The nine-membered ring system of the title compound is essentially planar. In the crystal, molecules are linked via C—HTrz⋯NTrz and C—HPyrm⋯NTrz (Trz = triazole and Pyrm = pyrimidine) hydrogen bonds together with weaker C—HPyrm⋯NPyrm hydrogen bonds to form layers parallel to ( 02). The layers are further connected by π–π-stacking interactions between the nine-membered ring system, forming oblique stacks along the a-axis direction.
02). The layers are further connected by π–π-stacking interactions between the nine-membered ring system, forming oblique stacks along the a-axis direction.
Keywords: crystal structure, triazole, pyrimidine, hydrogen bond, π⋯π-stacking, Hirshfeld surface analysis
Abstract
The nine-membered ring system of the title compound, C6H6N4, is essentially planar. In the crystal, molecules are linked via C—HTrz⋯NTrz and C—HPyrm⋯NTrz (Trz = triazole and Pyrm = pyrimidine) hydrogen bonds together with weaker C—HPyrm⋯NPyrm hydrogen bonds to form layers parallel to ( 02). The layers are further connected by π–π-stacking interactions between the nine-membered ring system [centroid–centroid = 3.7910 (8) Å], forming oblique stacks along the a-axis direction. The Hirshfeld surface analysis of the crystal structure indicates that the most important contributions for the crystal packing are from H⋯N/N⋯H (40.1%), H⋯H (35.3%), H⋯C/C⋯H (9.5%), N⋯C/C⋯N (9.0%), N⋯N (3.1%) and C⋯C (3.0%) interactions and that hydrogen-bonding and van der Waals interactions are the dominant interactions in the crystal packing. No significant C—H⋯π interactions are observed.
02). The layers are further connected by π–π-stacking interactions between the nine-membered ring system [centroid–centroid = 3.7910 (8) Å], forming oblique stacks along the a-axis direction. The Hirshfeld surface analysis of the crystal structure indicates that the most important contributions for the crystal packing are from H⋯N/N⋯H (40.1%), H⋯H (35.3%), H⋯C/C⋯H (9.5%), N⋯C/C⋯N (9.0%), N⋯N (3.1%) and C⋯C (3.0%) interactions and that hydrogen-bonding and van der Waals interactions are the dominant interactions in the crystal packing. No significant C—H⋯π interactions are observed.
Chemical context
In recent years, much attention has been paid to the development of new methods for the synthesis and investigation of biological and pharmacological properties of [1,2,4]triazolo[1,5-a]pyrimidine derivatives (Chebanov et al., 2010 ▸; Lahmidi et al., 2016a
▸,b
▸, 2018 ▸; Sedash et al., 2012 ▸). Thus, these compounds have also received successful applications for the preparation of new poly-condensed heterocycles (Beck et al., 2011 ▸). Among the various classes of nitrogen-containing heterocyclic compounds such as triazolopyrimidine derivatives display a broad spectrum of biological activities, including anti-inflammatory (Ashour et al., 2013 ▸), anticancer (Hoffmann et al., 2017 ▸) and antibacterial (Mabkhot et al., 2016 ▸) activities. In a continuation of our research on the elaboration of new methods for the synthesis of various heterocyclic systems, we investigated the reaction of bis(2-chloroethyl)amine hydrochloride with ethyl 2-(5-methyl-1-1,2,4-triazolo[1,5-a]pyrimidin-7-yl)acetate under phase-transfer catalysis conditions using tetra-n-butyl ammoniumbromide (TBAB) as catalyst and potassium carbonate as base to afford the title compound, 5-methyl-1,2,4-triazolo[1,5-a]pyrimidine, (I). We report herein its molecular and crystal structures along with the results of a Hirshfeld surface analysis.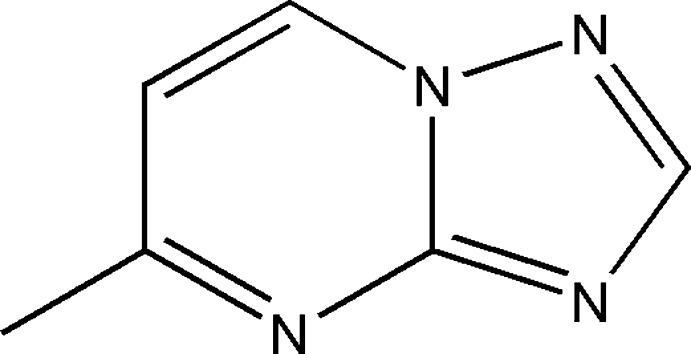
Structural commentary
In the title compound (Fig. 1 ▸), the nine-membered ring is planar to within 0.004 (1) Å (for atom C5), and the r.m.s. deviation of the fitted atoms is 0.009 Å. Methyl atom C6 is displaced by 0.032 (1) Å from the ring system.
