Abstract
Outcomes for patients with multiple myeloma (MM) have improved with the advent of novel therapies, however, real-world evidence of outcomes in clinical practice is scarce. We conducted a multi-center registry study to build a reliable picture of treatment and patient outcomes in Finland. The aim of this study was also to understand any methodological challenges in assessing treatment outcomes using disease registry data. Methods: We carried out a retrospective, observational study using data from the national Finnish Hematology Registry (FHR) to provide real-world evidence of outcomes for all adult patients diagnosed with and treated for MM between 2009–2013 at one of the six regional hospitals, with at least six months of recorded follow-up. Patients were identified within the FHR by applying eligibility criteria of a diagnosis of MM and verifiable records of medical treatment and lines of treatment during the study period. Patients receiving allogenic stem cell transplantation were excluded from the cohort, as were individuals who only had monoclonal gammopathy of undetermined significance diagnosis and patients who had not initiated treatment during this period. Kaplan Meier curves were used to calculate overall survival and time to next treatment. Stratification was carried out by drug status (conventional/novel) and by autologous stem cell transplant (ASCT) status. Results: A total of 321 patients met the inclusion criteria and were included in this study. Overall survival (OS) was longest in patients who received first-line novel therapy and ASCT (median not reached during 60-month follow-up) versus 46.2 months for novel first-line therapy without ASCT and 25.6 months for first-line conventional therapy without ASCT. Similarly, median time to next treatment were 33.9 months, 12.6 months and 7.8 months, respectively. Conclusions: The adoption of novel treatments in MM in Finland has had substantial impact on patient outcomes. Given the reality of complex treatment combinations for MM and relatively low patient numbers, assessing individual treatment effectiveness will require substantial cohort sizes and advanced, collaborative analytics on an international scale.
Introduction
Multiple myeloma (MM) is an incurable hematological malignancy mainly affecting the elderly [1]. In patients with MM, neoplastic plasma cells accumulate in the bone marrow and produce a monoclonal protein (paraprotein) which is detected in the blood and/or urine and causes organ or tissue impairment [2].
Over recent years, the use of novel agents (immunomodulatory drugs [lenalidomide, thalidomide and pomalidomide] and the proteasome inhibitor [bortezomib]) and autologous stem cell transplant (ASCT) in patients eligible for transplant has improved the outcomes of patients with MM around the world [2–6]. However, MM remains incurable. Retrospective data from the Swedish National Registry reveals that survival increased dramatically in younger patients (<65 years) between 1950 and 2005 with the greatest improvements seen between the 1990s and the 2000s [7] and that survival continues to improve with the increased use of novel agents in all age groups [5, 8].
European Society of Medical Oncology (ESMO) guidelines are regularly updated [9–15] and have consistently considered clinical condition (fitness) and age to be critical in deciding on treatment options. Patients aged <65 years or fit patients <70 years in good clinical condition are eligible for ASCT and novel chemotherapy, patients aged >65 years or patients ineligible for ASCT receive novel therapy if fit enough, or conventional chemotherapy if not. Novel therapies were first mentioned in the 2007 guidance [10]. Similarly, the treatment algorithm in Finland recommends treatment according to age. First-line treatment for younger patients is induction combination chemotherapy using novel agents, followed by ASCT. For older patients chemotherapy plus novel agents, if tolerated, is recommended without ASCT [6]. More recent treatment recommendations from Finland (2017) have raised the age threshold from 70 years; patients aged 70–75 years are eligible for transplant if fit enough, transplant ineligible patients aged up to 85 years who are fit enough for novel agents are treated with novel chemotherapy and unfit patients and those aged over 85 years are treated with conventional [16].
There are numerous first-line treatment options, regardless of whether patients are eligible for ASCT or not. Complexity emerges in the choice of further lines of treatment and sequencing of treatments. Treatment second-line and beyond includes the novel agents in various combinations. Treatment choice varies according to patient-specific characteristics, previous treatments (type, efficacy and tolerance), number of prior treatment lines, remaining treatment options and interval from the last therapy.
As new treatments become a part of clinical practice, the outcomes must be robustly evaluated outside the clinical trial setting to ensure that patient care is improved compared with existing treatments. Retrospective data collections are invaluable in assessing real-life outcomes. Cancer registries often rely on retrospective data collection, utilizing patient medical records to collect the key variables for clinical outcomes. Many cancer registries exist, especially in the Nordic countries, with detailed information captured at diagnosis and cause of death [17]. However, treatments and outcomes between diagnosis and death are rarely captured. As new therapies develop and require real-life comparisons, understanding the entire patient care trajectory is critical in establishing new treatment strategies and their timely reimbursement, if appropriate.
