Abstract
Anopheles gambiae, An. coluzzii and An. arabiensis are the three major vectors of malaria in Nigeria. These mosquitoes have developed resistance to different insecticides. Insecticides resistance intensity assay was recently introduced to provide insight into the potential operational significance of insecticide resistance. Here, we present data on pyrethroids resistance intensity and resistance mechanisms from six vector surveillance sites (Lagos, Ogun, Edo, Anambra, Kwara and Niger) in Nigeria. Adult Anopheles reared from larval collections were tested using WHO insecticides susceptibility protocol with 1x concentration of permethrin and deltamethrin followed with intensity assays with 5x and 10x concentrations of both insecticides. Synergistic and biochemical assays were carried out and underlying resistance mechanisms determined following standard protocols. Anopheles gambiae constituted >50% samples tested in five sites. Permethrin and deltamethrin resistance was observed at all the sites. The Kdt50 varied from 15 minutes (CI = 13.6–17.2) in deltamethrin to 42.1 minutes (CI = 39.4–44.1) in permethrin. For both insecticides, Kdt95 was >30 minutes with 25% to 87% post exposure mortality at the different sites. The West Africa knock down resistance (kdr-w) mechanism was found at each site. Resistant An. gambiae from Lagos, Ogun and Niger synergized prior to permethrin or deltamethrin exposure showed significant mortality (89–100%) compared to unsynergized mosquitoes (Lagos, p = 0.031; Ogun, p = 0.025; Niger, p = 0.018). Biochemical analyses revealed significant increased levels of P450 enzymes in resistant Anopheles gambiae from Lagos (p = 0.038); Ogun (p = 0.042) and Niger (p = 0.028) in addition to GST in Lagos (p = 0.028) and Ogun (p = 0.033). Overall, the results revealed high pyrethroid resistance associated with increased activities of metabolic enzymes (P450 + GST) in An. gambiae and An. coluzzii from Lagos and Ogun. The presence of kdr + P450 conferred moderate resistance whereas low resistance was the case where kdr was the sole resistance mechanism. Findings thus suggests that elevated levels of cytochrome P450 enzymes together with GST were responsible for high or severe pyrethroid resistance.
Introduction
The use of long-lasting insecticidal nets (LLINs) is one of the most effective measures for malaria control [1–2]. The basis of this intervention lies in the continuing susceptibility of Anopheles mosquito vectors to limited numbers of insecticides. Although new classes and combinations of insecticides are being pilot-tested [3], pyrethroid remains the only class currently in use for LLINs production. Over the past five decades, several accounts of reduced Anopheles susceptibility to insecticides, resistance to organophosphates, organochlorine and carbamates have been reported [4–5]. Resistance of Anopheles to pyrethroid insecticides is a much more recent development, first reported in Anopheles gambiae from Côte d'Ivoire [6] and now wide- spread in West, Central and East Africa [7–8]. Resistance is primarily due to target-site insensitivity arising from a single point mutation often referred to as knock down resistance (kdr) [9–11]. Metabolic resistance mechanisms which are principally associated with three enzyme families (cytochrome p450 mono-oxygenaeses (p450s), carboxylesterases (CEOEs) and glutathione-S-transferases (GSTs), have also been implicated in pyrethroid resistance in many sites in Africa [4,12].
Even though pyrethroid resistance is widespread, its impact on malaria control remains unclear. For instance, a just concluded review [13] was unable to determine if LLINs remain effective in the presence of resistance. To address the challenge of insecticides resistance, the World Health Organization (WHO) updated and expanded insecticide resistance test procedure to include resistance intensity assays [14]. It also initiated the Global Plan for Insecticide Resistance Management (GPIRM) [15] which outlined several strategies on how best to respond to resistance [16]. A key pillar in this effort among others, is timely surveillance for effective vector control and resistance management.
Pyrethroid resistance affecting both Anopheles gambiae and Anopheles coluzzii is widespread in Nigeria [17–20]. At least 18 out of the 36 States have reported pyrethroid resistance in Anopheles vectors [21]. The actual number and spread could be higher because, until recently, there was no in-country coordinated platform for insecticide resistance monitoring to present accurate data. Though indiscriminative use of agricultural insecticides has often been cited as a major factor responsible for the development of resistance [22], the rapid increase in the quantity of insecticides used for malaria control following the scale up of vector control activities have exerted significant increase in insecticide selection pressure in Anopheles populations evidenced by increased phenotypic resistance and kdr frequency [21]. The realization that in vector control, surveillance and information on resistance intensity could provide predictive value for decision making, the Nigerian Malaria Elimination Program recently established vector surveillance sites in the six geo-political zones. Here, we present data on levels of pyrethroid resistance with corresponding resistance mechanisms in populations of Anopheles gambiae from six vector surveillance sites in Nigeria.
