Abstract
Background
Innate immune responses of airway epithelium are important defences against respiratory pathogens and allergens. Newborn infants are at greater risk of severe respiratory infections compared to older infants, while premature infants are at greater risk than full term infants. However, very little is known regarding human neonatal airway epithelium immune responses and whether age-related morphological and/or innate immune changes contribute to the development of airway disease.
Methods
We collected nasal epithelial cells from 41 newborn infants (23 term, 18 preterm) within 5 days of birth. Repeat sampling was achieved for 24 infants (13 term, 11 preterm) at a median age of 12.5 months. Morphologically- and physiologically-authentic well-differentiated primary paediatric nasal epithelial cell (WD-PNEC) cultures were generated and characterised using light microscopy and immunofluorescence.
Results
WD-PNEC cultures were established for 15/23 (65%) term and 13/18 (72%) preterm samples at birth, and 9/13 (69%) term and 8/11 (73%) preterm samples at one-year. Newborn and infant WD-PNEC cultures demonstrated extensive cilia coverage, mucous production and tight junction integrity. Newborn WD-PNECs took significantly longer to reach full differentiation and were noted to have much greater proportions of goblet cells compared to one-year repeat WD-PNECs. No differences were evident in ciliated/goblet cell proportions between term- and preterm-derived WD-PNECs at birth or one-year old.
Conclusion
We describe the successful generation of newborn-derived WD-PNEC cultures and their revival from frozen. We also compared the characteristics of WD-PNECs derived from infants born at term with those born prematurely at birth and at one-year-old. The development of WD-PNEC cultures from newborn infants provides a powerful and exciting opportunity to study the development of airway epithelium morphology, physiology, and innate immune responses to environmental or infectious insults from birth.
Introduction
The airway epithelium provides a mechanical barrier to pathogen or particulate entry and plays a crucial role in initiating airway innate immune responses.[1] Respiratory disorders, such as asthma and cystic fibrosis, are associated with altered airway epithelial cell (AEC) immune responses and impaired barrier function.[2,3] Neonates have increased susceptibility to severe respiratory disease following infections, such as respiratory syncytial virus (RSV), compared to older infants and healthy adults.[4–6] Furthermore, the frequency of severe disease associated with infections is considerably higher in premature infants compared to those born at term.[7] Evidence suggests that asthma and other chronic respiratory disorders may begin in early life and it is possible airway innate immune responses undergo maturation in parallel with postnatal lung growth, differentiation and microbiome colonisation.[2,8] However, little is known regarding the development of airway epithelial morphology, physiology, and innate immune responses from very early in life.
Studies have demonstrated the successful use of both bronchial and nasal AEC cultures in investigating early life respiratory disorders.[9–12] However, undertaking bronchial brushings in very young infants is impractical, whereby acquiring samples could only be ethically conducted opportunistically when infants are intubated. The nasal passage provides an alternative source of AECs, accessible by less invasive means. Recent publications have highlighted the potential benefit of this technique in investigating early changes in immune function in cystic fibrosis (CF) infants, in the study of the nasal transcriptome of infants, and in the innate immune responses of the airway epithelium to allergens as part of asthma pathogenesis research.[11,13,14] To date, Miller et al is the only publication describing monolayer culture of nasal epithelial cells from newborn infants.[11]
A further development in AEC culture has been the creation of differentiated epithelial cell cultures via formation of an air-liquid interface. Indeed, we previously described the generation of well-differentiated paediatric nasal airway epithelial cell cultures (WD-PNECs) from older infants and demonstrated that this model reproduces many of the hallmarks of respiratory syncytial virus (RSV) cytopathogenesis seen in vivo.[10]
Here we report the first successful generation of WD-PNECs from infants at birth. We describe the methodology for culturing newborn AECs, including reviving them from frozen. As preterm infants are known to be at higher risk of severe respiratory tract infections, we compared characteristics of WD-PNECs derived from infants born at term and prematurely at birth and repeated at one-year-old. Our work presents an exciting opportunity to study ‘‘naive” human airway epithelial cells in very early life and to investigate the developmental morphology, physiology, and immunobiology of the airway epithelium over the first year of life. Indeed, study of the functional responses of these unique cultures to viral infection are underway and will be reported elsewhere.
Methods
Subjects and study design
Healthy newborn term infants, (37–42 weeks gestation) and preterm infants (28–34 weeks gestation) underwent a nasal brushing procedure up to 5 days after birth (median age 2 days, range 6 hours to 5 days) at the Royal Jubilee Maternity Hospital, Belfast. Infants with known severe congenital anomaly of the airway, immunodeficiency, or congenital heart disease at the time of recruitment were excluded. A repeat nasal brushing sample was taken from a subset of infants at one-year old and a medical questionnaire recording previous episodes of upper/lower respiratory tract symptoms and/or bronchiolitis was completed based on parent recall (see supporting information, S1 Fig).
Sampling of nasal epithelial cells
Nasal airway epithelial cells (AECs) were harvested from healthy, non-sedated neonates at the earliest opportunity post-delivery. Nasal sampling was performed either with the neonate lying in a parent’s arms or in a cot using the technique described by Miller et al.[11] In brief, the infant’s head was gently secured using one hand and a 2.7 mm diameter interdental brush (DentoCare Professional, London, UK) was introduced into each nostril (one brush/nostril) in turn and gently rotated twice against the medial aspect of the inferior turbinate.
We collected nasal AECs from 41 newborn infants (23 term, 18 preterm) within the first 5 days of life (median age 2 days, range 6 hours to 5 days) (Table 1). The procedure was well tolerated by all neonates with no adverse events such as overt bleeding noted.
