Abstract
Introduction and aim
Heart diseases, and in particular coronary artery disease (CAD), are the leading cause of death in Europe and it represents around 50% of overall mortality. Still many cardiac markers are under investigation with an aim to evaluate patients to different risk groups and predict more adequate cardio-cerebral events. Chemokines which regulate immune cell vascular chemotaxis, including CCL5/RANTES are points of great interest.
We hypothesized that chemokine RANTES level measured in peripheral blood may be associated with severity of atherosclerosis in patients with stable angina undergoing coronary angiography.
Material and methods
RANTES and IL-18 levels were measured by ELISA method. Classical and novel cardiovascular risk factors like brachial flow mediated dilation and intima-media thickness were analyzed.
Study included 62 consecutive patients with coronary atherosclerosis proven in coronary angiography, mean age 59.3 years (SD = 7.4), divided into group with lower amount of atherosclerosis, (Group I, 45 patients) and with severe coronary disease (Group II, 17 patients). Groups were well balanced for classic risk factors.
Results
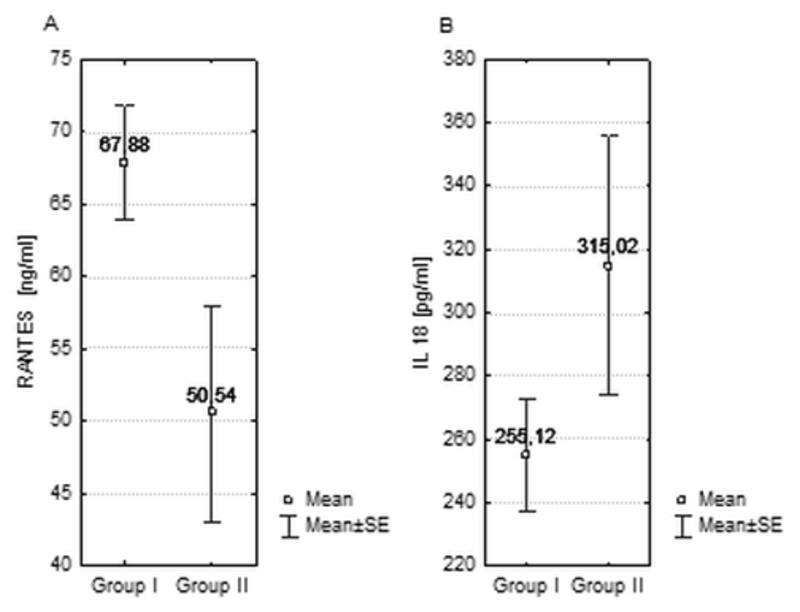
Mean RANTES level were significantly higher in patients in group I (67.9 ng/ml, SEM = 3.97) than group II (50.5 ng/ml, SEM = 7.49; p = 0.03). IL-18 levels were similar (255 pg/ml in group I and 315 pg/ml, SEM = 40.91 in group I, p = 0.12), as well as hsCRP concentration (3,45 SEM = 2.66 ng/ml and 4,69 ng/ml SEM= 1.64 ng/ml respectively; p = 0.47). FMD values have been significantly lower in group II than in group I (6.31; SEM = 0.61; vs 4.41; SEM = 0,56, respectively, p = 0.026).
No significant correlations between chemokine RANTES levels and IMT, FMD measurements have been found in both groups of patients (p > 0,05).
Conclusions
Chemokine RANTES level could become a useful marker of severity of coronary artery disease. Its lower levels were observed in patients with more diffuse disease. Elevated level of chemokine RANTES in patients with stable angina pectoris may evaluate patients to high risk group in plaque formation at early stages of atherosclerosis.
Introduction
Heart diseases, and in particular coronary artery disease (CAD), are the leading cause of death in Europe and it represents around 50% of overall mortality. (43% - 1 963 644 male and 55% - 2 307 945 female)(1). While classical risk factors for CAD are relatively well defined (1), they seem to be responsible for only part of the risk while remaining risk, often referred to as residual risk is less understood. Thus, it is of great importance to determine biomarkers of high cardiovascular risk. New risk markers including: inflammatory molecules (ex. CRP, selected interleukins), homocysteine, vWF, fibrynogen IMT (Intima Media Thickness) and calcium score are widely proven to predict adverse cardiovascular events (2,3). Aortic stiffnes also progresses with bigger extent of atherosclerosis (4), Still many cardiac markers are under investigation with an aim to evaluate patients to different risk groups and predict more adequate cardio-cerebral events.
