Abstract
Background
Fungal infections of the central nervous system (FICNS) are important causes of morbidity and mortality among immunocompromised pediatric patients. Standard diagnostic modalities lack the sensitivity for detecting and therapeutically monitoring these life-threatening diseases. Current molecular methods remain investigational. (1 → 3)-β- d -glucan (BDG) is a cell wall component found in several fungal pathogens, including Candida and Aspergillus spp. Detecting BDG in cerebrospinal fluid (CSF) may be an important approach for detecting and therapeutically monitoring FICNS. To date, there has been no study that has investigated the effectiveness of CSF BDG as a diagnostic and therapeutic marker of FICNS in children.
Methods
Serial BDG levels were measured in serum and CSF samples obtained from pediatric patients (aged 0–18 years) with a diagnosis of probable or proven Candida or Aspergillus CNS infection.
Results
Nine cases of FICNS were identified in patients aged 1 month to 18 years. Two patients were infected with an Aspergillus species, and 7 patients were infected with a Candida species. All the patients at baseline had detectable BDG in their CSF. Among 7 patients who completed therapy for an FICNS, all elevated CSF BDG levels decreased to <31 pg/mL. At the time of this writing, 1 patient was still receiving therapy and continued to have elevated BDG levels. One patient died from overwhelming disseminated candidiasis. The lengths of therapy for these 9 children ranged from 2 weeks to 28 months.
Conclusion
The BDG assay is useful in diagnosing and therapeutically monitoring Candida and Aspergillus CNS infections in pediatric patients.
Keywords: (1 → 3)-β- d -glucan , children, central nervous system, fungal infection
INTRODUCTION
Fungal infections of the central nervous system (FICNS) are important causes of morbidity and mortality among immunocompromised pediatric patients [ 1–4 ]. Hematogenous Candida meningoencephalitis (HCME) is a life-threatening infection in pediatric patients that is associated with seizures, intraventricular hemorrhage, cortical blindness, neurocognitive impairment, and the loss of developmental milestones [ 5 , 6 ]. Early diagnosis of HCME is difficult, and recurrence after the completion of antifungal therapy is common. Aspergillosis of the central nervous system (ACNS) is similarly difficult to diagnose and fraught with significant mortality rates, reported to be as high as 100% [ 7 ]. Morbidities associated with ACNS include hemorrhagic infarction, ventriculitis, meningitis, and subarachnoid hemorrhage.
Conventional culture-based approaches to testing cerebrospinal fluid (CSF) lack sensitivity for diagnosing and therapeutically monitoring these life-threatening diseases [ 8 , 9 ]. Current molecular methods remain investigational; however, fungal cell wall biomarkers such as (1→3)-β- d -glucan may provide an important approach for the detection and therapeutic monitoring of FICNS. Therefore, there is a critical need for understanding biomarkers that may be robustly used for the detection and therapeutic monitoring of CNS mycoses. The carbohydrate polymer (1→3)-β- d -glucan, which is expressed in the cell walls of Candida spp. and Aspergillus spp., has been widely used for diagnosing candidemia and invasive pulmonary aspergillosis, respectively [ 10–14 ]. However, little is known about the potential utility of (1→3)-β- d -glucan in diagnosing HCME and ACNS.
Our laboratory animal model of HCME demonstrated a high level of sensitivity and wide dynamic range of (1→3)-β- d -glucan in CSF, which has important implications for clinically diagnosing and monitoring the therapeutic response in patients with FICNS [ 15 , 16 ]. We therefore hypothesized that (1→3)-β- d -glucan in CSF has potential utility for detecting and therapeutically monitoring HCME and ACNS in immunocompromised pediatric patients. Herein, we present the cases of 9 patients in whom (1→3)-β- d -glucan was used for both detecting and therapeutically monitoring the management of HCME and ACNS.
PATIENTS AND METHODS
We conducted a multicenter retrospective study that included chart reviews of pediatric subjects, aged birth to 18 years, who had neurologic symptoms, who had a working diagnosis of HCME or ACNS, and whose CSF was evaluated for the detection of (1→3)-β- d -glucan. Patients were hospitalized at the Miller Children's and Women's Hospital Long Beach or the Weill Cornell Medical College–New York-Presbyterian Hospital between August 2009 and July 2012. The clinical study protocol for data collection was reviewed and approved by each institution's institutional review board.
Data Collection
Data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the Weill Cornell Medical College [ 17 ]. For each subject, we collected the following data: demographic characteristics, any underlying conditions, clinical presentation, diagnostic investigations (including cultures of blood, urine, tissue, and CSF), site of isolation, serial serum and CSF levels of (1→3)-β- d -glucan, diagnostic imaging, antimicrobial therapy, clinical course, and outcome.