Methods
Study areas
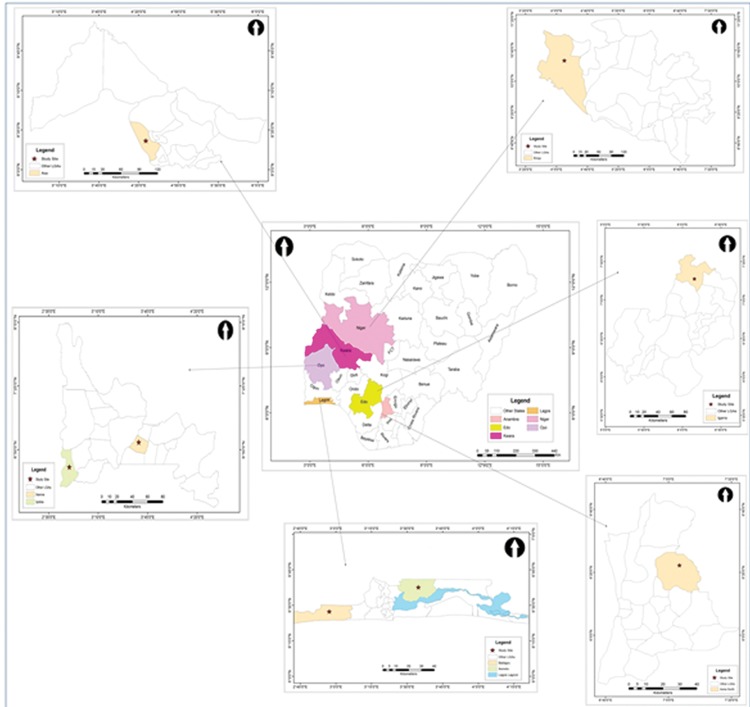
The study was carried out from May to July 2016 in six vector surveillance sites (Fig 1). The use of long lasting insecticidal nets is the main malaria vector control intervention in all the sites in addition to indoor residual spraying in Lagos.
Fig 1. Map of Nigeria showing the study sites in Lagos, Ogun, Edo, Anambra, Kwara and Niger States.
Mosquitoes collections
Anopheles larvae were collected from natural breeding sites by trained Entomology Technicians using standard method [23]. Larvae were sampled on four separate occasions over a period of three weeks. Mosquitoes were reared to adulthood under standard insectary condition (26–27°C and 76–82% relative humidity) at the Nigerian Institute of Medical Research and identified using morphological keys [24–25] and PCR assays [26–27],
Insecticide susceptibility tests with the standard diagnostic (1x) concentration
Insecticides susceptibility test was carried out using the standard WHO protocol [28] and insecticides test kits supplied by the Vector Control Research Unit, Universiti Sains Malaysia. Two-to three-day old non-blood-fed adult female Anopheles were tested. Five replicates of 20–25 mosquitoes were exposed to test papers impregnated with deltamethrin (0.05%) and permethrin (0.75%). Controls included batches of mosquitoes from each site exposed to untreated papers. The knock- down effect of each insecticide was recorded every 10 minutes over the one-hour exposure period. Mosquitoes were then transferred to a recovery tube and provided with 10% glucose solution. Final mortality was recorded 24 hours post-exposure. All batches of insecticide paper used were pre-tested on the Kisumu susceptible laboratory strain of An. gambiae known to be fully (100%) susceptible to pyrethroid. The tests were carried out at 26–27°C and 76–82% relative humidity.
Intensity assay
Based on the results of the susceptibility tests with 1x standard diagnostic concentration, intensity tests with x5 concentration of permethrin and deltamethrin were carried out at all the sites. The 10x concentrations of both insecticides was used where applicable according to the updated WHO protocols [14]. The 5x and 10x diagnostic concentrations were prepared using Technical-grade of permethrin and deltamethrin (Sigma-Aldrich) diluted in acetone and olive oil (1:1) as contained in the manufactural instruction to form a stock solution. For each insecticide concentration, 2ml of the stock was applied on filter papers (12 × 15 cm Whatman® no. 1: Whatman International Ltd., England). Filter papers treated with silicone was used as control. All filter papers were dried at room temperature for 24 hours prior to use.
Five replicates of 20–25 mosquitoes were tested for each insecticide concentration (where applicable). The exposures were carried out for the standard one hour period and final mortality recorded after 24-hours holding period during which 10% glucose solution was provided. All Anopheles gambiae s.l. tested (dead and live mosquitoes) were identified to species level using PCR assays [26–27]. Briefly, DNA was extracted from each mosquito as previously described [26]. PCR amplification was carried out using thermal cycler (Eppendorf, AG Hamburg, Germany) with an initial denaturation at 94 oC for 2 min, followed by 30 cycles of denaturation at 94 oC or 30 s annealing at 50 oC for 30 s and extension at 72 oC for 30 s with a final extension at 72 oC for 8 min. The amplified DNA was separated on 2.5% agarose gel, stained with 1% ethidium bromide and visualized on an ultraviolet violet trans-illuminator.
Determination of pyrethroid resistance mechanisms
The population of An. gambiae that survived the 1x standard diagnostic concentration of permethrin at each site was divided into two: the first subset was analyzed together with dead mosquitoes (n = 2038) to species level using PCR [26–27] and for the presence of the kdr mutation using allele-specific PCR diagnostic tests designed for the West and East African kdr mutations [9,11]. The second subset (n = 330: 50–60 families per site) was induced to lay eggs in the insectary and F1 progeny raised under standard insectary condition (26–27°C and 76–82% relative humidity) used for synergist and biochemical analyses.