Table 1. Perinatal and delivery characteristics of enrolled subjects.
| Term (23) | Preterm (18) | |
|---|---|---|
| Male (%) | 9 (39%) | 10 (56%) |
| Mean gestational age (range) | 279 days (260 to 291) | 229 days (207 to 244) |
| Mean birth weight (range) | 3396 g (2200 to 4210) | 1855 g (820 to 2540) |
| Caesarean section delivery | 3 (13%) | 9 (50%) |
| Siblings at home | ||
| 0 | 11 (48%) | 8 (44%) |
| 1 | 10 (43%) | 6 (33%) |
| 2 | 2 (9%) | 1 (6%) |
| >2 | 0 (0%) | 3 (17%) |
| Maternal smoking antenatally (%) | 3 (13%) | 4 (22%) |
| FH of asthma/atopy (%) | 11 (48%) | 7 (39%) |
FH = Family history
Nasal AECs from infants at one-year old were collected with the infant held in a parent’s arms. A cepillo cell sampler brush (Deltlab SLU, Barcelona, Spain) was introduced into each nostril in turn using the same technique as described above. Repeat sampling was achieved for 24 infants (13 term, 11 preterm) at a median age of 12.5 months (IQR: 12–14.75 months, range: 12–22 months) (Table 2). Follow-up was not achieved in 17 infants either because parents declined re-attendance or did not respond to re-attendance invitation. As for sampling at birth, the nasal brushing procedure was well tolerated by all infants with no adverse events.
Table 2. Clinical history of infants returning for repeat sampling after first year of life.
| Subject | Age (M) | Sex | Recurrent URTIs | Bronchiolitis | Atopy | Medications |
|---|---|---|---|---|---|---|
| FT | 16 | F | Y | N | N | None |
| FT | 18 | F | N | N | N | None |
| FT | 22 | F | Y | N | Y | ICS, β2 agonist |
| FT | 13 | F | Y | N | N | None |
| FT | 15 | M | N | N | N | None |
| FT | 15 | M | Y | Y (RSV) | Y | None |
| FT | 13 | F | N | N | N | None |
| FT | 15 | F | N | N | N | None |
| FT | 13 | F | N | N | N | None |
| FT | 14 | M | N | N | Y | None |
| FT | 12 | F | N | N | Y | None |
| FT | 14 | F | N | N | Y | None |
| FT | 12 | M | N | N | Y | None |
| PM (33wks, GA) | 12 | F | Y | Y (RSV) | N | None |
| PM (29wks, GA) | 12 | M | N | N | N | None |
| PM (33wks, GA) | 12 | M | N | N | N | None |
| PM (33wks, GA) | 12 | M | N | N | N | None |
| PM (34 wks, GA) | 13 | F | N | N | N | None |
| PM (29 wks, GA) | 12 | M | N | N | N | H2 antagonist |
| PM (32 wks, GA) | 12 | M | Y | N | N | None |
| PM (33 wks, GA) | 12 | F | N | N | Y | None |
| PM (33 wks, GA) | 12 | F | N | N | Y | None |
| PM (33 wks, GA) | 12 | F | N | N | Y | None |
| PM (33 wks, GA) | 12 | F | N | Y | Y | None |
FT = Full term, PM = Preterm, GA = gestational age, M = Months, RSV = Respiratory Syncytial Virus, URTI = Upper respiratory tract infection, ICS = inhaled corticosteroids
Processing of nasal samples and creation of WD-PNEC cultures
Each brush, with attached cells, was placed in sterile phosphate buffered saline (PBS) mixed with transport medium (DMEM, 0.1% Penicillin/streptomycin (1:1 V/V)). Paediatric nasal cells were removed from the brushes and expanded in airway epithelial cell basal medium supplemented with an airway epithelial cell growth medium supplement pack (Promocell, Heidelberg, Germany) using established methods.[9,15] Upon reaching 70–80% confluency, the cells were seeded at passage 3 onto collagen-coated 6 mm diameter Transwells (Corning, Tewksbury, Massachusetts) at a density of 1x105 cells/Transwell. After reaching confluency, air-liquid interface (ALI) conditions were established and maintained until complete differentiation occurred. Following complete differentiation, which was defined by extensive cilia coverage and mucus production under light microscopy, transepithelial electrical resistances (TEER) were measured, as described previously[9]
Immunofluorescence microscopy for WD-PNEC characterisation
For WD-PNEC cultures, representative Transwells were fixed in 4% (v/v) paraformaldehyde (PFA) for 30 min at room temperature (RT). Fixed Transwells were stored in 70% ethanol at +4°C until used. To prepare for immunofluorescence staining, ethanol was removed and cells rinsed twice with 200–300 μL PBS added to the apical surface (pH 7.4). Cells were permeabilised using 0.2% (v/v) Triton X-100 (Sigma-Aldrich, ST. Louis, Missouri) in PBS for 1 h at RT and subsequently blocked with 0.4% (w/v) bovine serum albumin (BSA) (Sigma-Aldrich, ST. Louis, Missouri) in PBS for 1 h at RT. Cultures were next stained for β-tubulin, Muc5Ac and nuclei (DAPI). Briefly, 200 μL rabbit anti-Muc5Ac antibody (ab78660, Abcam, Cambridge, UK) (1:100 dilution in 0.4% (w/v) BSA (Sigma-Aldrich, ST. Louis, Missouri) in PBS) was added and incubated overnight at 4°C. Cultures were washed 3 times with 200–300μL PBS added to the apical surface (pH 7.4) for 5 min at RT. Two hundred μL anti-rabbit secondary antibody (A11011, Alexa-Fluor 568, Invitrogen, Waltham, Massachusetts) was added (1:500 dilution in 0.4% (w/v) BSA (Sigma-Aldrich, ST. Louis, Missouri) in PBS) and incubated at 37°C for 1 h. Cultures were further washed 3 times with 200–300μL PBS added to the apical surface (pH 7.4), and 200 μL Cy3-conjugated mouse anti-β-tubulin antibody (ab11309, Abcam, Cambridge, UK) added (1:200 dilution in 0.4% (w/v) BSA (Sigma-Aldrich, ST. Louis, Missouri) in PBS) and incubated at 37°C for 1 h. Following further 3 x 200–300μL PBS (pH 7.4) washes added to the apical surface, nuclei were stained using DAPI-mounting medium (Vectashield, Vector Laboratories, Burlingame, California). Fluorescence was detected by confocal laser microscopy (TCS SP5 Leica, Germany) or by UV microscopy (Nikon Eclipse 90i, Nikon, Surrey, UK).
