Abstract
Background
Outpatient respiratory tract infections are the most common reason for antibiotic prescribing to children. Although prior studies suggest that antibiotic overuse occurs, patient-specific data or data exploring the variability and determinants of variability across practices and practitioners is lacking.
Methods
This study was conducted from a retrospective cohort of encounters to 25 diverse pediatric practices with 222 clinicians, from January 1 to December 31, 2009. Diagnoses, medications, comorbid conditions, antibiotic allergy, and demographic data were obtained from a shared electronic health record and validated by manual review. Practice-specific antibiotic prescription and acute respiratory tract infection diagnosis rates were calculated to assess across-practice differences after adjusting for patient demographics and clustering of encounters within clinicians.
Results
A total of 102 102 (28%) of 399 793 acute visits by 208 015 patients resulted in antibiotic prescriptions. After adjusting for patient age, sex, race, and insurance type, and excluding encounters by patients with chronic conditions, antibiotic prescribing by practice ranged from 18% to 36% of acute visits, and the proportion of antibiotic prescriptions that were broad-spectrum ranged from 15% to 58% across practices, despite additional exclusion of patients with antibiotic allergies or prior antibiotic use. Diagnosis of (Dx) and broad-spectrum antibiotic prescribing (Broad) for acute otitis media (Dx: 8%–20%; Broad: 18%–60%), sinusitis (Dx: 0.5%–9%; Broad: 12%–78%), Streptococcal pharyngitis (Dx: 1.8%–6.4%; Broad: 2%–30%), and pneumonia (Dx: 0.4%–2%; Broad: 1%–70%) also varied by practice ( P < 0.001 for all comparisons).
Conclusions
Antibiotic prescribing for common pediatric infections varied substantially across practices. This variability could not be explained by patient-specific factors. These data suggest the need for and provide high-impact targets for outpatient antimicrobial stewardship interventions.
Keywords: antimicrobials, children, outpatient, respiratory tract infection, variability
(See the Editorial Commentary by Hicks and Blaser on pages e136–e138.)
Antibiotics are the most common prescription drugs given to children [ 1 ], and outpatient acute respiratory tract infections (ARTIs) account for the vast majority of these prescriptions [ 2 ]. Although inappropriate antibiotic prescribing for viral infections has diminished over time, the use of broad-spectrum antibiotics has increased [ 3 ]. Professional guidelines, including recommendations from the American Academy of Pediatrics (AAP), support the treatment of most ARTIs with narrow-spectrum antibiotics [ 4 ].
Although national estimates have suggested inappropriate antibiotic use in children [ 5–9 ], administrative and survey data used for these studies lack (1) detailed, patient-specific clinical data, such as the presence of comorbid conditions, drug allergies, and prior antibiotic use, and (2) the ability to compare prescribing across practitioners and practice groups. Given the public health importance of judicious antibiotic use, comparing the management of common pediatric infections across practices would generate benchmarking data and help define high-impact targets for intervention. For example, variability in antibiotic prescribing relative to national guidelines within an electronically linked group of practices can highlight the need for antimicrobial stewardship, which has been shown to be effective in both inpatient [ 10 ] and outpatient [ 11 ] settings.
Therefore, we used one of the nation's largest pediatric healthcare networks and its shared, comprehensive electronic health record (EHR) to examine and compare antibiotic prescribing patterns across primary care pediatric practices.
METHODS
Data Source
Data came from the EHR of a pediatric healthcare network including 29 primary care pediatric practice sites located across southeastern Pennsylvania and southern New Jersey staffed by 222 pediatric practitioners. No urgent care, family medicine, or emergency medicine facilities or practitioners were included. Two clinician groups each staff 3 separate practice sites, creating 25 distinct practice groups (ranging from 5 to 18 clinicians). All practice sites used a common EHR (EpicCare, Epic Systems, Inc, Verona, WI) for charting and medication prescribing for all office and telephone encounters since 2004.
Data Collection
Patient level data extracted from the EHR included age, sex, race, insurance type, and antibiotic allergies. Visit level data included practice site; calendar month of contact; encounter type (office visit, telephone call, emergency department visit); purpose of the contact (preventive, nonpreventive); provider type (physician, nurse practitioner, trainee); provider sex, and years in practice; all International Classification of Disease, 9th Edition (ICD-9) codes associated with the encounter and on the active “problem list;” and all prescriptions generated during the encounter. Patients were identified with a practice if they had at least 2 encounters (office or telephone) at a practice site in the calendar year.
