Abstract
The explosion in novel cancer immunotherapies has resulted in extraordinary clinical successes in the treatment of multiple cancers. Checkpoint inhibitors (CPIs) that target negative regulatory molecules have become standard of care. However, with the growing use of CPIs, alone or in combination with chemotherapy, targeted therapies, or other immune modulators, a significant increase in immune-related adverse events (irAEs) has emerged. The wide-ranging and currently unpredictable spectrum of CPI-induced irAEs can lead to profound pathology and, in some cases, death. Growing evidence indicates that many irAEs are a consequence of a breakdown in self-tolerance, but the influence of genetics, the environment, and the mechanisms involved remains unclear. This review explores key questions in this emerging field, summarizing preclinical and clinical experiences with this new generation of cancer drugs, the growing understanding of the role of the immune response in mediating these toxicities, the relationship of CPI-induced autoimmunity to conventional autoimmune diseases, and insights into the mechanism of irAE development and treatment.
Keywords: immune-related adverse events, cancer immunotherapy, immune checkpoint inhibitors, autoimmunity, risk factors
Immune-related adverse events (irAEs) – the clinical experience
Cancer immunotherapies, including monoclonal antibodies, oncolytic viruses, and T-cell therapies, have yielded unprecedented clinical success in the treatment of advanced cancers. Immune checkpoint inhibitors (CPIs) antagonizing the negative regulatory molecules cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), programmed death-1 (PD-1), and programmed death-ligand 1 (PD-L1) have been approved by the FDA for a growing number of cancers and in some cases, are now being utilized as first-line treatments (1). However, with their increasing use, a significant increase in associated immunotoxicities, termed immune-related adverse events (irAEs) have been observed. These irAEs have been reported in nearly every organ system, often leading to profound pathology and, in some cases, death (2–4). Anti–CTLA-4 and anti–PD-1/PD-L1 therapies display distinct patterns of tissue-specific irAEs, indicative of their differential roles in the maintenance of tolerance (5). Overall, prevalence and severity of irAEs is greater with anti-CTLA-4 treatment than with anti-PD-1/PD-L1 treatments, however their use in combination exacerbates the frequency of irAEs. Ipilimumab (anti–CTLA-4) preferentially promotes colitis and hypophysitis, whereas while anti–PD-1 treatment is associated with higher rates of thyroiditis, and rare, but sometimes life-threatening, cases of pneumonitis and diabetes mellitus (Table 1)(2,6–8).
Table 1.
Unique characteristics of CPI-induced irAEs observed in the clinic that aid in understanding immune mechanisms
| irAE | Autoimmune vs Autoinflammatory | Preclinical and Translational Mechanistic Evidence | Severe irAE Treatment | Evidence of Improved Clinical Objective Response |
|---|---|---|---|---|
| Anti-CTLA-4 Predominant irAE | ||||
| Colitis | Autoinflammatory | T cell repertoire diversification (44), Treg depletion (32), increased IL-17 (46), increased neutrophil gene signature and infiltrate (74) | Corticosteroids, Infliximab, Vedolizumab (9) | Yes (57) |
| Hypophysitis | Autoimmune | CTLA4 expression on pituitary gland initiating activation of complement (10,37) | Hormone Replacement | Yes (56) |
| Anti-PD-1/PD-L1 Predominant irAE | ||||
| Diabetes Mellitus | Autoimmune | PD-L1 expression on stressed beta cells (35) | Insulin | Suggested with limited evidence (39) |
| Hypothyroidism | Autoimmune | Autoantibody amplification(6) | Hormone Replacement | Yes (6) |
| Pneumonitis | Ambiguous | Unknown | Corticosteroid, infliximab or cyclophosphamide, mycophenolate mofetil or IVIg (9) | Suggested with limited evidence (75) |
| Myocarditis | Autoimmune | Shared tumor and organ specific neoantigen (12) | Corticosteroids, IVIg, plasmapheresis (9) | Difficult to assess given irAE-related mortality |
| Melanoma Patients | ||||
| Vitiligo | Autoimmune | Shared tumor and self-antigens (55) | None | Yes (55) |
CTLA-4 – cytotoxic T lymphocyte–associated protein-4; irAE – immune-related adverse event; IVIg - intravenous immunoglobulin; PD-1 – programmed death-1; PD-L1 – programmed death-ligand 1; Treg – regulatory T cell.