There is particular focus on understanding the role of inflammation in cardiovascular disease. Cytokines are important regulators of leukocyte activation and migration to the sites of inflammation including vessels and the heart. Especially chemokines which regulate immune cell vascular chemotaxis are points of great interest. It has been shown that some of the chemokines such as CCL3/MIP1a, CCL5/ RANTES, and CCL18/PARC are expressed in atherosclerotic plaque (5–7), and cells within the plaque express CCL5/RANTES receptors such as: CCR5, CCR1, CCR3, CCR9 and DARC may express in different structures. The expression of DARC receptor is specific for the endothelium (8). Significant correlation between chemokine RANTES, as well as CCL18/PARC and CCL3/MIP-1α level and mortality risk in patients with acute coronary syndromes (including UA patients) has been demonstrated (9,10). Chemokine RANTES is increased in patients with myocardial infarction, especially complicated by left ventricular heart failure (LVEF <35%) (11). In animal studies a reduction of myocardial injury after infarction and reduction of heart failure was caused by after antiCCL5 mAb monoclonal antibody or RANTES inhibitors administration. The protective effect was observed due to the smaller migration of leukocytes to the sites of myocardial injury(12,13). Moreover high RANTES expression in perivascular adipose tissue plays an important role in vascular inflammatory cell recruitment which mediated endothelial dysfunction(14,15). In the ARIC study a significant correlation between RANTES level and IMT was found(16). On the other hand Cavusoglu et al. in evaluation of patients undergoing coronary angiography has shown that low RANTES level in peripheral blood is an independent predictor of mortality and MI in patients without previous MI. In the diabetes subgroup of patients similar correlation was also found(17). Thus, role of RANTES as a biomarker in CAD remains controversial.
We hypothesized that chemokine RANTES level measured in peripheral blood may be associated with severity of atherosclerosis in patients with stable angina undergoing coronary angiography.
Methods
Study population
Consecutive patients with stable angina who underwent coronary angiography in our Center were included in the study. Patients were qualified to coronary angiography based on clinical presentation and positive results of additional non invasive examiantions such as excersize stress test, imaging methods including dobutamine stress echo and SPECT (single photon emission tomography). Angina has been analyzed and estimated based on the Canadian Cardiovascular Classification (CCS).
The exclusion criteria included: age <40 years old, symptomatic heart failure, reduced left ventricular ejection fraction <45%, unstable angina or acute coronary syndrome in the last 6 months prior to admission, severe valvular heart disease, chronic inflammatory diseases. If not stated otherwise, data were presented as mean value with standard deviation.
This study complies with the guidelines in the Declaration of Helsinki. Ethics approval was granted by the Jagiellonian University Collegium Medicum ethics committee. After having supplied the complete information about the study to all the patients, prior to the inclusion into the study, patients’ written and signed consents were obtained.
Biochemical Analyses
Blood samples in all patients have been taken at the admission to the Department of Hemodynamics and Angiocardiography after obtaining informed consent and before administering any drugs related to coronary angiogrpahy. Samples were stored at the local laboratory in the temperature of -20 degree Celsius. Chemokine RANTES level in serum was measured by ELISA method and expressed in ng/ml. Anti-human RANTES ELISA kits (R&D Systems, Minneapolis, MN, USA) were used according to the manufacturers’ protocol.
Interleukin-18 level was measured by ELISA method. Human IL-18 Elisa Kit (MBL code no 7620) was used according to the manufacturers’ protocol Hs-CRP was assessed by Hitachi/Roche Cobas c system, using Cardiac C-Reactive Protein High Sensitivity Assay (Roche Diagnostics, Mannheim, Germany) according to supplier protocol.
All laboratory measurements were obtained in Pharmacology Department of the Jagiellonian University Collegium Medicum Hospital and the laboratory of the John Paul II Hospital in Cracow.
Coronary artery disease severity estimation
All coronary angiography procedures were performed in the Department of Hemodynamics and Angiocardiography Jagiellonian University Collegium Medicum in John Paul II Hospital in Cracow on the Siemens Coroscop. Right and left coronary arteries were analyzed. The degree of stenosis was assessed in optimal projections independently and separately by two operators. Stenosis of 50% was considered to be significant according to the ESC guidelines Significant narrowing in three or more arteries was considered as multivessel disease. Based on the number of significantly stenosed coronary arteries patients were divided into two groups: group I with ≤ 2 main coronary arteries (left anterior descendens coronary artery, circumflex coronary artery, right coronar artery) with significant stenosis and group II with with multivessel disease (all 3 main coronary arteries with significnt stenoses). Detailed information about the involvement of coronary arteries in study subjects is presented in table 1 in the results section.