Cohort Assembly
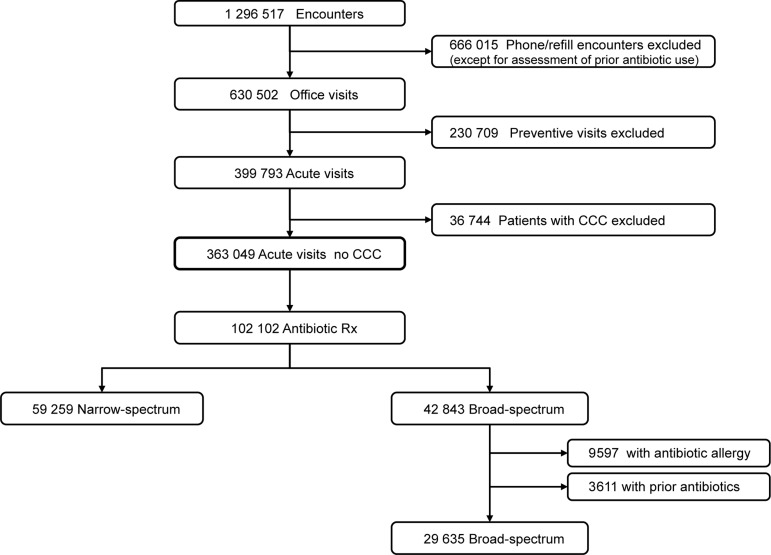
The initial cohort included all primary care office encounters within the network from January 1 through December 31, 2009 by children 18 years of age or younger (Figure 1 ). For encounter-level analyses, we excluded: (1) preventive encounters, identified by billing codes, to focus on “acute visits” at which the vast majority of antibiotics were prescribed; (2) encounters by children with complex chronic conditions (CCC) [ 12 ], to restrict the cohort to previously healthy children; and (3), when relevant, encounters by children with antibiotic allergies or by children who received an antibiotic prescription within the prior 3 months (including both office- and telephone-based prescribing). These exclusions avoided the influence of antibiotic prescribing decisions based upon antibiotic allergies, the treatment of recurrent disease, or consideration of the potential for resistant pathogens created by recent drug exposure.
Figure 1.
Network-wide patient encounters resulting in antibiotic prescriptions. CCC, complex chronic conditions.
Exposures
The primary exposure was an office-based acute visit to one of the 25 individual practice groups between January 1, 2009 and December 31, 2009.
Outcomes
The main outcome measures included: (1) antibiotic prescriptions, (2) broad-spectrum antibiotic prescriptions, and (3) encounter diagnoses. We defined antibiotic receipt as a prescription associated with an office visit for an oral antibacterial agent, including penicillin, amoxicillin, amoxicillin-clavulanate, cephalosporins, macrolides, trimethoprim-sulfamethoxazole, and clindamycin. Amoxicillin-clavulanate, second- and third-generation cephalosporins, and azithromycin (except for analyses of pneumonia) were considered broad-spectrum antibiotics, based upon AAP prescribing guidelines for bacterial ARTIs [ 4 ]; fluoroquinolones, tetracyclines, and linezolid were not included because they were used rarely for ARTIs.
Definition of Acute Respiratory Tract Infection Cases
Case definitions for bacterial ARTIs required (1) the specified ICD-9 code, including acute otitis media ([AOM] 382, 382.0, 382.00, 382.01, 382.02, 382.4, 382.9), sinusitis (461.8, 461.9, 473.9, 473.2, 473.1, 473.0), streptococcal pharyngitis (034.0, 462, 463), or pneumonia (482.9, 486, 485, 483.8, 481); (2) an associated antibiotic prescription; and (3) for Group A streptococcal pharyngitis, a positive rapid (available at point of care at all practices) or culture positive laboratory test. We similarly identified urinary tract infection (UTI) (599, 599.0, 788.41, 788.1, 590.1, 590.10, 590.11, 590.80), which represented a non-ARTI, nonsubjective diagnosis comparison. We excluded encounters with diagnosis codes for an additional bacterial infection, including UTI (except when UTI was the outcome), otitis externa, skin/soft tissue infection, Lyme disease, acne, chronic sinusitis, mycoplasma infection, staphylococcal infection, bite wound, oropharyngeal infection (other than streptococcal pharyngitis), streptococcal infection (without pharyngitis), pertussis, sexually transmitted infection, bone/joint infection, or bacterial gastroenteritis. Two individuals validated a 10% sample of these case definitions through iterative, manual chart review of fields collected electronically and by examining free text fields (eg, physical exam, assessment/plan).