Definitions
We defined “proven FICNS” as a positive CSF culture result or CNS tissue that yielded a pathogenic fungus, and “probable FICNS” was defined as a positive culture of a pathogenic fungus from a normally sterile site in a patient with clinical or radiological evidence of CNS infection in conjunction with a CSF (1→3)-β- d -glucan level of >31 pg/mL.
We used conservative cutoff values of (1→3)-β- d -glucan in serum and CSF (<31 pg/ml). To further validate this value, we assessed the normal concentrations of (1→3)-β- d -glucan in human CSF from 44 anonymized samples submitted as standard of care, and the result was <31 pg/mL. The actual mean and variation were calculated, and a standard slope curve with a y intercept was constructed to determine the Vmean (SoftMax Pro v3.1, Molecular Devices, Sunnyvale, CA). Among these specimens, the software-generated mean ± SD CSF (1→3)-β- d -glucan level was 1.77 ± 12.6 pg/mL (±2 SDs, 27 pg/mL), and the calculated mean of all the samples was 5.95 ± 9.0 pg/mL (±2 SDs, 24 pg/mL). Among all 44 CSF specimens, 34 (77%) had a (1→3)-β- d -glucan concentration of <10 pg/mL, and 10 (23%) had a concentration of ≥10 pg/mL.
Assays for detecting (1→3)-β- d -glucan levels in serum and CSF were performed by Beacon Diagnostics Laboratory (Associates of Cape Cod, Inc., East Falmouth, MA). CSF and serum samples were obtained, refrigerated at 4°C, and shipped overnight on dry ice to the Beacon Diagnostics Laboratory. The turnaround times varied between 48 and 96 hours.
(1→3)-β- d -Glucan is a cell wall component found in several fungal pathogens, including Candida spp . and Aspergillus spp. Lipopolysaccharide and (1→3)-β- d -glucan initiate the coagulation cascade in horseshoe crabs ( Limulus polyphemus and Tachypleus tridentatus ) by activating different serine protease zymogens, factors C and G, respectively [ 18 , 19 ]. Lipopolysaccharide specifically activates factor C, whereas (1→3)-β- d -glucan activates factor G. The specificity of (1→3)-β- d -glucan is ensured by using factor C-depleted L. polyphemus amebocyte lysate. The assays were performed according to the manufacturer's instructions. Briefly, 5-μL aliquots of plasma or CSF were added to duplicate wells of a 96-well microtiter plate and pretreated for 10 min at 37°C with 20 μl of an alkaline reagent (0.125 M KOH/0.6 M KCl). An aliquot of 25 μL of the standards (100–6.25 pg/mL pure pachyman and a linear β-glucan) was then added to each well. A 100-μL aliquot of Fungitell reagent (lyophilized β-glucan–specific Limulus amebocyte lysates) was reconstituted with 2.8 mL of glucan-free reagent-grade water, followed by 2.8 mL of Pyrosol reconstitution buffer (2 M Tris-HCl, pH 7.4), and 100 μL of this mixture was added to each sample. The plate was monitored at 405 nm (with 490-nm background subtraction) for 40 min at 37°C using an automated microplate reader equipped with KC4 software (Bio-Tek Instruments, Inc., Winooski, VT). The mean rate of optical-density change was determined for each well, and the glucan concentration was determined by comparing it to a standard curve, with correction for the 5-fold sample dilution relative to the standards. When the absorbance was outside the range of the standard curve, the serum or CSF samples were serially diluted in reagent-grade water and tested again. When serial data were available, the levels of (1→3)-β- d -glucan in CSF were plotted over time. Serum and CSF samples were obtained simultaneously when feasible.
RESULTS
Patient Population
The demographic characteristics, predisposing factors, and clinical presentations are shown in Table 1 . In each patient, we found evidence of invasive fungal infection and clinically or radiologically evident CNS abnormalities. The patients ranged in age from 1 month to 18 years (median age, 13 years). Predisposing factors for FICNS included prematurity (3 patients), acute leukemia (2 patients), CNS tumors (2 patients), neurologic trauma (1 patient), and Crohn disease (1 patient). Six of the 9 patients received immunosuppressive therapy or cytotoxic chemotherapy. Two patients were receiving antifungal prophylaxis in the setting of acute leukemia. None of the patients received antifungal agents as treatment. Clinical presentation for FICNS included fever, vomiting, headache, and seizures. Abnormalities revealed by diagnostic imaging in 7 patients included parenchymal lesions, ventriculitis, meningeal enhancement, CNS hemorrhage, and hydrocephalus. All cultures and biomarker testing were performed immediately after the development of CNS symptoms.