Study objectives
The objectives of this study are to investigate real-world MM treatment outcomes in Finland and to understand any methodological challenges in using retrospective registry data in assessing these specific outcomes.
Methods
The study design was non-interventional and retrospective. Available data were extracted from the Finnish Hematology Registry (FHR); patient medical records from six regional hospitals were used to fill and validate FHR data gaps. The FHR was established in 2009 where all diagnosed patients are asked to give their consent to be included, and thus the first patient consents were available from that year. Any data prior to late 2009 was thus inserted retrospectively, after the consent had been received. The FHR did not include any deceased patient data in the register and thus all the patients diagnosed prior to 2009 had to survive until late 2009 at least to be able to give a consent. As such, for the time-period prior to 2009, the FHR contained a selection bias due to excluded immortal time for many hospitals´ data entry, and, accordingly, the cohort entry date in this study was limited to those patients diagnosed from 2009 onward. Patients were identified within the FHR by applying eligibility criteria of a diagnosis of MM and verifiable records of medical treatment and lines of treatment during the study period. Patients’ data were screened for eligibility according to diagnosis, time period, age, medical treatment records and treatment line records.
Inclusion criteria were as follows: aged over 18 years, diagnosis of MM between 2009 and 2013 and at least 6 months of follow-up according to the protocol. Exclusion criteria for analysis were as follows: allogeneic SCT, monoclonal gammopathy of undetermined significance and patients who did not receive drug treatment.
Patients gave their informed consent before any patient data were entered into the FHR. The consent mentions that data from the registry may be used for collaborative projects with national study groups and commercial organisations. The FHR’s steering committee reviews research proposals on FHR data and the ethics committee for this project was Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital Region. The committee approved this study, with reference number was 68/13/03/00/2013.
Data collected included demographic data at the time of diagnosis, details of treatment (drug and ASCT) and treatment line, outcomes and date of last follow-up if the patient was still living.
Novel agents were defined as lenalidomide, thalidomide, bortezomib and pomalidomide. Line of treatment was defined in the protocol as one or more cycles of a planned treatment, e.g. induction followed by ASCT would count as one line of treatment and a new line of treatment was defined as modification of planned course of therapy to include other treatment agents (alone or in combination) as a result of disease progression, relapse, or toxicity.
Descriptive statistics and Kaplan Meier curves were used to calculate time to next treatment (TTNT) and overall survival (OS) in months for first-line and second-line treatment. Stratification was carried out by drug status (conventional/novel) and by ASCT status.
Results