Statistical Analysis
To estimate the degree of variation across practice sites while adjusting for differences in patient characteristics, we implemented fixed-effects logistic regression models with the outcomes of antibiotic prescribing, broad-spectrum antibiotic prescribing, and specific diagnoses; practice group as a fixed effect; and patient level characteristics (age, sex, Medicaid status, and race) as covariates. All models controlled for clustering by clinician within each site, because we suspected that prescribing varied also by clinician. Using predictive margins based on these regressions, we estimated standardized rates of antibiotic prescribing, broad-spectrum antibiotic prescribing, and diagnosis for each of the 25 practice groups. Wald tests were used to determine whether standardized differences in rates across practices were statistically significant.
Our method of estimating variation through model-based direct standardization (predictive margins) [ 13 ] assumes a finite number of practice groups and seeks not to rank practice groups but only to estimate standardized rates of prescribing for each practice, and generate confidence bounds for those rates, and describe the degree of variation across practice groups. To verify our chosen method of standardization, we implemented a patient-factor-adjusted, 3-level (visit, prescribing physician, and practice) hierarchical mixed-effects model using second-order Taylor series approximations for starting values; a Markov chain Monte Carlo algorithm with 50 000 preliminary iterations; and a sample of 1000 from an additional 5000 iterations to estimate the distributions of adjusted rates of prescribing for each of the 25 practices. From these distributions, we arrived at medians and 2.5th and 97.5th ordered values for measures of adjusted rates and their 95% confidence bounds (using MLwiN version 2.26 software [ 14 ]). Results from this alternative approach confirmed our reported results but were less conservative, showing even more variation across sites (data not shown). Analyses were performed using Stata 12.0 (College Station, TX).
The Children's Hospital of Philadelphia Committee for the Protection of Human Subjects approved this study.
RESULTS
Between January 1 and December 31, 2009, 222 clinicians treated 208 015 children across 25 practices (Table 1 ). The number of clinicians within practices ranged from 4 to 18, and the population of children served varied from 4286 to 17 135 patients. Practices varied markedly in racial characteristics (1%–96% black) and payer types (4%–72% Medicaid insurance). Of 1 296 517 total encounters, 666 015 were nonoffice-based encounters (eg, telephone triage, medication refills). After excluding preventive encounters (230 709), and encounters by children with CCC (36 744), 363 049 office-based nonpreventive encounters (acute visits) remained for the primary analyses (Figure 1 ).
Table 1.
Patient Encounters and Antibiotic Use by Practice, Sorted by % Broad-Spectrum Use