Figure 1.
The title molecule with the atom-labelling scheme and 50% probability ellipsoids.
Supramolecular features
In the crystal, C—HTrz⋯NTrz and C—HPyrm⋯NTrz (Trz = triazole and Pyrm = pyrimidine) hydrogen bonds (Table 1 ▸), together with weaker C—HPyrm⋯NPyrm hydrogen bonds, link the molecules, forming layers parallel to ( 02) (Fig. 2 ▸). The layers are further connected by π–π-stacking interactions between the nine-membered rings [centroid–centroid distance = 3.7910 (8) Å], forming oblique stacks along the a-axis direction (Fig. 3 ▸). No significant C—H ⋯ π interactions are observed.
02) (Fig. 2 ▸). The layers are further connected by π–π-stacking interactions between the nine-membered rings [centroid–centroid distance = 3.7910 (8) Å], forming oblique stacks along the a-axis direction (Fig. 3 ▸). No significant C—H ⋯ π interactions are observed.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯N1i | 1.016 (17) | 2.550 (19) | 3.4052 (18) | 141.5 (13) |
| C3—H3⋯N2vi | 0.979 (18) | 2.525 (18) | 3.4822 (18) | 165.8 (13) |
| C4—H4⋯N4viii | 0.946 (19) | 2.642 (19) | 3.5677 (17) | 165.9 (14) |
Symmetry codes: (i)  ; (vi)
; (vi)  ; (viii)
; (viii)  .
.
Figure 2.
The packing viewed along the a-axis direction giving a plan view of the layers. C—H⋯N hydrogen bonds are shown as black dashed lines and the orange dots mark the π–π stacking interactions.
Figure 3.
Packing seen along the b-axis direction giving a side view of the layers. Hydrogen bonds are depicted as in Fig. 2 ▸ and the π-stacking interactions are shown as orange dashed lines.
Hirshfeld surface analysis
In order to visualize the intermolecular interactions in the crystal of the title compound, a Hirshfeld surface (HS) analysis (Hirshfeld, 1977 ▸; Spackman & Jayatilaka, 2009 ▸) was carried out using CrystalExplorer17.5 (Turner et al., 2017 ▸). In the HS plotted over d norm (Fig. 4 ▸), the white surface indicates contacts with distances equal to the sum of the van der Waals radii, and the red and blue colours indicate distances shorter (in close contact) or longer (distinct contact), respectively, than the van der Waals radii (Venkatesan et al., 2016 ▸). The bright-red spots appearing near N2 and hydrogen atoms H2, H3 and H4 indicate their roles as the respective donors and/or acceptors in the dominant C—H⋯N hydrogen bonds; they also appear as blue and red regions corresponding to positive and negative potentials on the HS mapped over electrostatic potential (Spackman et al., 2008 ▸; Jayatilaka et al., 2005 ▸) as shown in Fig. 5 ▸. The blue regions indicate positive electrostatic potential (hydrogen-bond donors), while the red regions indicate negative electrostatic potential (hydrogen-bond acceptors). The shape-index of the HS is a tool to visualize π–π stacking by the presence of adjacent red and blue triangles; if there are no adjacent red and/or blue triangles, then there are no π–π interactions. Fig. 6 ▸ clearly suggest that there are π–π interactions present in the crystal structure of (I).
Figure 4.
View of the three-dimensional Hirshfeld surface of the title compound plotted over d norm in the range −0.1566 to 1.0057 a.u.
Figure 5.
View of the three-dimensional Hirshfeld surface of the title compound plotted over electrostatic potential energy in the range −0.0500 to 0.0500 a.u. using the STO-3 G basis set at the Hartree–Fock level of theory. Hydrogen-bond donors and acceptors are shown as blue and red regions around the atoms corresponding to positive and negative potentials, respectively.