Although increasing experience with these therapeutic regimens has improved diagnosis and monitoring of irAEs in clinical settings, limited mechanistic understanding remains a major obstacle for their clinical management. In some cases, irAEs are recognized as a consequence of an autoinflammatory response, driven by systemic activation of innate immunity and often treated with high-dose steroids and other anti-inflammatory drugs (9). In other cases, the irAEs are more likely autoimmune in nature, with the presence of autoantibodies and, in some cases, documented antigen-specific, memory T-cell responses indicative of adaptive immunity (6,10–12). These apparent autoimmune conditions raise a number of key questions. (i) Do CPI-induced autoimmune irAEs develop by unmasking underlying conventional autoimmune diseases, or do they represent an alternate process for initiation of autoimmunity? (ii) Are there conventional adaptive immune mechanisms involved, or are immunotherapies altering novel mechanisms of immune tolerance, inducing systemic or tissue-specific immunity? (iii) What are the genetic or environmental triggers that lead to this collateral damage? In this perspective, we will focus on a number of current efforts to answer these questions. A detailed understanding of the interplay between antitumor immunity and irAEs, preclinical and clinical investigations in irAE development, and methods of identifying patients at risk will be highlighted. Defining the mechanistic basis of irAEs induced by individual immune therapies will lead to more precise therapeutic strategies to dampen or prevent irAEs without impeding antitumor immunity.
Profiling autoimmunity in preclinical models
Preclinical studies examining the role of inhibitory checkpoints can play an important role in delineating clinically-relevant immune interactions (Fig. 1). Investigation of CTLA-4 and PD-1 blockade revealed that these molecules elicit differential effects on T-cell immunity. CTLA-4 predominantly alters CD4+ T-cell activation in the lymph nodes, whereas PD-1 mediates CD8+ T-cell activity in the tissue (5). CTLA-4–deficient mice rapidly succumb to lymphoproliferative disease with severe multi-organ failure (13,14). In contrast, mice with PD-1–deficiency have more delayed and restricted pathologies, with tissue-specific infiltration in a strain dependent-manner (15,16). In non-obese diabetic (NOD) mice, genetically susceptible to the development of autoimmune type 1 diabetes (T1D), anti–PD-1 rapidly induces diabetes, whereas anti–CTLA-4 only precipitates disease in neonates (17,18). Additional immune checkpoints targeted by either gene-knockout or antibody-directed therapies have been shown to abrogate tumor growth, leading to early phase clinical testing. However, targeting many of these molecules, including inhibitory receptors TIM-3, LAG-3, and TIGIT [reviewed by ref. (19)] have also been shown to potentiate autoimmunity in preclinical models, illustrating the range of tolerogenic mechanisms that contribute to control of a self-directed immune response. CPIs may also act on cells other than naïve and effector or memory T cells. For anti–CTLA-4, evidence in mouse models shows that these antagonists alter regulatory T-cell (Treg) numbers and function (20,21). For anti–PD-1/PD-L1 therapy, this remains unclear, but the PD-1 engagement may functionally control CD28 signaling, a critical co-stimulatory pathway in Tregs, suggesting they influence these cells as well (22,23).
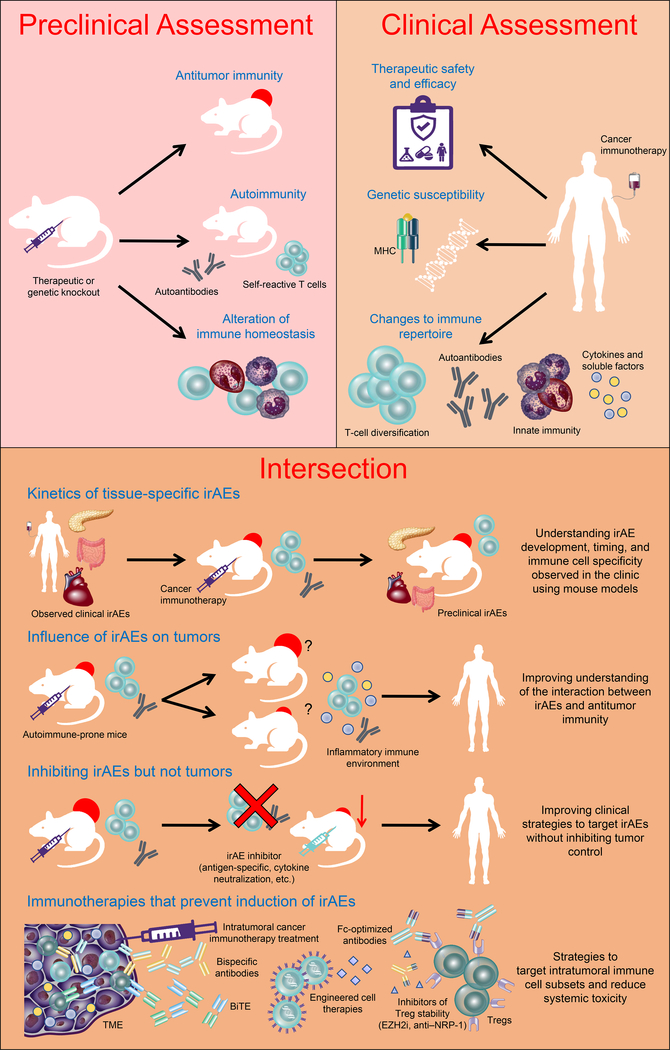
Figure 1. The intersection between preclinical and clinical studies.