| No. of Clinicians | Patients | Total Visits | Antibiotics (%) | Acute Visits (%) | Antibiotics, Acute (%) | % Broad, Acute |
|---|---|---|---|---|---|---|
| 5 | 5083 | 19 793 | 5356 (27) | 14 237 (72) | 5119 (36) | 63 |
| 6 | 7277 | 25 385 | 5931 (23) | 16 162 (64) | 5760 (36) | 62 |
| 6 | 4901 | 16 260 | 3697 (23) | 10 094 (62) | 3462 (34) | 54 |
| 7 | 7110 | 20 598 | 5235 (25) | 13 661 (66) | 5046 (37) | 54 |
| 9 | 10 197 | 32 059 | 6387 (20) | 21 508 (67) | 6162 (29) | 51 |
| 15 | 17 135 | 49 375 | 11 031 (22) | 30 677 (62) | 10 514 (34) | 49 |
| 9 | 11 513 | 40 441 | 10 513 (26) | 29 984 (74) | 10 110 (34) | 47 |
| 7 | 6266 | 23 800 | 5288 (22) | 16 333 (69) | 5038 (31) | 46 |
| 5 | 4821 | 14 668 | 3345 (23) | 9620 (66) | 3124 (32) | 45 |
| 10 | 10 756 | 30 492 | 5983 (20) | 18 646 (61) | 5723 (31) | 44 |
| 7 | 9219 | 28 985 | 5932 (20) | 19 171 (66) | 5699 (30) | 43 |
| 4 | 4286 | 14 494 | 3068 (21) | 9570 (66) | 2946 (31) | 41 |
| 7 | 5881 | 21 149 | 5042 (24) | 13 658 (65) | 4781 (35) | 41 |
| 5 | 6644 | 20 283 | 3115 (15) | 12 207 (60) | 2982 (24) | 38 |
| 5 | 5869 | 18 063 | 3652 (20) | 12 251 (68) | 3508 (29) | 33 |
| 7 | 6109 | 22 273 | 4000 (18) | 14 990 (67) | 3833 (26) | 33 |
| 8 | 8497 | 28 648 | 4351 (15) | 19 784 (69) | 4110 (21) | 33 |
| 6 | 9911 | 29 391 | 4907 (17) | 19 159 (65) | 4673 (24) | 32 |
| 7 | 7305 | 23 818 | 3634 (15) | 14 927 (63) | 3473 (23) | 28 |
| 11 | 6615 | 19 326 | 2453 (13) | 11 524 (60) | 2299 (20) | 27 |
| 16 | 9561 | 25 342 | 3570 (14) | 14 425 (57) | 3336 (23) | 24 |
| 10 | 11 013 | 29 797 | 3499 (12) | 17 072 (57) | 3339 (20) | 21 |
| 16 | 9950 | 24 504 | 2513 (10) | 13 629 (56) | 2252 (17) | 18 |
| 18 | 11 319 | 26 048 | 2343 (9) | 12 591 (48) | 2077 (17) | 18 |
| 16 | 10 787 | 25 510 | 2322 (9) | 13 913 (55) | 2052 (15) | 16 |
| 222 | 208 015 | 630 502 | 117 167 (19) | 399 793 (63) | 111 418 (28) | 42 |
Antibiotic Prescribing Across Practices
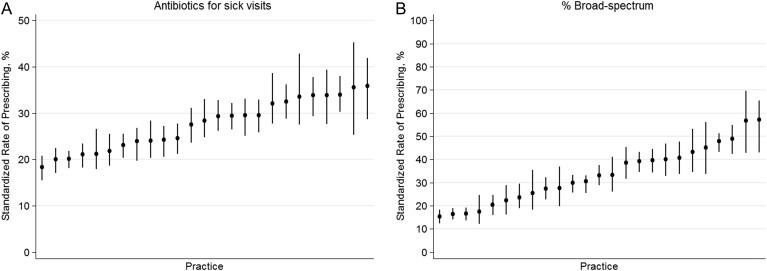
Of 363 049 acute office visits by children without CCC, 102 102 (28%) encounters resulted in an antibiotic prescription. However, after adjusting for patient age, sex, race, and insurance type, standardized rates of antibiotic prescribing at acute office visits ranged from 18% to 36% across practices (Figure 2 A; P < .001). Of children who received antibiotics, 42 843 (42%) were prescribed broad-spectrum drugs. After excluding patients with antibiotic allergy and those with prior antibiotic use and adjusting for patient age, sex, race, and insurance type, standardized broad-spectrum prescribing rates (ie, when an antibiotic was prescribed, how often was it a broad-spectrum agent) ranged from 15% to 57% across practices (Figure 2 B; P < .001).
Figure 2.
(A) Proportion of acute (nonpreventive) encounters at which any antibiotics were prescribed. (B) Proportion of antibiotics prescribed at acute (nonpreventive) encounters that were broad-spectrum. Bars represent 95% confidence intervals for the practice rates.