Table 1.
Demographics, Predisposing Factors, and Clinical Presentation
| Patient No. | Age | Sex | Race | Predisposing Factor(s) | Neutropenia (<500/mm 3 ) | Immunosuppressive Therapy | Clinical Presentation | Radiological Findings at Diagnosis | Radiological Finding(s) at the End of Therapy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 mo | M | White | Prematurity, VPS | No | Inhaled steroids | Fever, vomiting | Persistent third and lateral ventricular dilatation | Meningeal enhancement, hemorrhage |
| 2 | 18 y | M | White | Prolonged antibiotic therapy, Crohn disease, thrush | No | 6MP | Fever, vomiting, headache, altered mental status, decreased activity | No abnormalities | Not performed |
| 3 | 14 y | F | Other | ALL, prolonged antibiotic therapy | Yes | Parenteral steroids, cyclophosphamide, cytarabine, doxorubicin, methotrexate, PEG-asparaginase, vincristine | Fever, headache, forgetfulness | Parenchymal lesions | Mild global loss of cerebral volume |
| 4 | 13 y | M | White | AML | Yes | Cytarabine, l -asparaginase, sorafenib | Fever, vomiting, headache, altered mental status, behavioral changes, decreased activity | Diffuse prominence of cerebral ventricles and sulci | Patient died |
| 5 | 4 y | M | Other | Medulloblastoma VPS, prolonged antibiotic therapy | No | Parenteral steroids, vincristine | Fever, seizures, behavioral changes, abnormal posturing, altered mental status, decreased activity | Hydrocephalus, infected hematoma | Meningeal enhancement, parenchymal lesions |
| 6 | 2.5 mo | F | White | Prematurity | No | None | Apnea, bradycardia |
CNS hemorrhage, bilateral
renal fungal balls |
Normal kidneys and CNS |
| 7 | 1 mo | M | White | Prematurity | No | None | Apnea, hypotension | Hemorrhage a | Mild bilateral hydrocephalus, periventricular leukomalacia |
| 8 | 18 mo | F | White | Neurologic trauma, VPS | No | None | Fever, altered mental status | Parenchymal lesions, ventriculitis, meningeal enhancement, infarction | Not applicable |
| 9 | 16 y | M | White | Germinoma, VPS, prolonged antibiotic therapy | No | Parenteral steroids, carboplatin, etoposide | Fever, seizures, headache, poor appetite, decreased activity | Ventriculitis, meningeal enhancement, hemorrhage | Resolution of ventriculitis and meningeal enhancement |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; 6MP, 6-mercaptopurine; VPS, ventriculoperitoneal shunt.
a The hemorrhage was already present at the prenatal ultrasound.
Microbiology
The microbiologic characteristics and fungal biomarkers of the patient population are presented in Table 2 . Among the 9 patients who had detectable (1→3)-β- d -glucan levels in their CSF, 4 patients had candidemia, 2 had biopsy-proven cerebral infection (1 case each of candidiasis and aspergillosis), 1 had growth from the CSF, 1 had candiduria, and 1 was diagnosed with probable pulmonary aspergillosis. Among the organisms cultured, there were 4 isolates of Candida albicans , 2 of Candida krusei , and 1 each of Candida parapsilosis and Aspergillus fumigatus . One immunocompromised patient was diagnosed with probable invasive pulmonary aspergillosis, defined by pulmonary infiltrates and elevated serum galactomannan antigen levels.
Table 2.
