Abstract
Purpose
The aim was to investigate the association between pre-diagnostic intakes of polyphenol classes (flavonoids, lignans, phenolic acids, stilbenes and other polyphenols) in relation to breast cancer survival (all-cause and breast cancer-specific mortality).
Methods
We used data from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Pre-diagnostic usual diet was assessed using dietary questionnaires, and polyphenol intakes were estimated using the Phenol-Explorer database. We followed 11,782 breast cancer cases from time of diagnosis until death, end of follow-up or last day of contact. During a median of 6 years, 1,482 women died (753 of breast cancer). We related polyphenol intake to all-cause and breast cancer-specific mortality using Cox proportional hazard models with time since diagnosis as underlying time and strata for age and country.
Results
Among postmenopausal women, an intake of lignans in the highest versus lowest quartile was related to a 28% lower risk of dying from breast (adjusted model: HR, quartile 4 vs. quartile 1, 0.72, 95% CI: 0.53;0.98). In contrast, in premenopausal women, a positive association between lignan intake and all-cause mortality was found (adjusted model: HR, quartile 4 vs. quartile 1, 1.63, 95% CI 1.03;2.57). We found no association for other polyphenol classes.
Conclusions
Intake of lignans before breast cancer diagnosis may be related to improved survival among postmenopausal women, but may on the contrary worsen the survival for pre-menopausal women. This suggests that the role of phytoestrogens in breast cancer survival is complex and may be dependent of menopausal status.
Keywords: Breast cancer, Survivorship, Polyphenols, phytoestrogens, lignans
Introduction
The high prevalence of breast cancer in Western societies is partly attributable to long relative survival periods [1, 2]; accordingly, there is a large interest in initiatives aimed at optimizing health of breast cancer survivors. Cancer survivors are highly motivated to initiate dietary changes [3]. However, at present, there is not sufficient evidence to make special dietary recommendations to breast cancer survivors, and cancer survivors are advised to follow the general advice for cancer prevention [4].
There are indications of foods rich in dietary fiber and soy products being associated with lower risk of all-cause mortality among women diagnosed with breast cancer [4]. These foods are also characterized as being rich in polyphenols, secondary plant metabolites, which have received a lot of attention mainly due to their antioxidant properties [5]. They are a large family of heterogeneous compounds that are divided into five main classes based on their chemical structure i.e. flavonoids, phenolic acids, stilbenes, lignans, and other polyphenols [6].
Flavonoids are found especially in fruits, fruit juices, wine and tea [6, 7]. They been shown in in vivo and in vitro to depress angiogenesis and delay tumor growth [8, 9]. Phenolic acids are found primarily in coffee, tea and red wine and to a lesser extent in vegetables and fruits [6]. They have been shown to have anti-inflammatory and anti-oxidative properties [10, 11]. Stilbenes, found particularly in red wine, have been shown to have anti-aromatase activity [12]. Lignans are found especially in flaxseed, whole grains and vegetables, and lignans are converted to the estrogen-like compounds enterolactone and enterodiol during digestion [13].
In epidemiological studies, especially lignan and flavonoids have been studied, and intake of these has been found to be associated with lower incidence of breast cancer [14, 15], but the evidence is not entirely consistent [16]. However, some of these studies suggested effects may be more relevant in the progression of already established tumors [17, 18] and thereby more relevant for cancer prognosis rather than incidence. In an American study following breast cancer cases from date of diagnosis until death or end of follow-up, postmenopausal women with a high reported pre-diagnostic dietary intake of flavones and isoflavones had a lower all-cause mortality risk [19]. A meta-analysis found that soy intake was associated with lower risk of breast cancer recurrence and better survival [20]. Moreover, a meta-analysis of studies on lignan intake and blood concentrations of enterolactone, an inverse association among postmenopausal women was found both in relation to all-cause mortality and breast cancer-specific mortality [21]. Further epidemiological studies are needed to confirm the hypothesized associations between lignans and flavonoids in relation to improved survival in women diagnosed with breast cancer, as well as to see if other polyphenol classes play a role.
The objective of the proposed study was to investigate the association between estimated dietary intakes of polyphenol classes (flavonoids, phenolic acids, stilbenes, lignans, and other polyphenols) and survival after breast cancer diagnosis (all-cause mortality and breast cancer-specific mortality). We hypothesized that high intakes before the breast cancer diagnosis are associated with lower all-cause and breast-cancer specific mortality. We used data from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, where there is large variability in polyphenol intake and sources. Usual dietary polyphenol intake was estimated using the comprehensive Phenol-Explorer database [22–24].
Methods
Study population
The EPIC study is a large, multicenter cohort study that includes more than half a million participants (367 903 women). The cohort comprises of 23 centers in Denmark, France, Greece, Germany, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom. Most participants were recruited from the general population [25].
At recruitment (years 1993–1999), lifestyle questionnaires, dietary questionnaires, and anthropometric measurements were collected from the participants. Excluded from the present study where those with prevalent cancer diagnosis at recruitment (n=19,853) or those missing diagnosis or censoring date (n=2,892) leaving 345,158 women. Of those, 11,914 women were diagnosed with breast cancer between recruitment and end of follow-up for cancer incidence (2004–2009, depending on center). Of those, women with missing information on dietary information (n=123) or where there was uncertainty of whether their cancer was benign or malignant (n=9) were excluded and consequently 11,782 women were included in the present study. Breast cancer diagnosis was ascertained through linkage with registers in most countries, or using a combination of methods including health insurance records, cancer and pathology registries or active follow-up [25].
This study was approved by the Ethical Review Board at the International Agency for Research on Cancer (IARC). All participants provided informed consent.
Follow-up for vital status
Women diagnosed with breast cancer were followed from date of diagnosis until censoring, which were identified as date of death, last date of contact or end of follow-up for vital status (2006–2010, depending on center). Information on vital status and movement of participants was obtained through record linkage with the municipal and national mortality registries most countries or through a combination of methods, including health insurance records, cancer and pathology registries, and active follow-up of study subjects and their next-of-kin [26]. The outcome of the study was mortality (all-cause or breast cancer-specific). Breast cancer-specific death was assigned based on the underlying cause of death, which was coded according to the 10th revision of the International Classification of Disease, Injuries and Causes of Death (ICD-10).