The study was carried out at six sites in Finland (Helsinki University Hospital, Turku University Hospital, Kuopio University Hospital, Tampere University Hospital, Satakunta Central Hospital, Kainuu Central Hospital) where the majority of patients with newly diagnosed MM are treated in Finland. Data from 321 patients diagnosed and treated between 2009–2013 and who met the study inclusion criteria were analyzed. It should be noted that although the FHR is a national, population-based quality, treatment and research registry, input is not fully comprehensive and verifiable at all hospitals, since there are around 380 new MM cases annually in Finland including smoldering MM [18]. Median follow-up time (i.e. person time) for included patients was 25 months, with a maximum follow-up of 60 months.
Demographics at diagnosis
The demographics at diagnosis are shown in Table 1, stratified by first-line treatment choice.
Table 1. Demographic details of patients included in the study.
| Non-ASCT | ASCT | Total | |||
|---|---|---|---|---|---|
| Treatment at first line | Novel | Conventional | Novel + ASCT | ||
| Patients, n | 161 | 46 | 114 | 321 | |
| Male, n (%) | 72 (45%) | 21 (46%) | 45 (51%) | 138 (43%) | |
| Median age at diagnosis, years (range) | 69 (45–83) | 77.5 (47–89) | 61 (42–70) | 66 (42–89) | |
| Age distribution, n (%) | |||||
| <40 years | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 40–49 years | 3 (2%) | 2 (4%) | 4 (4%) | 9 (3%) | |
| 50–59 years | 18 (11%) | 0 (0%) | 36 (32%) | 54 (17%) | |
| 60–64 years | 29 (18%) | 2 (4%) | 47 (41%) | 78 (24%) | |
| 65–69 years | 32 (20%) | 4 (9%) | 26 (23%) | 62 (19%) | |
| 70–74 years | 41 (25%) | 9 (20%) | 1 (1%) | 51 (16%) | |
| ≥ 75 years | 38 (24%) | 29 (63%) | 0 (0%) | 67 (21%) | |
| Type of MM, n (%) | |||||
| IgA | 21 (13%) | 6 (13%) | 15 (13%) | 42 (13%) | |
| IgD | 0 (0%) | 1 (2%) | 1 (1%) | 2 (1%) | |
| IgG | 58 (36%) | 15 (33%) | 53 (46%) | 126 (39%) | |
| Bence Jones | 37 (23%) | 9 (19%) | 27 (24%) | 73 (23%) | |
| Missing | 45 (28%) | 15 (33%) | 18 (16%) | 78(24%) | |
| Osteolytic bone lesions, n (%) | |||||
| 0 | 48 (30%) | 15 (33%) | 26 (23%) | 89 (28%) | |
| 1 | 18 (11%) | 3 (6%) | 10 (9%) | 31 (10%) | |
| >1 | 85 (53%) | 23 (50%) | 74 (65%) | 182 (57%) | |
| Missing | 10 (6%) | 5 (11%) | 4 (3%) | 19 (6%) | |
| Laboratory values at diagnosis, median | |||||
| Creatinine, μmol/l | 87 (2% missing) |
86 (0% missing) |
79 (3% missing) |
84 (2% missing) |
|
| Beta-2 microglobulin (β2m), mg/l | 3.25 (35% missing) |
4.45 (57% missing) |
2.9 (28% missing) |
3.2 (36% missing) |
|
| Hemoglobin (Hb), g/l | 107 (0% missing) |
103 (0% missing) |
110.5 (2% missing) |
108 (1% missing) |
|
| Durie-Salmon, n (%) | |||||
| I | 13 (8%) | 3 (7%) | 13 (11%) | 29 (9%) | |
| II | 21 (13%) | 5 (11%) | 24 (21%) | 50 (16%) | |
| III | 22 (14%) | 7 (15%) | 31 (27%) | 60 (19%) | |
| Missing | 105 (65%) | 31 (67%) | 46 (40%) | 182 (57%) | |
First-line treatment choice
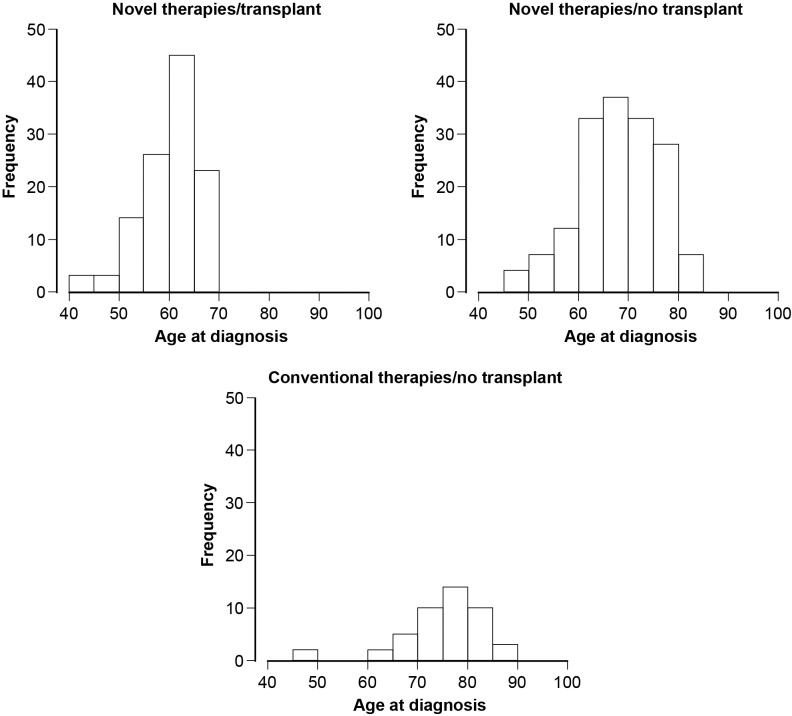
Overall, almost 90% of patients received novel therapies (275/321, 86%). In accordance with the Finnish advice at the time, age was the key determinant of treatment (novel or conventional agents, ASCT or not, Fig 1).
Fig 1. First-line treatment choice by age at diagnosis.
