Abstract
Background
Treatment of metastatic prostate cancer is associated with high personal and economic burden. Recently, new treatment options for castration-resistant prostate cancer became available with promising survival advantages. However, cost-effectiveness of those new treatment options is sometimes ambiguous or given only under certain circumstances. The aim of this study was to systematically review studies on the cost-effectiveness of treatments and costs of castration-resistant prostate cancer (CRPC) and metastasizing castration-resistant prostate cancer (mCRPC) on their methodological quality and the risk of bias.
Methods
A systematic literature search was performed in the databases PubMed, CINAHL Complete, the Cochrane Library and Web of Science Core Collection for costs-effectiveness analyses, model-based economic evaluations, cost-of-illness analyses and budget impact analyses. Reported costs were inflated to 2015 US$ purchasing power parities. Quality assessment and risk of bias assessment was performed using the Consolidated Health Economic Evaluation Reporting Standards checklist and the Bias in Economic Evaluations checklist, respectively.
Results
In total, 38 articles were identified by the systematic literature search. The methodological quality of the included studies varied widely, and there was considerable risk of bias. The cost-effectiveness treatments for CRPC and mCRPC was assessed with incremental cost-effectiveness ratios ranging from dominance for mitoxantrone to $562,328 per quality-adjusted life year gained for sipuleucel-T compared with prednisone alone. Annual costs for the treatment of castration-resistant prostate cancer ranged from $3,067 to $77,725.
Conclusion
The cost-effectiveness of treatments of CRPC strongly depended on the willingness to pay per quality-adjusted life year gained/life-year saved throughout all included costs-effectiveness analyses and model-based economic evaluations. High-quality cost-effectiveness analyses based on randomized controlled trials are needed in order to make informed decisions on the management of castration-resistant prostate cancer and the resulting financial impact on the healthcare system.
Introduction
Among men, prostate cancer is the most commonly diagnosed cancer in developed countries and the second most commonly diagnosed cancer worldwide. In 2015, approximately 1.6 million new prostate cancer cases occurred worldwide [1]. Furthermore, prostate cancer was associated with incidence rates of 70 and 15 per 100,000 population and mortality rates of 10 and 7 per 100,000 population in developed countries and developing countries in 2012, respectively [1]. Since 2005, the incidence rate of prostate cancer increased by 66% due to an aging and growing population, and prostate cancer was associated with 6.3 million DALYs globally in 2015 [1]. Besides the increasing disease burden, the economic burden of prostate cancer needs to be considered in particular. For example, in the European Union, prostate cancer has been associated with high total economic costs (€8.4 billion) in 2009, consisting of healthcare costs (€5.4 billion) including medication costs (€3.1 billion), informal care costs (€1.9 billion) and costs due to productivity losses attributable to mortality (€0.7 billion) [2].
It is well known that with increasing incidence rates and rising costs of cancer treatments, a major, even potentially unsustainable, economic burden will affect the governments or public health services [3]. In the past ten years, the number of new effective cancer treatments significantly increased and treatment costs have risen significantly relative to the gross domestic product [4]. Such a significant economic burden might exacerbate in the future, as recently new treatment options for advanced prostate cancer have become available. Therefore, it is pivotal to consider the relationship between additional effects of those new prostate cancer treatments and economic burden to society very carefully [5].
Prostate cancer is mainly treated surgically by radical prostatectomy, hormonally by suppression of endogenous androgens and by definitive radiotherapy [6–9]. However, a considerable amount of prostate cancers become castration resistant/hormone refractory (CRPC; 10–20% within 5 years) and metastasizing (mCRPC; 33% within 2 years of CRPC diagnosis) [10–12]. CRPC is defined as advanced prostate cancer associated with disease progression following surgical or pharmaceutical castration (i.e. continuous rise in serum prostate-specific antigen PSA levels, and/or appearance of new metastases) [11]. Until recently, effective treatment options for CRPC were scarce, however, since 2004, options for treatment of CRPC have impressively evolved [13–19].