Table 1.
Group I and II baseline characteristics and CAD risk factors, pharmacotherapy
| mean (+/-) | |||
|---|---|---|---|
| group I | group II | p | |
| Male:female ratio (%) | 28:17 (62.2%) | 14:3 (82.3%) | 0.13 |
| age [years old] | 58.5 (1.95) | 61.4 (1.92) | 0.18 |
| waist diameter[cm] | 98.71 (1.40) | 104.88 (2.43) | 0.04 |
| BMI [kg/m2] | 28.95 (0.46) | 29.66 (1.01) | 0.53 |
| Positive family history for CAD | 24 (54.5%) | 8(46.6%) | 0.60 |
| hipercholesterolemia during statin or fibrate treatment or LDL >3 mmol/l | 44 (97.78%) | 16 (94.12%) | 0.48 |
| Diabetes | 7 (15.56%) | 6 (35.29%) | 0.10 |
| smoking | 25 (55.56%) | 11 (64.71%) | 0.51 |
| hypertension | 39 (86.67%) | 16 (94.12%) | 0.66 |
| Overweight or obesity | 42 (93.33%) | 15 (88.24%) | 0.61 |
| Total cholesterol [mmol/l] | 5.02 (0.18) | 4.55 (0.20) | 0.14 |
| LDL [mmol/l] | 2.92 (0.15) | 2.69 (0.17) | 0.40 |
| HDL [mmol/l] | 1.23 (0.05) | 1.21 (0.07) | 0.77 |
| TG [mmol/l] | 1.63 (0.12) | 1.37 (0.23) | 0.30 |
| CCS: | |||
| CCS I | 5 (11.11%) | 2 (11.76%) | 0.94 |
| CCS II | 24 (53.33%) | 7 (41.18%) | 0.39 |
| CCS III | 4 (8.89%) | 8 (47.06%) | < 0,001 |
| Non-specific symptoms | 12 (26.67%) | 0 (%) | 0.02 |
| pharmacotherapy: | |||
| acetylosalicylic acid | 41 (91.1%) | 16 (94.1%) | 0.70 |
| ACEI | 38 (84.4%) | 15 (88.2%) | 0.71 |
| statin | 35 (77.8%) | 14 (82.4%) | 0.69 |
| beta-blocker | 30 (66.7%) | 11 (64.7%) | 0.88 |
| nitrate | 20 (44.4%) | 12 (70.6%) | 0.07 |
| Ca-blocker | 11 (24.4%) | 7 (41.2%) | 0.20 |
| diuretic | 12 (26.7%) | 3 (17.6%) | 0.46 |
| fibrate | 3 (6.7%) | 1 (5.9%) | 0.91 |
| ARB | 2 (4.4%) | 0 (0.0%) | 0.38 |
BMI body mass index; CAD coronary artery disease; CCS Canadian Cardiovascular Society scale; ACEI angiotensin convering enzyme inhibitor; ARB angiotensin receptor blocker
Risk factors
Cardiovascular risk factors were assessed according to the current ESC guidelines (1). A current smoker was defined as having smoked at least for 1 month during the previous 12 months; all others were classified as non-smokers. Body mass index (BMI) was calculated as weight [kg]/height [m]2. Based on BMI value, patients were categorized as normal weight (BMI < 25 kg/m2), overweight (BMI >25 kg/m2 and < 30 kg/m2), and obese (BMI > 30 kg/m2).
Brachial flow mediated dilatation
Brachial flow mediated dilatation was obtained according to the Brachial Artery Reactivity Task Force indications(18). As previously described (4) measurements were taken in patients who have eaten their last meal and have been administrated medication at least 12 hours before the measurement. All measurements were obtained in the same quiet conditions (patient lying in horizontal position, right arm). Diameter of the brachial artery was measured at 1 to 2 cm above the elbow in diastole (measurement was synchronized with ECG) before ischemia of the right arm and after cuff deployment. Diameter was defined as the distance between the nearest and further m line (border between middle layer and adventitia obtained in 2D presentation). Results were taken from mean values of 5 consecuitive heart beats. Ischemia was induced due to inflation of the standard blood preasure cuff inflated to 250 mmHg for 5 minutes. Endothelial dependent (bFMD), as well as the independent/nitrate mediated (bEID/NMD) brachial artery dilatation were analized (18).