Synergist assay with 4% Piperonyl Butoxide (PBO) was performed along with permethrin [29–30]. The F1 progenies that survived 1x concentration of permethrin were further sub-divided into two sub sets. A subset was exposed to 4% PBO prior to permethrin exposure. The second subset was exposed to permethrin only. To determine the role of metabolic degradation as a mechanism for pyrethroid resistance, mortality rate was compared between PBO exposed and unexposed mosquitoes. To investigate the relative role of specific metabolic pathways inhibited by this synergist, enzyme assays were carried out on live mosquitoes not exposed to insecticides were used to measure esterase, glutathione S-transferase (GST) and cytochrome P450 monooxygenase activities as previously described [30]. The same mosquitoes tested for P450 monooxygenase and GST were also tested for esterases activities. All mosquitoes tested were identified to species level by PCR [26–27].
Data analysis
The knock-down times for 50 and 95% (Kdt50 and Kdt95) mosquito exposed was analyzed using probit-log [31] with MedCalc statistical software version 17.1. Following insecticides tests and PCR identification, data were segregated and analyzed separately for each species using the updated WHO guideline [14]:
98–100% mortality at 1x dose confirms resistance
98–100% mortality at 5× dose indicates a low resistance intensity. Not necessary to assay at the 10× dose.
< 98% mortality at 5× dose indicates a moderate resistance intensity. Further test at 10× dose needed.
98–100% mortality at 10× dose confirms a moderate resistance intensity.
< 98% mortality at 10× dose indicates high resistance intensity.
Enzymatic activity of P450 oxidases, GST and esterases were analyzed and compared with the Kisumu reference Anopheles strain using ANOVA (F-statistic) with significant p-values < 0.05.
Ethics
The study was approved by the Research Ethics Review Committee and Institutional Review Board of the Nigerian Institute of Medical Research with protocol approval ref. No. IRB/14/257.
Results
Proportion of Anopheles gambiae, An. coluzzii and An. arabiensis in the study sites
The species composition of Anopheles gambiae s.l. found in this study sites did not differ from previous records with An. gambiae constituting >50% at each site except in Edo where An. coluzzii represented 65% (Table 1). Wherever present, An. arabiensis was < 20% of the Anopheles tested. Overall, there were mixed populations of An. gambiae and An. coluzzii in Lagos, Ogun, Edo, Kwara and Niger but a pure collection of An. gambiae in Anambra. No hybrid form of these species was found.
Table 1. Number of Anopheles gambiae s.l. tested with proportion of An. gambiae, An. coluzzii and An. arabiensis.
| State: (site) | N | Number and proportion (%) | ||
|---|---|---|---|---|
| An. gambiae ss. | An. coluzzii | An. arabiensis | ||
| Lagos (Ikorodu-Badagry) | 680 | 405 (59.6) | 275 (40.4) | - |
| Ogun (Ipokia-Irolu) | 600 | 332 (55.3) | 207 (34.5) | 61 (10.2) |
| Edo (Igara) | 420 | 147 (35.0) | 273 (65.0) | - |
| Anambra (Achalla) | 540 | 530 (98.1) | - | - |
| Niger (New Bussa) | 540 | 315 (58.3) | 125 (23.2) | 100 (18.5) |
| Kwara (Ayede) | 480 | 300 (62.5) | 125 (26.0) | 55 (11.5) |
Status of pyrethroid resistance at the study sites
The Kisumu strain used as control was fully susceptible to the standard (1x) diagnostic concentration of permethrin (0.75%) and deltamethrin (0.05%) with 50% knock down recorded within 10 minutes’ exposure to both insecticides and 100% mortality 24-hours’ post exposure. In contrast An. gambiae s.l. from the six sites were resistant to permethrin and deltamethrin. The knock-down times for 50% mosquitoes (Kdt50) varied from 15 minutes (CI = 13.6–17.2) in Anopheles exposed to deltamethrin in Edo to 42.1 minutes (CI = 39.4–44.1) in mosquitoes exposed to permethrin in Lagos. For both insecticides, Kdt95 was > 30 minutes at each site. The 24-hour post exposure mortality from the six sites was 25 to 85% in permethrin (Table 2) and 30 to 87% in deltamethrin (Table 3). The lowest mortality (< 17.0%) and thus highest resistant was recorded in mosquitoes exposed to permethrin in Lagos. Mortality in the control was < 5% for each test.
Table 2. Mortality of Anopheles gambiae s.l. after 24-hr post exposure to 1x, 5x and 10x concentrations of permethrin in WHO bioassays and associated resistance intensity based on WHO classification [14].