Cancer immunotherapies undergo preclinical screening for their potential suitability for clinical use, with considerations to their ability to mediate antitumor immunity and autoimmunity determined. Following translation to clinical utility, the ability to answer clinical questions relating to response to therapy and safety profile is critical for clinical success. Many of these questions cannot be answered using available clinical parameters alone and rely on revisiting preclinical models. This represents an intersection between preclinical and clinical studies to improve our understanding of therapy-induced phenomena, such as the development of irAEs, to improve mechanistic understanding and clinical management. Ag: antigen; BiTE: Bispecific T-cell engager; EZH2i: enhancer of zeste homolog 2 inhibitor; irAEs: immune-related adverse events; MHC: major histocompatibility complex; NRP1: neuropilin 1; TME: tumor microenvironment; Treg: regulatory T cell.
The potential for improved antitumor immunity through targeting multiple, non-redundant immunomodulatory pathways has led to early phase clinical trials for a variety of combinatorial strategies. These combination treatments may improve the efficacy of cancer immunotherapy but may also amplify irAEs. Examining gene-targeted mice with multiple immunoregulatory molecule deficiencies may assist in recognizing potential pathologies and mechanisms for organ-specific disruption of tolerance in response to immunotherapy combinations. PD-1 and VISTA dual-knockout C57BL/6 (B6) mice display increased immune infiltration into tissues, whereas CD96 and PD-1 double-deficient mice have minimal impact on immune homeostasis or pathologies (24,25). Combined loss of PD-1 and LAG-3 results in lethal autoimmunity in mice (26,27). Despite differential effects on autoimmunity, each molecule in combination with anti–PD-1 enhances antitumor immunity (24,26,28). Thus, co-targeting complementary pathways of immune regulation can clearly impact tumor immunity, but the frequency and severity of irAEs remains to be evaluated fully in clinical trials. Although mouse models may suggest an augmentation in the frequency of irAEs in combination studies, clinical efforts may not reflect this. Anti–LAG-3 plus nivolumab (anti–PD-1) demonstrates a similar safety profile to nivolumab alone (29). Nonetheless, animal models provide a valuable tool for interrogation of organ-specific tolerogenic mechanisms maintained by different immunoregulatory molecules.
The study of CPI-induced toxicity has been limited in many clinical settings due to the rarity of specific events and absence of appropriate tissue material. Animal models have been limited to strains such as B6 and BALB/c mice, which are generally resistant to autoimmune sequelae. Thus, the adverse effects of CPIs have benefited from animal studies that have compromised regulatory pathways. CPI treatment of mice, in which Tregs have been transiently depleted to heighten immune cell activation and lower the threshold to overcome immune tolerance, have been used to increase the risk for immunotoxicity (30). Using a short-term Treg depletion strategy, individual antibodies that antagonize inhibitory and agonize stimulatory immune modulators have been compared. As a single agent, both anti–PD-1 antagonism and agonistic anti-CD137 treatment enhance antitumor activity. However, anti-CD137 treatment leads to more severe toxicities than anti–PD-1, consistent with clinical observations, particularly for hepatoxicity (31). Antibody-mediated neutralization of tumor necrosis factor (TNF)-α reverses anti-CD137–induced irAEs without impeding antitumor immunity (30). These results suggest that animal models may not only be useful in determing irAE risk but may also facilitate development of combinations that can avoid toxicities while maximizing antitumor activity. B6 mice expressing humanized CTLA-4 identified human CTLA-4 monoclonal antibodies with reduced irAEs compared to clinically approved ipilimumab without compromising tumor control (32). Ipilimumab-mediated antitumor immunity in mouse models expressing human CTLA-4 or human Fcγ receptors was attributed to Treg depletion in the tumor (20,32). Although limited evidence exists for Treg depletion in anti–CTLA-4–treated cancer patients, ipilimumab patients with Fcγ receptor polymorphisms have improved survival outcomes in tumors with high mutational burden (20). Further testing of novel immunotherapy combinations in these Treg-compromised models will determine antitumor efficacy versus immunotoxicity in these autoimmune-prone settings.