Clinical characteristics
Most centers collected information from pathology reports on tumor estrogen (ER) status, on the available laboratory methods, and on quantification descriptions used to determine receptor status. To standardize the quantification of receptor status among the EPIC centers, the following criteria for a positive receptor status were used: ≥10% cells stained, any 'plus-system' description, ≥20 fmol/mg, an Allred score of ≥3, an immunoreactive score (IRS) ≥2, or an H-score ≥10 [27–30]. No information was available on whether diagnosis was detected as a result of screening or not. Furthermore, no data on treatment as well as recurrence was available. Both invasive and in situ cases were included in the present study.
Assessment of dietary intake and lifestyle factors
Dietary intake reflecting the habitual intake over the previous 12 months before recruitment, on average 6 years before diagnosis, was assessed using country- or center-specific dietary questionnaires [25].
Intakes of polyphenols were estimated using the Phenol-Explorer database. In brief, Phenol-Explorer contains data on 502 polyphenol compounds in 452 foods collected from 638 scientific peer-review articles [22]. The content of polyphenols was expressed in mg/100g fresh food weight. In the present study, intakes of 419 different polyphenols were used, and these were grouped into five classes according to their chemical structure: flavonoids, phenolic acids, stilbenes, lignans, and other polyphenols. The “other polyphenols” class consisted of a heterogeneous class of polyphenols not belonging to any of the four previous classes and included e.g. tyrosol and alkylphenols (mainly alkylresorcinols). The effect of food processing on the polyphenol content was taken into account using retention factors [24]. Full details of the Phenol-Explorer calculations have been published previously [31].
Lifestyle questionnaires were used to assess lifestyle information such as smoking, physical activity, and socioeconomic characteristics. Furthermore, the questionnaires included information about reproductive history, menopausal status and use of exogenous hormones for contraception and postmenopausal replacement therapy. Height, weight, and waist and hip circumference were measured by trained personnel in most centers [25].
Information on menopausal status was available only at recruitment. Women that underwent bilateral ovariectomy were regarded as postmenopausal (n=336), and women that were reported as being premenopausal at recruitment but reported to be former (n=53) or current users (n=16) of hormone replacement therapy were regarded as postmenopausal. Furthermore, women that were reported to be perimenopausal at recruitment (n=2,654) were considered postmenopausal, because menopausal status was assessed at recruitment and most of the perimenopausal women would be expected to have entered menopause before diagnosis of breast cancer.
Statistical methods
The association between polyphenol intake and all-cause or breast cancer-specific mortality was investigated using Cox Proportional Hazard Models with time since diagnosis as underlying time scale. Time since diagnosis was defined as time from date of breast cancer diagnosis until date of death (all-cause or breast cancer-specific mortality, in the latter analyses women who died of other causes were censored at date of death) or until last date of contact or end of follow-up for vital status (2006-2010, depending on center). The model was stratified by 5-year age intervals and country (thus allowing for separate underlying hazards by age group and country). For the UK cohorts, the two study centers were included as two different “countries” due the overrepresentation of health-conscious people and vegetarians in the Oxford cohort.
Analyses were conducted for the different exposures including the polyphenol classes: flavonoids, phenolic acids, stilbenes, and lignans. The class “Other polyphenols” was studied as subclasses only due to the heterogeneity of this class. We assessed subclasses of flavonoids, phenolic acids and other polyphenols because they include a range of compounds with rather different structures and possible health effects. Altogether 27 sub-classes were evaluated with respect to all-cause and breast cancer mortality. For these sub-classes analyses, multiple testing was taken into account. The significance level calculated to p<0.0005 instead of p<0.05 using a Bonferroni correction. In all other analyses, P-values < 0.05 were considered statistical significant.
In the Cox Proportional Hazard models, all exposures were log2 transformed, meaning that the continuous risk estimates were expressed for doublings in intakes. The associations for polyphenol classes were further expressed as quartiles based on the intakes among all participants. Before entering polyphenol classes, polyphenol subclasses, and linear potential confounders into the model, the linearity of the association was evaluated using linear splines. No departures from linearity were found.
All models were stratified by menopausal status (premenopausal, postmenopausal) due to the proposed difference in disease etiology, and thus potentially also survival [4].
The results are presented as hazard ratios (HR) with 95% confidence intervals (CI), for both crude (model 1) and adjusted models (models 2 and 3). Adjustments were made for the following lifestyle factors (model 2): alcohol intake (abstainer yes/no, g/day continuous), BMI (kg/m2, continuous), use of hormone replace therapy (ever, never, unknown/missing), schooling (none, primary school, technical/professional school, secondary school, longer education incl. university, not specified/missing), smoking status (never, former, current), and physical activity according to the Cambridge index (inactive, moderately inactive, moderately active, active) [32]. Secondly (model 3), adjustments were further made for the following clinical disease characteristics: estrogen receptor status (ER+, ER-, or unknown/missing), cancer stage (in situ; localized; metastatic; metastatic regional; metastatic distant; unknown/missing), and grading of the tumor (well differentiated, moderately differentiated, poor differentiated, undifferentiated, B-cell, unknown/missing), The covariates were included in the models based on a priori assumptions. All analyses were mutually adjusted meaning that analyses of e.g. flavonoids were adjusted for other polyphenol classes (phenolic acids, stilbenes, lignans, other polyphenols).
Another analysis was made where dietary fiber intake was adjusted for to see if this attenuated the association. Furthermore, adjustment for year of diagnosis was conducted since breast cancer treatment and thus survival has improved with time. Sensitivity analyses excluding women with missing information on clinical characteristics (estrogen receptor status, tumor stage and tumor grade) of the tumor were also conducted. Sensitivity analyses stratifying by time since dietary information was collected before diagnosis (0-2 year, >2 years) were also conducted. Sensitivity analyses were conducted excluding perimenopausal, and furthermore analyses were performed where only women under the age of 50 years at diagnosis were regarded as pre-menopausal.
Effect modifications by disease characteristics were investigated using a Wald’s test for the following factors: estrogen receptor status (ER+, ER-), tumor stage (localized, metastatic), tumor grade (well differentiated, moderately differentiated, poor differentiated), BMI group (BMI≤25 kg/m2, 25–30 kg/m2, >30 kg/m2), alcohol intake (abstainers; low-to-moderate consumers ≤12g/day; heavy consumers >12 g/day) and smoking status (never, former, current). In these analyses, the polyphenol intakes were included as continuous variable.
SAS® statistical software release 9.3 was used for all statistical analyses. The PHREG procedure was used for the Cox proportional hazards models and the UNIVARIATE and FREQ procedures for the descriptive analyses.