Figure 6.
Hirshfeld surface of the title compound plotted over shape-index.
The overall two-dimensional fingerprint plot, Fig. 7 ▸(a), and those delineated into H⋯N/N⋯H, H⋯H, H⋯C/C⋯H, N⋯C/C⋯N, N⋯N and C⋯C contacts (McKinnon et al., 2007 ▸) are illustrated in Fig. 7 ▸(b)–(g), respectively, together with their relative contributions to the Hirshfeld surface. The most important interaction is H⋯N/N⋯H, contributing 40.1% to the overall crystal packing, which is reflected in Fig. 7 ▸(b) as a pair of characteristic wings with the tips at d e + d i = 2.40 Å arising from the C—H⋯N hydrogen bonds (Table 1 ▸) as well as from the H⋯N/N⋯H contacts (Table 3 ▸). The split thin and thick pair of wings with the tips at d e + d i ∼2.23 Å in Fig. 7 ▸(c), arise from the short interatomic H⋯H contacts, which make a 35.3% contribution to the HS and are seen as widely scattered points of high density arising from the large hydrogen content of the molecule. In the absence of C—H⋯π interactions, the pair of wings in the fingerprint plot delineated into H⋯C/C⋯H contacts (9.5% contribution to the HS) have a nearly symmetrical distribution of points, Fig. 7 ▸(d), with the tips at d e + d i ∼2.77 Å. The N⋯C/C⋯N [Fig. 7 ▸(e)] and N⋯N [Fig. 7 ▸(f)] contacts make contributions of 9.0 and 3.1%, respectively, to the HS and have widely scattered distributions of points. Finally, the C⋯C [Fig. 7 ▸(g)] contacts (3.0% contribution to the HS) have a symmetrical distribution of points, with the tip at d e = d i = 1.69 Å.
Figure 7.
The full two-dimensional fingerprint plots for the title compound, showing (a) all interactions, and delineated into (b) H⋯N/N⋯H, (c) H⋯H, (d) H⋯C/C⋯H, (e) N⋯C/C⋯N, (f) N⋯N and (g) C⋯C interactions. The d i and d e values are the closest internal and external distances (in Å) from given points on the Hirshfeld surface contacts.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | C6H6N4 |
| M r | 134.15 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 150 |
| a, b, c (Å) | 3.7910 (2), 18.0092 (10), 9.0069 (5) |
| β (°) | 101.704 (2) |
| V (Å3) | 602.14 (6) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 0.82 |
| Crystal size (mm) | 0.29 × 0.18 × 0.13 |
| Data collection | |
| Diffractometer | Bruker D8 VENTURE PHOTON 100 CMOS |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.67, 0.90 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 4567, 1205, 1102 |
| R int | 0.074 |
| (sin θ/λ)max (Å−1) | 0.626 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.044, 0.113, 1.10 |
| No. of reflections | 1205 |
| No. of parameters | 116 |
| H-atom treatment | All H-atom parameters refined |
| Δρmax, Δρmin (e Å−3) | 0.20, −0.20 |
The Hirshfeld surface representations with the function d norm plotted onto the surface are shown for the H⋯N/N⋯H, H⋯H, H⋯C/C⋯H, N ⋯ C/C⋯N, N⋯N and C⋯C interactions in Fig. 8 ▸(a)–(f), respectively.
Figure 8.
The Hirshfeld surface representations with the function d norm plotted onto the surface for (a) H⋯N/N⋯H, (b) H⋯H, (c) H⋯C/C⋯H, (d) N⋯C/C⋯N, (e) N⋯N and (f) C⋯C interactions.
The Hirshfeld surface analysis confirms the importance of H-atom contacts in establishing the packing. The large number of H⋯N/N⋯H, H⋯H and H⋯C/C⋯H interactions suggest that van der Waals interactions and hydrogen bonding play the major roles in the crystal packing (Hathwar et al., 2015 ▸).
Database survey
Two structures have previously been reported in which the title compound, (I), is present as a ligand (L), namely [Fe(L)2(SCN)2(H2O)2] (Bigini Cingi et al., 1986 ▸) and [Cu(μ-L)2(SCN)]n (Cornelissen et al., 1989 ▸), but to the best of our knowledge, the molecule itself has not previously been structurally characterized.