Outcomes
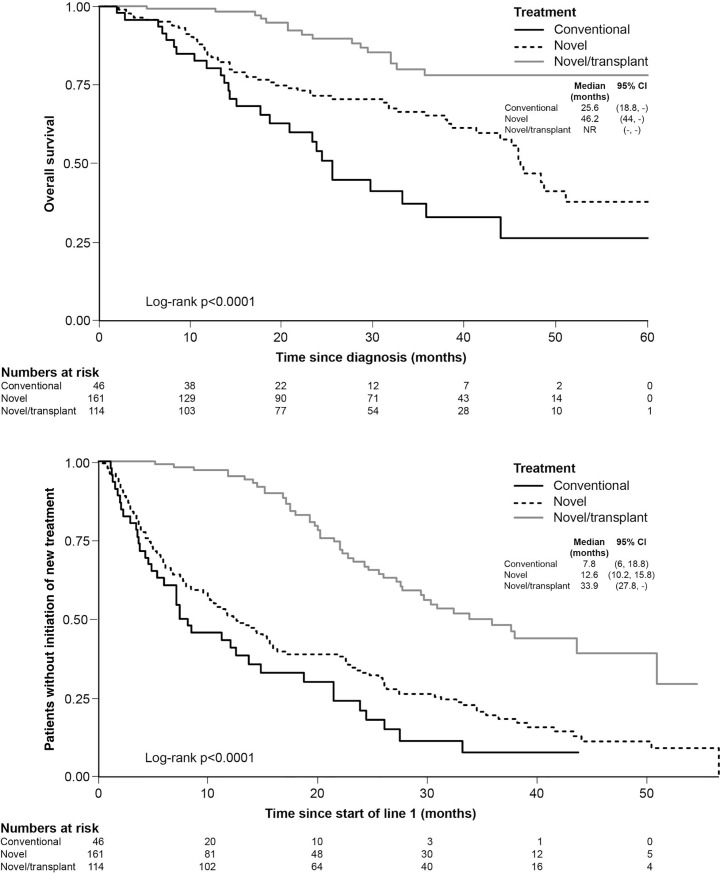
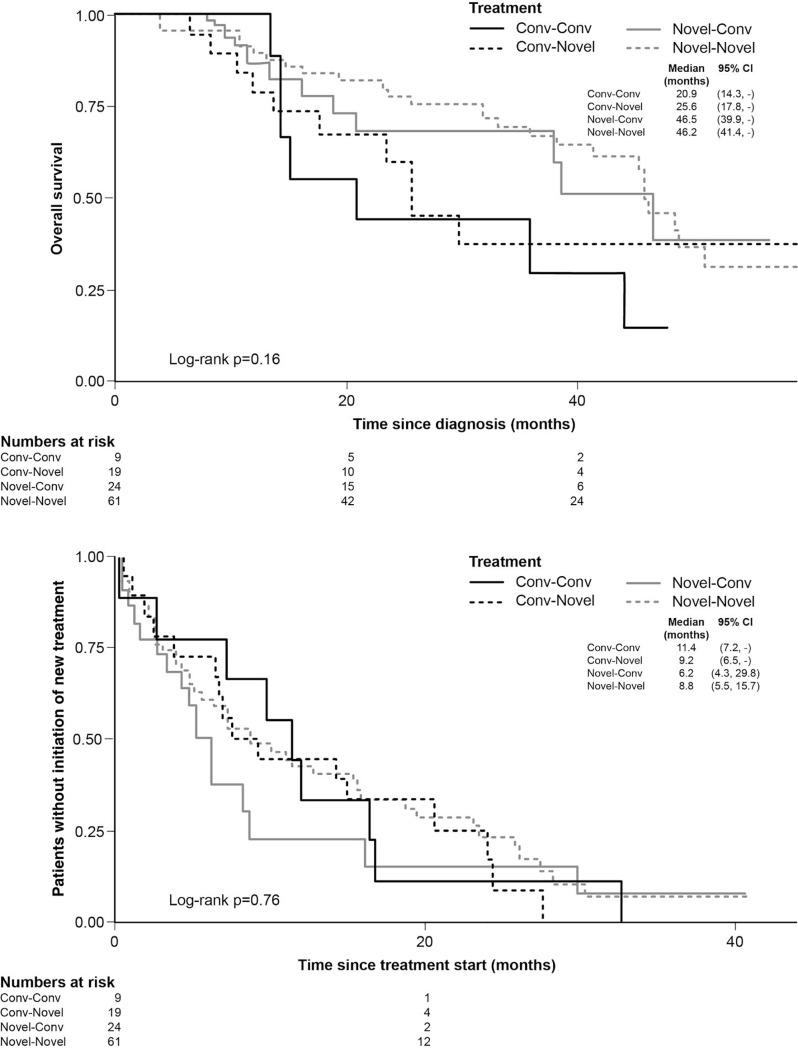
Patients with transplant and novel agents in first-line treatment had the best outcomes (OS and TTNT), followed by novel agents alone and finally conventional agents alone (Fig 2). Limited data was available for non-transplanted patients who had received two lines of treatment. For non-transplanted patients, there was a trend towards extended OS in patients who received novel agents in first line and novel or conventional agents in second line (median OS 46.2 months for novel followed by novel and 46.5 months for novel followed by conventional), versus those who received conventional agents in first line and novel agents in second line (median OS 25.6 months) or conventional agents in both treatment lines (median OS 20.6 months), illustrating the importance of early treatment with novel agents (Fig 3, top). No significant differences in TTNT in second-line were observed between the different treatment groups (Fig 3, bottom). It should be noted that patient numbers are too low for meaningful comparisons.
Fig 2.