Cells from one representative Transwell for each culture were trypsinised and the cell suspension was either smeared onto two microscope slides or 200–250 μL of cell suspension was added to a cytofunnel (EZ single cytofunnel, Thermo Fisher Scientific, Waltham, Massachusetts) and spun at 100 rpm (Using Thermo Shandon Cytospin 4 Cytocentrifuge) for 4 min onto a microscope slide. Smeared and cytospun slides were then fixed in 4% (v/v) PFA for 15 min at RT. Fixed slides were stored in the dark at -20°C until immunofluorescence was performed. Slides were stained either for ciliated cells (anti-β-tubulin) or goblet cells (anti-Muc5Ac). In brief, cells were permeabilised using 0.2% (v/v) Triton X-100 (Sigma-Aldrich, ST. Louis, Missouri) in PBS (pH 7.4) for 30 min at RT and subsequently blocked with 0.4% (w/v) bovine serum albumin (BSA) (Sigma-Aldrich, ST. Louis, Missouri) in PBS (pH 7.4) for 30 min at RT. One slide was stained for Muc5Ac by addition of 100 μL rabbit anti-Muc5Ac antibody (ab78660, abcam) (1:100 dilution in 0.4% (w/v) BSA (Sigma-Aldrich, ST. Louis, Missouri) in PBS) incubated overnight at 4°C, followed by 3 x 200 μL PBS (pH 7.4) washes for 5 min at RT before addition of 100 μL anti-rabbit secondary antibody (A11011, Alexa-Fluor 568, Invitrogen, Waltham, Massachusetts) (1:500 dilution in 0.4% (w/v) BSA (Sigma-Aldrich, ST. Louis, Missouri) in PBS) at 37°C for 1 h. A second slide was stained for β-tubulin by addition of 100 μL Cy3-conjugated mouse anti-β-tubulin antibody (ab11309, Abcam, Cambridge, UK) (1:200 dilution in 0.4% (w/v) BSA (Sigma-Aldrich, ST. Louis, Missouri) in PBS) incubated at 37°C for 1 h. Both slides next underwent 3 x 200 μL PBS (pH 7.4) washes and nuclei were stained using DAPI-mounting medium (Vectashield, Vector Laboratories, Burlingame, California). Quantification of ciliated, goblet and total DAPI+ cell numbers was undertaken for each slide by counting under fluorescent microscopy (Nikon Eclipse 90i; Nikon, Surrey, UK) and the proportion of ciliated and goblet cells relative to total DAPI+ cell numbers was determined.
Freezing and defrosting of harvested nasal epithelial cells
Expanded paediatric nasal cells were trypsinised at passage 3, added to low glucose DMEM containing 5% (v/v) foetal bovine serum (FBS) and centrifuged for 5 min at 129 x g. Following supernatant removal, the resulting cell pellet was re-suspended in monolayer medium (epithelial cell basal growth medium, Promocell, Heidelberg, Germany) containing 10% FBS and 10% dimethylsulfoxide (DMSO) (Sigma-Aldrich, ST. Louis, Missouri) to give a final concentration of 1 x 106 cells/mL. One mL aliquots of the cell suspension in cryovials were placed into an isopropanol cell freezing apparatus (Mr Frosty, Nalgene, Thermo Fisher Scientific, Waltham, Massachusetts) at RT and transferred to -80°C for 24 h, before being stored long-term in the gaseous phase of a liquid Nitrogen (N2) tank.
To resuscitate frozen primary nasal epithelial cells, vials were removed from the liquid N2, defrosted rapidly in a water bath at 37°C and the contents centrifuged at 129 x g for 5 min. The resulting cell pellet was re-suspended in monolayer medium (epithelial cell basal growth medium, Promocell, Heidelberg, Germany), transferred to a collagen-coated 75 cm2 flask (Thermo Fisher Scientific, Waltham, Massachusetts) and incubated at 37°C in 5% CO2. The generation of WD-PNEC cultures then proceeded as described above.
Statistical analysis
Data are presented as means ± standard deviation (SD) and median, interquartile range (IQR) and range for skewed data. Statistical analysis was performed by a student’s paired or unpaired t-test unless otherwise stated. Statistical significance was set at a p-value less than 0.05 (* p < 0.05 or **p <0.01). Data were analyzed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA).
Ethics statement
Written informed consent was obtained from parents at recruitment. Study was approved by the Office for Research Ethics Committee Northern Ireland (ORECNI), (REC reference 14/NI/0056).