Diagnosis of Acute Respiratory Tract Infections Across Practices
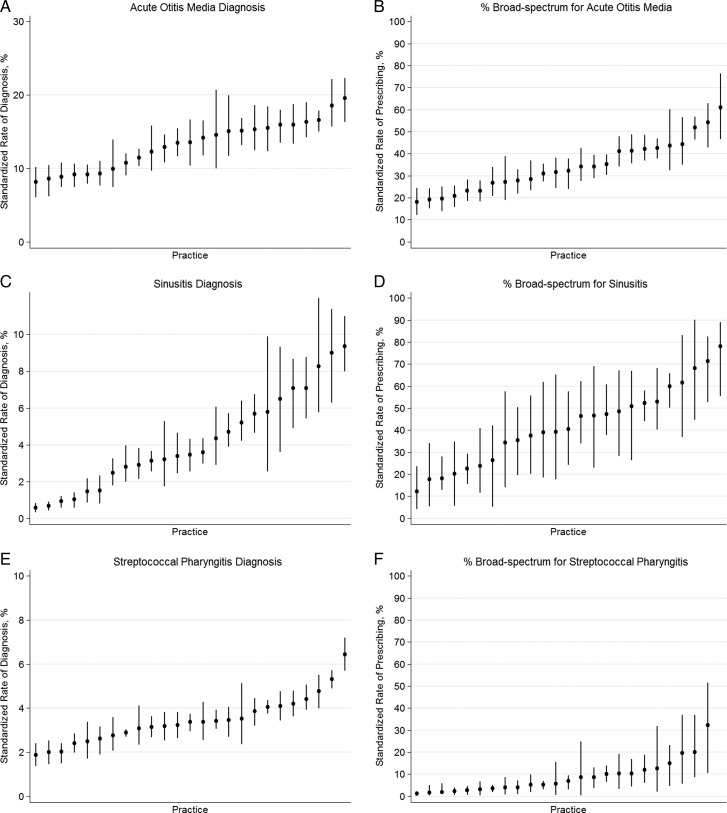
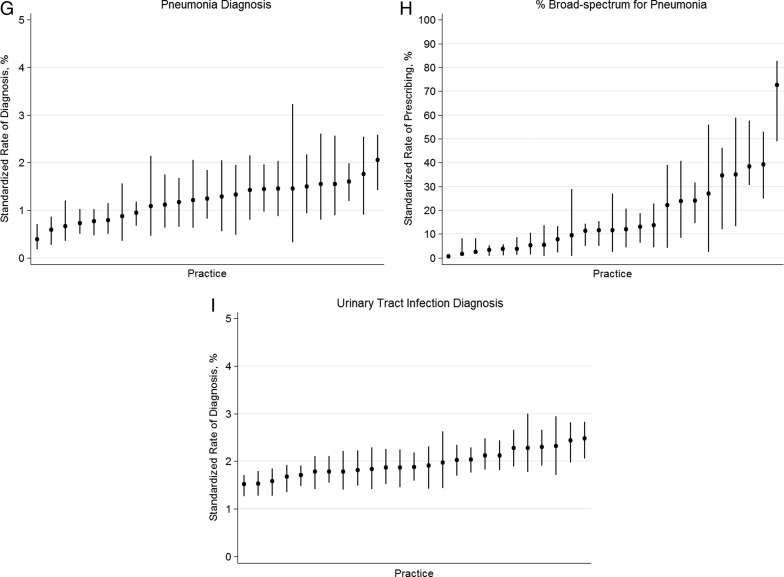
Given the variability in antibiotic use across practices, and because antibiotic prescribing should follow a diagnosis of a specific bacterial infection, we examined across-practice ARTI diagnosis rates. After standardizing for patient age, sex, race, and insurance type, and accounting for clustering of clinicians within a practice, the rate of diagnosis per acute visit of AOM (8%–20%), sinusitis (0.5%–9%), streptococcal pharyngitis (1.8%–6.4%), and pneumonia (0.4%–2%) varied significantly across practices (Figures 3 A, C, E, G; P < .001 for all comparisons). Furthermore, when an ARTI diagnosis was assigned and an antibiotic given, the proportion of broad-spectrum antibiotic prescribing for AOM (18%–60%), sinusitis (12%–78%), streptococcal pharyngitis (2%–30%), and pneumonia (1%–70%) differed significantly across practices (Figures 3 B, D, F, H; P < .001 for all comparisons), despite the additional exclusion of children with prior antibiotic use and antibiotic allergies. However, diagnosis rates of UTI were less variable across practices (1.5%–2.8%; Figure 3 I).
Figure 3.
Proportion of acute encounters resulting in a diagnosis of acute otitis media (A), sinusitis (C), streptococcal pharyngitis (E), pneumonia (F), and urinary tract infection (I). Proportion of encounters receiving broad-spectrum antibiotics for acute otitis media (B), sinusitis (D), streptococcal pharyngitis (F), and pneumonia (H). Bars represent 95% confidence intervals for practice rates. Y-axis ranges for diagnosis rates are scaled to correspond to the relative proportion of pediatric visits for each condition based on previous national sampling estimates (eg, acute otitis media accounts for approximately 30% of antibiotic prescribing).