For the treatment of patients with non-metastatic CRPC, observation with continued androgen deprivation therapy is currently recommended by guidelines [9, 20–22]. However, apalutamide and enzalutamide recently showed and increased metastasis-free survival and time to symptomatic progression as compared with placebo [23, 24]. For the treatment of patients with asymptomatic or minimally symptomatic mCRPC with good performance status, the androgen receptor targeting therapies abiraterone and enzalutamide [14, 25], chemotherapy with docetaxel [19] or the immunotherapeutic sipuleucel-T [16] are recommended [9, 20, 21, 26, 27]. For patients with symptomatic mCRPC with good performance status, treatment with docetaxel is preferred [19]. For patients with symptomatic mCRPC with poor performance status, treatment with abiraterone, enzalutamide or docetaxel can be considered if feasible. Abiraterone, enzalutamide [13] or chemotherapy with cabazitaxel [15] can be offered to patients with prior docetaxel therapy. For patients with symptomatic metastatic disease limited to the bone, treatment with the radionuclide radium-223 is recommended [17]. Furthermore, bone protective agents should be offered to patients with mCRPC and skeletal metastases to prevent osseous complications. External beam radiotherapy should be considered for localized symptomatic bone metastases [22, 28].
Besides docetaxel, which has proven life-prolonging efficacy for patients with mCRPC, also the therapeutic agents abiraterone, cabazitaxel, enzalutamide, the immunotherapeutic sipuleucel-T and the radionuclide radium-223 showed significant survival advantages [16, 17, 29]. Nevertheless, within the last decade, it has been acknowledged that cost-effectiveness of those therapeutic agents is given only under certain circumstances [30–36]. A recent review examined cost-effectiveness studies in the field of metastatic prostate cancer [37]. The review analyzed 12 studies, focusing on hormonal therapy and 19 studies, focusing on chemotherapy, immunotherapy and medication on cancer-induced bone loss. Single fraction radiotherapy and enzalutamide were mostly considered cost-effective for patients with prior docetaxel treatment, and zoledronic acid was recommended for treatment of patients with symptoms from bone metastases [37]. However, this review did not specifically focus on castration resistant/hormone refractory prostate cancer for which recently new effective, yet high-cost treatment options have become available. Furthermore, this review did not assess the risk of bias specific to economic evaluations. To our knowledge, currently no review systematically examined cost-effectiveness studies or cost studies in the field of CRPC and mCRPC. Therefore, it is important to focus on those new effective, yet high-cost treatment options for CRPC and mCRPC in order to facilitate an informed policy decision making. Therefore, the aim of this study is to systematically review studies on the cost-effectiveness of treatments and costs of CRPC and mCRPC on behalf of their methodological quality and the risk of bias.
Materials and methods
Literature search and selection criteria
A systematic literature search was conducted in the databases PubMed, CINAHL Complete, the Cochrane Library (including the National Health Service Economic Evaluations Database, the HTA Database and the Database of Abstracts of Reviews of Effects) and Web of Science Core Collection in September 2017 and was updated in June 2018 to minimize time lag of this review [38]. The following search strategy was used: (neoplasm* OR cancer*) AND (castration resistant OR androgen insensitive OR hormone refractory) AND (prostatic OR prostate) AND (cost* OR economic OR burden* OR marginal analysis OR benefit*). No restrictions were defined according to publication year. Reviews were excluded during eligibility assessment, but screened for further eligible studies. A further manual search for eligible studies has been performed in included studies.
Search results were first independently screened for relevance of title and abstract by two authors (TG and AD). A third author (JD) was involved to reach consensus on disagreement. Second, those articles that were deemed relevant were considered in full text. Articles were excluded if
they were protocols, letters, editorials, commentaries, conference abstracts, case reports or reviews,
the study had other objectives than model-based economic evaluations (MEE), cost-effectiveness analyses (CEA), cost-of-illness analyses (COIA) or budget-impact-analyses (BIA) in CRPC and mCRPC, and if
the full text was not available in English or German.
Full text examination has been conducted independently by two authors (TD and AD). A third author (JD) was involved to reach consensus on disagreement.
Data extraction and adjustment
Data on study characteristics (e.g. study type, sample size, study perspective, included cost categories), effects, costs, incremental cost-effectiveness ratios (ICERs) and probabilities of cost-effectiveness were extracted and entered into spreadsheets independently by two authors (TG and AD). Included studies were classified as CEA, MEE, COIA or BIA. CEA were defined as economic evaluation using patient-level data, whereas MEE were defined as economic evaluation using decision analytic modelling [39]. Costs were classified into two different perspectives of economic evaluations: the payer’s perspective (e.g. health maintenance organization or NHS) or the societal perspective (costs for productivity losses additionally), according to the consolidated health economic evaluation reporting standards (CHEERS) checklist [40].
Costs reported in the studies were assigned to the following categories: treatment/medication (e.g. chemotherapy, radiation therapy, hormonal therapy, medication administration, health monitoring and adverse events therapy), hospital care (including inpatient care, emergency room visits and treatments), physician and non-physician outpatient care, nursing care, transportation and indirect costs. Furthermore, cost data were inflated to the year 2015 using country-specific gross domestic product inflation rates and converted to international dollars using purchasing power parity (PPP) rates [41]. If no year for the calculation of costs was denoted, the year of study publication was used as base year.