Although the manipulation of standard mouse strains has been useful, developing tumor models in mouse strains prone to autoimmune and other immune-related toxicities will provide additional benefits. NOD mice are predisposed to developing autoimmune manifestations and multiple genetic loci that precipitate autoimmunity have been identified [reviewed by ref. (33)]. Some insulin-dependent diabetes (Idd) susceptibility loci prevent anti–PD-1–induced diabetes (34). Autoimmune mice allow for detailed investigations of target expression during autoimmune and antitumor immune conditions. PD-L1 expression is increased with accumulation of immune infiltrate in the islets of Langerhans in NOD mice, suggesting that the PD-1/PD-L1 axis may be critical for disarming autoreactive T cells and preventing T1D onset (35). Diabetes in NOD mice can be induced months after tolerance induction using anti–PD-1 and anti–PD-L1 (36). Ectopic CTLA-4 expression on pituitary gland endocrine cells may be the direct target of antibody-mediated hypophysitis following anti–CTLA-4 treatment through activation of complement (10,37). Thus, it will be essential to develop tumor models in autoimmune-prone strains to enable interrogation of the autoimmune and antitumor immune interface and provide insights into the clinical manifestations of irAEs, as well as various genetic and environmental parameters that better define the human experience (Fig. 1).
Comparing conventional and immunotherapy-induced autoimmunity in patients
Determining whether irAEs develop similarly to conventional forms of spontaneous autoimmunity and, therefore, share common features such as autoantibody presentation, autoreactive T cells, and underlying genetic risk factors is of great interest. For conventional T1D, autoantibody positivity for islet-associated antigens is present in over 90% of cases at diagnosis (38), but in CPI-induced diabetes, the same autoantibodies are positive in less than half the cases (39). Similarly, patients with rheumatological irAEs are negative for rheumatoid factor and cyclic citrullinated protein antibodies and frequently have low or negative titers for anti-nuclear antibodies at presentation (40). In contrast, thyroid antibodies are associated with thyroid irAEs induced by anti–PD-1 treatment in non-small cell lung cancer (NSCLC), suggesting that humoral immunity may play a role in some forms of irAEs (6).
When autoantibodies related to conventional disease are not present, screening for alternate antibodies may both assist with defining disease and providing mechanistic insight by identifying functionally significant pathways for irAEs. In patients receiving intralesional Bacillus Calmette–Guérin (BCG) and ipilimumab for melanoma, an increased number of autoantibody specificities was found in individuals with severe irAEs (41). Similarly, interrogation of global antibody profiles prior to CPI treatment identified toxicity-associated antibody signatures that were heightened in patients that developed severe irAEs (42). Minimal signature or functional overlap was identified between different CPI treatment groups, including monotherapy versus combination anti–PD-1 and anti–CTLA-4 treatment (42). This finding also highlights the potential variation in the mechanism by which autoimmune sequelae develop in response to CPI treatment but should be replicated with separation of irAEs based on organ pathology rather than severity alone. Decreased circulating B cells after CPI treatment correlates with time to onset and severity of immunotoxicity, irrespective of site, with cellular changes preceding clinical manifestation of irAEs (43). However, whether reduced proportions of B cells reflect egress to tissues, differentiation, or apoptotic programs and the subsequent effect on antibody production is not known.
Alterations to the T-cell repertoire in response to CPI treatment have been reported to correlate with both therapeutic response and severity of irAEs. An increased number of expanded CD8+ T-cell clones in the periphery of prostate cancer patients receiving ipilimumab correlated with severe irAEs (44). Another study confirmed that patients who develop irAEs display increased T-cell diversification in the periphery two weeks after treatment initiation with ipilimumab and GM-CSF (11). Determining pathogenic antigen recognition versus bystander expansion of T-cell clones is of major interest and may represent an opportunity to develop tolerogenic therapies in the form of antigen-specific Tregs and peptide-based approaches to be given prophylactically to at-risk individuals. Similarly, the presence of previously defined autoreactive T cells from spontaneous autoimmune disease has not been assessed in CPI-treated patients developing irAEs.
Major histocompatibility complex (MHC) genes [human leukocyte antigens (HLA)] are known to confer risk to the development of many spontaneously occurring autoimmune diseases. In hypophysitis, HLA-markers DQ8 and DR53 are associated with sporadic, but not anti-CTLA-4 treatment-associated disease (45). In CPI-induced diabetes, the T1D high-risk HLA allele, DR4, is significantly more prevalent than reported frequencies in USA Caucasians and individuals who develop spontaneous T1D (39). As the association between individual irAEs and genetic variation, including HLA type, are discovered, it may become clinically useful to carry out genetic risk screening prior to choosing an appropriate CPI treatment plan if multiple treatment lines have similar antitumor efficacy but are disparate for an individual’s risk of irAE.
Serum cytokine levels may also provide predictive value and mechanistic insight for patient susceptibility to CPI-induced irAEs. Pre-existing, circulating IL17 may predict which ipilimumab-treated melanoma patients could develop severe diarrhea and colitis (46). IL17 plays a critical role in the pathogenesis of inflammatory immune-mediated diseases, including various forms of arthritis and bowel diseases. Gastrointestinal irAEs are the most common co-occurring immunotoxicities in CPI-treated patients with inflammatory arthritic irAEs (40). Although defining a correlation between multiple irAEs requires a greater number of patients, it may suggest a link between IL17-driven pathogenesis and the type of autoimmune sequelae that co-occur in response to CPI treatment, with potential for a targeted approach to relieve irAEs.