Synthesis and crystallization
To a solution of ethyl-2-{5-methyl-1-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl}acetate (1.00 g, 4.5 mmol) in DMF (25 ml) was added 2eq of bis(2-chloroethyl)amine hydrochloride (1.61g, 9 mmol), potassium carbonate (1.37 g, 9.9 mmol) and a catalytic amount of tetra-n-butylammonium bromide. The mixture was stirred at 353.15 K for 24 h. The solution was filtered and the solvent was removed under reduced pressure. The residue obtained was dissolved in dichloromethane and purified by column chromatography (EtOAc/Hexane, 1:9 v:v). The title compound was obtained as colourless crystals in 40% yield.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. H atoms were located in a difference Fourier map and were freely refined.
Table 2. Selected interatomic distances (Å).
| N1⋯C2i | 3.4051 (19) | C1⋯C4iii | 3.5667 (19) |
| N2⋯C2ii | 3.385 (2) | C2⋯C6vii | 3.5715 (18) |
| N3⋯C3iii | 3.4163 (19) | C2⋯C2i | 3.595 (2) |
| N4⋯C5iii | 3.4314 (17) | C4⋯C5ii | 3.4986 (19) |
| N4⋯C4iii | 3.4177 (19) | C1⋯H6B iv | 2.94 (3) |
| N1⋯H6B iv | 2.85 (2) | C6⋯H6C iii | 2.98 (3) |
| N1⋯H2i | 2.553 (18) | H2⋯C6vii | 2.773 (16) |
| N1⋯H6C v | 2.86 (3) | H2⋯H6B vii | 2.58 (3) |
| N2⋯H3vi | 2.525 (18) | H2⋯H6C vii | 2.48 (3) |
| N4⋯H4v | 2.641 (18) | H6A⋯H4v | 2.59 (3) |
| N4⋯H6B iv | 2.84 (3) | H6B⋯H6C iii | 2.47 (4) |
| C1⋯C3iii | 3.4166 (19) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  ; (vii)
; (vii)  .
.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989018016225/lh5886sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018016225/lh5886Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989018016225/lh5886Isup3.cdx
Supporting information file. DOI: 10.1107/S2056989018016225/lh5886Isup4.cml
CCDC reference: 1879279
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| C6H6N4 | F(000) = 280 |
| Mr = 134.15 | Dx = 1.480 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54178 Å |
| a = 3.7910 (2) Å | Cell parameters from 3969 reflections |
| b = 18.0092 (10) Å | θ = 4.9–74.7° |
| c = 9.0069 (5) Å | µ = 0.82 mm−1 |
| β = 101.704 (2)° | T = 150 K |
| V = 602.14 (6) Å3 | Column, colourless |
| Z = 4 | 0.29 × 0.18 × 0.13 mm |
Data collection
| Bruker D8 VENTURE PHOTON 100 CMOS diffractometer | 1205 independent reflections |
| Radiation source: INCOATEC IµS micro-focus source | 1102 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.074 |
| Detector resolution: 10.4167 pixels mm-1 | θmax = 74.7°, θmin = 4.9° |
| ω scans | h = −4→4 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −22→21 |
| Tmin = 0.67, Tmax = 0.90 | l = −11→10 |