Microbiology, Fungal Markers, Treatment Regimens, and Outcome
| Patient No. | Organism | Site of Isolation | CSF WBC Count at Diagnosis (per μL) |
Baseline BDG (pg/mL)
|
End of Therapy BDG (pg/mL)
|
Antifungal Agents Used For Therapy | Combination Therapy | Duration of Combination Therapy (mo) | Duration of Total Therapy (mo) | Patient Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | CSF | Serum | CSF | |||||||||
| 1 | Aspergillus spp. | CNS tissue | 405 | 203 | 230 | 31 | 31 | LAMB, VRC, FLC, CFG | Echino plus triazole | 1.5 | 2 | Alive |
| 2 | Candida albicans | Blood | 3 | 2824 | 87 | 31 | 31 | FLC, MFG | Echino plus triazole | 0.5 | 3 | Alive |
| 3 | Candida krusei | Blood | 1 | 744 | 152 | 31 | 31 | LAMB, VRC, FLC, CFG | Echino plus triazole, Echino plus polyene | 1 | 5 | Alive |
| 4 | Candida krusei | Blood | 0 | 43 830 | 1158 | 21 734 | NA | LAMB, VRC, MFG | Echino plus triazole, triple | 0.5 | 0.5 | Death |
| 5 | Candida albicans | CNS tissue | 107 | 31 | 185 | 463 | 451 | LAMB, FLC, CFG | Echino plus triazole | 1 | Therapy ongoing | Alive |
| 6 | Candida albicans | Blood, urine | 2 | >500 a | >500 a | 31 | 31 | AMBD, FLC | Triazole plus polyene | 1 | 6 | Alive |
| 7 | Candida parapsilosis | Urine | 22 | >500 a | 361 | 31 | 31 | AMBD, FLC | None | 0 | 2.7 | Alive |
| 8 | Candida albicans | CSF | 82 | 167 | 86 | 31 | 31 | LAMB, FLC | Triazole plus polyene | 1 | 5 | Alive |
| 9 | Aspergillus fumigatus | CNS tissue | 30 | 31 | 987 | 31 | 31 | LAMB, VRC, CFG, PSC | Echino plus triazole, Echino plus polyene, triple | 22.7 | 23.7 | Alive |
Abbreviations: AMBD, amphotericin B deoxycholate; BDG, (1→3)-β- d -glucan; CFG, caspofungin; Echino, echinocandin; FLC, fluconazole; LAMB, liposomal amphotericin B; MFG, micafungin; PSC, posaconazole; triple, triple combination; WBC, white blood cell; VRC, voriconazole.
a BDG level not quantified.
(1→3)-β- d -Glucan . Levels in CSF and Serum
All 9 patients had a detectable level of (1→3)-β- d -glucan in their CSF at baseline, whereas 7 patients had an elevated (1→3)-β- d -glucan level in their serum at baseline (Table 2 ). Among the 7 patients who completed therapy for FICNS, elevated CSF (1→3)-β- d -glucan levels decreased to <31 pg/mL. One patient (patient 5), who at the time of this writing was still receiving antifungal therapy for CNS candidiasis, continued to have elevated CSF (1→3)-β- d -glucan levels of 451 pg/mL; this patient was considered to have chronic CNS infection related to the presence of an indwelling CSF shunt apparatus. The ninth patient (patient 4), who died from overwhelming disseminated candidiasis, had only 1 CSF sample, in which the (1→3)-β- d -glucan level was 1158 pg/mL.
CSF (1→3)-β- d -glucan levels exceeded the serum (1→3)-β- d -glucan levels in both cases of ACNS (Table 2 ). Among the 7 cases of CNS candidiasis, CSF (1→3)-β- d -glucan levels exceeded the serum (1→3)-β- d -glucan levels only in a patient with ventriculoperitoneal shunt infection. Conversely, serum (1→3)-β- d -glucan levels exceeded the CSF (1→3)-β- d -glucan levels in 4 patients with candidemia and 1 with candiduria.
The median baseline CSF (1→3)-β- d -glucan level was 230 pg/mL (range, 86–1158 pg/mL). After antifungal therapy, the median level declined to <31 pg/mL (range, <31 to >500 pg/mL). The median baseline serum (1→3)-β- d -glucan level was 203 pg/mL (range, <31 to 43 830 pg/mL), and after antifungal therapy, the median level declined to <31 pg/mL (range, <31 to 21 734 pg/mL).
Treatment and Outcome
The time courses of (1→3)-β- d -glucan levels in CSF and serum for each case during antifungal therapy are presented in Figures 1 and 2 , respectively. The pattern of clearance of the CSF (1→3)-β- d -glucan levels correlated with a favorable therapeutic outcome in each successfully treated patient. The data further revealed that serum (1→3)-β- d -glucan levels decreased to <31 pg/mL while the CSF (1→3)-β- d -glucan levels were persistently elevated in patients with active infection, which underscores the more predictive value of serial CSF (1→3)-β- d -glucan measurements in patients with CNS infection.
Figure 1.