Taken together, these data suggest that for autoimmune irAEs, T cells and antibodies recognizing autoantigens may, at least in part, be responsible for immunotherapy-induced pathologies, but in many cases, display alternate features compared to conventional autoimmune diseases. However, a more complete interrogation of these immune processes will be necessary in the human setting by initiating dedicated efforts to collect clinical and mechanistic data longitudinally, starting prior to immunotherapy commencement to understand key parameters of disease etiology and mechanistic considerations.
Environmental influences on irAEs
Growing evidence suggests that an individual’s gut microflora can alter immune homeostasis and tolerance. Both microbial diversity and abundance may impact the development of immunotoxicity and antitumor immune responses to CPI treatment by altering the immune composition in the tumor and periphery (47–50). In preclinical models, the presence of certain species of Bacteroides and Burkholderiales not only potentiate anti–CTLA-4 tumor control, but also reduce the severity of experimental colitis following CTLA-4 blockade (51). Similarly, enrichment of the Bacteroidetes phylum was identified in colitis-free, ipilimumab-treated melanoma patients (49,52), whereas the Firmicutes phylum was enriched preceding ipilimumab in patients developing colitis (49). Administration of antibiotics, which potently disrupt commensal microbiota, prior to CPI treatment reduces survival benefit for renal cell carcinoma and NSCLC patients (53) and may further highlight an interaction among microbiota, antitumor immunity, and irAEs. Mechanistically, microbial changes may alter immune responses through cross-reactivity and mimicry of endogenous molecules or tumor-derived neoantigens, promotion of metabolic activity, or influencing recruitment and polarization of inflammatory immune cells. Understanding the impact of the microbiome on the immune response will be essential in enabling the treatment of dysbiosis and to reestablish equilibrium of the protective gut microflora to limit irAEs. It will also be equally critical to determine the influence of non-gastrointestinal microbiota and whether anti–PD-1/PD-L1–driven irAEs are influenced by microbial composition and other environmental factors.
Treatment of irAEs without impeding antitumor immunity
Studies of differential treatment responses based on irAE development have been conflicting, with some reporting improved response in patients who develop irAEs (4,54) and others that report no difference (3). Investigation of the effect of individual irAEs within different cancer subtypes may provide more clarity. Vitiligo occurs preferentially in melanoma patients, highlighting a crossover between antitumor immunity and autoimmunity, with hypopigmentation correlated to improved outcome to immunotherapy (55). This is likely due to an interaction between self-antigens that persist in the tumor, which also become an immune target in non-malignant, pigmented cells. The presence of autoimmune sequelae, such as hypophysitis in patients treated with ipilimumab for melanoma (56), thyroid dysfunction following pembrolizumab in NSCLC patients (6), and gastrointestinal irAEs for CPI treatment of advanced malignancy (57) all reveal favorable survival outcomes despite therapeutic intervention and without clear indication for the shared response. Understanding the mechanism by which autoimmunity and tumor immunity provide interactive responses will assist in developing therapeutic interventions to moderate autoimmune irAEs, without impacting the antitumor immune response.
The mainstay of irAE treatment is corticosteroids of varying doses, depending on the severity of the irAE (9,58). Some high-grade or corticosteroid-refractory irAEs are treated with additional immunosuppressive agents (9,58). Because corticosteroids act as a systemic immunosuppressive agent through induction of apoptosis of lymphocytes among other mechanisms, it has been theorized that corticosteriods may attenuate the antitumor immune response initiated after CPI treatment. Although multiple studies have suggested that corticosteroids do not worsen overall survival or other cancer outcome measures (3,4), these studies have not adequately accounted for the possibility that the response benefit from the irAEs was attenuated by the use of corticosteroids or other immunosuppressive agents. In a study of melanoma patients with ipilimumab-induced hypophysitis, patients treated with low-dose steroids, rather than high-dose steroids, had a significantly better melanoma response to ipilimumab with no difference in clinical benefit for treatment of hypophysitis. This study suggests that the high-dose corticosteroids used to treat irAEs may attenuate the improved response that the irAE portends. Additionally, NSCLC patients receiving corticosteroids at baseline experienced an inferior treatment response to PD-1/PD-L1 inhibitors compared to patients not on chronic corticosteroids (59), suggesting that steroid treatment may be detrimental to CPI-induced antitumor responses.