Results
WD-PNEC cultures from preterm and term newborn infants demonstrate similar differentiation schedules and success rates
Primary monolayers were successfully established in 18 term and 15 preterm infants (80%). One sample failed to grow due to fungal contamination during culture. For the remaining samples, culture failure was due to insufficient cells harvested following brushing. Following establishment of primary monolayer culture, the subsequent success rate of differentiation into WD-PNECs was 28/33 (85%). Of the cultures failing to achieve differentiation no obvious cause was evident, but it may be due to low sample yield resulting in cell division beyond the upper limit at which successful cell differentiation can occur. Although numbers were low, there was no correlation evident between maternal antenatal smoking and cell culture failure. Overall, the rate of success for complete differentiation from initial sample acquisition was 15/23 (65%) for recruited term neonates and 13/18 (72%) for recruited preterm neonates. WD-PNEC differentiation was established by the formation of extensive cilial coverage in all quadrants of each Transwell with clear mucous production and no holes evident, as observed under light microscopy. No significant difference was noted in the duration of time required to achieve differentiation in term (median 77.5 days; IQR: 74 to 94, range: 64 to 97 days) and preterm (median 80 days; IQR: 70 to 88, range 61 to 133 days) cultures.
Term- and preterm-derived newborn WD-PNEC cultures are morphologically indistinguishable
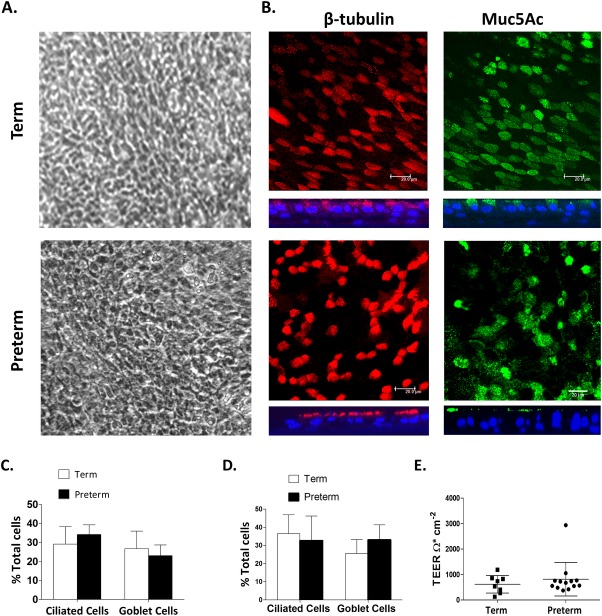
Newborn term and preterm WD-PNEC cultures were indistinguishable under phase-contrast light microscopy (Fig 1A). Fluorescent microscopy of fixed and stained cultures confirmed the formation of multi-layered pseudostratified cultures containing ciliated and goblet cells (Fig 1B). The mean proportions of ciliated cells in fixed and stained Transwells were similar in term- and preterm-derived WD-PNEC cultures (29.2% and 34.2%, respectively), as were the goblet cell proportions (26.7% and 23%, respectively) (Fig 1C). These proportions were comparable to mean proportions of ciliated and goblet cells observed in representative trypsinised Transwell smears and cytospins (Fig 1D). Tight junction integrity, as demonstrated by robust transepithelial electrical resistance (TEERs) of ≥300 Ω.cm-2, was evident for most Transwells in both term and preterm derived WD-PNEC cultures, with no significant difference between cohorts (Fig 1E). Three term WD-PNEC cultures had TEERs ≤300 Ω.cm-2; however these cultures demonstrated extensive apical cilia coverage and obvious mucous production with no holes evident under phase-contrast light microscopy.
Fig 1. Morphology and differentiation status of newborn term and preterm WD-PNEC cultures.
Cultures were monitored by (A) phase-contrast microscopy (magnification x20) or (B) confocal microscopy after staining for β-tubulin (ciliated cell marker) (red), Muc5Ac (goblet cell marker) (green), or nuclei (DAPI) (blue). For (B), square panels represent en face images, whereas rectangular panels represent orthogonal sections, with the apical side at the top (magnification x63, with x1.5 digital zoom, bar = 20 μm). (C) Transwell cultures from term and preterm donors (n = 4 each) were fixed and stained for ciliated or goblet cells. Images from 5 fields/Transwell were taken at magnification x60 and ciliated, goblet cell and total DAPI+ cell numbers were counted using fluorescent microscopy. The % ciliated and goblet cells were determined relative to total DAPI+ cell numbers. Data are presented as mean (±SD). (D) Transwells cultures were trypsinized, contents fixed onto slides by smearing or use of cytospin funnels, as described, and stained for ciliated (anti–β-tubulin) and goblet (anti-Muc5Ac) and total DAPI+ cells (n = 9 term, n = 10 preterm). Ciliated and goblet cell numbers were expressed as a percentage of the total DAPI+ cell numbers. Data presented as mean (±SD). (E) Transepithelial electrical resistances (TEER) of neonatal term- and preterm-derived WD-PNECs. Measured by EVOM epithelial voltometer and presented as mean (±SD) Ohm.cm-2.
Nasal AECs from one-year old infants achieve complete differentiation faster than those from newborn infants
Primary monolayers were successfully established in 10/13 (77%) term and 9/11 (82%) preterm of repeat nasal brushing samples. Following establishment of primary monolayer culture the subsequent success rate of differentiation into WD-PNECs was 17/19 (89%). Of the two cultures failing to achieve differentiation no obvious cause was evident. Overall, the rate of success for differentiation from initial sample acquisition was 9/13 (69%) for one-year term infants and 8/11 (73%) for one-year preterm infants. Importantly, the time to achieve complete culture differentiation was significantly shorter for one-year cohort samples (median 63.5 days; IQR 49 to 72 days) than for birth cohort samples (median 80 days; IQR 72 to 133 days), p = 0.0001 (2-tailed Mann-Whitney U).