Results
11,782 women were followed since date of diagnosis of breast cancer for a median of 6 years (Supplementary figure 1). During this time, 1,482 women died including 753 deaths due to breast cancer. Characteristics of women and vial status are shown in Table 1. Women who died before end of follow-up had more severe disease. Furthermore, slightly more of the deceased women were smokers at recruitment (before diagnosis of the disease).
Table 1.
Clinical and lifestyle characteristics of women diagnosed with breast cancer by vital status
| All cases | Deceased | ||
|---|---|---|---|
| n=11,782 | All causes n=1,482 | Breast cancer n=753 | |
| Follow-up time used in study: Time from diagnosis until censoring, years, Median (P5; P95) | 6.3 (1.1–13.2) | 4.1 (0.5–10.8) | 3.3 (0.3–9.1) |
| Information from time of diagnosis of breast cancer | |||
| Age at diagnosis, years, median (P5–P95) | 59 (46–73) | 60 (45–76) | 58 (41–74) |
| Receptor status1 | |||
| ER+, % (n) | 51% (6,043) | 38% (567) | 34% (258) |
| ER-, % (n) | 13% (1,489) | 20% (302) | 23% (173) |
| Unknown/missing, % (n) | 36% (4,250) | 41% (613) | 43% (322) |
| Stage of tumor2 | |||
| In situ, % (n) | 8% (911) | 3% (38) | 1% (6) |
| Localized, % (n) | 36% (4,270) | 24% (361) | 20% (148) |
| Metastatic, % (n) | 2% (310) | 6% (92) | 10% (73) |
| Metastatic regional, % (n) | 14% (1,618) | 22% (329) | 19% (146) |
| Metastatic distant, % (n) | 1% (84) | 4% (60) | 5% (41) |
| Unknown/missing, % (n) | 39% (4,589) | 41% (602) | 45% (339) |
| Grading of the tumor3 | |||
| Well differentiated, % (n) | 9% (1,046) | 2% (30) | 1% (9) |
| Moderately differentiated, % (n) | 20% (2,376) | 14% (212) | 14% (106) |
| Poor/undifferentiated, % (n) | 18% (2,065) | 24% (347) | 22% (167) |
| Unknown/missing, % (n) | 53% (6,295) | 60% (893) | 63% (471) |
| Recruitment information (prior to diagnosis of breast cancer) | |||
| Menopausal status¥ | |||
| Premenopausal, % (n) | 24% (2,804) | 20% (295) | 25% (186) |
| Postmenopausal, % (n) | 76% (8,978) | 80% (1,187) | 75% (567) |
| Use of Hormone Replacement Therapy | |||
| Ever, % (n) | 34% (3,969) | 30% (440) | 28% (209) |
| Never, % (n) | 59% (7,002) | 63% (928) | 65% (490) |
| Unknown/missing, % (n) | 7% (811) | 7% (114) | 7% (54) |
| BMI, kg/m2, median (P5–P95) | 24 (19–33) | 25 (19–35) | 25 (20–36) |
| Highest education level | |||
| None, n (%) | 3% (284) | 3% (41) | 4% (29) |
| Primary school completed, n (%) | 23% (2,669) | 26% (381) | 28% (209) |
| Technical/professional school, n (%) | 21% (2,520) | 20% (295) | 24% (184) |
| Secondary school, n (%) | 25% (2,958) | 24% (365) | 18% (133) |
| Longer education, incl. university, n (%) | 24% (2,847) | 21% (307) | 18% (140) |
| Not specified, n (%) | 4% (504) | 6% (93) | 8% (58) |
| Smoking status | |||
| Never, n (%) | 54% (6,413) | 52% (772) | 51% (387) |
| Former, n (%) | 24% (2,857) | 24% (350) | 25% (185) |
| Current, n (%) | 19% (2,188) | 22% (326) | 22% (168) |
| Unknown, n (%) | 3% (324) | 2% (34) | 2% (13%) |
| Cambridge physical activity index | |||
| Inactive, n (%) | 20% (2,381) | 27% (397) | 27% (206) |
| Moderately inactive, n (%) | 34% (3,958) | 34% (504) | 32% (239) |
| Moderately active, n (%) | 23% (2,637) | 18% (266) | 17% (126) |
| Active, n (%) | 14% (1,694) | 14% (204) | 16% (120) |
| Missing, n (%) | 9% (1,112) | 7% (111) | 8% (62) |
| Alcohol abstainers | 14% (1,683) | 16% (238) | 18% (132) |
| Alcohol intake (g/day)† | 6.4 (0.4–36.9) | 5.8 (0.4–37.9) | 5.4 (0.4–36.3) |
| Intake of dietary fiber (g/day) | 21.2 (11.6–35.7) | 21.2 (10.7–36.8) | 21.0 (10.1–36.8) |
| Total energy intake (kcal/day) | 1909 (1131; 3002) | 1914 (1128–2988) | 1852 (1110–2916) |
Among users only
Perimenopausal women were considered as postmenopausal
ER+: Estrogen receptor positive
ER- : Estrogen receptor negative
The total polyphenol intake varied considerably between countries (Table 2), with highest intakes in UK and Denmark and lowest in Spain, Norway and Greece. The intake of flavonoids was especially high in UK, whereas phenolic acids were especially high in Denmark. Stilbenes intake was highest in France and Denmark, and lignans intake in Italy.
Table 2.
Polyphenols intake among women diagnosed with breast cancer measured at baseline, total and by country.