To determine the contribution of these variable ARTI diagnosis rates on the previously identified across-practice differences in antibiotic prescribing, we further adjusted the standardized antibiotic prescribing rates by each practice's combined ARTI diagnosis rate (AOM, sinusitis, streptococcal pharyngitis, and pneumonia, which account for approximately 80% of indications for antibiotic prescribing to children). This adjustment largely attenuated the across-practice variability in overall antibiotic prescribing (from 18%–36% to 24%–32%) but had no effect on broad-spectrum antibiotic prescribing across practices (from 15%–57% to 17%–56%).
DISCUSSION
Using a comprehensive EHR from a single, unified pediatric healthcare network, we identified wide variation across primary care practices in the management of common infections in otherwise healthy children. Significant differences were observed across practices in overall antibiotic prescribing rates and preference for broad-spectrum agents, as well as in diagnosis rates and treatment choices for individual ARTIs, and were unexplained by patient-level differences. Although prior studies using national estimates suggested that both overall and broad-spectrum antibiotic prescribing rates were unnecessarily high [ 1–3 ], our access to more granular data elements (allergies, comorbid conditions, prior antibiotic use) and practice-specific comparisons provides more definitive evidence that a substantial proportion of antibiotic prescribing is likely inappropriate.
The observed variability in antibiotic prescribing and ARTI diagnosis rates is notable, particularly given the extensive exclusions and adjustments used to standardize encounters across practices. These data suggest that, compared with a child seeking care at a low-antibiotic use practice, a similar child visiting a high use practice is twice as likely to receive an antibiotic prescription at any acute visit and, when given an antibiotic, 4 times as likely to receive a broad-spectrum agent. In addition, practices with higher rates of overall antibiotic prescribing tended to be the same practices with higher rates of broad-spectrum antibiotic prescribing, suggesting a general practice-level propensity toward more aggressive antimicrobial use. Differences also were observed in ARTI diagnosis rates. The rate of sinusitis varied 10-fold across practices, and both streptococcal pharyngitis (which benefits from rapid, point-of-care testing available at all practice sites) and AOM (the most common indication for antibiotics in children) varied approximately 3-fold across practice groups. In addition, it is important to note that these differential rates of diagnosis accounted for only some of the antibiotic prescribing variability. Even when considering specific diagnoses in isolation, the chances of receiving a broad-spectrum antibiotic depended upon the practice visited, which ranged from 2% to 30% for strep throat, 18% to 60% for AOM, 12% to 78% for sinusitis, and 1% to 70% for pneumonia.
We suspect that the observed across-practice variability was driven by a combination of clinician- and practice-level factors, rather than by patient mix. First, we analyzed nearly 400 000 acute visits to more than 200 clinicians across 25 practice groups, providing substantial statistical power for comparing outcomes. Second, data were collected during the same calendar year, avoiding seasonal differences in the incidence of ARTI and the potential influence of epidemics. Third, we excluded children with conditions that might influence the threshold for diagnosis or the class of antibiotic chosen, including those with chronic health conditions, prior antibiotic use (to avoid prescribing decisions based on the concern for antibiotic-resistant pathogens as well as to exclude children who might be returning to the office for treatment failure and, thus, the need for an alternative antibiotic choice), or antibiotic allergy. Fourth, we adjusted for factors that might impact the incidence of disease (age) or choice of antibiotic (insurance type) as well as sex and race. Fifth, we chose conditions that were not only common but have evidence-based treatment guidelines that, when managed in the outpatient setting, do not call for differences in antibiotic choice according to severity of illness. Thus, comparisons included similar, previously healthy children with homogenous clinical presentations, differing only by the pediatric practice/clinician. Although we were unable to assess potential practice/clinician-level factors that might account for these differences, awareness of prescribing guidelines or parental pressure to prescribe (real or perceived) might have contributed.