| 4567 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.044 | All H-atom parameters refined |
| wR(F2) = 0.113 | w = 1/[σ2(Fo2) + (0.0458P)2 + 0.1643P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.10 | (Δ/σ)max < 0.001 |
| 1205 reflections | Δρmax = 0.20 e Å−3 |
| 116 parameters | Δρmin = −0.20 e Å−3 |
| 0 restraints | Extinction correction: SHELXL2018 (Sheldrick, 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.021 (4) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.7561 (3) | 0.42236 (6) | 0.88570 (13) | 0.0322 (3) | |
| N2 | 0.4407 (3) | 0.49411 (6) | 0.69436 (15) | 0.0341 (3) | |
| N3 | 0.3764 (3) | 0.42019 (6) | 0.66430 (13) | 0.0281 (3) | |
| N4 | 0.5532 (3) | 0.30294 (6) | 0.77958 (13) | 0.0277 (3) | |
| C1 | 0.5676 (3) | 0.37780 (7) | 0.78059 (15) | 0.0269 (3) | |
| C2 | 0.6678 (4) | 0.49082 (7) | 0.82759 (17) | 0.0340 (4) | |
| H2 | 0.776 (5) | 0.5371 (9) | 0.883 (2) | 0.037 (4)* | |
| C3 | 0.1587 (4) | 0.38918 (7) | 0.54119 (15) | 0.0313 (3) | |
| H3 | 0.025 (5) | 0.4230 (9) | 0.465 (2) | 0.037 (4)* | |
| C4 | 0.1409 (4) | 0.31397 (7) | 0.53851 (16) | 0.0302 (3) | |
| H4 | −0.006 (5) | 0.2889 (10) | 0.456 (2) | 0.039 (4)* | |
| C5 | 0.3442 (3) | 0.27179 (7) | 0.66009 (15) | 0.0279 (3) | |
| C6 | 0.3279 (4) | 0.18893 (7) | 0.65408 (19) | 0.0344 (4) | |
| H6A | 0.462 (6) | 0.1656 (13) | 0.749 (3) | 0.066 (6)* | |
| H6B | 0.431 (6) | 0.1705 (12) | 0.576 (3) | 0.071 (7)* | |
| H6C | 0.095 (7) | 0.1716 (12) | 0.629 (3) | 0.073 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0381 (6) | 0.0233 (5) | 0.0321 (6) | −0.0020 (4) | 0.0000 (5) | −0.0010 (4) |
| N2 | 0.0434 (7) | 0.0197 (5) | 0.0367 (6) | −0.0011 (4) | 0.0026 (5) | 0.0000 (4) |
| N3 | 0.0324 (6) | 0.0222 (5) | 0.0281 (6) | −0.0001 (4) | 0.0024 (5) | 0.0005 (4) |
| N4 | 0.0311 (6) | 0.0221 (5) | 0.0289 (6) | −0.0004 (4) | 0.0040 (5) | −0.0003 (4) |
| C1 | 0.0297 (6) | 0.0224 (6) | 0.0280 (7) | 0.0001 (4) | 0.0041 (5) | 0.0011 (4) |
| C2 | 0.0414 (8) | 0.0221 (6) | 0.0360 (8) | −0.0023 (5) | 0.0020 (6) | −0.0019 (5) |
| C3 | 0.0337 (7) | 0.0304 (7) | 0.0281 (7) | 0.0005 (5) | 0.0020 (5) | 0.0005 (5) |
| C4 | 0.0319 (7) | 0.0289 (7) | 0.0284 (7) | −0.0030 (5) | 0.0026 (5) | −0.0029 (5) |
| C5 | 0.0284 (6) | 0.0250 (6) | 0.0308 (7) | −0.0016 (5) | 0.0074 (5) | −0.0022 (5) |
| C6 | 0.0386 (8) | 0.0244 (7) | 0.0393 (8) | −0.0022 (5) | 0.0058 (7) | −0.0043 (5) |
Geometric parameters (Å, º)
| N1—C1 | 1.3329 (17) | C3—C4 | 1.3560 (18) |
| N1—C2 | 1.3545 (17) | C3—H3 | 0.979 (18) |
| N2—C2 | 1.329 (2) | C4—C5 | 1.4241 (19) |
| N2—N3 | 1.3703 (15) | C4—H4 | 0.946 (19) |
| N3—C3 | 1.3607 (17) | C5—C6 | 1.4940 (18) |
| N3—C1 | 1.3775 (17) | C6—H6A | 1.00 (2) |