| State (site) | Permethrin diagnostic concentration (%) | ||||
|---|---|---|---|---|---|
| 1x (0.75) | 5x (3.75) | 10x (7.5) | |||
| Lagos: Ikorodu-Badagry | No. exposed | 120 | 100 | 100 | High |
| Kdt50 (CI) minutes | 42.1 (39.4–44.1) | 24.2 (22.1–27.2) | 18.8(17.1–21.5) | ||
| Kdt95 (CI) minutes | > 60 | 30.5 (27.5 32.1) | 23.2 (21.1–25.8) | ||
| 24-h % mortality | 16.7 | 55.0 | 89.0 | ||
| Ogun: Ipokia-Irolu | No. exposed | 100 | 100 | 100 | High |
| Kdt50 (CI) minutes | 38.9 (37.2–41.2) | 22.6 (21.2–26.5) | 16.7(15.8–18.6) | ||
| Kdt95 (CI) minutes | 49.6 (47.2–53.4) | 29.5 (28.5 32.2) | 23.5 (22.5–25.9) | ||
| 24-h % mortality | 32(32.0) | 66(66.0) | 95(95.0) | ||
| Edo: Igara | No. exposed | 100 | 100 | NA* | Low |
| Kdt50 (CI) minutes | 15.9 (12.7–18.2) | 13.2 (12.2–15.5) | |||
| Kdt95 (CI) minutes | 30.4 (27.2–32.4) | 26.5 (25.3–28.6) | |||
| 24-h % mortality | 85.0 | 100 | |||
| Anambra: Achalla | No. exposed | 100 | 100 | NA* | Low |
| Kdt50 (CI) minutes | 20.9 (18.8–22.2) | 18.2 (16.1–20.5) | |||
| Kdt95 (CI) minutes | 38.4 (36.2–41.4) | 28.5 (28.0–30.6) | |||
| 24-h % mortality | 80 | 99 | |||
| Niger: New Bussa | No. exposed | 100 | 120 | 110 | Moderate |
| Kdt50 (CI) minutes | 27.5 (25.2–30.2) | 21.6 (20.2–23.5) | 15.8(14.1–17.7) | ||
| Kdt95 (CI) minutes | 38.5 (37.2–41.6) | 28.6 (27.5 32.0) | 20.2 (18.1–22.2) | ||
| 24-h % mortality | 65 | 87.5 | 98.2 | ||
| Kwara: Ayede | No. exposed | 120 | 120 | NA* | Low |
| Kdt50 (CI) minutes | 18.8 (16.5–21.2) | 16.8 (15.0–19.1) | |||
| Kdt95 (CI) minutes | 36.5 (35.2–38.1) | 27.5 (26.2–29.4) | |||
| 24-h % mortality | 89(74.2) | 118(98.3) | |||
*NA (Not applicable): 98–100% mortality at 5× dose indicates a low resistance intensity. Not necessary to assay at 10x dose.
Table 3. Mortality of Anopheles gambiae s.l. after 24-hr post exposure to 1x, 5x and 10x concentrations of deltamethrin in WHO bioassays and associated resistance intensity based on WHO classification [14].
| Sites | Deltamethrin diagnostic concentration (%) | ||||
|---|---|---|---|---|---|
| 1x (0.05) | 5x (0.25) | 10x (0.5) | |||
| Lagos: Ikorodu Badagry | No. exposed | 120 | 120 | 120 | High |
| Kdt50 (CI) minutes | 25.8 (23.7–27.2) | 18.5 (16.1–21.2) | 13.2(12.1–15.8) | ||
| Kdt95 (CI) minutes | 38.5 (37.1–40.1) | 29.2 (27.4 31.5) | 22.1 (19.0–24.6) | ||
| 24-h % mortality | 30.0 | 70.8 | 93.3 | ||
| Ogun: Ipokia-Irolu | No. exposed | 100 | 100 | 100 | Moderate |
| Kdt50 (CI) minutes | 30.8 (27.2–33.2) | 20.5 (18.1–23.5) | 14.5(12.0–16.2) | ||
| Kdt95 (CI) minutes | 42.2 (40.1–44.4) | 27.4 (25.2 29.0) | 21.1 (19.9–23.7) | ||
| 24-h % mortality | 41.0 | 75.0 | 99.0 | ||
| Edo: Igara | No. exposed | 120 | 100 | NA* | Low |
| Kdt50 (CI) minutes | 15.0 (13.6–17.2) | 13.0 (12.8–15.1) | |||
| Kdt95 (CI) minutes | 30.8 (27.0–32.2) | 25.8 (24.3–27.4) | |||
| 24-h % mortality | 87.5 | 100 | |||
| Anambra: Achalla | No. exposed | 120 | 120 | NA* | Low |
| Kdt50 (CI) minutes | 18.5 (16.8–21.2) | 16.2 (14.1–17.9) | |||
| Kdt95 (CI) minutes | 32.4 (30.2–33.1) | 25.2 (24.3–27.6) | |||
| 24-h % mortality | 76.7 | 98.3 | |||
| Niger: New Bussa | No. exposed | 120 | 120 | 110 | High |
| Kdt50 (CI) minutes | 25.3 (24.2–27.2) | 19.6 (17.4–22.5) | 13.8(11.8–15.4) | ||
| Kdt95 (CI) minutes | 39.2 (37.0–42.8) | 27.6 (25.5 28.8) | 19.5 (18.0–21.2) | ||
| 24-h % mortality | 61.7 | 85.0 | 94.5 | ||
| Kwara: Ayede | No. exposed | 120 | 120 | NA* | Low |
| Kdt50 (CI) minutes | 17.8 (15.2–19.8) | 14.2 (12.5–16.1) | |||
| Kdt95 (CI) minutes | 30.4 (28.2–32.1) | 26.4 (24.9–29.1) | |||
| 24-h % mortality | 76.7 | 100 | |||
*NA (Not applicable): 98–100% mortality at 5× dose indicates a low resistance intensity. Not necessary to assay at 10x dose.