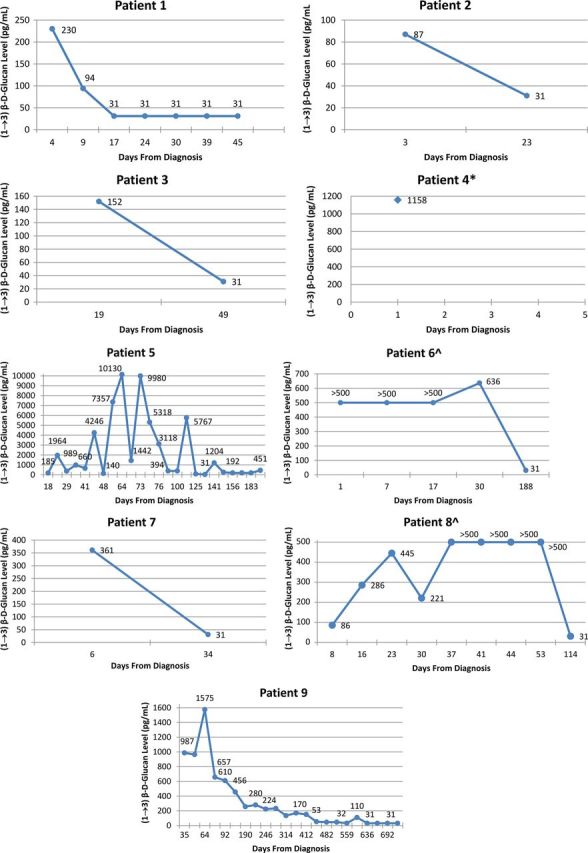
Serial levels of (1→3)-β- d -glucan in the cerebrospinal fluid (CSF). *Patient 4 died before another CSF sample was collected; ^serial (1→3)-β- d -glucan levels for patients 6 and 8 are marked as >500 pg/mL because the levels were not quantified.
Figure 2.
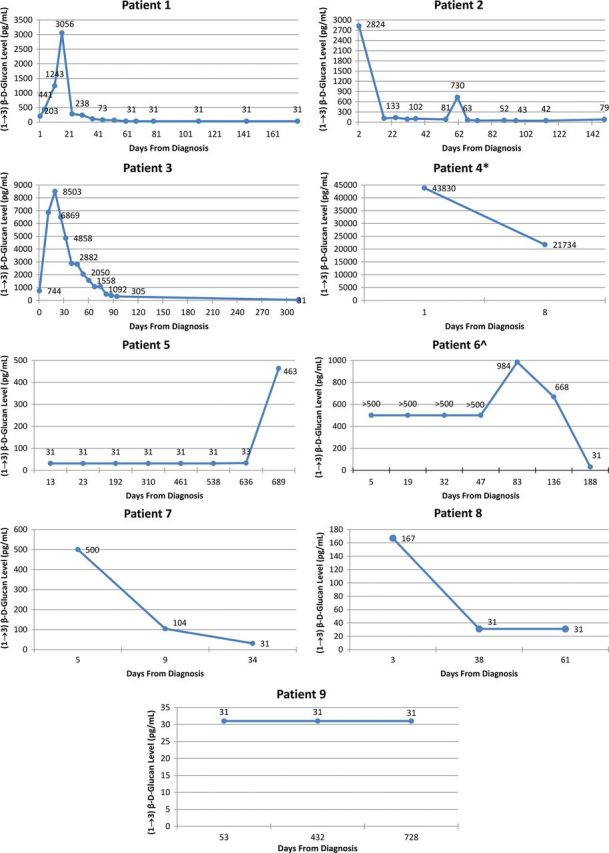
Serial levels of (1→3)-β- d -glucan in serum. *Patient 4 died before another serum sample was collected; ^serial (1→3)-β- d -glucan levels for patient 6 are marked as >500 pg/mL because the levels were not quantified.
The predominant antifungal therapy regimen consisted of an echinocandin and a triazole. The selection of antifungal agents alone or in combination was determined by individual unit practice. The duration of therapy was adjusted according to therapeutic response of CSF (1→3)-β- d -glucan levels. All but 1 of the patients survived. Among those who survived, 5 patients (63%) had neurologic sequelae, most likely as a result of their FICNS. However, 3 of the patients had other factors that may have contributed to the neurologic sequelae observed: severe prematurity (patients 6 and 7) and preceding severe neurologic trauma (patient 8) (Tables 1 and 2 ).
DISCUSSION
Diagnosing invasive fungal infections in the immunocompromised pediatric population continues to be challenging and problematic, given the prolonged time to culture positivity, the low sensitivity of blood cultures related to the smaller volumes of blood collected for culture, and the increasing use of antifungal prophylaxis. Patients at risk for invasive fungal infections include solid organ transplant recipients, hematopoietic stem cell transplant recipients, patients with a hereditary or acquired immunodeficiency, patients with connective tissue disorders, patients who receive immunosuppressive therapy, and patients with prolonged neutropenia and/or neutrophil dysfunction.