Intima-media thickness
Intima-media thickness was measured by linear head of the ultrasonography at 8 MHz frequency. Both right and left common carotid arteries were analyzed in two projections: anterior and postero-lateral in systole. In order to visualize arterial wall both projections were taken with ultrasonography head placed parallel to the artery. Measurements were recorded in highest resolution to and best parts, without artefacts were considered for analysis (all measurements were synchronized with ECG. Estimated value was mean from 12 measurements obtained 1 cm below carotid bulb, excluding parts with atherosclerotic plaques. (19,20).
Statistical analysis
Characteristics of patients were analyzed by means od descriptive statistics. If not mentioned otherwise, data were presented as mean value with standard deviation. Continue values were compared with two-way t-test, dichotomous values were compared using chi-square test. Correlation coeficients were obtained by Pearson’s method. All analyses were made using StatSoft Statitica software. In all analyses assumed 95% confidence interval (α = 0.05).
Results
62 consecutive patients with stable angina, aged 59.3 ± 7.4 y.o. (from 46 to 78) were enrolled. Group I consisted of 45 patients: 12 patients with single vessel stenosis of more than 50%, 12 with two vessel disease and 21 without significant stenoses in coronary arteries. Group II conisted of 17 patients of which two patients had signifficant left main disease. The prevalence of cardiovascular risk factors and treatment were similar in both groups despite waist circumference which was higher in group II. Symptoms were more severe in group II (p <0.001). Baseline characteristics of patients were summarized in tables 1 and 2.
Table 2.
Number of coronary areries significantly stenosed in group I and II. Left anterior descending (LAD), circumflex artery (Cx), right coronary artery (RCA), left main coronary artery (LMCA).
| stenosis | Group I (N = 45) |
Group II (N = 17) |
All (N = 62) |
|---|---|---|---|
| No signifficant | 21 (46.7%) | 0 (0.0%) | 21 (33.9%) |
| isolated LAD | 6 (13.3%) | 0 (0.0%) | 6 (9.7%) |
| isolated Cx | 2 (4.4%) | 0 (0.0%) | 2 (3.2%) |
| isolated RCA | 4 (8.9%) | 0(0.0%) | 4 (6.5%) |
| LAD + Cx | 2 (4.4%) | 0 (0.0%) | 2 (3.2%) |
| LAD + RCA | 4 (8.9%) | 0 (0.0%) | 4 (6.5%) |
| Cx + RCA | 5 (11.1%) | 0 (0.0%) | 5 (8.1%) |
| LMCA | 0 (0%) | 2*(6.7%) | 2 (3.2%) |
| LAD + Cx + RCA | 0 (0.0%) | 17 (100%) | 17 (27.4%) |
in both patients significant stenosis of LAD, Cx and RCA was present
Correlations between chemokine RANTES and IL-18 level in group I and II
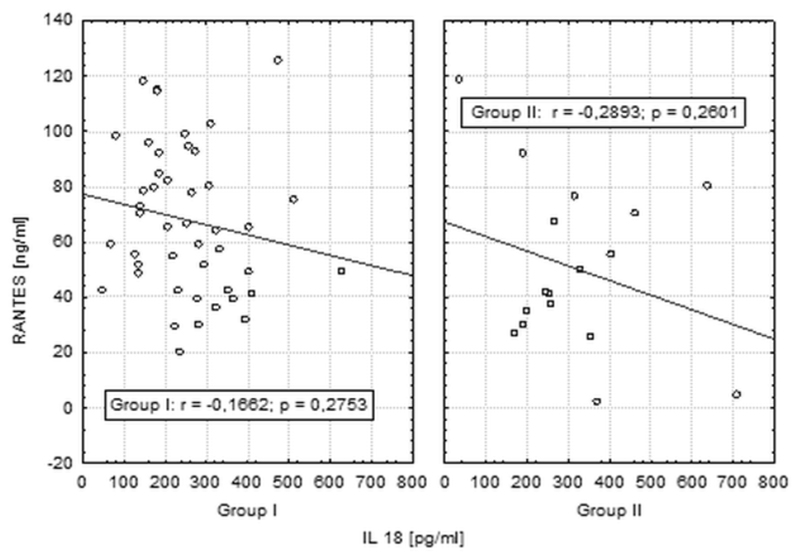
Mean RANTES level were significantly higher in patients in group I than group II (respectively 67.9 ng/ml, SEM = 3.97vs 50.5 ng/ml, SEM = 7.49; p = 0.03). No significant difference in IL-18 measurements has been noticed between the groups (mean IL-18 level in 255 pg/ml in group II and 315 pg/ml, SEM = 40.91 in group I, p = 0.12) as shown in Figure 1. We did not observe any correlation between RANTES and IL-18 levels (Figure 2).