Resistance intensity
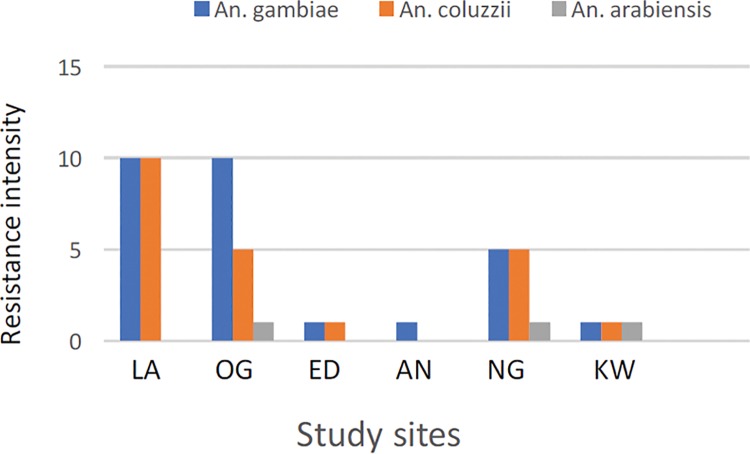
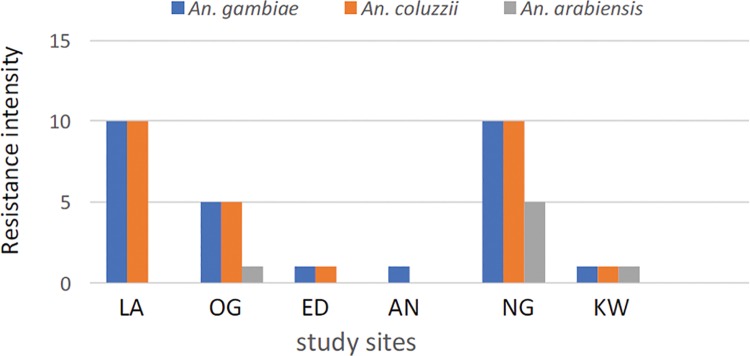
The results of the intensity assays with permethrin and deltamethrin are presented in Tables 2 and 3. The Kdt50 and Kdt95 values for the two insecticides indicate differential tolerance levels of An. gambiae s.l. to pyrethroid in the study sites. More than two-fold increase (from 1x to 10x) in the tolerance level to permethrin and deltamethrin was observed at three sites (Lagos, Ogun and Niger). Based on the expanded WHO classification, there was high permethrin and deltamethrin resistance in Lagos, moderate deltamethrin and high permethrin resistance in Ogun but moderate permethrin and high deltamethrin resistance in Niger. In contrast, resistance was low for both insecticides in Edo, Anambra and Kwara (Tables 2 and 3). Species specific analysis revealed high permethrin and deltamethrin resistance in An. gambiae and An. coluzzii from Lagos (Fig 2) in addition to deltamethrin in Niger (Fig 3). Where present, percentage mortality data of An. arabiensis against 5x concentration of both pyrethroid insecticides showed low resistance (S1 and S2 Tables).
Fig 2. Permethrin resistance intensity in Anopheles gambiae, An. coluzzii and An. arabiensis from Lagos (LA), Ogun (OG), Edo (ED), Anambra (AN), Niger (NG) and Kwara (KW).
Fig 3. Deltamethrin resistance intensity in Anopheles gambiae, An. coluzzii and An. arabiensis from Lagos (LA), Ogun (OG), Edo (ED), Anambra (AN), Niger (NG) and Kwara (KW).
Pyrethroid resistance mechanisms
The West Africa Kdr mutation (Kdr-w) was confirmed in An. gambiae at all the study sites with frequencies ranging from 55% in Anambra to 88% in Niger (S3 Table). The overall kdr-w frequency was significantly higher only in Niger (χ2 = 0.379, df = 5, P = 0.024). The East African mutation (Kdr-e) was absent in the mosquitoes tested.