| All (n=11,782) | France (n=3,256) |
Italy (n=1,062) |
Spain (n=507) |
UK GP (n=484) |
UK HC (n=1019) |
NL (n=931) |
Greece (n=201) |
Germany (n=852) |
Sweden (n=1,117) |
Denmark (n=1,354) |
Norway (n=999) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total polyphenol intake | 1121 (439;2226) |
1332 (562;2549) |
1075 (434;1426) |
690 (266;1400) |
1523 (778;2367) |
1441 (650;2303) |
1158 (655–1789) |
790 (326;1492) |
1056 (530;1907) |
826 (404;1558) |
1560 (818; 2485) |
646 (246;1093) |
| Flavonoids | 444 (120–1260) |
530 (189–1240) |
415 (166–806) |
292 (82–657) |
935 (359–1512) |
864 (323–1533) |
511 (177–1003) |
266 (102–556) |
427 (149–1054) |
260 (84–646) |
527 (142–1433) |
179 (62–397) |
| Phenolic acids | 539 (141–1408) |
677 (167–1806) |
380 (120–754) |
319 (67–854) |
363 (188–1068) |
507 (129–1061) |
584 (252–950) |
432 (106–1049) |
497 (190–1070) |
483 (165–1054) |
880 (305–1545) |
365 (65–856) |
| Stilbenes | 0.6 (0.0–8.1) |
1.3 (0.1 9.8) |
0.4 (0.0–8.6) |
0.1 (0.0–5.9) |
0.0 (0.0–2.9) |
0.4 (0.0–6.6) |
0.2 (0.0–3.8) |
0.2 (0.0–2.4) |
0.8 (0.1–5.9) |
0.3 (0.0–2.9) |
1.5 (0.1–8.5) |
0.5 (0.0–2.8) |
| Lignans | 1.4 (0.7–4.6) |
1.6 (1–4) |
3.3 (1.4–11.9) |
1.5 (0.8–2.9) |
1.6 (0.8–3.0) |
1.7 (0.9–3.0) |
0.9 (0.7–1.7) |
2.2 (1.2–11.0) |
1.2 (0.7–5.4) |
1.1 (0.6–2.3) |
1.4 (0.7–3.0) |
1.0 (0.5–1.9) |
| Other polyphenols | 38 (12–97) |
25 (8–63) |
35 (18–71) |
28 (11– 83) |
36 (8–109) |
36 (10–99) |
41 (17–68) |
65 (36–122) |
62 (25–119) |
42 (20–88) |
69 (30–121) |
40 (15–65) |
All values are in mg/day and are presented as medians (P5–P95)
UK GP– United Kingdom cohort representing general population
UK HC– United Kingdom health conscious cohort
NL: The Netherlands
P5– 5th percentile, P95– 95th percentile
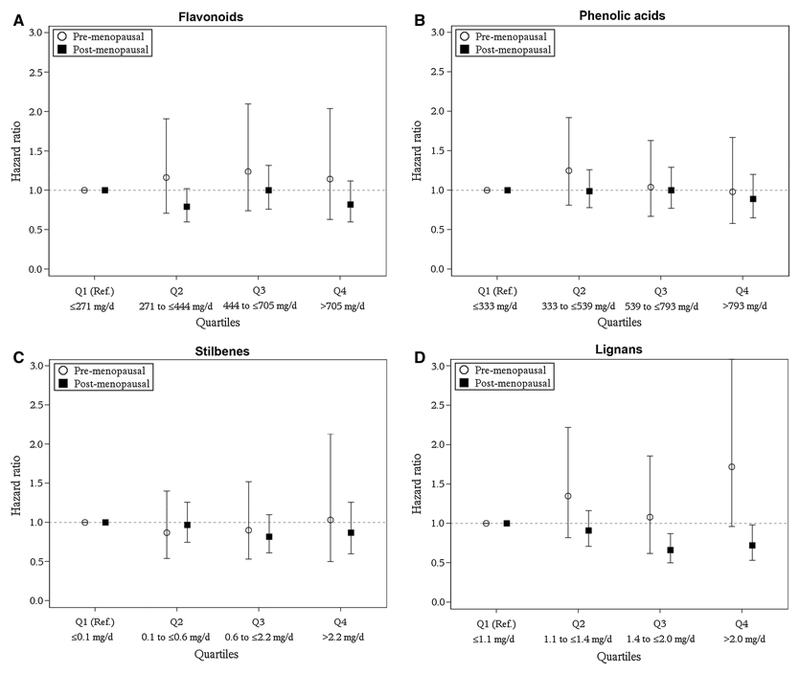
For premenopausal women, no association was observed between polyphenols classes and all-cause or breast cancer-specific mortality (Table 3), except for the lignans class, where higher intakes were significantly associated with higher risk of all-cause mortality (adjusted model: HR, pr. doubling, 1.26, 95% CI: 1.05;1.51) and non-significantly with higher risk of breast cancer-specific mortality (adjusted model: HR, pr. doubling, 1.24, 95% CI: 0.98;1.58). For postmenopausal women, intake of lignans was associated with lower risk of breast cancer-specific mortality (adjusted model: HR, pr. doubling, 0.83, 95% CI: 0.72;0.96), and no association was found for any of the other polyphenol classes. The same tendency was found when investigating the association between quartiles of polyphenol class intake and breast cancer specific mortality (Figure 1 and Supplementary table 1).
Table 3.
Association between estimated intake of polyphenol classes with all-cause mortality and breast cancer specific mortality according to menopausal status among women diagnosed with breast cancer – The European Prospective Investigation into Cancer and Nutrition (EPIC) cohort
| Premenopausal | Postmenopausal | |||
|---|---|---|---|---|
| Endpoint (deceased/cases) | All-cause mortality (295/2,804) |
BC-specific mortality (186/2,804) |
All-cause mortality (1,187/8,978) |
BC-specific mortality (/8,978) |
| HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
|
| Flavonoids | ||||
| Minimally adjusted (model 1)* | 0.95 (0.81–1.10) |
1.05 (0.86–1.28) |
0.99 (0.92–1.07) |
1.01 (0.91–1.13) |
| Adjusted for lifestyle factors (model 2)† | 0.98 (0.84–1.14) |
1.11 (0.91–1.36) |
1.01 (0.94–1.09) |
1.02 (0.92–1.14) |
| Adjusted for lifestyle and clinical factors (model 3)‡ | 0.94 (0.80–1.10) |
1.02 (0.83–1.26) |
1.02 (0.95–1.10) |
1.04 (0.93–1.15) |
| Phenolic acids | ||||
| Minimally adjusted (model 1)* | 1.11 (0.97–1.28) |
1.08 (0.90–1.28) |
1.01 (0.94–1.08) |
0.98 (0.89–1.09) |
| Adjusted for lifestyle factors (model 2)† | 1.08 (0.94–1.25) |
1.04 (0.87–1.25) |
0.97 (0.91–1.04) |
0.97 (0.87–1.08) |
| Adjusted for lifestyle and clinical factors (model 3)‡ | 1.04 (0.90–1.20) |
1.00 (0.83–1.21) |
0.97 (0.91–1.05) |
0.99 (0.89–1.10) |
| Stilbenes | ||||
| Minimally adjusted (model 1)* | 1.01 (0.96–1.06) |
0.98 (0.93–1.05) |
0.98 (0.96–1.00) |
0.97 (0.95–1.00) |
| Adjusted for lifestyle factors (model 2)† | 0.99 (0.92–1.06) |
0.99 (0.91–1.08) |
0.99 (0.96–1.01) |
0.98 (0.95–1.02) |
| Adjusted for lifestyle and clinical factors (model 3)‡ | 0.99 (0.92–1.06) |
0.99 (0.91–1.08) |
0.97 (0.95–1.00) |
0.97 (0.94–1.00) |
| Lignans | ||||
| Minimally adjusted (model 1)* | 1.22 (1.02–1.46) |
1.23 (0.97–1.55) |
0.95 (0.86–1.05) |
0.85 (0.73–0.98) |
| Adjusted for lifestyle factors (model 2)† | 1.24 (1.03–1.49) |
1.24 (0.98–1.58) |
0.96 (0.88–1.06) |
0.86 (0.74–0.99) |
| Adjusted for lifestyle and clinical factors (model 3)‡ | 1.26 (1.05–1.51) |
1.24 (0.98–1.58) |
0.94 (0.86–1.04) |
0.83 (0.72–0.96) |
Hazard ratios (HR) are expressed as pr. doubling (log2) in intake
Model 1: Not adjusted, but age and country is taken into account by creating strata for country and 5-year age group.