Other immunosuppressive agents used as second- or third-line therapies for specific irAEs include infliximab, vedolizumab, mycophenolate mofetil, cyclophosphamide, IVIg, plasmapheresis, and others (9,58), with mounting evidence for the safety and efficacy of these medications for treatment of irAEs. Infliximab neutralizes TNFα (57,60), whereas vedolizumab is a monoclonal antibody against α4β7 integrin, expressed primarily on a subset of CD4+ T cells in the gut (61), allowing for irAE treatment to be relatively gut-specific. Both have been used for the treatment of steroid-refractory enterocolitis, and some experts recommend early use of infliximab (3,4,60). Two small studies (57,60) comparing cancer outcomes of patients with enterocolitis requiring infliximab in addition to high-dose corticosteroids to patients treated with high-dose corticosteroids alone did not identify a survival difference. However, it was suggested that shortened corticosteroid treatment alongside infliximab may reduce infection risk and improve immune recovery.
Organ-specific and systemic immune responses are not just limited to cancer patients receiving CPIs or other immunomodulatory therapeutics. Cancer patients receiving chimeric antigen receptor (CAR) T-cell therapy can also develop cytokine release syndrome, which has some similarities to CPI-induced irAEs and may provide insights into the overall irAE experience. In the CAR T-cell therapy setting, anti-IL6/IL6 receptor agents are used in many patients prior to use of systemic corticosteroids because the latter may reduce the efficacy of the CAR T-cell therapy (62). The use of IL6 inhibition for CPI-induced irAEs is not common, but case reports of successful prevention and treatment of irAEs with use of IL6 blockade exist (63–65). Evidence for improved antitumor immune responses with IL6 blockade in combination with PD-1 blockade in preclinical mouse models has also been reported (66). As CAR T-cell therapies become more widely used in the cancer setting, including for solid tumors, it is likely that the number and kind of irAEs will increase, indicating a need to develop animal models to better understand and treat the irAEs (67,68).
Cancer immunotherapeutic strategies that prevent induction of irAEs
Although improved mechanistic understanding to monitor and treat irAEs will enhance clinical management of immunotherapy-treated patients, development of therapeutic strategies that induce tumor-specific immunity without the threat of systemic irAEs will provide greatest clinical benefit (Fig. 1). Targeting intratumoral Tregs without peripheral depletion may improve therapeutic specificity. Fc-optimization of a depleting anti-CD25 enhanced intratumoral Treg depletion and, when combined with anti–PD-1, augmented tumor eradication (69). However, a reduction in peripheral Tregs remained evident, highlighting a need for identification of markers that define tumor-infiltrating Tregs. Modulating Treg stability may also promote antitumor immunity with increased therapeutic safety. Targeting Treg-derived EZH2 or NRP1, both having high expression in tumor-infiltrating Tregs, bolster production of proinflammatory cytokines, leading to antitumor immunity without autoimmune consequences (70,71). Inhibitors of the epigenetic modifier EZH2 are in early phase clinical trials as anti-cancer agents, and their impact on immune cell function should be evaluated.
Bispecific antibodies, and alternate antibody-based structures, that target multiple tumor and immune components simultaneously may selectively enhance localized, tumor-targeted immune activity with reduced risk of irAEs. In the clinic, reversible autoinflammatory toxicities, but not autoimmunity, have been observed in some patients when co-targeting T cells by CD3 engagement alongside tumor antigens. Refined marker selection will be critical to ensure their clinical utility. Dual immunomodulators targeting inhibitory PD-1 and LAG-3, or targeting stimulatory OX40 and inhibitory CTLA-4 are entering early phase clinical testing (72), providing an opportunity to compare their therapeutic safety and efficacy to monoclonal antibody combination treatment. In addition to multi-arm antibody approaches, engineered cell therapies may assist in fine-tuning immune cell responses to provide greater therapeutic control. Orthogonalization of ligand and receptor interactions, which retain native receptor signaling but limit negative pleiotropic, off-target effects, may be employed for maximizing the potential of adoptive cell therapies and was achieved for the IL2/IL2Rβ interaction (73). Selection of the route for therapeutic administration may also alter the onset of irAEs. With increasing interest in intratumoral therapies and observed abscopal effects, it will be important to learn if more localized immunotherapies impact irAEs.
Concluding remarks
The presence of irAEs is indicative of an inflammatory immune response and is precipitated due to potential cross-reactivity between tumor and self, underlying predisposition to autoimmunity, or collateral damage from cytokine-induced inflammation. Improving our mechanistic understanding of factors that lead to immunotherapy-induced autoimmune pathologies may facilitate identification of biomarkers to predict or monitor onset of disease, enabling clinicians to more accurately treat autoimmune irAEs in immunotherapy-treated cancer patients. Knowledge of those cancer patients with higher susceptibility for irAEs will provide improved clinical care and heightened monitoring to prevent complications from the development of potentially life-threatening irAEs and limit the impact of treatment disruption to an individual’s cancer care. As we improve our understanding of how each CPI treatment disrupts immune tolerance, we may be able to identify mechanisms to direct patients towards their most appropriate treatment type or engineer alternative therapies that control or prevent tissue inflammation without exacerbating tumor growth to provide optimal antitumor immunity with minimal irAEs for greatest clinical efficacy and safety.