OS (top) and TTNT (bottom) by first-line treatment choice (transplant and non-transplant patients).
Fig 3.
OS (top) and TTNT (bottom) by first-and second-line treatment choice (non-transplant patients only).
Discussion
This study shows that clinical practice in Finland between 2009 and 2013 broadly reflected guidelines [6, 14], with the majority of younger patients receiving ASCT and novel therapies first-line and older patients receiving chemotherapy alone. Overall, most patients received novel agents as first-line treatment (86%). Similar results were seen in a retrospective US-based study which used data from the National Cancer Institute Surveillance, Epidemiology and End Results cancer registries. Of the patients diagnosed with MM in 2007 (n = 742) in the US study, 75.5% received a novel agent within 12 months of diagnosis [19].
The treatment choices were reflected as poorer outcomes for older patients receiving conventional therapy in Finland between 2009 and 2013. However, data from several recent studies have shown that use of novel agents in older people improves OS. Retrospective data from the national Swedish registry (diagnosed with MM between 2000 and 2011) revealed that in the elderly the use of novel agents first-line without ASCT resulted in a significantly longer median OS versus conventional therapies (4.9 years versus 2.3 years, p<0.0001) [5]. A retrospective study carried out in the US reported the outcomes of 117 elderly MM patients aged over 70 years who did not undergo ASCT [20]. A considerable proportion of these patients (83%) received novel agents which resulted in improved outcomes; patients receiving a two-drug regimen had median OS of 7.1 years and median OS was not reached in those receiving three- or four-drug regimens.
The results from the present national registry study revealed that the best outcomes were seen, as expected, with novel agents plus ASCT as first-line treatment. We provide real-world evidence that early use of novel therapy improves outcomes, confirming the findings of other retrospective studies from the Nordics [8, 21] and elsewhere [22, 23]. Our real-world OS results are comparable to those reported in many studies in young and elderly MM cohorts [24], although longer OS have been observed in selected phase 3 trials [25].
The outcome results from the FHR data presented here are hampered by challenges and pitfalls in collecting and analyzing the available retrospective registry data. Lack of complete patient records made it impossible to extract treatment outcomes from the 1990s to the early 2000s from the FHR. Accordingly, exclusion of this period leads to a substantial underestimation of the impact of novel treatment options. However, it is clear that outcomes have improved over time; a study published in 1999 showed that MM patients under 70 years in Finland and treated with chemotherapy only had a median OS of 49 months [26]. Shifts in standard treatment regimens over time make comparing outcomes between time periods difficult, but median OS in the present study was not reached in the population who received ASCT and novel therapies, with a follow-up of 60 months.
Although the Nordic countries are well known for their solid tumor registries, registries in hematology are less well-established and there is a paucity of real-world retrospective studies looking at treatment patterns in patients with MM over time in the Nordics. Several large studies from the US provide real-world evidence of variation in treatment options and in treatment sequencing, as seen in the present study. They show that bortezomib is the mainstay of treatment from first to third-line treatment, with second-generation novel agents (carfilzomib and pomalidomide) used as later lines of therapy since 2013. Numerous treatment options are used in first-line and beyond, with varying treatment sequences [27–29].
Real-world evidence is important for evaluating on-going practice, including the use of new agents in different treatment lines, and is crucial to facilitate patient access to new treatments, developing criteria for stopping one treatment and starting the next, understanding treatment sequencing and informing clinical trial design. There is a paucity of adequate real-world data, which could be utilized by national and local authorities in making decisions on treatment access.
However, there are limitations and methodological challenges in using registries to provide real-world evidence in hematological cancers. The key limitation is the quality of data collection and the heterogeneity of data available to collect from everyday clinical practice. When collecting real-world data, it is essential that key source data is standardized within patient records and can be easily transferred to the registry and collected within a normal clinical practice setting. At present standardization is not commonplace and many key outcome variables are currently retrieved from free-text fields. Standardized data collection systems would also ensure that users input all the essential data, including cytogenetics and risk grouping, which would minimize missing data within the registry. In the present study, bias in treatment selection and definition of treatment lines, missing data such as cytogenetic data and low patient numbers in further treatment lines due to deficiencies in the follow-up data made accurate stratified multivariate adjusted models impossible to generate. Also, not automating and requiring data entry in to a registry leads to a potential patient selection bias. In 2013 for example, 54 MM patients from the Helsinki centers were entered into the FHR while the mandatory Finnish Cancer Registry (with information on diagnosis but neither treatment nor outcomes) reported 114 incident MM cases from the entire Helsinki region. Smaller clinics, treating older MM patients who do not receive ASCT, are less likely to enter data into the FHR according to national experts. This bias could lead to an overestimation of the OS among MM patients in the current study. Table 2 lists potential solutions to the challenges faced in using real-world retrospective registry data in this disease area.
