Alcohol. 1997;14:125–130. doi: 10.1016/s0741-8329(96)00115-2. doi:10.1016/S0741-8329(96)00115-2. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ. Acute alcohol tolerance in social drinkers: Changes in subjective effects dependent on the alcohol dose and prior alcohol experience. Alcohol. 1997;14:373–378. doi: 10.1016/s0741-8329(96)00186-3. doi:10.1016/S0741-8329(96)00186-3. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Kerr JS, Sherwood N. The effects of alcohol and other drugs on psychomotor performance and cognitive function. Alcohol and Alcoholism. 1991;26:71–79. Retrieved from http://alcalc.oxfordjournals.org/ [PubMed] [Google Scholar]
- Hodges H, Allen Y, Sinden J, Mitchell SN, Arendt T, Lantos PL, et al. The effects of cholinergic drugs and cholinergic-rich foetal neural transplants on alcohol-induced deficits in radial maze performance in rats. Behavioral Brain Research. 1991;43:7–28. doi: 10.1016/s0166-4328(05)80048-8. doi:10.1016/S0166-4328(05)80048-8. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcoholism: Clinical & Experimental Research. 2000;24:789–794. doi: 10.1111/j.1530-0277.2000.tb02057.x. [PubMed] [Google Scholar]
- Hu Z.-J., Bai L, Tizabi Y, Southerland W. Computational modeling study of human nicotinic acetylcholine receptor for developing new drugs in the treatment of alcoholism. Journal of Interdisciplinary Science: Computational Life Sciences. 2009;1:254–262. doi: 10.1007/s12539-009-0052-7. doi: 10.1007/s12539-009-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Rose GL, Callas PW. Nicotine is more reinforcing in smokers with a past history of alcoholism than in smokers without this history. Alcoholism: Clinical & Experimental Research. 2000;24:1633–1638. doi: 10.1111/j.1530-0277.2000.tb01964.x. [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: Onset, persistence and trajectories of use across two samples. Addiction. 2002;97:517–531. doi: 10.1046/j.1360-0443.2002.00082.x. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Park A. Drinking among college students. Consumption and consequences. Recent Developments in Alcoholism. 2005;17:85–117. doi: 10.1007/0-306-48626-1_5. Retrieved from http://www.springer.com/series/6939. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA. Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol and Alcoholism. 2006;41:486–493. doi: 10.1093/alcalc/agl049. doi:10.1093/alcalc/agl049. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Probabilities of alcohol high-risk drinking, abuse or dependence estimated on grounds of tobacco smoking and nicotine dependence. Addiction. 2003;98:805–814. doi: 10.1046/j.1360-0443.2003.00381.x. doi: 10.1046/j.1360-0443.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Sherwood N, Hindmarch I. Separate and combined effects of the social drugs on psychomotor performance. Psychopharmacology. 1991;104:113–119. doi: 10.1007/BF02244564. doi: 10.1007/BF02244564. [DOI] [PubMed] [Google Scholar]
- King A, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology (Berl) 2009;207:107–117. doi: 10.1007/s00213-009-1638-9. doi: 10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcoholism: Clinical & Experimental Research. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. doi:10.1097/01.ALC.0000158839.65251.FE. [DOI] [PubMed] [Google Scholar]
- Korkosz A, Scinska A, Taracha E, Plaznik A, Kukwa A, Kostowski W, et al. Nicotine-induced conditioned taste aversion in the rat: Effects of ethanol. European Journal of Pharmacology. 2006;537:99–105. doi: 10.1016/j.ejphar.2006.03.023. doi:10.1016/j.ejphar.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Korkosz A, Taracha E, Plaznik A, Wrobel E, Kostowski W, Bienkowski P. Extended blockade of the discriminative stimulus effects of nicotine with low doses of ethanol. European Journal of Pharmacology. 2005;512:165–172. doi: 10.1016/j.ejphar.2005.02.026. doi:10.1016/j.ejphar.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Korkosz A, Zatorski P, Taracha E, Plaznik A, Kostowski W, Bienkowski P. Ethanol blocks nicotine-induced seizures in mice: Comparison with midazolam and baclofen. Alcohol. 2006;40:151–157. doi: 10.1016/j.alcohol.2006.12.001. doi: 10.1016/j.alcohol.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol's acute effects in men. Drug and Alcohol Dependence. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. doi:10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Belger A, Kirino E, Gore J, McCarthy G. Ketamine effects on the cortical processing of novelty in humans assessed with fMRI. Society for Neuroscience Abstracts. 1998;24:104–107. [Google Scholar]