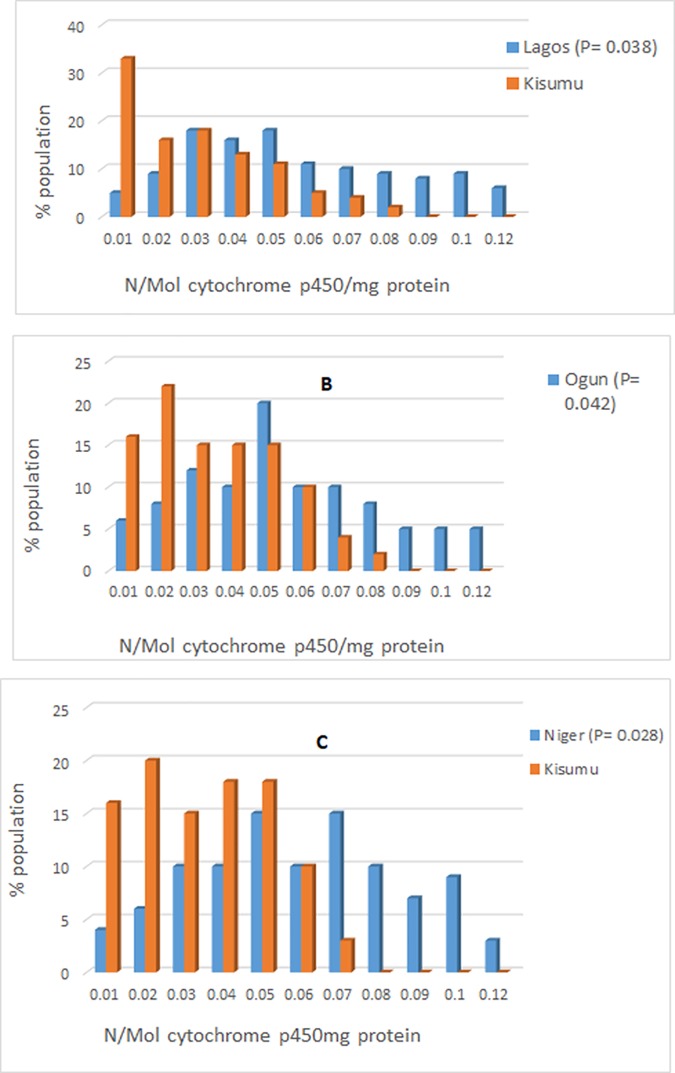
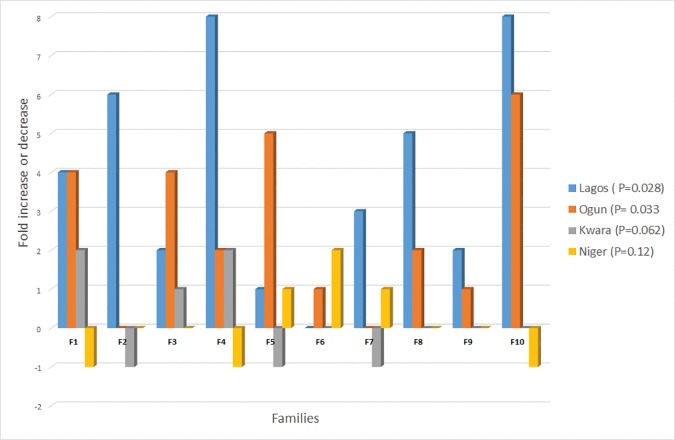
Resistant An. gambiae populations from Lagos, Ogun and Niger synergized prior to permethrin or deltamethrin exposure showed significant mortality (89–100%) compared to unsynergized mosquitoes (Lagos, p = 0.031; Ogun, p = 0.025; Niger p = 0.018) (S4 and S5 Tables). Biochemical analysis revealed increased levels of P450 activity (Fig 4) with mean level of monooxygase > 0.06 N/mol p450 protein compared with the standard Kisumu strain (Lagos, F = 3.48, df = 39, p = 0.038; Ogun, F = 4.25, df = 39, p = 0.042; Niger, F = 5.40, df = 39, p = 0.028). The mean P450 activity in resistant An. gambiae from the remaining sites were like that of the Kisumu strain. Furthermore, GST activities (Fig 5) were significantly higher in resistant populations of An. gambiae from Lagos (p = 0.028) and Ogun (P = 0.033) compared to that of the reference Kisumu strain. In contrast, the mean esterase activity in mosquitoes from all the sites were like the Kisumu reference strain indicating the absence of esterase as a resistance mechanism in the mosquito populations tested.
Fig 4.
Mean level of monooxygenase in pyrethroid resistant Anopheles gambiae from Lagos (A), Ogun (B) and Niger (C) in relation to the susceptible Kisumu strain.
Fig 5. Percentage increase or decrease in GST activity level in families of Anopheles gambiae from Lagos, Ogun, Kwara and Niger relative to the baseline Kisumu level set at zero.
Discussion
The study set out to determine pyrethroid resistance intensity in areas where Anopheles gambiae is resistant to insecticides. The species composition of An. gambiae s.l. found in this study did not differ from previous records [17–19] with a predominance of An. gambiae and An. coluzzii. The high pyrethroid resistance intensity in An. gambiae from Lagos, Ogun and Niger is not surprising considering the prevailing insecticide selection pressure on vector populations following the rapid scale up and use of pyrethroid-based vector control interventions and agricultural usage of pyrethroid insecticides in these areas. Similar resistance intensity (10x) was recently reported in different laboratory strains of An. gambiae from Southern Africa [32].
The present results clearly show the differential effect of the same class of insecticide on three Anopheles species: An. gambiae, An. coluzzii and An. arabiensis. In previous studies [17–18], we investigated pyrethroid susceptibility levels of An. gambiae and An. arabiensis from Lagos and Niger and found that An. arabiensis was fully susceptible to permethrin and deltamethrin. This contrast the present observations in Kwara and Niger where An. arabiensis is resistant to deltamethrin. Pyrethroid resistance in An. arabiensis is not common in Nigeria [19] and there is currently no published information on its spread nor underlying resistance mechanisms. Despite the low resistance, pyrethroid resistance in An. arabiensis could be much wider spread than previously thought.