Table 2. Challenges in using a retrospective study design to understand real-world experience and potential solutions.
| Issue | Solution |
|---|---|
| Cancer registries traditionally set up for solid tumors | Design cancer registries specifically for hematological tumors |
| Data quality heterogeneous–incomplete or relevant data not collected | Predetermined outcome parameters defined Structured patient data from electronic journals to enable direct derivation |
| Potential patient selection bias | Automated data extractions based upon diagnostic criteria and permissions granted |
| Lack of accurate progression data | TTNT potentially more reliable |
| Low patient numbers, especially in later treatment lines | Share data across countries International registry Common data model |
| Complex treatment pathway | Predetermined outcome parameters defined Use clinical support decision tools to record practice prospectively |
Treatment line definition differs in real-life clinical practice, with some clinicians adding on new treatments to deepen response for example, while still considering it part of the current treatment line, while another clinician, or the retrospective record, might classify that as a new treatment line. Although treatment lines were defined in the protocol, it was up to practicing clinicians to document line of treatment as per their standard practice, introducing variations and potential uncertainty.
Similar data limitations have been observed in a recent Dutch study which compared data from a bortezomib clinical trial in MM to real-world data. The real-world data was limited by missing data in patients’ records, lack of information on prognostic factors e.g. biomarkers or measures of frailty and variation in treatment patterns [30].
Outcome data from this study focuses on first and second-line treatments; data from multiple lines of therapy was limited by small patient numbers and is excluded from the analysis. We believe that it would be more valuable to be able to follow the whole patient journey. Clinical decision support tools are available and are used in the Nordics in other disease areas, for example prostate cancer [31] and human immunodeficiency virus [32], and a similar approach for MM would be helpful. Such tools not only allow longitudinal treatments and patient responses to be collected and displayed, but also make comprehensive patient overview easier for the treating physician and their team. Importantly for real-world studies, these tools drive the standardization of variables in clinical practice.
Progression free survival (PFS) is the usual measure of progression in clinical trials, but is not considered an outcome in clinical practice. PFS is very difficult to determine accurately from patient records, since it is generally confirmed by monitoring, and a patient may have progressed prior to scans being carried out. In MM specifically, some agents are not given until progression, additionally, some clinicians do not treat until progression in order to avoid treatment resistance or to reduce costs. In our study, progression data was missing from many clinical records. Therefore, we considered that TTNT might be a more objective measure [3]. However, TTNT is not without limitations and does not always accurately reflect treatment effectiveness since the reasons for starting a new therapy are not always related to disease progression and may vary between different centers. Indeed, in the present study, TTNT seemed to be shorter for non-transplanted patients receiving novel treatments in both first and second line than those receiving only conventional treatment in both first and second-line, although patient numbers were small. This is possibly due to these patients having more aggressive or fast progressing disease, though it also may indicate a change in clinical practice in that novel treatments are started earlier than conventional treatments, which clinicians generally initiate once end-organ damage has been incurred.
Collaboration across countries would be valuable to increase patient numbers needed for more robust analyses; however, it will be important to ensure data quality across countries and that differences in treatment guidelines are understood. Europe’s Innovative Medicines Initiative (IMI) project HARMONY (Healthcare alliance for resourceful medicines offensive against neoplasms in hematology) will do just this with its pan-European stakeholder network, creating the largest ever synchronized data source for hematology outcomes [33]. HARMONY will use real-life data to define relevant clinical endpoints and disease outcomes applicable to clinicians and policy makers, increasing the understanding of treatment outcomes in MM, while supporting the optimal treatment choices provided to patients over the course of their disease.
Conclusion
Registry data shows that the adoption of novel treatments in MM has had substantial impact on patient outcomes and that age is the key determinant of treatment choice and OS. In Finland between 2009 and 2013, most younger (<70 years) patients received ASCT and novel therapies as first-line treatment and had far superior OS and TTNT versus those patients who did not receive this combination treatment. Of patients who did not receive ASCT, those who received novel therapies had superior outcomes to those who received conventional therapies alone. Since 2013, MM treatment has progressed substantially with additional novel agents currently in use.
In future, given the reality of complex treatment combinations and sequencing, together with relatively low patient numbers, assessing individual treatment effects will require substantial cohorts and advanced, collaborative analytics on an international scale, such as those planned for hematology in the IMI HARMONY project. Knowledge of real-life treatment outcomes can facilitate patient access to novel treatments and should be used to inform and improve trial design. Trial endpoints should be feasible to assess in clinical practice settings, so that proper real-world comparisons can be made. There is a clear need for real-world data to shed light on everyday practice and outcomes outside of clinical trials.