WD-PNEC cultures derived from one-year old infants demonstrate significantly reduced goblet cell content compared to their paired newborn-derived WD-PNECs
One-year WD-PNEC cultures were indistinguishable from newborn-derived cultures under phase-contrast light microscopy. As for newborn-derived WD-PNECs, there was no difference observed between the proportions of ciliated and goblet cells in term and preterm-derived cultures at one year (Fig 2). However, we observed a significant decrease in mean goblet cell proportions at one-year compared to newborn-derived WD-PNECs for both term (11.7% vs 25.5%; p = 0.0003) and preterm cohorts (8.9% vs 33.1%; (p<0.0001) when trypsinised Transwell cell smear proportions were examined (Fig 2D). We were not successful in achieving paired birth and one-year-old samples for all recruited infants due to drop out in follow-up and difficulties in achieving successful differentiation as detailed above. Cohorts were supplemented with data from WD-PNECs from donors which successfully grew and differentiated at birth but not at one year. Subgroup analysis of paired birth and one-year samples only also demonstrated no difference in ciliated cell proportions but a significant decrease in mean goblet cell proportions at one-year (p <0.005) (data not shown).
Fig 2. Morphology and differentiation status of birth and one-year repeat WD-PNEC cultures.
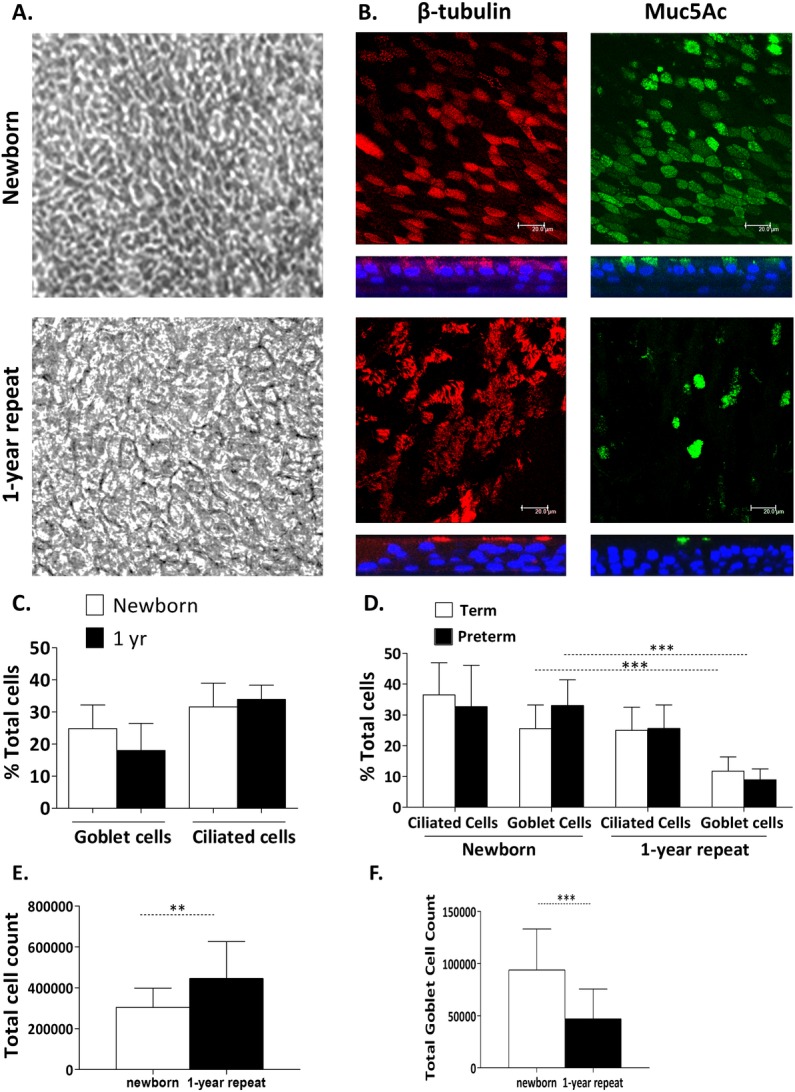
Cultures were monitored by (A) phase-contrast microscopy (magnification x20) or (B) confocal microscopy after staining for β-tubulin (ciliated cell marker) (red), Muc5Ac (goblet cell marker) (green), or nuclei (DAPI) (blue). For (B), square panels represent en face images, whereas rectangular panels represent orthogonal sections, with the apical side at the top (magnification x63, with x1.5 digital zoom, bar = 20 μm). (C) Transwell cultures from birth and 1-year donors (n = 8 each) were fixed and stained for ciliated, goblet and total DAPI+ cells. Images from 5 fields/Transwell were taken at x60 magnification and ciliated, goblet and total DAPI+ cell numbers were counted using fluorescent microscopy. The % ciliated and goblet cells were determined relative to total DAPI+ cell numbers. (D) Representative preterm and term Transwell cultures (newborn term n = 9, preterm n = 10, one-year term n = 9, preterm n = 6 donors) were trypsinised, contents fixed onto slides by smearing or use of cytospin funnels, as described, and stained for ciliated (anti–β-tubulin), goblet (anti-Muc5Ac), and total DAPI+ cells. Ciliated and goblet cell numbers were expressed as mean (±SD) % proportion of total DAPI+ cell numbers for each cohort. *** unpaired t-test, p<0.0001. (E) Transwell cultures were trypsinized and total cell count performed by trypan blue exclusion using a haemocytometer. Total cell numbers are presented as mean (±SD) for newborn (n = 17) and 1-year cohorts (n = 15), p = 0.0087 (unpaired t-test of difference in means). (F) Combined total goblet cell numbers were determined for trypsinised representative Transwell cultures. Data plotted for newborn (n = 17) and 1-year (n = 15) cohorts as mean (±SD) for each culture cohort. ***unpaired t-test of difference in means, p = 0.0005.