The evaluation of a patient with suspected FICNS should include diagnostic imaging and a lumbar puncture. However, normal CSF parameters (white blood cell count and glucose and protein levels) are found in half of the infants with culture-proven candidal meningitis; therefore, normal CSF parameters in a pediatric patient do not exclude candidal meningitis [ 20–22 ]. FICNS should be suspected in immunocompromised pediatric patients who have headache, seizure, focal neurologic deficits, and/or an associated mental status change despite broad-spectrum antimicrobial therapy. Prolonged placement of an external ventricular catheter may increase the risk for FICNS. Low birth weight with candidemia, traumatic open head injury, near-drowning, and a history of systemic corticosteroid therapy should also be considered high-risk conditions that prompt a neurologic evaluation.
Candida spp . , Aspergillus spp., and related fungal pathogens contain (1→3)-β- d -glucan as a major component in their cell walls. Circulating (1→3)-β- d -glucan has been shown to precede fever and clinical signs and symptoms of invasive fungal infections in immunocompromised adults [ 13 ]. Several studies have demonstrated the potential role of the (1→3)-β- d -glucan assay in screening children at risk for invasive fungal infection [ 23–25 ]. Additional studies have shown the improved sensitivity of the (1→3)-β- d -glucan assay for detecting invasive fungal infections when there are 2 consecutive positive results [ 13 , 14 , 26 ].
In a recent study, Petraitiene et al. [ 15 ] investigated the expression of (1→3)-β- d -glucan in CSF and plasma in an experimental model of nonneutropenic rabbits with HCME treated with micafungin and amphotericin B. The study revealed a direct quantitative relationship between CSF (1→3)-β- d -glucan levels and cerebral tissue concentrations of C albicans . Although the study revealed that (1→3)-β- d -glucan levels in CSF were predictive of therapeutic response, the clearance of C albicans from blood cultures was not predictive of the eradication of organisms from the CNS. The authors also reported that the levels of (1→3)-β- d -glucan in plasma were lower than the levels in simultaneously obtained CSF samples. To our knowledge, there have been no clinical studies that evaluated the therapeutic response of (1→3)-β- d -glucan levels in infants and children with FICNS or addressed the role of CSF (1→3)-β- d -glucan as a marker in this population to determine the length of therapy. Because the length of antifungal therapy in most cases of FICNS is still uncertain and based on clinical judgment, the use of CSF (1→3)-β- d -glucan measurements may better inform this decision. Since the completion of this study, Litvintseva et al. [ 27 ] reported the utility of measuring CSF (1→3)-β- d -glucan levels in diagnosing and therapeutically monitoring patients suffering from Exserohilum rostratum CNS infection after the nationwide outbreak of contaminated methylprednisolone, which further substantiates its utility in the management of FICNS.
Consistent with the observations in an experimental HCME model, the clinical study reported herein revealed that the clearance of (1→3)-β- d -glucan levels from serum was not always predictive of the resolution of an FICNS. Indeed, in our study, the CSF (1→3)-β- d -glucan levels continued to be elevated in 5 patients (patients 1, 3, 5, 8, and 9) and prompted continuation of antifungal therapy. These findings are consistent with those of the rabbit model of HCME, in which the serum (1→3)-β- d -glucan levels responded promptly to antifungal therapy, whereas the CSF (1→3)-β- d -glucan levels remained elevated as a reflection of persistent CNS infection. One of the advantages of serial (1→3)-β- d -glucan levels from the CSF is that it enables one to individualize the length of therapy for each patient. In this study, each patient was managed uniquely on the basis of the decline in the CSF (1→3)-β-D-glucan level to <31 pg/mL, and treatment ranged from 2 to 28 months. This strategy, therefore, can potentially expose each patient to the correct amount of the antifungal agent to clear the FICNS, thereby decreasing potential toxicity or antifungal resistance.
Serum and CSF samples were obtained simultaneously when feasible; however, because access to CSF samples was limited, more serum samples were obtained. Moreover, when a serum (1→3)-β- d -glucan level was >500 pg/mL, the CSF was considered likely to be positive and, thus, was not sampled. We based our therapeutic end point (a CSF (1→3)-β- d -glucan level of <31 pg/mL) on our laboratory animal studies and our unpublished pediatric clinical observations. When their (1→3)-β- d -glucan CSF level was <31 pg/mL, laboratory animals with successfully treated HCME consistently had culture-negative cerebral and cerebellar tissues. Pediatric patients under our care, and in whom a possible invasive fungal infection was ruled out, consistently showed CSF (1→3)-β- d -glucan levels of <31 pg/mL and no clinical or radiological evidence of FICNS (our unpublished data). Thus, when a CSF (1→3)-β- d -glucan level in any of our patients with FICNS declined to <31 pg/mL, antifungal therapy was then subsequently discontinued after a finite period of treatment consolidation ranging from 2 to 6 weeks, depending on the primary physician's judgment.