Acknowledgments
Financial Support: A.Y. was supported by a NHMRC C.J. Martin Fellowship (1143981). Z.Q. was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR001871. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Research support by the Parker Institute for Cancer Immunotherapy and the Sean N. Parker Autoimmune Research Laboratory.
Footnotes
Conflicts of Interest:
JAB is a consultant for Juno, a Celgene company; a stock holder and member of the Board of Directors on Rheos Medicines, a stock holder and member of the Scientific Advisory Boards of Pfizer Center for Therapeutic Innovation, Vir Therapeutics, Arcus Biotherapeutics, Quentis Therapeutics, Solid Biosciences, and Celsius Therapeutics. JAB owns stock in MacroGenics Inc., Viacyte Inc., and Kadmon Holdings. AY and ZQ none to disclose
References
- 1.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 2016;13(6):394 doi 10.1038/nrclinonc.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016;13(8):473–86 doi 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 3.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33(28):3193–8 doi 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017;35(7):785–92 doi 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 5.June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nature Medicine 2017;23(5):540–7 doi 10.1038/nm.4321. [DOI] [PubMed] [Google Scholar]
- 6.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28(3):583–9 doi 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 2010;13(1):29–38 doi 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 8.Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother 2017;66(11):1399–410 doi 10.1007/s00262-017-2033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C 3rd, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5(1):95 doi 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014;6(230):230ra45 doi 10.1126/scitranslmed.3008002. [DOI] [PubMed] [Google Scholar]
- 11.Oh DY, Cham J, Zhang L, Fong G, Kwek SS, Klinger M, et al. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res 2017;77(6):1322–30 doi 10.1158/0008-5472.CAN-16-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. The New England journal of medicine 2016;375(18):1749–55 doi 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3(5):541–7. [DOI] [PubMed] [Google Scholar]
- 14.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995;270(5238):985–8. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999;11(2):141–51. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291(5502):319–22 doi 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 17.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. The Journal of experimental medicine 2003;198(1):63–9 doi 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. The Journal of experimental medicine 1998;187(3):427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016;44(5):989–1004 doi 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 2018;33(4):649–63 e4 doi 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013;1(1):32–42 doi 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 22.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017;355(6332):1428–33 doi 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017;355(6332):1423–7 doi 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci U S A 2015;112(21):6682–7 doi 10.1073/pnas.1420370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harjunpaa H, Blake SJ, Ahern E, Allen S, Liu J, Yan J, et al. Deficiency of host CD96 and PD-1 or TIGIT enhances tumor immunity without significantly compromising immune homeostasis. Oncoimmunology 2018;7(7):e1445949 doi 10.1080/2162402X.2018.1445949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012;72(4):917–27 doi 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med 2011;208(2):395–407 doi 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blake SJ, Stannard K, Liu J, Allen S, Yong MC, Mittal D, et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov 2016;6(4):446–59 doi 10.1158/2159-8290.CD-15-0944. [DOI] [PubMed] [Google Scholar]
- 29.Ascierto PA, Melero I, Bhatia S, Bono P, Sanborn RE, Lipson EJ, et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J Clin Oncol 2017;35:(suppl; abstr 9520, 2017 ASCO Annual Meeting). [Google Scholar]
- 30.Liu J, Blake SJ, Harjunpaa H, Fairfax KA, Yong MC, Allen S, et al. Assessing Immune-Related Adverse Events of Efficacious Combination Immunotherapies in Preclinical Models of Cancer. Cancer Res 2016;76(18):5288–301 doi 10.1158/0008-5472.CAN-16-0194. [DOI] [PubMed] [Google Scholar]
- 31.Yonezawa A, Dutt S, Chester C, Kim J, Kohrt HE. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin Cancer Res 2015;21(14):3113–20 doi 10.1158/1078-0432.CCR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du X, Liu M, Su J, Zhang P, Tang F, Ye P, et al. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res 2018;28(4):433–47 doi 10.1038/s41422-018-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005;23:447–85 doi 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 34.Kochupurakkal NM, Kruger AJ, Tripathi S, Zhu B, Adams LT, Rainbow DB, et al. Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS One 2014;9(2):e89561 doi 10.1371/journal.pone.0089561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. Beta cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell metabolism 2017;25(3):727–38 doi 10.1016/j.cmet.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. The Journal of experimental medicine 2006;203(12):2737–47 doi 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, et al. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am J Pathol 2016;186(12):3225–35 doi 10.1016/j.ajpath.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab 2010;95(1):25–33 doi 10.1210/jc.2009-1365. [DOI] [PubMed] [Google Scholar]