Although our primary goal was to compare antibiotic prescribing across practices, the observed differences demanded an evaluation of ARTI diagnosis rates. Because the 4 bacterial ARTIs examined account for more than 80% of antibiotic prescribing in pediatric primary care [ 2 ], differences in the rate of diagnosis of these conditions could explain differences in overall prescribing rates. Consistent with this, the adjustment for overall antibiotic prescribing rates by bacterial ARTI diagnosis rates largely attenuated differences in total antibiotic prescribing. However, it is unclear whether the desire to prescribe (and justify the use of) an antibiotic led to more permissive use of ARTI diagnoses, or if true differences in perceived infections contribute to prescribing differences. The exclusions and adjustments outlined above favor the former explanation, because there should not be significant differences in the true prevalence of ARTIs across practices after comparing encounters standardized by patient-level factors. In support of this hypothesis, we found significant differences in across-practice diagnosis rates of ARTIs, which rely on a largely subjective mode of diagnosis, but a relatively uniform rate for UTIs, which benefit from a more objective (ie, laboratory-based) diagnosis. However, this potential explanation applies only to the comparison of total antibiotic use per sick visit and not to the choice of antibiotic (ie, broad-spectrum vs narrow spectrum), either overall or within specific ARTIs. Accordingly, differences in antibiotic selection (given an antibiotic prescription) were unaffected by adjustment by ARTI type.
Although these analyses do not identify the appropriate rates of antibiotic prescribing and diagnosis of ARTIs, it is likely that lower rates of broad-spectrum antibiotic prescribing and ARTI diagnosis are closer to the ideal. Regarding antibiotic choice, narrow-spectrum antibiotics are indicated (and recommended by the AAP) for the ARTIs targeted in this study. Because AOM and sinusitis are often self-limiting and the rate of “true” streptococcal pharyngitis is influenced by the rate of streptococcal testing, lower rates of diagnosis and antibiotic use for these conditions—as practiced by some clinician groups in this network—is likely safe and appropriate and might serve as achievable benchmarks for the management of ARTIs in outpatient children; however, rigorously performed comparative effectiveness studies comparing outcomes between variable prescribing and diagnosis strategies are necessary to confirm this hypothesis. These future studies will be particularly informative given the emergence of more permissive antibiotic recommendations for common conditions including AOM and sinusitis.
The implications of antibiotic overuse are profound. Of more than 100 000 antibiotic prescriptions to children in this single, unified pediatric care network, approximately 40% were broad-spectrum. Thus, a modest 10% reduction in off-guideline prescribing would result in approximately 4000 fewer unnecessary broad-spectrum antibiotic prescriptions, and, assuming a mean duration of 10 days, approximately 40 000 fewer antibiotic days. If applied to the approximately 70 million antibiotic prescriptions to US children annually [ 1 ], approximately half of which are broad-spectrum, 3.5 million fewer broad-spectrum prescriptions and 35 million fewer antibiotic days would occur. Therefore, to the extent that our sample from a single network might apply to other primary care pediatrics practices, our findings merit sustained intervention. The variability in the diagnosis and treatment of high-frequency presentations, nonadherence to prescribing guidelines, and the uptake of primary care EHRs create an ideal setting to improve the management of these common conditions. Variability in prescribing likely reflects opportunities to reduce excessive antibiotic use [ 11 , 15 ].
This study had limitations. First, the results from any single geographical area might not be generalizable. However, this large network spans urban, rural, and suburban settings across two states in both academic and community practice environments, many of which were previously private-practice groups, and the overall proportion of encounters resulting in antibiotic prescriptions (28%) mirrors prior national estimates [ 1–3 ]. Second, data were collected from an EHR using ICD-9 codes to identify ARTIs. To ameliorate this, we used multifactorial case definitions (eg, streptococcal pharyngitis required an acute pharyngitis code, antibiotic receipt, and a positive laboratory test) to reduce misclassification, and we applied consistent case definitions across practices. Third, because diagnosis and antibiotic prescribing are linked, it is unclear whether the desire to prescribe antibiotics might be driving the increased rate of diagnosis. Therefore, our case definitions for ARTIs of interest included children with both an ICD-9 code and an antibiotic prescription. These definitions were designed primarily to examine the choice of antibiotic (narrow or broad) given an antibiotic prescription or diagnosis. Furthermore, since some codes (ie, pneumonia, sinusitis) might be applied to children with either presumed viral or bacterial disease, the inclusion of an antibiotic served to isolate those with presumed bacterial infection (and the subsequent antibiotic choice given this assumption). Fourth, when prescribing occurs in a complex care setting wherein panels of patients are seen by a single clinician and clinicians are clustered by practice sites, quantifying variation can be challenging. Nevertheless, the observed variation certainly exceeded what one would expect at random, even when our variance estimates accounted for the hierarchical data structure.