Acknowledgments
We would like to thank Mirjam Barendse for her excellence and dedication in driving the development of the study protocol. We would also like to thank Johan Aschan, Frans Soltoft and Johan Liwing for their analytic insights and support. Janssen would like to acknowledge the patients, the doctors and the nurses whose lives and efforts made this evaluation possible.
Abbreviations
- ASCT
Autologous stem cell transplant
- ESMO
European Society of Medical Oncology
- FHR
Finnish Hematology Registry
- HARMONY
Healthcare alliance for resourceful medicines offensive against neoplasms in hematology)
- IMI
Innovative Medicines Initiative
- MM
Multiple myeloma
- OS
Overall survival
- PFS
Progression free survival
- TTNT
Time to next treatment
- TTP
Time to progression
Data Availability
As data contain patient information, permission to access the data must be granted by the Finnish Hematology Register steering committee in accordance with the informed consent collected. Requests can be submitted to Chief Physician Perttu Koskenvesa, HUCH perttu.koskenvesa@hus.fi, specifying study number MMY6062 in the request.
Funding Statement
This research is sponsored by Janssen Pharmaceuticals. The funder provided support in the form of salaries for authors AL, PCK, TMJ, FS, MaP. This was a company sponsored study conducted in collaboration with the Finnish researchers, where both parties were involved in the study design, analysis and publication as per ICMJE standards. Data collection was carried out at the Finnish hospitals, with financial support from the funder. Janssen funded JB Medical Ltd to provide medical writing support, TD is a paid employee of JB Medical and carried out the work. JB Medical did not have any additional role in the study design, data collection and analysis or decision to publish.
References
- 1.Bird JM, Owen RG, D'Sa S, Snowden JA, Pratt G, Ashcroft J, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol 2011;154:32–75. 10.1111/j.1365-2141.2011.08573.x [DOI] [PubMed] [Google Scholar]
- 2.Smith D, Yong K. Multiple myeloma. BMJ 2013;346:f3863 10.1136/bmj.f3863 [DOI] [PubMed] [Google Scholar]
- 3.Willenbacher E, Weger R, Rochau U, Siebert U, Willenbacher W. Real-world use of 3rd line therapy for multiple myeloma in Austria: An Austrian Myeloma Registry (AMR) analysis of the therapeutic landscape and clinical outcomes prior to the use of next generation myeloma therapeutics. PLoS One 2016;11:e0147381 10.1371/journal.pone.0147381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sant M, Minicozzi P, Mounier M, Anderson LA, Brenner H, Holleczek B, et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol 2014;15:931–942. 10.1016/S1470-2045(14)70282-7 [DOI] [PubMed] [Google Scholar]
- 5.Liwing J, Uttervall K, Lund J, Aldrin A, Blimark C, Carlson K, et al. Improved survival in myeloma patients: starting to close in on the gap between elderly patients and a matched normal population. Br J Haematol 2014;164:684–693. 10.1111/bjh.12685 [DOI] [PubMed] [Google Scholar]
- 6.Silvennoinen R, Putkonen M, Pelliniemi T-T, Säily M, Anttila P, Remes K, et al. Treatment algorithm for myeloma [Finnish]. 2016. [Google Scholar]
- 7.Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol 2010;28:830–834. 10.1200/JCO.2009.25.4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund J, Uttervall K, Liwing J, Gahrton G, Alici E, Aschan J, et al. Addition of thalidomide to melphalan and prednisone treatment prolongs survival in multiple myeloma—a retrospective population based study of 1162 patients. Eur J Haematol 2014;92:19–25. 10.1111/ejh.12213 [DOI] [PubMed] [Google Scholar]
- 9.Harrouseau JL, Greil R, Kloke O. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of multiple myeloma. Ann Oncol 2005;16 Suppl 1:i45–47. [DOI] [PubMed] [Google Scholar]
- 10.Harrosseau JL. Multiple myeloma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2007;18 Suppl 2:ii44–46. [DOI] [PubMed] [Google Scholar]
- 11.Harousseau JL, Dreyling M. Multiple myeloma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008;19 Suppl 2:ii55–57. [DOI] [PubMed] [Google Scholar]
- 12.Harousseau JL, Dreyling M. Multiple myeloma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20 Suppl 4:97–99. [DOI] [PubMed] [Google Scholar]
- 13.Harousseau JL, Dreyling M. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v155–157. [DOI] [PubMed] [Google Scholar]
- 14.Moreau P, San Miguel J, Ludwig H, Schouten H, Mohty M, Dimopoulos M, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi133–137. [DOI] [PubMed] [Google Scholar]
- 15.Moreau J, San Miguel P, Sonneveld MV, Mateos E, Zamagni H, Avet-Loiseau R, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv52–iv61. 10.1093/annonc/mdx096 [DOI] [PubMed] [Google Scholar]
- 16.Treatment recommendation of the Finnish Myeloma Group (FMG), 2017 [Finnish] http://www.hematology.fi/fi/hoito-ohjeet/veritaudit/plasmasolutaudit/myelooma/hoito/kansalliset-hoitosuositukset-fmg. Accessed 17 Jan 2018.