We noted the total number of cells per Transwell culture was higher for the one-year versus newborn cohort (Fig 2E). Therefore, to determine if the observed differences in goblet cell proportions could be explained by differences in total cell numbers within pseudostratified cultures we re-analysed the data as total goblet cell numbers for each donor. Consistent with the proportion data, combined total goblet cell count for all newborn-derived WD-PNECs (mean = 93,797 cells; SD = 39427) was double that of one-year derived-WD-PNECs (mean = 46,969; SD = 28607: t-test of difference in means, p = 0.0014) (Fig 2F). These data suggest the observed decrease in goblet cell proportions in one-year-derived WD-PNECs is not simply the result of increased total WD-PNEC culture cell numbers in the one-year cohort.
We did not find any significant correlation between parental reports of recurrent URTIs and goblet cell proportions. However, due to the relatively small sample size in our current study there are insufficient cohort numbers and reports of recurrent infections to allow us to draw any robust conclusions with in vivo clinical characteristics, such as correlations between mucociliary differentiation and susceptibility to respiratory infection.
Nasal primary AECs successfully differentiate after storage in liquid nitrogen and are morphologically indistinguishable from freshly differentiated AECs
We next sought to determine if nasal AECs could be successfully frozen and stored, as this vastly increases the versatility of this model for future research into the origins of airway disease. Newborn and one-year old nasal samples stored in liquid N2 for approximately one year were defrosted as described and expanded to enable Transwell seeding and differentiation.
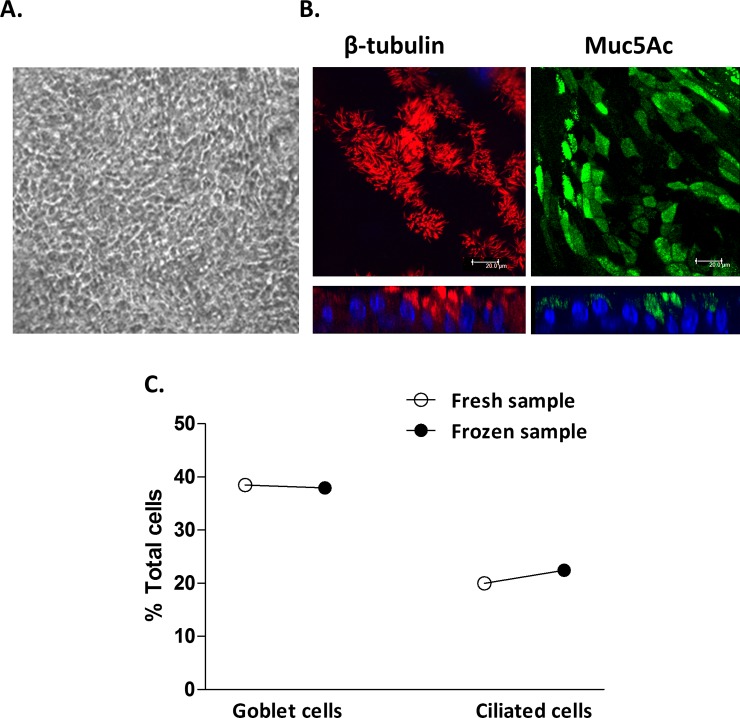
Both samples successfully differentiated in a similar fashion, requiring one additional week to achieve complete differentiation compared to fresh counterparts. Differentiation was evidenced by the formation of extensive cilial coverage in all quadrants of each Transwell with clear mucous production under light microscopy (Fig 3A). The extra week was due to additional time needed to enable cell expansion in monolayers prior to Transwell seeding.
Fig 3. Morphology and differentiation status of WD-PNEC cultures derived from epithelial cells frozen at passage 3.
Cultures were visualised by (A) phase-contrast microscopy (magnification x20) or (B) confocal microscopy after staining for β-tubulin (ciliated cell marker) (red), Muc5Ac (goblet cell marker) (green), or nuclei (DAPI) (blue). For (B), square panels represent en face images, whereas rectangular panels represent orthogonal sections, with the apical side at the top (magnification x63, with x1.5 digital zoom, bar = 20 μm). (C) Representative Transwell cultures derived from n = 1 newborn donor either differentiated “fresh” or following a period of storage in liquid nitrogen “frozen” were fixed and stained for ciliated, goblet and total DAPI+ cells. Images from 5 fields/Transwell were taken at x60 magnification and ciliated, goblet and total DAPI+ cell numbers were counted using fluorescent microscopy. The % ciliated and goblet cells were determined relative to total DAPI+ cell numbers.
Transepithelial electrical resistances (TEERs) >300 Ohm.cm-2 were recorded for Transwells in both cultures indicating robust epithelial cell tight junction formation. As described above for freshly processed nasal epithelial cells, fluorescent microscopy of fixed and stained WD-PNEC cultures derived from frozen cells confirmed extensive ciliated and goblet cell contents (Fig 3B). Importantly, proportions of goblet and ciliated cells in frozen samples were very similar to those from the same donor cultured without freezing, with the unusually high goblet cell content from this donor being reproduced (Fig 3C). While this was limited to cells from a single donor, our data demonstrate a proof of principle for the use of freezing newborn nasal epithelial cells for subsequent culture, differentiation and experimentation.
Discussion
Very little is known regarding the development of the human airway epithelium in early life. We believe this is the first study describing the successful differentiation and characterisation of human newborn AECs and represents a unique model for studying early life airway epithelium morphology, physiology and innate immune responses.