Figure 1.
Mean levels of RANTES (A) and interleukin 18 (B) in groups I and II.
Figure 2.
Correlations between RANTES and IL-18 in groups I and II.
Similarly, hsCRP levels didn’t differ significantly between group I and group II (3,45+/- 2.66 ng/ml vs 4,69 ng/ml +/- 1.64 ng/ml; p = 0.47).
FMD and IMT in the study group
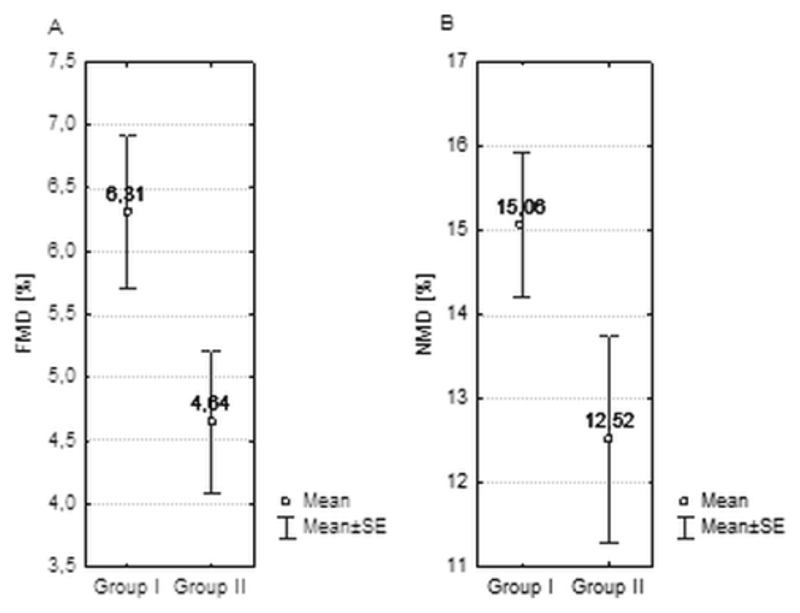
FMD values have been significantly lower in group II (respectively 6.31; SEM = 0.61; vs 4.41; SEM = 0,56 p = 0.026). In NMD measurements no significant differences have been noticed.
No significant differences in mean IMT measurements between groups have been shown (0.91mm vs. 0.85mm) (table 3 and figure 3).
Table 3.
FMD, NMD and IMT mean values in both groups.
| parameter | mean (SEM) | ||
|---|---|---|---|
| Group I | Group II | p | |
| FMD (%) | 6.31 (0.61) | 4.41 (0.56) | 0.0259 |
| NMD (%) | 15.06 (0.86) | 12.52 (1.23) | 0.1028 |
| IMT (mm) | 0.85 (0.03) | 0.91 (0.17) | 0.3700 |
Figure 3.
Mean FMD (panel A) and NMD (panel B) measurements in group I and II.