- 39.Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018. doi 10.2337/dbi18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76(1):43–50 doi 10.1136/annrheumdis-2016-209595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Da Gama Duarte J, Parakh S, Andrews MC, Woods K, Pasam A, Tutuka C, et al. Autoantibodies May Predict Immune-Related Toxicity: Results from a Phase I Study of Intralesional Bacillus Calmette-Guerin followed by Ipilimumab in Patients with Advanced Metastatic Melanoma. Front Immunol 2018;9:411 doi 10.3389/fimmu.2018.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gowen MF, Giles KM, Simpson D, Tchack J, Zhou H, Moran U, et al. Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J Transl Med 2018;16(1):82 doi 10.1186/s12967-018-1452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018;128(2):715–20 doi 10.1172/JCI96798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci USA 2016;113(42):11919–24 doi 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heaney AP, Sumerel B, Rajalingam R, Bergsneider M, Yong WH, Liau LM. HLA Markers DQ8 and DR53 Are Associated With Lymphocytic Hypophysitis and May Aid in Differential Diagnosis. J Clin Endocrinol Metab 2015;100(11):4092–7 doi 10.1210/jc.2015-2702. [DOI] [PubMed] [Google Scholar]
- 46.Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, et al. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:39 doi 10.1186/s40425-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359(6371):97–103 doi 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359(6371):104–8 doi 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28(6):1368–79 doi 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 50.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359(6371):91–7 doi 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 51.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350(6264):1079–84 doi 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016;7:10391 doi 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018;29(6):1437–44 doi 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol 2018;4(3):374–8 doi 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015;33(7):773–81 doi 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 56.Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018. doi 10.1002/cncr.31629. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer 2018;6(1):37 doi 10.1186/s40425-018-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2(10):1346–53 doi 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 59.Arbour KC, Mezquita L,Long N,Rizvi H,Auclin E,Ni A, et al. Deleterious effect of baseline steroids on efficacy of PD-(L)1 blockade in patients with NSCLC. J Clin Oncol 2018;36(suppl; abstr 9003, 2018 ASCO Annual Meeting). [DOI] [PubMed] [Google Scholar]
- 60.Arriola E, Wheater M, Karydis I, Thomas G, Ottensmeier C. Infliximab for ipilimumab-related colitis. Clin Cancer Res 2015;21(24):5642–3 doi 10.1158/1078-0432.CCR-15-2471. [DOI] [PubMed] [Google Scholar]
- 61.Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017;66(5):581–92 doi 10.1007/s00262-017-1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018;15(1):47–62 doi 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uemura M, Trinh VA, Haymaker C, Jackson N, Kim DW, Allison JP, et al. Selective inhibition of autoimmune exacerbation while preserving the anti-tumor clinical benefit using IL-6 blockade in a patient with advanced melanoma and Crohn’s disease: a case report. J Hematol Oncol 2016;9(1):81 doi 10.1186/s13045-016-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017;76(12):2061–4 doi 10.1136/annrheumdis-2017-211560. [DOI] [PubMed] [Google Scholar]
- 65.Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, Walker PR. Interleukin-6 as one of the potential mediators of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint blockade: evidence from a case report. Acta Oncol 2018;57(5):705–8 doi 10.1080/0284186X.2017.1406668. [DOI] [PubMed] [Google Scholar]
- 66.Bialkowski L, Van der Jeught K, Bevers S, Tjok Joe P, Renmans D, Heirman C, et al. Immune checkpoint blockade combined with IL-6 and TGF-beta inhibition improves the therapeutic outcome of mRNA-based immunotherapy. Int J Cancer 2018;143(3):686–98 doi 10.1002/ijc.31331. [DOI] [PubMed] [Google Scholar]
- 67.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24(6):731–8 doi 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018;24(6):739–48 doi 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 69.Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory t cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 2017;46(4):577–86 doi 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D, Quiros J, Mahuron K, Pai CC, Ranzani V, Young A, et al. Targeting EZH2 reprograms intratumoral regulatory T cells to enhance cancer immunity. Cell Rep 2018;23(11):3262–74 doi 10.1016/j.celrep.2018.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, et al. Interferon-gamma drives Treg fragility to promote anti-tumor immunity. Cell 2017;169(6):1130–41 e11 doi 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dahlen E, Veitonmaki N, Norlen P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother 2018;6(1):3–17 doi 10.1177/2515135518763280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sockolosky JT, Trotta E, Parisi G, Picton L, Su LL, Le AC, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 2018;359(6379):1037–42 doi 10.1126/science.aar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shahabi V, Berman D, Chasalow SD, Wang L, Tsuchihashi Z, Hu B, et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 2013;11:75 doi 10.1186/1479-5876-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35(7):709–17 doi 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]