As described by Miller et al, we found the nasal brushing procedure to be safe and well tolerated with similar success rates for newborn and older infant samples. This indicated consistency of sampling and culture technique. Once newborn AEC monolayer cultures were successfully established, generation of morphologically-authentic WD-PNECs was achieved with a high success rate (85%). However, 13 of 41 newborn samples failed to successfully achieve WD-PNEC differentation. Eight of these were due to failure to procure nasal basal cells at sampling or concomitant bacterial or fungal contamination, thereby obviating the establishment of AEC monolayers. Five successfully established monolayer cultures failed to differentiate, with no obvious cause, although it is possible this was due to low initial nasal cell yield during brushing. Increasing experience over time in the nasal sampling technique resulted in higher initial nasal cell yields, with concomitant greater success in establishing monolayer cultures and subsequent differentiation.
WD-PNECs generated from term and preterm newborn infants were morphologically and physiologically indistinguishable, differentiating in a similar time frame. This indicates no obvious impact of gestational age on AEC growth and differentiation. However, intriguingly, we detected morphological and differentiation differences between WD-PNECs generated from newborn and older infants, suggesting age-related developmental changes occur in nasal AECs during the first year of life.
We noted differences in the speed at which full WD-PNEC differentiation was achieved between newborn and one-year samples. This may represent increased capacity for nasal AEC proliferation and differentiation at one-year compared to the neonatal period. This is consistent with previous reports, in which differences in cell proliferation speed and functionality between neonatal, later postnatal age and adulthood were observed in other epithelial tissues, including mouse gastrointestinal epithelium and human keratinocytes.[16,17] Nasal sampling in newborn infants was limited by their comparatively smaller nasal passages, which in principle could have result in reduced initial AEC basal cell yields, thereby increasing time to achieve sufficient cell numbers for differentiation. However, we observed cell adherence to collagen-coated plastic from the brushes and found no obvious differences between brush samples from newborns and one-year olds. Furthermore, the time taken to the first passage of the cells in monolayers was similar for both newborns and 1 year old infants (data not shown), suggesting similar quantities of basal cells were obtained by the nasal brushes from both cohorts.
Proportions of ciliated and goblet cells in the older infant cohort were similar to previous work in WD-PNECs from older infants.[10] In contrast, we observed significantly higher proportions of goblet cells in the newborn-derived WD-PNECs compared to one-year WD-PNECs. This is a valuable finding that was not previously described. The greater number of total cells in WD-PNECs derived from older infants was reflected in more cell layers evident in orthogonal sections, which may in part explain the relative decrease in proportions of goblet cells at one year. However, total numbers of goblet cells were also reduced at one-year with no reciprocal increase in ciliated cell numbers evident. Presently we do not have additional data on other cell types present that may account for this difference and further work is needed to explain the reduced goblet cell content finding.
Respiratory viral infections result in excess mucous production, which contributes to the pathogenesis of respiratory viral diseases.[18] Thus, our finding of increased goblet cell content in newborn airways could contribute to increased mucous plug formation during viral infections and may explain, in part, the increased frequency of severe respiratory viral disease in very young infants. Indeed, there is some evidence to support such a hypothesis in the neonatal mouse model, where increased levels of Muc5Ac expression detected at baseline in neonatal versus adult mice was shown to further increase in response to human rhinovirus infection.[19,20] Further work to determine the mechanisms contributing to this increase may yield new insights into the development of diseases such as asthma, in which goblet cell hyperplasia is a feature.
One potential limitation with our model is the use of nasal rather than bronchial AECs and there is ongoing debate regarding the validity of nasal AECs as surrogates to investigate lung disease.[21] Studies of airway inflammation in adult populations demonstrated that nasal AECs cannot substitute for bronchial AECs, such as in COPD.[22] However, indistinguishable epithelial morphology was reported in paired nasal and bronchial AEC cultures from both adults and children, while more recent work in paediatric asthma, atopy and respiratory syncytial virus infection demonstrated that nasal AECs act as reasonable surrogates for lower airway AECs.[10,23–25] Evidently, as discussed earlier, bronchial sampling is not appropriate in newborn infants who are otherwise well. Moreover, use of bronchial AECs would not permit repeated sampling within the same healthy subject, which is one of the most attractive exploitations of our WD-PNEC model.
In conclusion, we present the first description of morphologically- and physiologically-authentic WD-PNEC cultures generated from term and preterm newborn infants. We demonstrated this to be a safe, minimally invasive method that can be performed consistently with high rates of success. We also demonstrated the feasibility of sequential sampling of subjects for future research studies. Accordingly, our model of the neonatal airway presents a unique opportunity to study differences contributing to the increased severity of respiratory infections seen in this age group compared to older infants. Furthermore, the successful protocol for freezing and later reviving nasal AECs offers a unique opportunity to store ‘‘naıve” AECs indefinitely. This provides exciting opportunities for birth cohort studies, in which differentiation may be performed at later dates once absence/presence of particular respiratory diseases, such as asthma, has been established. Thus, newborn WD-PNECs represent a unique opportunity to study the development of human airway morphology, physiology and innate immune responses from birth, and have significant and exciting potential applications in elucidating the early origins of respiratory disease.
Supporting information
(DOCX)
Acknowledgments
We are extremely grateful to the parents and infants whose participation in this study made it possible. We would also like to thank Isobel Douglas, research nurse, for assistance in training in the technique of nasal brushing sampling.
Data Availability
Data from this study has been uploaded to a stable, public repository and can be accessed using the following DOI: 10.17034/d34301e3-20ae-4d26-a7d0-a2a5bf0b5371.