Correlations between endothelial function (FMD), IMT and chemokine RANTES level in group I and II
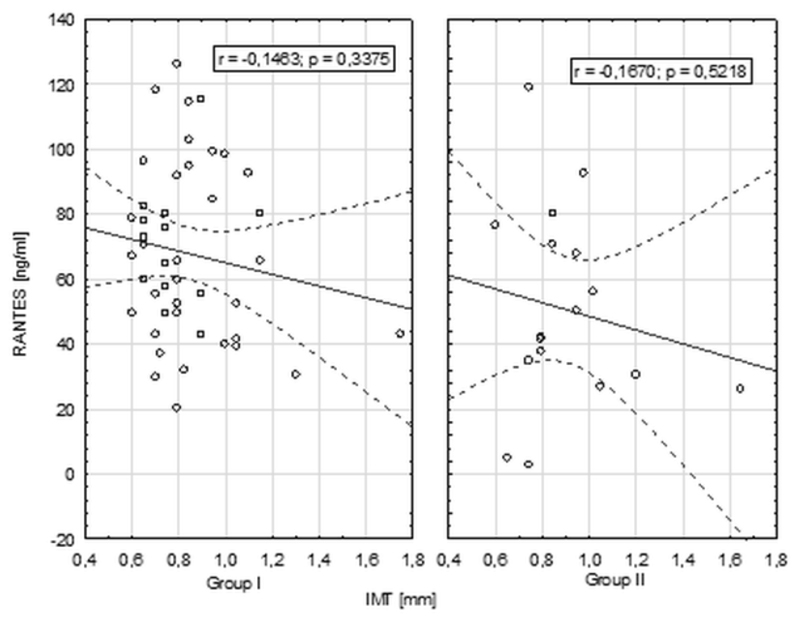
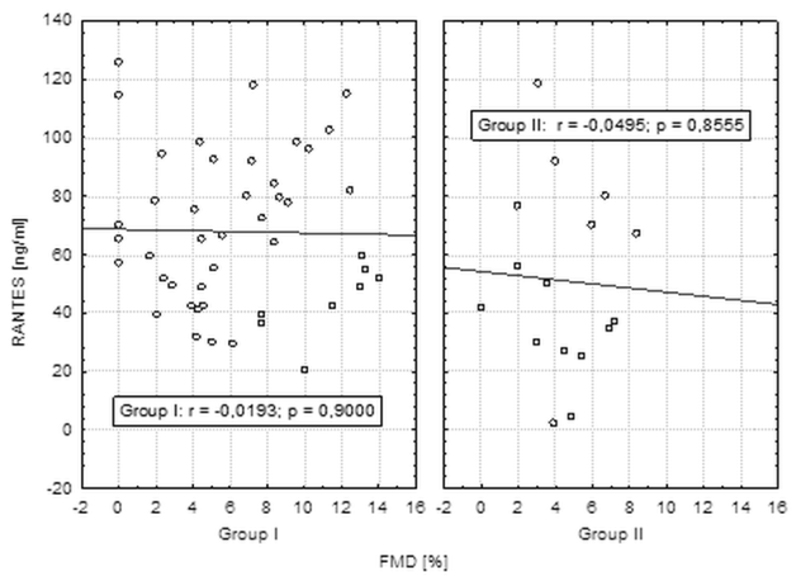
No significant correlations between chemokine RANTES levels and IMT, FMD measurements have been found in both groups of patients (Figure 4 and 5).
Figure 4.
Correlation between chemokine RANTES levels and IMT measurements.
Figure 5.
Correlations between chemokine RANTES and FMD level in groups I and II.
Discussion
Among the large group of inflammatory molecules Hs-CRP has been the best studied inflammatory molecule of the “new” biomarkers. It provides clear and predictive value beyond that of traditional risk factors as a marker of cardiovascular risk in potentially healthy individuals and in CAD patients(21–23). In our study no significant correlations were found probably due to relative small groups sizes. Other inflammatory molecules have also been proposed as biomarkers in CAD, and some of them including IL-6, IL-18, intracellular adhesion molecule [ICAM]-1 have proven to provide some prognostic information beyond that of CRP(24–26). In our study we have evaluated IL-18 level and found no significant relationship between IL-18 serum level and the severity of atherosclerosis. Furthermore, no significant correlations with chemokine RANTES level have been found.
However, data on chemokine RANTES serum level and the correlations are equivocal. Chemokine RANTES/CCL5 role in atherosclerosis is confirmed, and recent research focuses on its gene polimorphisms and their predictive role as a risk factor of coronary artery disease (27,28). Similar role of severity predictor would play increased mRNA expression of catecholamine-synthesizing enzymes in heart failure (29). According to previous studies, low serum RANTES levels were associated with more advanced coronary atherosclerosis and were an independent predictor of poor outcome in terms of cardiovascular mortality and acute myocardial infarction, in numerous cases associated with reperfusion induced arrhytmia(17,30,31). On the other hand Kraaijeveld et al. showed, that higher RANTES levels were observed in patients with persistent angina, compared to those without clinical symptoms(10). There were also positive correlations of RANTES levels and volume of an atherosclerotic plaque in patients with carotid atherosclerosis published(32).
In our study we have discovered, that more severe coronary atherosclerosis, assessed in coronary angiography, is correlated with lower RANTES levels. Direct mechanism of this phenomenon is not well explained. We suspect, that RANTES serum levels decrease together with progression of coronary atherosclerosis. According to the research done by Guzik T. et al., this might be due to migration of chemokine RANTES to the inside of arterial wall or atherosclerotic plaque in patients with severe atherosclerosis [data not published]. Due to that phenomenon its level in peripheral blood would decrease. Similar effect was recently discovered by Herder et el. in histological studies of atherosclerotic plaques removed during carotid endarterectomy, where higher RANTES levels were observed in more unstable lesions, compared to more calcified ones(32). We suspect that some undiscovered phenomenon intercellular interaction level may play a role, just like conexin alterations influence pathogenesis of atrial arrhytmia(33).