On the basis of previous laboratory work and previous clinical experience with FICNS, we used serum and CSF (1→3)-β- d -glucan levels as a standard of care in the management of these patients who were hospitalized between 2009 and 2012. Indeed, before the use of CSF (1→3)-β- d -glucan measurements, we observed relapses in the treatment of other patients with HCME and ACNS. We did not observe such relapses in those we managed by serially measuring CSF (1→3)-β- d -glucan levels.
As a helpful procedural point, to minimize contamination, it has been our practice to use chlorhexidine swabs to cleanse the involved area before peripheral venipuncture, blood draw from a central venous catheter, or in preparation for lumbar puncture. Testing of our chlorhexidine swabs revealed no detectable (1→3)-β- d -glucan. When drawing blood specimens or obtaining CSF, we recommend sending the last blood or CSF samples obtained for the (1→3)-β- d -glucan assay.
This study had several limitations. The number of patients was relatively small, and CSF sampling was limited in 2 patients because of their prematurity. However, given the infrequency of HCME and ACNS in children, we were able to focus on an uncommon but life-threatening infection, and the diagnostic trends of the serial CSF (1→3)-β- d -glucan levels were highly consistent. Although the study lacks robust CSF and serum cutoff data in a large pediatric population, we provide an analysis of otherwise normal CSF using a possible cutoff value of <31 pg/ml. Additional studies are needed to elucidate the CSF and serum cutoff values and the sensitivity and specificity of the (1→3)-β- d -glucan assay in diagnosing and treating FICNS in the pediatric population.
In summary, we have shown that the (1→3)-β- d -glucan assay is useful in diagnosing and therapeutically monitoring FICNS in pediatric patients. The CSF (1→3)-β- d -glucan assay will have its greatest utility in patients suspected of having FICNS in the setting of candiduria in preterm infants and of candidemia or pulmonary aspergillosis in all patients. This report lays the conceptual framework for a future prospective multicenter study of FICNS in children.
Acknowledgments
Financial support. This study was supported in part by Clinical and Translational Science Center grant support (CTSC GRANT UL1-RR024996). T. J. W. is a Scholar of the Henry Schueler Foundation and a Scholar of the Sharp Family Foundation in Pediatric Infectious Diseases.
Potential conflicts of interest. T. J. W. receives support from the Save Our Sick Kids Foundation, the Henry Schueler Foundation (Scholar in Mucormycosis), and the Sharpe Family Foundation (Scholar in Pediatric Infectious Diseases) and research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Novartis, Merck, ContraFect, and Pfizer; he has served as a consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. M. A. F. is an employee of Associates of Cape Cod, Inc., the manufacturer and marketer of Fungitell, a (1→3)-β- d -glucan in vitro diagnostic kit.
All other authors report no conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Abbasi S , Shenep JL , Hughes WT , Flynn PM . Aspergillosis in children with cancer: a 34-year experience . Clin Infect Dis 1999. ; 29 : 1210 – 9 . [DOI] [PubMed] [Google Scholar]
- 2. Lin SJ , Schranz J , Teutsch SM . Aspergillosis case-fatality rate: systematic review of the literature . Clin Infect Dis 2001. ; 32 : 358 – 66 . [DOI] [PubMed] [Google Scholar]
- 3. Dotis J , Iosifidis E , Roilides E . Central nervous system aspergillosis in children: a systematic review of reported cases . Int J Infect Dis 2007. ; 11 : 381 – 93 . [DOI] [PubMed] [Google Scholar]
- 4. Faix RG , Chapman RL . Central nervous system candidiasis in the high-risk neonate . Semin Perinatol 2003. ; 27 : 384 – 92 . [DOI] [PubMed] [Google Scholar]
- 5. Benjamin DK Jr , Stoll BJ , Fanaroff AA et al. . Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months . Pediatrics 2006. ; 117 : 84 – 92 . [DOI] [PubMed] [Google Scholar]
- 6. Benjamin DK Jr , Stoll BJ , Gantz MG et al. . Neonatal candidiasis: epidemiology, risk factors, and clinical judgment . Pediatrics 2010. ; 126 : e865 – 73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCarthy M , Rosengart A , Schuetz AN et al. . Mold infections of the central nervous system . N Engl J Med 2014. ; 371 : 150 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Creger RJ , Weeman KE , Jacobs MR et al. . Lack of utility of the lysis-centrifugation blood culture method for detection of fungemia in immunocompromised cancer patients . J Clin Microbiol 1998. ; 36 : 290 – 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berenguer J , Buck M , Witebsky F et al. . Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis . Disseminated versus single-organ infection. Diagn Microbiol Infect Dis 1993. ; 17 : 103 – 9 . [DOI] [PubMed] [Google Scholar]