Funding Statement
This work was supported by The Wellcome Trust [104516/Z/14/Z]; this work was conducted as part of a research program supported by funding from the HSC R&D Division, Northern Ireland [RRG 9.44]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parker D, Prince A. Innate Immunity in the Respiratory Epithelium. Am J Respir Cell Mol Biol. 2011;45(18):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–19. 10.1111/j.1600-065X.2011.01030.x [DOI] [PubMed] [Google Scholar]
- 3.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2013;18(4):509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkhaug IM, Inchley CS, Aamodt G, Ånestad G, Nystad W, Nakstad B. Infectious burden of respiratory syncytial virus in relation to time of birth modifies the risk of lower respiratory tract infection in infancy: the Norwegian Mother and Child Cohort. Pediatr Infect Dis J. 2013;32(6):e235–41. 10.1097/INF.0b013e31828ab9ff [DOI] [PubMed] [Google Scholar]
- 5.Battersby AJ, Gibbons DL. The gut mucosal immune system in the neonatal period. Pediatr Allergy Immunol. 2013;24(5):414–21. 10.1111/pai.12079 [DOI] [PubMed] [Google Scholar]
- 6.Marodi L. Neonatal Innate Immunity to Infectious Agents. Infect Immun. 2006;74(4):1999–2006. 10.1128/IAI.74.4.1999-2006.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauroux B, Gouyon J-B, Roze J-C, Guillermet-Fromentin C, Glorieux I, Adamon L, et al. Respiratory morbidity of preterm infants of less than 33 weeks gestation without bronchopulmonary dysplasia: a 12-month follow-up of the CASTOR study cohort. Epidemiol Infect. 2014;142(7):1362–74. 10.1017/S0950268813001738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez F. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2016;375:871–8. 10.1056/NEJMra1603287 [DOI] [PubMed] [Google Scholar]
- 9.Villenave R, Thavagnanam S, Sarlang S, Parker J, Douglas I, Skibinski G, et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc Natl Acad Sci U S A. 2012;109(13):5040–5. 10.1073/pnas.1110203109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo-Parke H, Canning P, Douglas I, Villenave R, Heaney LG, Coyle P V, et al. Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am J Respir Crit Care Med. 2013. October 1;188(7):842–51. 10.1164/rccm.201304-0750OC [DOI] [PubMed] [Google Scholar]
- 11.Miller D, Turner SW, Spiteri-Cornish D, McInnes N, Scaife A, Danielian PJ, et al. Culture of airway epithelial cells from neonates sampled within 48-hours of birth. PLoS One. 2013;8(11):e78321 10.1371/journal.pone.0078321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker J, Sarlang S, Thavagnanam S, Williamson G, O’Donoghue D, Villenave R, et al. A 3-D well-differentiated model of pediatric bronchial epithelium demonstrates unstimulated morphological differences between asthmatic and nonasthmatic cells. Pediatr Res. 2010;67(1):17–22. 10.1203/PDR.0b013e3181c0b200 [DOI] [PubMed] [Google Scholar]
- 13.Mosler K, Coraux C, Fragaki K, Zahm J. Feasibility of nasal epithelial brushing for the study of airway epithelial functions in CF infants. J Cyst Fibros. 2008;7:44–53. 10.1016/j.jcf.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 14.Chu C-Y, Qiu X, Wang L, Bhattacharya S, Lofthus G, Corbett A, et al. The Healthy Infant Nasal Transcriptome: A Benchmark Study. Sci Rep. Nature Publishing Group; 2016;6:33994 10.1038/srep33994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broadbent L, Villenave R, Hong G-P, Douglas I, Shields MD, Power UF. In Vitro Modeling of RSV Infection and Cytopathogenesis in Well-Differentiated Human Primary Airway Epithelial Cells (WD-PAECs) In: Tripp RA, Jorquera P, editors. Human Respiratory Syncytial Virus. Springer; 2016. p. 199–139. [DOI] [PubMed] [Google Scholar]
- 16.Krejčí E, Kodet O, Szabo P, Borskỳ J, Smetana K, Grim M, et al. In vitro differences of neonatal and later postnatal keratinocytes and dermal fibroblasts. Physiol Res. 2015;64(4):561–9. [DOI] [PubMed] [Google Scholar]
- 17.Mateu R, Ẑivicová V, Krejí ED, Grim M, Strnad H, Vlek A, et al. Functional differences between neonatal and adult fibroblasts and keratinocytes: Donor age affects epithelial-mesenchymal crosstalk in vitro. Int J Mol Med. 2016;38(4):1063–74. 10.3892/ijmm.2016.2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stokes KL, Currier MG, Sakamoto K, Lee S, Collins PL, Plemper RK, et al. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol. 2013;87(18):10070–82. 10.1128/JVI.01347-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014;134(2):429–39. 10.1016/j.jaci.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider D, Hong JY, Popova AP, Bowman ER, Linn MJ, McLean AM, et al. Neonatal rhinovirus infection induces mucous metaplasia and airways hyperresponsiveness. J Immunol. 2012;188(6):2894–904. 10.4049/jimmunol.1101391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush A. Nosing out the future asthmatic? Allergy. 2000;55:685–7. [DOI] [PubMed] [Google Scholar]
- 22.Comer DM, Elborn JS, Ennis M. Comparison of Nasal and Bronchial Epithelial Cells Obtained from Patients with COPD. PLoS One. 2012;7(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mcdougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, Walsh GM. Nasal Epithelial Cells as Surrogates for Bronchial Epithelial Cells in Airway Inflammation Studies. Am J Respir Crit Care Med. 2008;39(19):560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thavagnanam S, Parker JC, McBrien ME, Skibinski G, Shields MD, Heaney LG. Nasal epithelial cells can act as a physiological surrogate for paediatric asthma studies. PLoS One. 2014;9(1):e85802 10.1371/journal.pone.0085802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole A, Urbanek C, Eng C, Schageman J, Jacobson S, O’Connor BP, et al. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J Allergy Clin Immunol. 2014;133(3):670–8.e12. 10.1016/j.jaci.2013.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data from this study has been uploaded to a stable, public repository and can be accessed using the following DOI: 10.17034/d34301e3-20ae-4d26-a7d0-a2a5bf0b5371.