Model2: Adjusted for lifestyle factors including alcohol (abstainer and intake g/day), BMI, HRT use, Schooling, smoking status, physical activity index, and the polyphenol classes are further adjusted for intake of other polyphenol classes (mutual adjustment). Further, strata are made for country and 5-year age group
Model 3: Adjusted for above mentioned lifestyle factors and also the following clinical factors: ER receptor status, cancer stage and grading of tumor. Further, strata are made for country and 5-year age group
95%CI– 95% confidence intervals, ER– estrogen receptor, HRT– hormone replacement therapy
Figure 1.
Hazard ratios of the association between intake of polyphenols classes (A: Flavonoids, B: Phenolic acids, C: Stilbenes, D: Lignans) and breast cancer-specific mortality among pre- and postmenopausal women, respectively, diagnosed with breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Values are also shown in Supplementary table 5.
Quartile 1 is reference. Q– quartile, Ref– reference.
All analyses are adjusted for lifestyle and clinical factors including alcohol (abstainer and intake g/day), BMI, HRT use, Schooling, smoking status, physical activity index, ER receptor status, cancer stage, grading of tumor. Further, strata are made for country and 5-year age group
The significant association with lignan intake persisted for both pre- and postmenopausal women when adjusting for dietary fiber intake (data not shown). Excluding women with missing information on clinical characteristics or adjusting for calendar time of diagnosis did not modify the results (data not shown). Excluding women that were perimenopausal at recruitment (rather than including them as postmenopausal) and excluding premenopausal women that were older than 50 at diagnosis did not change the results (data not shown). Lastly, stratifying by time from dietary assessment to diagnosis (0-2 years or >2 years), did not seem to change the results either (data not shown). No consistent signs of effect modification by lifestyle or clinical characteristics were found (data not shown).
For the polyphenol sub-classes (Table 4), no statistically significant associations were found in relation to all-cause and breast cancer-specific mortality after Bonferroni adjustment for multiple testing (significance level p=0.0005). For postmenopausal women, there was non-significant association between intake of alkylphenols and risk of all-cause mortality and breast cancer-specific mortality (adjusted model, breast cancer-specific mortality: HR, pr. doubling, 0.94, 95% CI: 0.90;0.98, p=0.0015).
Table 4.
Association between estimated intake of subclasses of flavonoids, phenolic acids and other polyphenols with all-cause mortality and breast cancer specific mortality among pre- and postmenopausal women respectively diagnosed with breast cancer – The European Prospective Investigation into Cancer and Nutrition (EPIC) cohort
| Premenopausal | Postmenopausal | |||
|---|---|---|---|---|
| All-cause mortality | BC-specific mortality | All-cause mortality | BC-specific mortality | |
| HR (95% CI) p-value |
HR (95% CI) p-value |
HR (95% CI) p-value |
HR (95% CI) p-value |
|
| deceased/cases | 295/2,804 | 186/2,804 | 1,187/8,978 | 567/8,978 |
| Flavonoid subclasses | ||||
| Flavanols | 0.99 (0.88–1.12) p=0.87 |
1.07 (0.91–1.26) p=0.40 |
0.99 (0.93–1.05) p=0.69 |
0.96 (0.89–1.05) p=0.39 |
| Anthocyanins | 1.02 (0.93–1.11) p=0.72 |
1.05 (0.94–1.17) p=0.41 |
1.00 (0.97–1.03) p=0.96 |
0.99 (0.94–1.05) p=0.79 |
| Chalcones | 0.99 (0.98–1.00) p=0.0095 |
0.98 (0.97–1.00) p=0.0212 |
1.00 (0.99–1.00) p=0.53 |
1.00 (0.99–1.00) p=0.33 |
| Dihydrochalcones | 1.01 (0.98–1.04) p=0.55 |
1.02 (0.98–1.05) p=0.39 |
1.00 (0.99–1.01) p=0.77 |
1.00 (0.98–1.01) p=0.66 |
| Dihydroflavonols | 1.00 (0.98–1.02) p=0.94 |
1.00 (0.98–1.02) p=0.96 |
1.00 (0.99–1.01) p=0.87 |
1.00 (0.99–1.01) p=0.97 |
| Flavanones | 1.00 (0.94–1.07) p=0.94 |
1.02 (0.94–1.12) p=0.61 |
0.98 (0.95–1.00) p=0.08 |
0.99 (0.95–1.04) p=0.76 |
| Flavones | 0.91 (0.79–1.05) p=0.18 |
0.96 (0.81–1.15) p=0.68 |
0.95 (0.92–1.00) p=0.0287 |
0.96 (0.90–1.02) p=0.16 |
| Flavonols | 0.99 (0.86–1.13) p=0.85 |
1.03 (0.86–1.22) p=0.76 |
0.95 (0.89–1.02) p=0.15 |
0.93 (0.85–1.02) p=0.11 |
| Isoflavonoids | 1.00 (0.98–1.03) p=0.71 |
1.00 (0.97–1.02) p=0.78 |
1.00 (0.99–1.01) p=0.73 |
1.00 (0.98–1.01) p=0.66 |
| Phenolic acid subclasses | ||||
| Hydroxybenzoic | 0.96 (0.88–1.05) p=0.40 |
0.96 (0.86–1.07) p=0.45 |
0.99 (0.95–1.03) p=0.66 |
0.97 (0.92–1.03) p=0.37 |
| Hydroxycinnamic | 1.00 (0.90–1.13) p=0.94 |
0.99 (0.85–1.14) p=0.85 |
0.96 (0.90–1.02) p=0.15 |
0.96 (0.88–1.05) p=0.34 |
| Hydroxyphenylacetic | 0.97 (0.91–1.03) p=0.27 |
0.96 (0.89–1.04) p=0.27 |
0.98 (0.96–1.00) p=0.0311 |
0.99 (0.96–1.02) p=0.45 |
| Hydroxyphenylpropanoic | 1.00 (0.99–1.01) p=0.77 |
1.00 (0.99–1.02) p=0.69 |
1.00 (0.99–1.00) p=0.41 |
1.00 (0.99–1.01) p=0.79 |
| Other polyphenol subclasses | ||||
| Alkylmethoxyphenols | 1.01 (0.94–1.08) p=0.84 |
0.99 (0.91–1.08) p=0.84 |