- Lezak M. Neuropsychological assessment. Oxford, UK: Oxford University Press; 1983. [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical & Experimental Research. 1993;93:8–15. doi: 10.1111/j.1530-0277.1993.tb00739.x. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status as a clinical indicator for alcohol misuse in US adults. Archives of Internal Medicine. 2007;167:716–721. doi: 10.1001/archinte.167.7.716. Retrieved from http://archinte.ama-assn.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, Shi J. Alcohol expectancy increases positive responses to cigarettes in young, escalating smokers. Psychopharmacology. 2010;210:355–364. doi: 10.1007/s00213-010-1831-x. doi: 10.1007/s00213-010-1831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: Interactions with alcohol use. Psychopharmacology. 1987;93:8–15. doi: 10.1007/BF02439579. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- Michel C, Battig K. Separate and combined psychophysiological effects of cigarette smoking and alcohol consumption. Psychopharmacology. 1989;97:65–73. doi: 10.1007/BF00443415. doi: 10.1007/BF00443415. [DOI] [PubMed] [Google Scholar]
- Mungas D. Differential clinical sensitivity of specific parameters of the Rey Auditory-Verbal Learning Test. Journal of Consulting & Clinical Psychology. 1983;51:848–855. doi: 10.1037//0022-006x.51.6.848. Retrieved from http://www.apa.org/pubs/journals/ccp/index.aspx. [DOI] [PubMed] [Google Scholar]
- National Advisory Council on Alcohol Abuse and Alcoholism. Recommended council guidelines on ethyl alcohol administration in human experimentation—Revised May 2005. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Retrieved from http://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm. [Google Scholar]
- Perkins KA, Fonte C, Grobe JE. Sex differences in the acute effects of cigarette smoking on the reinforcing value of alcohol. Behavioural Pharmacology. 2000;11:63–70. doi: 10.1097/00008877-200002000-00007. Retrieved from http://journals.lww.com/behaviouralpharm/pages/default.aspx. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, DiMarco A, Grobe JE, Scierka A, Stiller RL. Subjective and cardiovascular response to nicotine combined with alcohol in male and female smokers. Psychopharmacology (Berl) 1995;119:205–212. doi: 10.1007/BF02246162. doi: 10.1007/BF02246162. [DOI] [PubMed] [Google Scholar]
- Perrino A, Acampora G, Ralevski E, Limoncelli D, Edgecombe J, Petrakis I. Examining NMDA and GABA mechanisms using pain response paradigms with alcohol, ketamine and thiopental. Alcoholism: Clinical & Experimental Research. 2008;32:105. Retrieved from http://www.blackwellpublishing.com/journal.asp?ref=0145-6008. [Google Scholar]
- Prendergast ML, Podus D, Change E, Urada D. The effectiveness of drug abuse treatment: A meta-analysis of comparison group studies. Drug and Alcohol Dependence. 2002;67:53–72. doi: 10.1016/s0376-8716(02)00014-5. doi:10.1016/S0376-8716(02)00014-5. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcoholism: Clinical & Experimental Research. 1999;23:617–623. doi: 10.1111/j.1530-0277.1999.tb04163.x. [PubMed] [Google Scholar]
- Ramchandani VA, Flury L, Morzorati S, Kareken D, Blekher T, Foroud T, et al. Recent drinking history: Association with family history of alcoholism and the acute response to alcohol during a 60mg% clamp. Journal of Studies on Alcohol. 2002;63:734–744. doi: 10.15288/jsa.2002.63.734. Retrieved from http://www.jsad.com/ [DOI] [PubMed] [Google Scholar]
- Rey A. En l’examen clinique en psychologie. Paris, France: Presses Universitaries de France; 1964. [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-alcohol interactions and cognitive function in rats. Pharmacology, Biochemistry and Behavior. 2002;72:865–872. doi: 10.1016/s0091-3057(02)00762-1. doi:10.1016/S0091-3057(02)00762-1. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine & Tobacco Research. 2004;6:133–144. doi: 10.1080/14622200310001656957. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-verbal Learning Test performance of patients with and without memory impairment. Journal of Clinical Psychiatry. 1984;40:785–787. doi: 10.1002/1097-4679(198405)40:3<785::aid-jclp2270400325>3.0.co;2-4. doi: 10.1002/1097-4679(198405)40:3<785::AID-JCLP2270400325>3.0.CO;2–4. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Geisser ME, Randall DM, Georgemiller RJ. Alternate form reliability and equivalency of the Rey Auditory Verbal Learning Test. Journal of Clinical and Experimental Neuropsychology. 1986;8:611–616. doi: 10.1080/01688638608405179. doi: 10.1080/01688638608405179. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Harrison D. Alternate forms of the AVLT: A procedure and test of form equivalency. Archives of Clinical Neuropsychology. 1990;5:405–410. doi: 10.1016/0887-6177(90)90018-K. [PubMed] [Google Scholar]