Most insecticide-resistance monitoring projects emphasize resistance detection, identification of underlying resistance mechanisms and spread. The Kdr-mediated resistance is one of the most common mechanisms reported in pyrethroid resistance Anopheles populations. The role of metabolic detoxification of pyrethroids is increasingly being investigated in Anopheles across different sites in Africa. The study revealed a significant increase in the levels of P450 and GST activities in An. gambiae and An. coluzzii from Lagos and Ogun which consolidated findings from previous study [33] on multiple pyrethroid resistance mechanisms in An. gambiae from Nigeria. Thus, the operational significance of resistance could hinge on interplay between different resistance mechanisms in the vector population. Interestingly, in Edo and Anambra where kdr is present as the sole resistance mechanism, it appears to be associated with low resistance in the Anopheles populations. In all, three scenarios emerged from the study. In Lagos and Ogun with high pyrethroid resistance intensity, kdr plus metabolic: P450s and GST are the main mechanisms; moderate to high pyrethroid resistance intensity was observed in Niger in the presence of P450 plus Kdr; lastly, kdr was the only resistance mechanism in Edo and Anambra where permethrin and deltamethrin resistance was low. Although the operational significance of the severe pyrethroid resistance recorded in the study is uncertain, similar pyrethroid resistance phenotype in southern African An. funestus has been shown to have serious operational implications for malaria vector control in that region [34–36]. Therefore, a much more detailed entomological and epidemiological investigations will be required to unravel the impact of high resistance intensity on malaria control in Nigeria.
Summarily, the study revealed a probable association between resistance intensity and multiple resistance mechanisms and suggest that elevated levels of P450 together with GST were responsible for high pyrethroid resistance. Accordingly, resistance intensity could provide useful information on plausible mechanism developed by the local malaria vectors to combat pyrethroid insecticides.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Entomology Technicians at the Vector Control Research Laboratory of the Nigerian Institute of Medical Research and field workers that participated in the sample collection in Lagos, Ogun, Edo, Anambra, Niger and Kwara States.
Data Availability
All relevant data are within the paper and its Supporting Information files. Detailed statistical analysis is included. A supplementary file containing four tables: S1, S2, S3 and S4 Tables is attached.
Funding Statement
The study was partly supported by Vestergaard Frandsen (EA) Ltd through research grant No. IRB/11/168 and the World Health Organization Special Program for Research and Training in Tropical Diseases (TDR) through Grant Ref. No. B20388 to TSA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2016) Integrated Vector Management (IVM) Geneva: World Health Organization; http://www.who.int/neglected_diseases/vector_ecology/ivm_concept/en/. [Google Scholar]
- 2.WHO (2015) Core vector control methods. Geneva: World Health Organization; http://www.who.int/malaria/areas/vector_control/core_methods/en/. [Google Scholar]
- 3.WHO (2006) Pesticides and their application for the control of vectors and pests of public health importance 6th ed Geneva: World Health Organization; 2006. [Google Scholar]
- 4.Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Ann Rev Entomol 45: 371–391. [DOI] [PubMed] [Google Scholar]
- 5.Mittal PK, Adak T, Sharma VP (1991) Acute toxicity of certain organochlorine, organophosphorus, synthetic pyrethroid and microbial insecticides to the mosquito, fish: Gambusia affinis (Baird and Girard). Indian J Mal 28: 167–170. [PubMed] [Google Scholar]
- 6.Elissa N, Mouchet J, Riviere F, Meunier JY, Yao K (1993) Resistance of Anopheles gambiae s.s. to pyrethroids in Côte d'Ivoire. Ann Soc Belg Med Trop 73::291–294. [PubMed] [Google Scholar]
- 7.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V (2011) Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27: 91–98. 10.1016/j.pt.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 8.Chandre F, Frederic D, Sylvie M, Cecile B, Carnevale P, Guillet P (1999) Pyrethroid cross resistance spectrum among populations of Anopheles gambiae s.s. from Côte d’Ivoire. J Am Cont Ass Inc 15: 53–59. [PubMed] [Google Scholar]
- 9.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. (1998) Molecular characterization of pyrethroid knock down resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol 71: 79–84. [DOI] [PubMed] [Google Scholar]
- 10.Liu N (2015) Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Ann Rev Entomol 60: 537–559. [DOI] [PubMed] [Google Scholar]
- 11.Santolamazza F, Calzetta M, Etang J, Barrese E, Dia I, Caccone A, et al. (2008) Distribution of knockdown resistance mutations in Anopheles gambiae (Diptera: Culicidae) molecular forms in West and Central Africa. Mal J 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemingway J, Hawkes NJ, McCarroll L, Ranson H (2004) The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol 34: 653–665. 10.1016/j.ibmb.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 13.Alout H, Labbe P, Chandre F, Cohuet A (2017) Malaria Vector Control still matters despite insecticides resistance. Trends Parasitol 33: 610–618. 10.1016/j.pt.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 14.WHO (2016) Test procedures for insecticide resistance monitoring in malaria vector mosquitoes 2nd ed Geneva: World Health Organization. [Google Scholar]