0.98 (0.96–1.00) p=0.08 |
0.99 (0.96–1.02) p=0.58 |
| Alkylphenols | 0.94 (0.85–1.03) p=0.19 |
0.94 (0.82–1.07) p=0.36 |
0.96 (0.93–0.98) p=0.0016 |
0.94 (0.90–0.98) p=0.0015 |
| Cucurminoids | 1.01 (0.99–1.03) p=0.40 |
1.01 (0.99–1.03) p=0.49 |
1.00 (0.99–1.01) p=0.86 |
1.00 (0.99–1.01) p=0.59 |
| Furanocoumarins | 1.01 (0.99–1.03) p=0.36 |
1.01 (0.99–1.04) p=0.44 |
1.00 (0.99–1.00) p=0.31 |
0.99 (0.98–1.00) p=0.11 |
| Hydroxybenzaldehydes | 0.93 (0.87–1.00) p=0.05 |
0.95 (0.87–1.03) p=0.21 |
0.98 (0.96–1.00) p=0.10 |
0.98 (0.95–1.02) p=0.32 |
| Hydroxybenzoketones | 0.99 (0.98–1.00) p=0.06 |
0.99 (0.97–1.00) p=0.05 |
1.00 (1.00–1.01) p=94 |
1.00 (0.99–1.01) p=0.64 |
| Hydroxycinnamaldehydes | 1.01 (1.00–1.02) p=0.20 |
1.01 (1.00–1.03) p=0.16 |
1.00 (0.99–1.00) p=0.44 |
1.00 (0.99–1.00) p=0.38 |
| Hydroxycoumarins | 1.00 (0.98–1.03) p=0.73 |
1.02 (0.99–1.05) p=0.29 |
1.00 (0.99–1.01) p=0.31 |
1.00 (0.98–1.01) p=0.73 |
| Hydroxyphenylpropenes | 0.99 (0.98–1.00) p=0.0244 |
0.99 (0.97–1.01) p=0.30 |
1.00 (1.00–1.01) p=0.62 |
1.00 (0.99–1.01) p=0.78 |
| Methoxyphenols | 1.00 (0.98–1.02) p=0.99 |
1.02 (0.99–1.05) p=0.15 |
1.00 (0.99–1.01) p=0.49 |
1.00 (0.99–1.01) p=0.57 |
| Naphtoquinones | 1.00 (0.98–1.01) p=0.42 |
1.01 (0.99–1.04) p=0.26 |
1.00 (1.00–1.01) p=0.75 |
1.00 (0.99–1.01) p=0.92 |
| Phenolic terpenes | 0.99 (0.98–1.01) p=0.41 |
0.99 (0.98–1.01) p=0.31 |
1.00 (0.99–1.01) p=0.70 |
1.00 (0.99–1.00) p=0.29 |
| Tyrosols | 0.95 (0.85–1.05) p=0.30 |
0.94 (0.82–1.09) p=0.42 |
0.98 (0.96–1.00) p=0.0204 |
0.98 (0.95–1.01) p=0.25 |
| Other_PP | 1.01 (0.91–1.12) p=0.85 |
0.98 (0.87–1.12) p=0.81 |
0.95 (0.91–1.00) p=0.0341 |
0.95 (0.89–1.02) p=0.15 |
Hazard ratios (HR) are expressed as pr. doubling (log2) in intake
All analyses are adjusted for lifestyle and clinical factors including alcohol (abstainer and intake g/day), BMI, HRT use, Schooling, smoking status, physical activity index, ER receptor status, cancer stage, grading of tumor. Further, strata are made for country and 5-year age group
HR– hazard rate, 95%CI– 95% confidence intervals, ER– estrogen receptor, HRT– hormone replacement therapy
None were statistically significant (p<0·0005 after Bonferroni correction for multiple comparisons)
Discussion
In this large prospective European study of almost 12,000 women diagnosed with breast cancer, we observed that pre-diagnostic intake of lignans was associated with lower risk of dying of breast cancer among postmenopausal women. We observed the opposite among premenopausal women, with higher intakes of lignans being associated with higher all-cause mortality. We found no association for other polyphenol classes (flavonoids, phenolic acids, stilbenes, other polyphenols) for either post or premenopausal women.
There are several weaknesses of our study, which need to be considered. First, dietary intake and lifestyle were assessed at recruitment long before diagnosis (median 6 years), and it is possible that in the course of the time since diagnosis women have changed dietary and lifestyle habits. This could be a problem if for instance the women with severe disease had changed their habits to a greater or lesser extent than those with less severe disease. However, stratifying by time from dietary assessment to diagnosis did not seem to change the results. We had no information on whether the breast cancer was detected as a result of breast cancer screening or not, and thus lead time bias cannot be ruled out [33]. Further, we had no data on treatment, which is problematic since treatment has much larger effect on survival than diet and lifestyle is expected to have [4]. However, we expect that clinical characteristics such as estrogen receptor status and tumor stage and tumor grade are surrogate variables. Information on menopausal status was available at recruitment only, and not at diagnosis. Many of the women that were premenopausal most likely entered menopause either around diagnosis or during time from diagnosis to end of follow-up. This is problematic since menopausal status may affect disease etiology, and thus potentially also survival [4]. However, we undertook sensitivity analyses where we excluded women that were premenopausal at recruitment but older than 50 years at diagnosis, and found no difference. Another study limitation pertains to the assessment of the polyphenol intake from dietary questionnaires using the comprehensive Phenol-Explorer database. Dietary assessment from questionnaires is prone to measurement errors [34], which may lead to underestimation of the true association. Furthermore, we cannot rule out that the observed association with lignans is in fact not due to these compounds, but rather other strongly correlated constituents (e.g. dietary fiber, vitamins and minerals) found in the same foods. A model where adjustment for total dietary fiber was made yielded similar results. We have thoroughly adjusted the analyses for potential confounders, but residual confounding cannot be ruled out.