In conclusion, we observed wide variation in diagnosis and management behaviors across primary care pediatric practices, despite adjustment through exclusion and regression for patient clinical and demographic factors that might influence antibiotic choice or diagnosis rates. When variation of this magnitude is observed within primary care practice, benchmarking data can be used to inform outpatient antimicrobial stewardship interventions targeting specific practices, providers, and conditions.
Acknowledgments
We thank Lihai Song (Healthcare Analytics Unit, CHOP Center for Pediatric Clinical Effectiveness) for preparing the data for analysis. We also thank the network of primary care clinicians and their patients and families for contributions to clinical research through the Pediatric Research Consortium at The Children's Hospital of Philadelphia.
Financial support . This work was supported by the US Agency for Health Care Research and Quality contract no. HHSA2900200710013.
Potential conflicts of interest . All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Chai G, Governale L, McMahon AW, et al. Trends of outpatient prescription drug utilization in US children, 2002–2010 . Pediatrics 2012. ; 130 : 23 – 31 . [DOI] [PubMed] [Google Scholar]
- 2. Hersh AL, Shapiro DJ, Pavia AT, Shah SS . Antibiotic prescribing in ambulatory pediatrics in the United States . Pediatrics 2011. ; 128 : 1053 – 61 . [DOI] [PubMed] [Google Scholar]
- 3. Grijalva CG, Nuorti JP, Griffin MR . Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings . JAMA 2009. ; 302 : 758 – 66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Committee on Infectious Diseases, A.A.o.P. In: Pickering LK , ed. Principles of Appropriate Use for Upper Respiratory Tract Infections . Red Book: 2009 Report of the Committee on Infectious Diseases , 28 Ed . Elk Grove Village, IL: : American Academy of Pediatrics; ; 2012. ; pp 802 – 805 . [Google Scholar]
- 5. Nyquist AC, Gonzales R, Steiner JF, Sande MA . Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis . JAMA 1998. ; 279 : 875 – 7 . [DOI] [PubMed] [Google Scholar]
- 6. Nash DR, Harman J, Wald ER, Kelleher KJ . Antibiotic prescribing by primary care physicians for children with upper respiratory tract infections . Arch Pediatr Adolesc Med 2002. ; 156 : 1114 – 9 . [DOI] [PubMed] [Google Scholar]
- 7. Gonzales R, Malone DC, Maselli JH, Sande MA . Excessive antibiotic use for acute respiratory infections in the United States . Clin Infect Dis 2001. ; 33 : 757 – 62 . [DOI] [PubMed] [Google Scholar]
- 8. McCaig LF, Besser RE, Hughes JM . Trends in antimicrobial prescribing rates for children and adolescents . JAMA 2002. ; 287 : 3096 – 102 . [DOI] [PubMed] [Google Scholar]
- 9. Linder JA, Bates DW, Lee GM, Finkelstein JA . Antibiotic treatment of children with sore throat . JAMA 2005. ; 294 : 2315 – 22 . [DOI] [PubMed] [Google Scholar]
- 10. Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship . Clin Infect Dis 2007. ; 44 : 159 – 77 . [DOI] [PubMed] [Google Scholar]
- 11. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial . JAMA 2013. ; 309 : 2345 – 52 . [DOI] [PubMed] [Google Scholar]
- 12. Feudtner C, Christakis DA, Connell FA . Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997 . Pediatrics 2000. ; 106 : 205 – 9 . [PubMed] [Google Scholar]
- 13. Korn EL, Graubard BI . Analysis of Health Surveys . New York: John Wiley & Sons, Inc; ; 1999. . [Google Scholar]
- 14. Rashbash J, Steele F, Browne WJ, Goldstein H . A User's Guide to MLwiN . Centre for Multilevel Modelling. UK: University of Bristol; ; 2012. . Available at: http://www.bristol.ac.uk/cmm/software/mlwin/download/2-26/manual-web.pdf . Accessed June 2013 . [Google Scholar]
- 15. Linder JA, Schnipper JL, Tsurikova R, et al. Electronic health record feedback to improve antibiotic prescribing for acute respiratory infections . Am J Manag Care 2010. ; 16 : e311 – 9 . [PubMed] [Google Scholar]