- 10. Montagna MT , Coretti C , Lovero G et al. . Diagnostic performance of 1→3-beta-d-glucan in neonatal and pediatric patients with candidemia . Int J Mol Sci 2011. ; 12 : 5871 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odabasi Z , Mattiuzzi G , Estey E et al. . Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome . Clin Infect Dis 2004. ; 39 : 199 – 205 . [DOI] [PubMed] [Google Scholar]
- 12. Ostrosky-Zeichner L , Alexander BD , Kett DH et al. . Multicenter clinical evaluation of the (1→3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans . Clin Infect Dis 2005. ; 41 : 654 – 9 . [DOI] [PubMed] [Google Scholar]
- 13. Pazos C , Ponton J , Del Palacio A . Contribution of (1→3)-beta-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan . J Clin Microbiol 2005. ; 43 : 299 – 305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Senn L , Robinson JO , Schmidt S et al. . 1,3-Beta-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia . Clin Infect Dis 2008. ; 46 : 878 – 85 . [DOI] [PubMed] [Google Scholar]
- 15. Petraitiene R , Petraitis V , Hope WW et al. . Cerebrospinal fluid and plasma (1→3)-beta-d-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis . Antimicrob Agents Chemother 2008. ; 52 : 4121 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hope WW , Petraitis V , Petraitiene R et al. . The initial 96 hours of invasive pulmonary aspergillosis: histopathology, comparative kinetics of galactomannan and (1→3) beta-d-glucan and consequences of delayed antifungal therapy . Antimicrob Agents Chemother 2010. ; 54 : 4879 – 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris PA , Taylor R , Thielke R et al. . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support . J Biomed Inform 2009. ; 42 : 377 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohki M , Nakamura T , Morita T , Iwanaga S . A new endotoxin sensitive factor associated with hemolymph coagulation system of horseshoe crab ( Limulidae ) . FEBS Lett 1980. ; 120 : 217 – 20 . [DOI] [PubMed] [Google Scholar]
- 19. Hossain MA , Miyazaki T , Mitsutake K et al. . Comparison between Wako-WB003 and Fungitec G tests for detection of (1→3)-beta-d-glucan in systemic mycosis . J Clin Lab Anal 1997. ; 11 : 73 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faix RG . Systemic Candida infections in infants in intensive care nurseries: high incidence of central nervous system involvement . J Pediatr 1984. ; 105 : 616 – 22 . [DOI] [PubMed] [Google Scholar]
- 21. Fernandez M , Moylett EH , Noyola DE , Baker CJ . Candidal meningitis in neonates: a 10-year review . Clin Infect Dis 2000. ; 31 : 458 – 63 . [DOI] [PubMed] [Google Scholar]
- 22. Cohen-Wolkowiez M , Smith PB , Mangum B et al. . Neonatal Candida meningitis: significance of cerebrospinal fluid parameters and blood cultures . J Perinatol 2007. ; 27 : 97 – 100 . [DOI] [PubMed] [Google Scholar]
- 23. Montagna MT , Coretti C , Lovero G et al. . Diagnostic performance of 1→3-beta-d-glucan in neonatal and pediatric patients with candidemia . Int J Mol Sci 2011. ; 12 : 5871 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mackay CA , Ballot DE , Perovic O . Serum 1,3-betaD-glucan assay in the diagnosis of invasive fungal disease in neonates . Pediatr Rep 2011. ; 3 : e14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mularoni A , Furfaro E , Faraci M et al. . High levels of beta-d-glucan in immunocompromised children with proven invasive fungal disease . Clin Vaccine Immunol 2010. ; 17 : 882 – 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamoth F , Cruciani M , Mengoli C et al. . beta-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3) . Clin Infect Dis 2012. ; 54 : 633 – 43 . [DOI] [PubMed] [Google Scholar]
- 27. Litvintseva AP , Lindsley MD , Gade L et al. . Utility of (1–3)-beta-d-glucan testing for diagnostics and monitoring response to treatment during the multistate outbreak of fungal meningitis and other infections . Clin Infect Dis 2014. ; 58 : 622 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]