We found no data in the literaure corelating directly the chemokine RANTES level with the FMD and IMT measurements. In our study we evaluated this relationship and no significant correlations between RANTES serum levels and endothelial disfunction measured by FMD and IMT measurements were found. Although they seem to apear in higher levels in patients with severe atherosclerosis but the significance of the results may be impaired due to relative small groups sizes. Further studies on large patient population might provide some new insights to this analysis.
Study limitations
As coronarography is an invasive procedure, low number of patients were enrolled, causing widening of confidence intervals, and in some cases, probably loss of statistical significance of observed differences. Study limitations include also the semi-quantitive way of assessment of atherosclerosis extent, based only on number of diseased vessels, and lack of more detailed information on lesions composition in particular patients. Importance of cardiovascular disease as an increasing worldwide problem and some promising results of this study should lead to further research in chemokine RANTES evaluation as a molecule presenting in the early stages of cardiovascular disease and qualifying patients to high risk group with CAD.
Conclusions
Chemokine RANTES level could become a useful marker of severity of coronary artery disease. Its lower levels were observed in patients with more diffuse disease. Elevated level of chemokine RANTES in patients with stable angina pectoris may evaluate patients to high risk group in plaque formation at early stages of atherosclerosis.
References
- 1.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by re. Eur Heart J. 2012;33(13):1635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 2.Podolec P, Kopeć G, Pajak A, et al. Polish forum for prevention guidelines on cardiovascular risk assessment. Kardiol Pol. 2007;65(1):100–4. [PubMed] [Google Scholar]
- 3.Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012) : the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice. Int J Behav Med. 2012;19(4):403–88. doi: 10.1007/s12529-012-9242-5. [DOI] [PubMed] [Google Scholar]
- 4.Kopeć G, Podolec P, Podolec J, Rubiś P, Zmudka K, Tracz W. Atherosclerosis progression affects the relationship between endothelial function and aortic stiffness. Atherosclerosis. 2009;204(1):250–4. doi: 10.1016/j.atherosclerosis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox JN, Nelken NA, Coughlin SR, Gordon D, Schall TJ. Local expression of inflammatory cytokines in human atherosclerotic plaques. J Atheroscler Thromb. 1994;1(Suppl 1):S10–3. doi: 10.5551/jat1994.1.supplemment1_s10. [DOI] [PubMed] [Google Scholar]
- 6.Reape TJ, Rayner K, Manning CD, et al. Expression and cellular localization of the CC chemokines PARC and ELC in human atherosclerotic plaques. Am J Pathol. 1999;154(2):365–74. doi: 10.1016/S0002-9440(10)65283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hägg DA, Olson FJ, Kjelldahl J, et al. Expression of chemokine (C-C motif) ligand 18 in human macrophages and atherosclerotic plaques. Atherosclerosis. 2009;204(2):e15–20. doi: 10.1016/j.atherosclerosis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien AD, Standiford TJ, Christensen PJ, Wilcoxen SE, Paine R. Chemotaxis of alveolar macrophages in response to signals derived from alveolar epithelial cells. J Lab Clin Med. 1998;131(5):417–24. doi: 10.1016/s0022-2143(98)90142-1. [DOI] [PubMed] [Google Scholar]
- 9.de Jager SCA, Bongaerts BWC, Weber M, et al. Chemokines CCL3/MIP1α, CCL5/RANTES and CCL18/PARC are independent risk predictors of short-term mortality in patients with acute coronary syndromes. PLoS One. 2012;7(9):e45804. doi: 10.1371/journal.pone.0045804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraaijeveld AO, de Jager SCA, de Jager WJ, et al. CC chemokine ligand-5 (CCL5/RANTES) and CC chemokine ligand-18 (CCL18/PARC) are specific markers of refractory unstable angina pectoris and are transiently raised during severe ischemic symptoms. Circulation. 2007;116(17):1931–41. doi: 10.1161/CIRCULATIONAHA.107.706986. [DOI] [PubMed] [Google Scholar]