| N4—C5 | 1.3245 (17) | C6—H6B | 0.94 (3) |
| N4—C1 | 1.3492 (17) | C6—H6C | 0.92 (3) |
| C2—H2 | 1.016 (17) | ||
| N1···C2i | 3.4051 (19) | C1···C4iii | 3.5667 (19) |
| N2···C2ii | 3.385 (2) | C2···C6vii | 3.5715 (18) |
| N3···C3iii | 3.4163 (19) | C2···C2i | 3.595 (2) |
| N4···C5iii | 3.4314 (17) | C4···C5ii | 3.4986 (19) |
| N4···C4iii | 3.4177 (19) | C1···H6Biv | 2.94 (3) |
| N1···H6Biv | 2.85 (2) | C6···H6Ciii | 2.98 (3) |
| N1···H2i | 2.553 (18) | H2···C6vii | 2.773 (16) |
| N1···H6Cv | 2.86 (3) | H2···H6Bvii | 2.58 (3) |
| N2···H3vi | 2.525 (18) | H2···H6Cvii | 2.48 (3) |
| N4···H4v | 2.641 (18) | H6A···H4v | 2.59 (3) |
| N4···H6Biv | 2.84 (3) | H6B···H6Ciii | 2.47 (4) |
| C1···C3iii | 3.4166 (19) | ||
| C1—N1—C2 | 102.64 (11) | N3—C3—H3 | 117.3 (10) |
| C2—N2—N3 | 101.05 (10) | C3—C4—C5 | 120.13 (12) |
| C3—N3—N2 | 127.88 (11) | C3—C4—H4 | 120.6 (11) |
| C3—N3—C1 | 122.05 (11) | C5—C4—H4 | 119.3 (11) |
| N2—N3—C1 | 110.07 (11) | N4—C5—C4 | 122.68 (12) |
| C5—N4—C1 | 116.45 (11) | N4—C5—C6 | 117.78 (12) |
| N1—C1—N4 | 128.43 (12) | C4—C5—C6 | 119.54 (12) |
| N1—C1—N3 | 109.26 (11) | C5—C6—H6A | 112.4 (14) |
| N4—C1—N3 | 122.30 (12) | C5—C6—H6B | 111.1 (13) |
| N2—C2—N1 | 116.97 (12) | H6A—C6—H6B | 106.4 (19) |
| N2—C2—H2 | 122.2 (10) | C5—C6—H6C | 112.2 (14) |
| N1—C2—H2 | 120.8 (10) | H6A—C6—H6C | 111.5 (19) |
| C4—C3—N3 | 116.38 (12) | H6B—C6—H6C | 103 (2) |
| C4—C3—H3 | 126.3 (10) | ||
| C2—N2—N3—C3 | 179.68 (13) | N3—N2—C2—N1 | 0.14 (17) |
| C2—N2—N3—C1 | 0.03 (14) | C1—N1—C2—N2 | −0.24 (17) |
| C2—N1—C1—N4 | 179.97 (13) | N2—N3—C3—C4 | −179.91 (12) |
| C2—N1—C1—N3 | 0.24 (14) | C1—N3—C3—C4 | −0.30 (18) |
| C5—N4—C1—N1 | −179.67 (12) | N3—C3—C4—C5 | −0.18 (19) |
| C5—N4—C1—N3 | 0.04 (17) | C1—N4—C5—C4 | −0.53 (17) |
| C3—N3—C1—N1 | −179.85 (12) | C1—N4—C5—C6 | 178.84 (11) |
| N2—N3—C1—N1 | −0.18 (14) | C3—C4—C5—N4 | 0.6 (2) |
| C3—N3—C1—N4 | 0.39 (18) | C3—C4—C5—C6 | −178.74 (13) |
| N2—N3—C1—N4 | −179.93 (11) |
Symmetry codes: (i) −x+2, −y+1, −z+2; (ii) x−1, y, z; (iii) x+1, y, z; (iv) x, −y+1/2, z+1/2; (v) x+1, −y+1/2, z+1/2; (vi) −x, −y+1, −z+1; (vii) −x+1, y+1/2, −z+3/2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···N1i | 1.016 (17) | 2.550 (19) | 3.4052 (18) | 141.5 (13) |
| C3—H3···N2vi | 0.979 (18) | 2.525 (18) | 3.4822 (18) | 165.8 (13) |
| C4—H4···N4viii | 0.946 (19) | 2.642 (19) | 3.5677 (17) | 165.9 (14) |
Symmetry codes: (i) −x+2, −y+1, −z+2; (vi) −x, −y+1, −z+1; (viii) x−1, −y+1/2, z−1/2.
Funding Statement
This work was funded by National Science Foundation grant 1228232. Tulane University grant . Hacettepe University Scientific Research Project Unit grant 013 D04 602 004 to Tuncer Hökelek.