- 15.WHO (2012) Global plan on insecticide resistance management in malaria vectors (GPIRM) Geneva: World Health Organization; 2012. www.who.int/malaria/publications/aoz/gpirm/en/. [Google Scholar]
- 16.Mnzava AP, Knox TB, Temu EA, Trett A, Fornadel C, Hemingway J, et al. (2015) Implementation of the global plan for insecticide resistance management in malaria vectors: progress, challenges and the way forward. Mal J 14:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awolola TS, Brooke BD, Hunt RH, Coetzee M (2002) Resistance of the malaria vector Anopheles gambiae to pyrethroid insecticides in Nigeria. Ann Trop Med Parasitol 96: 849–852. 10.1179/000349802125002581 [DOI] [PubMed] [Google Scholar]
- 18.Awolola TS, Oduola AO, Oyewole IO, Obansa JB, Amajoh CN, Koekemoer LL, Coetzee M (2007) Dynamics of knockdown pyrethroid insecticide resistance alleles in a field population of Anopheles gambiae s.s. in southwestern Nigeria’. J Vect Borne Dis 44: 181–188. [PubMed] [Google Scholar]
- 19.Kristan M, Fleischmann H, della Torre A, Stich A, Curtis CF (2002) Pyrethroid resistance/susceptibility and differential urban/rural distribution of Anopheles arabiensis and Anopheles gambiae s.s. malaria vectors in Nigeria and Ghana. Med Vet Entomol 17: 326–332. [DOI] [PubMed] [Google Scholar]
- 20.Okorie PN, McKenzie FE, Ademowo OG, Bockarie M, Hope LK (2011) Nigeria Anopheles Vector Database: An Overview of 100 Years’ Research. PLoS ONE 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awolola TS, Adeogun AO, Olojede JB, Oduola AO, Oyewole IO, Amajoh CN (2014) Impact of PermaNet 3.0 on entomological indices in an area of pyrethroids resistant Anopheles gambiae in South Western Nigeria. Parasit Vectors 7: 236 10.1186/1756-3305-7-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asogwa EU, Dongo LN (2009) ‘Problems associated with pesticide usage and application in Nigerian cocoa production: A review’. Afr J Agr Res 4: 675–683. [Google Scholar]
- 23.Service MW (1977) A critical review of procedures for sampling populations of adult mosquitoes. Bull Ent Res 67: 343–382. [Google Scholar]
- 24.Gillies MT, De Meillon B (1968) The Anophelinae of Africa south of the Sahara. Johannesburg: Publ S Afr Inst Med Res. no. 54. [Google Scholar]
- 25.Gillies MT, Coetzee M (1987) A supplement to the Anophelinae of Africa south of the Sahara. Publ S Afr Inst Med no. 55. [Google Scholar]
- 26.Scott JA, Brogon WG, Collins FH (1993) Identification of single specimen of the Anopheles gambiae complex by polymerase chain reaction. Am J Trop Med Hyg 49: 520–529. [DOI] [PubMed] [Google Scholar]
- 27.Fanello C, Santolamazza F, Della Torre A (2002) Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol 16: 461–464. [DOI] [PubMed] [Google Scholar]
- 28.WHO (1998) World Health Organization. Test procedures for insecticide resistance, bio efficacy and persistence of insecticides on treated surfaces: In Report of WHO Informal Consultation 1998: WHO/CDS/CPC/MAL/98.12. 1998.
- 29.Brogdon WG, McAllister JC (1998) Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Control Assoc 14: 159–166. [PubMed] [Google Scholar]
- 30.Brooke BD, Kloke G, Hunt RH, Koekemoer LL, Temu EA, Taylor ME, et al. (2001) Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus. Bull Entomol Res 91: 265–272. [DOI] [PubMed] [Google Scholar]
- 31.Finney DJ (1971) Probit analysis Vol. 63, 3rd edition 32E 5th St. New York, Cambridge: Cambridge University Press. [Google Scholar]
- 32.Venter N, Oliver SV, Muleba M, Davies C, Hunt RH, Koekemoer LL, et al. (2017) Benchmarking insecticide resistance intensity bioassays for Anopheles malaria vector species against resistance phenotypes of known epidemiological significance. Parasit Vectors 10: 198 10.1186/s13071-017-2134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awolola TS, Oduola OA, Strode C, Koekemoer LL, Brooke B, Ranson H (2009) Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans R Soc Trop Med Hyg 103: 1139–1145. 10.1016/j.trstmh.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 34.Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M (2000) Anopheles funestus resistant to pyrethroids insecticides in South Africa. Med Vet Entomol 14:181–189. [DOI] [PubMed] [Google Scholar]
- 35.Coetzee M, Kruger P, Hunt RH, Durrheim DN, Urbach J, Hansford CF (2013) Malaria in South Africa: 110 years of learning to control the disease. S Afr Med J 103: 770–778. 10.7196/samj.7446 [DOI] [PubMed] [Google Scholar]
- 36.Strode C, Donegan S, Garner P, Enayati AA, Hemingway J (2014) The impact of pyrethroid resistance on the efficacy of insecticide-treated Bed nets against African anopheline mosquitoes: systematic review and meta-analysis. PLoS Med 11: 3–e1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Detailed statistical analysis is included. A supplementary file containing four tables: S1, S2, S3 and S4 Tables is attached.