- 11.Parissis JT, Adamopoulos S, Venetsanou KF, Mentzikof DG, Karas SM, Kremastinos DT. Serum profiles of C-C chemokines in acute myocardial infarction: possible implication in postinfarction left ventricular remodeling. J Interferon Cytokine Res. 2002;22(2):223–9. doi: 10.1089/107999002753536194. [DOI] [PubMed] [Google Scholar]
- 12.Montecucco F, Braunersreuther V, Lenglet S, et al. CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. Eur Heart J. 2012;33(15):1964–74. doi: 10.1093/eurheartj/ehr127. [DOI] [PubMed] [Google Scholar]
- 13.Braunersreuther V, Steffens S, Arnaud C, et al. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler Thromb Vasc Biol. 2008;28(6):1090–6. doi: 10.1161/ATVBAHA.108.165423. [DOI] [PubMed] [Google Scholar]
- 14.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–60. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58(4):591–610. [PubMed] [Google Scholar]
- 16.Virani SS, Nambi V, Hoogeveen R, et al. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Eur Heart J. 2011;32(4):459–68. doi: 10.1093/eurheartj/ehq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavusoglu E, Eng C, Chopra V, Clark LT, Pinsky DJ, Marmur JD. Low plasma RANTES levels are an independent predictor of cardiac mortality in patients referred for coronary angiography. Arterioscler Thromb Vasc Biol. 2007;27(4):929–35. doi: 10.1161/01.ATV.0000258789.21585.76. [DOI] [PubMed] [Google Scholar]
- 18.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 19.Megnien JL, Simon A, Gariepy J, et al. Preclinical changes of extracoronary arterial structures as indicators of coronary atherosclerosis in men. J Hypertens. 1998;16(2):157–63. doi: 10.1097/00004872-199816020-00005. [DOI] [PubMed] [Google Scholar]
- 20.Bots ML, Grobbee DE, Hofman A, Witteman JCM. Common carotid intima-media thickness and risk of acute myocardial infarction: the role of lumen diameter. Stroke. 2005;36(4):762–7. doi: 10.1161/01.STR.0000158924.71069.94. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N Engl J Med. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 23.Zebrack JS, Muhlestein JB, Horne BD, Anderson JL. C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol. 2002;39(4):632–7. doi: 10.1016/s0735-1097(01)01804-6. [DOI] [PubMed] [Google Scholar]
- 24.Biasucci LM, Liuzzo G, Fantuzzi G, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99(16):2079–84. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- 25.Hulthe J, McPheat W, Samnegård A. Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6. Atherosclerosis. 2006;188(2):450–4. doi: 10.1016/j.atherosclerosis.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet (London, England) 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Jia Y-J, Li X-L, et al. RANTES gene G-403A polymorphism and coronary artery disease: a meta analysis of observational studies. PLoS One. 2012;7(10):e47211. doi: 10.1371/journal.pone.0047211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Hu X, Zhang S, Xu X, Wang J. Association of the CCR5Δ32 polymorphism and its ligand RANTES-403G/A polymorphism with coronary artery disease: a meta-analysis. Thromb Res. 2013;131(3):e77–84. doi: 10.1016/j.thromres.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Tomaszek A, Kiczak L, Bania J, et al. Increased gene expression of catecholamine-synthesizing enzymes in adrenal glands contributes to high circulating catecholamines in pigs with tachycardia-induced cardiomyopathy. J Physiol Pharmacol. 2015;66(2):227–31. [PubMed] [Google Scholar]
- 30.Rothenbacher D, Müller-Scholze S, Herder C, Koenig W, Kolb H. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler Thromb Vasc Biol. 2006;26(1):194–9. doi: 10.1161/01.ATV.0000191633.52585.14. [DOI] [PubMed] [Google Scholar]
- 31.Szepesi J, Acsai K, Sebok Z, et al. Comparison of the efficiency of Na+/Ca2+ exchanger or Na+/H+ exchanger inhibition and their combination in reducing coronary reperfusion-induced arrhythmias. J Physiol Pharmacol. 2015;66(2):215–26. [PubMed] [Google Scholar]
- 32.Herder C, Peeters W, Illig T, et al. RANTES/CCL5 and risk for coronary events: results from the MONICA/KORA Augsburg case-cohort, Athero-Express and CARDIoGRAM studies. PLoS One. 2011;6(12):e25734. doi: 10.1371/journal.pone.0025734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tribulova N, Egan Benova T, Szeiffova Bacova B, Viczenczova C, Barancik M. New aspects of pathogenesis of atrial fibrillation: remodelling of intercalated discs. J Physiol Pharmacol. 2015;66(5):625–34. [PubMed] [Google Scholar]