- 17.Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint A, et al. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol 2010;49:725–736. 10.3109/02841861003782017 [DOI] [PubMed] [Google Scholar]
- 18.Cancer statistics. https://syoparekisteri.fi/tilastot/tautitilastot/. Accessed 17 Jan 2018.
- 19.Warren JL, Harlan LC, Stevens J, Little RF, Abel GA. Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States. J Clin Oncol 2013;31:1984–1989. 10.1200/JCO.2012.46.3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonomo L, Lue J, Jagannath S, Chari A. The outcomes of newly diagnosed elderly multiple myeloma patients treated at a single U.S. institution. Cancer Med 2016;5:500–505. 10.1002/cam4.620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uttervall K, Admasie J, Alici E, Lund J, Liwing J, Aschan J, et al. A combination regimen of bortezomib, cyclophosphamide and betamethasone gives quicker, better and more durable response than VAD/CyBet regimens: results from a Swedish retrospective analysis. Acta Haematol 2013;130:7–15. 10.1159/000345422 [DOI] [PubMed] [Google Scholar]
- 22.Mian M, Tinelli M, Turri G, Meneghini V, Pescosta N, Berno T, et al. Bortezomib, thalidomide and lenalidomide: Have they really changed the outcome of multiple myeloma? Anticancer Res 2016;36:1059–1065. [PubMed] [Google Scholar]
- 23.Kim K, Lee JH, Kim JS, Min CK, Yoon SS, Shimizu K, et al. Clinical profiles of multiple myeloma in Asia-An Asian Myeloma Network study. Am J Hematol 2014;89:751–756. 10.1002/ajh.23731 [DOI] [PubMed] [Google Scholar]
- 24.Mateos MV, Ocio EM, Paiva B, Rosinol L, Martinez-Lopez J, Blade J, et al. Treatment for patients with newly diagnosed multiple myeloma in 2015. Blood Rev 2015;29:387–403. 10.1016/j.blre.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 25.Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood 2015;125:3076–3084. 10.1182/blood-2014-09-568915 [DOI] [PubMed] [Google Scholar]
- 26.Finnish Leukaemia Group. Long-term survival in multiple myeloma: a Finnish Leukaemia Group study. Br J Haematol 1999;105:942–947. [DOI] [PubMed] [Google Scholar]
- 27.Jagannath S, Roy A, Kish J, Lunacsek O, Globe D, Eaddy M, et al. Real-world treatment patterns and associated progression-free survival in relapsed/refractory multiple myeloma among US community oncology practices. Expert Rev Hematol 2016;9:707–717. 10.1080/17474086.2016.1195254 [DOI] [PubMed] [Google Scholar]
- 28.Song X, Cong Z, Wilson K. Real-world treatment patterns, comorbidities, and disease-related complications in patients with multiple myeloma in the United States. Curr Med Res Opin 2016;32:95–103. 10.1185/03007995.2015.1105202 [DOI] [PubMed] [Google Scholar]
- 29.Potluri R, Farr AM, Hirji I, Davis C, Bhandari H, Oukessou A. Treatment sequencing patterns and costs of care in patients with relapsed/refractory multiple myeloma. Value Health 2015;18:A450. [Google Scholar]
- 30.Gaultney JG, Franken MG, Uyl-de Groot CA, Redekop WK, Huijgens PC, van der Holt B, et al. Experience with outcomes research into the real-world effectiveness of novel therapies in Dutch daily practice from the context of conditional reimbursement. Health Policy 2015;119:186–194. 10.1016/j.healthpol.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 31.Regionala Cancercentrum I Samverkan. Patientöversikt prostatacancer PPC [Finnish]. 2016.
- 32.Gisslen M, Svedhem V, Lindborg L, Flamholc L, Norrgren H, Wendahl S, et al. Sweden, the first country to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS)/World Health Organization (WHO) 90-90-90 continuum of HIV care targets. HIV Med 2017;18:305–307. 10.1111/hiv.12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HARMONY Healthcare alliance for resourceful medicines offensive against neoplasms in hematology. http://www.imi.europa.eu/projects-results/project-factsheets/harmony. Accessed 17 Jan 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As data contain patient information, permission to access the data must be granted by the Finnish Hematology Register steering committee in accordance with the informed consent collected. Requests can be submitted to Chief Physician Perttu Koskenvesa, HUCH perttu.koskenvesa@hus.fi, specifying study number MMY6062 in the request.