Our study also has several strengths. We used data from ten European countries, including women with very different dietary intakes and therefore very different polyphenol intakes both in quantity and type which were estimated from the very comprehensive Phenol-Explorer database. Furthermore we had a large sample size and thus were able to conduct sub analyses according to clinical and lifestyle characteristics. We have almost complete morbidity and mortality information as well as information about important potential confounders of relevance in relation to breast cancer survival including detailed clinical information. Furthermore, our dietary and lifestyle information is a combination of subjectively reported habits assembled before diagnosis of breast cancer and administrative unbiased information on the outcome in broad terms (both morbidity and mortality) which is of outmost importance in an epidemiological study.
Biological evidence supports a role of lignans in breast cancer development and prognosis. After ingestion, lignans are converted into enterolignans (enterolactone and enterodiol) by the colonic microbiota. Especially enterolactone has been found to bind weakly to estrogen receptors, to have estrogenic effects in cultured cells and to modulate the response to endogenous estrogens [35, 36]. Animal models support that these factors may play an important role in prevention of cancer at the early stages leading to lower cancer incidence as well as in the progression of already established tumors [17, 37]. Five studies on blood levels of enterolactone or lignan intake in relation to breast cancer survival in postmenopausal women has been summarized in a meta-analysis, and an inverse association was found with both all-cause and breast–cancer specific mortality [21]. In Asia, the main phytoestrogen source is isoflavones (polyphenol class flavonoids) from soy products. A meta-analysis found indications of an inverse association between soy intake and breast cancer recurrence and mortality [20]. In this meta-analysis, the inverse association was especially found in the studies made in Asian populations, whereas studies based on western populations more often observed null results [20]. While many of the included studies did not present results stratified by menopausal status, a Chinese prospective study found lower risk of recurrence among postmenopausal women only, and not among premenopausal women [38]. Lignans are the main phytoestrogen source in western countries [31, 39], and thus a more relevant phytoestrogen to study in a westerns population.
The timing of exposure to these estrogen-like compounds has been pointed out as crucial in recent research [40]. It has been suggested that estrogen exposure stimulates growth of breast cancer cells in women who just entered menopause, whereas from five years and longer after menopause it triggers apoptosis and thereby death of breast cancer cells [41]. Many of the women included in the present study who were premenopausal at recruitment had a median age of 51 years at time of diagnosis, and thus many of them must have entered menopause within five years or so. This period might, as mentioned, be a critical period in which estrogen-like compounds might have adverse effects in relation to breast cancer progression. In a meta-analysis of lignans in relation to all-cause and breast-cancer specific mortality, an inverse association was only found for postmenopausal women [21]. Thus, both biological mechanisms and evidence from RCT and observational studies suggest that the effects of lignans, and other dietary phytoestrogens, on breast cancer survival may differ according to menopausal status.
Conclusions
In conclusion, we found that higher pre-diagnostic lignan intake was associated with a better survival of postmenopausal women diagnosed with breast cancer, and, in contrast, the opposite was seen for premenopausal women. However, no associations were detected for any of the other classes of polyphenols. The role of phytoestrogens in breast cancer survival is complex, and menopausal status is important to take into account. More research is needed before giving recommendations to breast cancer survivors regarding dietary intake of phytoestrogens such as lignans.
Supplementary Material
Acknowledgements
The authors thank Bertrand Hemon, Katja Boll and Nick Martinussen for help with data management.
This work was funded by Innovation Fund Denmark (ELIN: 0603-00580B).
The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (France); German Cancer Aid, German Cancer Research Center, Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports, Netherlands Cancer Registry, LK Research Funds, Dutch Prevention Funds, Dutch Zorg Onderzoek Nederland, World Cancer Research Fund, Statistics Netherlands (The Netherlands); ERC-2009-AdG 232997, the Norwegian Research Council, Extrastiftelsen Helse og Rehabiliering med Extra-midler (Norway); Health Research Fund, Regional Governments of Andalucía, Asturias, Basque Country, Murcia (no. 6236) and Navarra, RETIC (RD06/0020/0091 and RD12/0036/0018) (Spain); Swedish Cancer Society, Swedish Scientific Council and Regional Government of Skåne and Västerbotten (Sweden); and Cancer Research UK, Medical Research Council (UK).
Abbreviations
- EPIC
The European Prospective Investigation into Cancer and Nutrition
- ICD-10
the 10th revision of the International Classification of Disease, Injuries and Causes of Death
- ER
estrogen receptor
- HR
hazard ratio
- CI
confidence interval
- UK
United Kingdom
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. JCO.2005.01.230 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund. Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Breast Cancer Survivors. [Accessed 1 October 2015];2014 http://www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf.