- Shiffman S. Tobacco “chippers”—Individual differences in tobacco dependence. Psychopharmacology. 1989;97:539–547. doi: 10.1007/BF00439561. doi: 10.1007/BF00439561. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA. Smoking patterns and dependence: Contrasting chippers and heavy smokers. Journal of Abnormal Psychology. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: Subjective and cognitive effects. Health Psychology. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. Retrieved from http://www.health-psych.org/ [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb D, Hatsukami DK. Labetalol treatment enhances the attention of tobacco withdrawal symptoms by nicotine in abstinent smokers. Nicotine & Tobacco Research. 2003;5:947–953. doi: 10.1080/14622200310001615312. doi: 10.1080/14622200310001615312. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Yoo S, Culligan K, Kosten T. Effects of tiagabine in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2005;181:504–510. doi: 10.1007/s00213-005-0010-y. doi 10.1007/s00213-005-0010-y. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Mouratidis M, Kosten T. Effects of topiramate in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2006;184:645–651. doi: 10.1007/s00213-005-0296-9. doi: 10.1007/s00213-005-0296-9. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M, Heil S, Kruger M, Collins K, Buck P, Zawacki T, et al. A three-stage alcohol clamp procedure in human subjects. Alcoholism: Clinical & Experimental Research. 2002;26:1479–1483. doi: 10.1097/01.ALC.0000034038.41972.36. doi: 10.1111/j.1530-0277.2002.tb02446.x. [DOI] [PubMed] [Google Scholar]
- Taslim N, Al-Rejaie S, Dar MS. Attenuation of ethanol-induced ataxia by alpha(4)beta(2) nicotinic acetylcholine receptor subtype in mouse cerebellum: A functional interaction. Neuroscience. 2008;157:204–213. doi: 10.1016/j.neuroscience.2008.08.046. doi:10.1016/j.neuroscience.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Taslim N, Dar MS. The role of nicotinic acetylcholine receptor (nAChR) α7 subtype in the functional interaction between nicotine and ethanol in mouse cerebellum. Alcoholism: Clinical & Experimental Research. 2011;35:540–549. doi: 10.1111/j.1530-0277.2010.01371.x. doi: 10.1111/j.1530-0277.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Manaye KF, Taylor RE. Nicotine blocks ethanol-induced apoptosis in primary cultures of rat cerebral cortical and cerebellar granule cells. Neurotoxicity Research. 2005;7:319–322. doi: 10.1007/BF03033888. doi: 10.1007/BF03033888. [DOI] [PubMed] [Google Scholar]
- Tracy HAJ, Wayner MJ, Armstrong DL. Nicotine blocks ethanol and diazepam impairment of air righting and ethanol impairment of maze performance. Alcohol. 1999;18:123–130. doi: 10.1016/s0741-8329(98)00074-3. doi:10.1016/S0741-8329(98)00074-3. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. State-dependent effects of alcohol on explicit memory: The role of semantic associations. Psychopharmacology (Berl) 2000;149:98–106. doi: 10.1007/s002139900349. doi: 10.1007/s002139900349. [DOI] [PubMed] [Google Scholar]
- Weissenborn T, Duka T. Acute alcohol effects on cognitive function in social drinkers: Their relationship to drinking habits. Psychopharmacology (Berl) 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Weitzman ER, Chen YY. The co-occurrence of smoking and drinking among young adults in college: National survey results from the United States. Drug and Alcohol Dependence. 2005;80:377–386. doi: 10.1016/j.drugalcdep.2005.05.008. doi:10.1016/j.drugalcdep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Action of ethanol on responses to nicotine from cerebellar Purkinje neurons: Relationship to methyllycaconitine (MLA) inhibition of nicotine responses. Neurochemistry International. 1999;35:185–194. doi: 10.1016/s0197-0186(99)00060-1. doi:10.1016/S0197-0186(99)00060-1. [DOI] [PubMed] [Google Scholar]