References
- Ashour, H., Shaaban, O., Rizk, O. & El-Ashmawy, I. M. (2013). Eur. J. Med. Chem. 62, 341–351. [DOI] [PubMed]
- Beck, H. P., DeGraffenreid, M., Fox, B., Allen, J. G., Rew, Y., Schneider, S., Saiki, A. Y., Yu, D., Oliner, J. D., Salyers, K., Ye, Q. & Olson, S. (2011). Bioorg. Med. Chem. Lett. 21, 2752–2755. [DOI] [PubMed]
- Biagini Cingi, M., Manotti Lanfredi, A. M., Tiripicchio, A., Cornelissen, J. P., Haasnoot, J. G. & Reedijk, J. (1986). Acta Cryst. C42, 1296–1298.
- Brandenburg, K. & Putz, H. (2012). DIAMOND, Crystal Impact GbR, Bonn, Germany.
- Bruker (2016). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chebanov, V. A., Gura, K. A. & Desenko, S. M. (2010). Top. Heterocycl. Chem. 23, 41–84.
- Cornelissen, J. P., De Graaff, R. A. G., Haasnoot, J. G., Prins, R., Reedijk, J., Biagini-Cingi, M., Manotti-Lanfredi, A. M. & Tiripicchio, A. (1989). Polyhedron, 8, 2313–2320.
- Hathwar, V. R., Sist, M., Jørgensen, M. R. V., Mamakhel, A. H., Wang, X., Hoffmann, C. M., Sugimoto, K., Overgaard, J. & Iversen, B. B. (2015). IUCrJ, 2, 563–574. [DOI] [PMC free article] [PubMed]
- Hirshfeld, H. L. (1977). Theor. Chim. Acta, 44, 129–138.
- Hoffmann, K., Wiśniewska, J., Wojtczak, A., Sitkowski, J., Denslow, A., Wietrzyk, J., Jakubowski, M. & Łakomska, I. (2017). J. Inorg. Biochem. 172, 34–45. [DOI] [PubMed]
- Jayatilaka, D., Grimwood, D. J., Lee, A., Lemay, A., Russel, A. J., Taylor, C., Wolff, S. K., Cassam-Chenai, P. & Whitton, A. (2005). TONTO - A System for Computational Chemistry. Available at: http://hirshfeldsurface.net/
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Lahmidi, S., El Hafi, M., Moussaif, A., Benchidmi, M., Essassi, E. M. & Mague, J. T. (2018). IUCrData, 3, x181280.
- Lahmidi, S., Sebbar, N. K., Boulhaoua, M., Essassi, E. M., Mague, J. T. & Zouihri, H. (2016a). IUCrData, 1, x160870.
- Lahmidi, S., Sebbar, N. K., Harmaoui, A., Ouzidan, Y., Essassi, E. M. & Mague, J. T. (2016b). IUCrData, 1, x161946.
- Mabkhot, Y. N., Alatibi, F., El-Sayed, N. N. E., Kheder, N. A. & Al-Showiman, S. (2016). Molecules, 21, 1036–1045. [DOI] [PMC free article] [PubMed]
- McKinnon, J. J., Jayatilaka, D. & Spackman, M. A. (2007). Chem. Commun. pp. 3814. [DOI] [PubMed]
- Sedash, Y. V., Gorobets, N. Y., Chebanov, V. A., Konovalova, I. S., Shishkin, O. V. & Desenko, S. M. (2012). RSC Adv. 2, 6719–6728.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, M. A. & Jayatilaka, D. (2009). CrystEngComm, 11, 19–32.
- Spackman, M. A., McKinnon, J. J. & Jayatilaka, D. (2008). CrystEngComm, 10, 377–388.
- Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D. & Spackman, M. A. (2017). CrystalExplorer17. The University of Western Australia.
- Venkatesan, P., Thamotharan, S., Ilangovan, A., Liang, H. & Sundius, T. (2016). Spectrochim. Acta Part A, 153, 625–636. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989018016225/lh5886sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018016225/lh5886Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989018016225/lh5886Isup3.cdx
Supporting information file. DOI: 10.1107/S2056989018016225/lh5886Isup4.cml
CCDC reference: 1879279
Additional supporting information: crystallographic information; 3D view; checkCIF report