- 5.Zamora-Ros R, Touillaud M, Rothwell JA, Romieu I, Scalbert A. Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. Am J Clin Nutr. 2014;100:11–26. doi: 10.3945/ajcn.113.077743. ajcn.113.077743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Jimenez J, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J Agric Food Chem. 2010;58(8):4959–69. doi: 10.1021/jf100128b. [DOI] [PubMed] [Google Scholar]
- 7.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 8.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52(5):507–26. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Archives of toxicology. 2014;88:1803–1853. doi: 10.1007/s00204-014-1330-7. [DOI] [PubMed] [Google Scholar]
- 10.Nagasaka R, Chotimarkorn C, Shafiqul IM, Hori M, Ozaki H, Ushio H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem Biophys Res Commun. 2007;358:615–619. doi: 10.1016/j.bbrc.2007.04.178. [DOI] [PubMed] [Google Scholar]
- 11.Kylli P, Nousiainen P, Biely P, Sipila J, Tenkanen M, Heinonen M. Antioxidant potential of hydroxycinnamic acid glycoside esters. J Agric Food Chem. 2008;56(12):4797–805. doi: 10.1021/jf800317v. [DOI] [PubMed] [Google Scholar]
- 12.Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, Van den Berg M. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro. 2014;28:1215–1221. doi: 10.1016/j.tiv.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- 14.Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr. 2010;92:141–153. doi: 10.3945/ajcn.2009.28573. [DOI] [PubMed] [Google Scholar]
- 15.Takemura H, Sakakibara H, Yamazaki S, Shimoi K. Breast cancer and flavonoids - a role in prevention. Curr Pharm Des. 2013;19:6125–6132. doi: 10.2174/1381612811319340006. CPD-EPUB-20130219-10 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Zamora-Ros R, Ferrari P, Gonzalez CA, Tjonneland A, Olsen A, Bredsdorff L, Overvad K, Touillaud M, Perquier F, Fagherazzi G, Lukanova A, et al. Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Res Treat. 2013;139:163–176. doi: 10.1007/s10549-013-2483-4. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Wang L, Thompson LU. Flaxseed and its components reduce metastasis after surgical excision of solid human breast tumor in nude mice. Cancer Lett. 2006;234:168–175. doi: 10.1016/j.canlet.2005.03.056. S0304-3835(05)00331-9 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Hui C, Yujie F, Lijia Y, Long Y, Hongxia X, Yong Z, Jundong Z, Qianyong Z, Mantian M. MicroRNA-34a and microRNA-21 play roles in the chemopreventive effects of 3,6-dihydroxyflavone on 1-methyl-1-nitrosourea-induced breast carcinogenesis. Breast cancer research : BCR. 2012;14:R80. doi: 10.1186/bcr3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Gaudet MM, Abrahamson PE, Bell P, Schroeder JC, Teitelbaum SL, Neugut AI, et al. Dietary flavonoid intake and breast cancer survival among women on Long Island. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2285–92. doi: 10.1158/1055-9965.EPI-07-0245. [DOI] [PubMed] [Google Scholar]
- 20.Fritz H, Seely D, Flower G, Skidmore B, Fernandes R, Vadeboncoeur S, Kennedy D, Cooley K, Wong R, Sagar S, Sabri E, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013;8:e81968. doi: 10.1371/journal.pone.0081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seibold P, Vrieling A, Johnson TS, Buck K, Behrens S, Kaaks R, Linseisen J, Obi N, Heinz J, Flesch-Janys D, Chang-Claude J. Enterolactone concentrations and prognosis after postmenopausal breast cancer: assessment of effect modification and meta-analysis. Int J Cancer. 2014;135:923–933. doi: 10.1002/ijc.28729. [DOI] [PubMed] [Google Scholar]
- 22.Neveu V, Perez-Jimenez J, Vos F, Crespy V, du CL, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothwell JA, Urpi-Sarda M, Boto-Ordonez M, Knox C, Llorach R, Eisner R, Cruz J, Neveu V, Wishart D, Manach C, Andres-Lacueva C, et al. Phenol-Explorer 2.0: a major update of the Phenol-Explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database (Oxford) 2012:bas031. doi: 10.1093/database/bas031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothwell JA, Perez-Jimenez J, Neveu V, Medina-Remon A, M'hiri N, Garcia-Lobato P, Manach C, Knox C, Eisner R, Wishart DS, Scalbert A. Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) 2013:bat070. doi: 10.1093/database/bat070. bat070 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 26.Dik VK, Murphy N, Siersema PD, Fedirko V, Jenab M, Kong SY, Hansen CP, Overvad K, Tjonneland A, Olsen A, Dossus L, et al. Prediagnostic intake of dairy products and dietary calcium and colorectal cancer survival--results from the EPIC cohort study. Cancer Epidemiol Biomarkers Prev. 2014;23:1813–1823. doi: 10.1158/1055-9965.EPI-14-0172. [DOI] [PubMed] [Google Scholar]
- 27.Flowers JL, Burton GV, Cox EB, McCarty KS, Sr, Dent GA, Geisinger KR, McCarty KS., Jr Use of monoclonal antiestrogen receptor antibody to evaluate estrogen receptor content in fine needle aspiration breast biopsies. Ann Surg. 1986;203:250–254. doi: 10.1097/00000658-198603000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 29.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 30.McCann J. Better assays needed for hormone receptor status, experts say. J Natl Cancer Inst. 2001;93:579–580. doi: 10.1093/jnci/93.8.579. [DOI] [PubMed] [Google Scholar]
- 31.Zamora-Ros R, Knaze V, Rothwell JA, Hemon B, Moskal A, Overvad K, Tjonneland A, Kyro C, Fagherazzi G, Boutron-Ruault MC, Touillaud M, et al. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. 2015 doi: 10.1007/s00394-015-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.InterAct Consortium. Peters T, Brage S, Westgate K, Franks PW, Gradmark A, Tormo Diaz MJ, Huerta JM, Bendinelli B, Vigl M, Boeing H, et al. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur J Epidemiol. 2012;27:15–25. doi: 10.1007/s10654-011-9625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox B, Sneyd MJ. Bias in breast cancer research in the screening era. Breast. 2013;22:1041–1045. doi: 10.1016/j.breast.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 34.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst. 2011;103:1086–1092. doi: 10.1093/jnci/djr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 36.Penttinen P, Jaehrling J, Damdimopoulos AE, Inzunza J, Lemmen JG, van der Saag P, Pettersson K, Gauglitz G, Makela S, Pongratz I. Diet-derived polyphenol metabolite enterolactone is a tissue-specific estrogen receptor activator. Endocrinology. 2007;148:4875–4886. doi: 10.1210/en.2007-0289. en.2007-0289 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Chen J, Thompson LU. The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenograftsis attributed to both its lignan and oil components. Int J Cancer. 2005;116:793–798. doi: 10.1002/ijc.21067. [DOI] [PubMed] [Google Scholar]
- 38.Kang X, Zhang Q, Wang S, Huang X, Jin S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ. 2010;182:1857–1862. doi: 10.1503/cmaj.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S.women: the Framingham study. J Nutr. 2002;132(2):276–82. doi: 10.1093/jn/132.2.276. [DOI] [PubMed] [Google Scholar]
- 40.Jordan VC. Avoiding the bad and enhancing the good of soy supplements in breast cancer. J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju233. pii dju233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shike M, Doane AS, Russo L, Cabal R, Reis-Filho JS, Gerald W, Cody H, Khanin R, Bromberg J, Norton L. The effects of soy supplementation on gene expression in breast cancer: a randomized placebo-controlled study. J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju189. pii: dju189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.