ICERs reported in the studies were generally assigned to either additional cost per quality-adjusted life year (QALY) gained or per life-year saved. Probabilities of cost-effectiveness were defined as the probability that a treatment/medication was cost-effective compared with an alternative treatment/medication or placebo given a maximum acceptable ratio of cost per QALY gained or per life-year saved. A low probability of cost-effectiveness was defined as a probability of around 50% assuming a symmetric distribution of the incremental net benefit of the treatment/medication and the alternative treatment/medication or placebo [42].
Quality assessment
The methodological quality of CEA/MEE included in this review was assessed using the CHEERS checklist [40, 43]. The CHEERS checklist consists of 24 items arranged in the groups: title and abstract, introduction, methods, results, discussion and other. For the assessment of the methodological quality of COIA, a checklist explicitly developed for the evaluation of COIA has been used [44]. The quality checklist consists of 22 items arranged in the six groups: scope, general economic criteria, calculation of costs, study design and analysis, presentation of results, and discussion. For the assessment of the methodological quality of BIA, currently no valid and reliable instrument exists.
The risk of bias of CEA/MEE was assessed using the Bias in Economic Evaluation (ECOBIAS) checklist [45]. The ECOBIAS checklist consists of 11 items in an overall checklist for bias in economic evaluations and of 11 items in a checklist for model-specific aspects of bias in economic evaluations arranged in three groups: bias related to structure, bias related to data and bias related to consistency. For the assessment of risk of bias of COIA and BIA, currently no valid and reliable instruments exist.
All studies were independently assessed for methodological quality and risk of bias by two authors (TG and JD), and the assessments were compared with each other. Any disagreements were resolved through discussion. The methodical quality and the risk of bias were reported per item. A summary score was not calculated, as weights for different items are non-existent [46]. Thus, no weighting of the studies’ effects, costs, incremental cost-effectiveness ratios and probabilities of cost-effectiveness has been performed.
Results
Search results
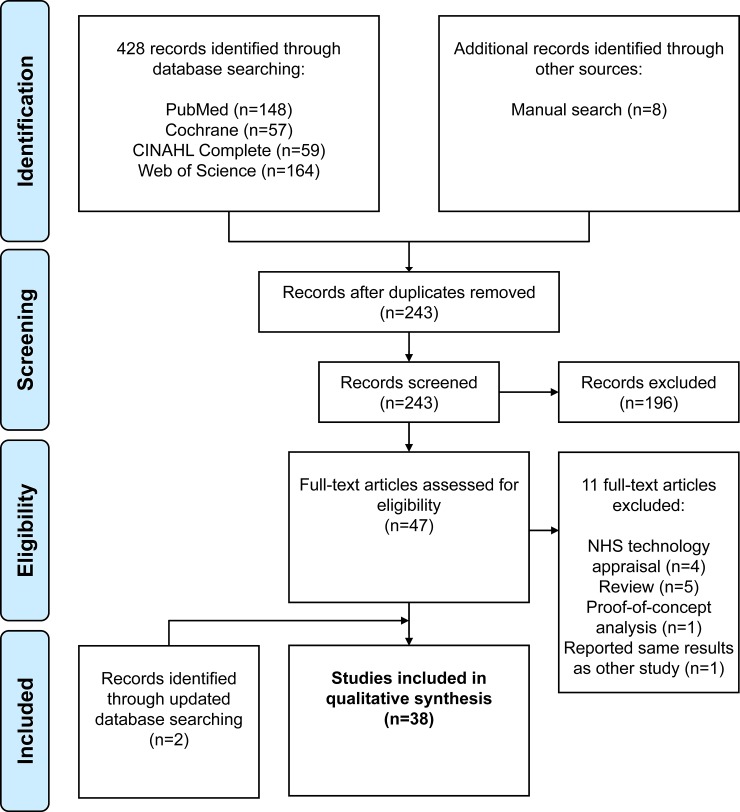
In total, 436 articles were identified by database searching and manual search. Based on title and abstract screening for relevance, 193 duplicates and 196 non-relevant articles were removed (65 protocols, editorials, commentaries or conference abstracts; 120 articles with other study objectives; six articles with full text not available in English or German; and six reviews). A reference list of the excluded non-relevant articles can be found in the S2 File. From the remaining 47 potentially relevant articles, full texts were retrieved and examined for relevance. Based on the full text examination, 11 articles were rejected as they did not meet inclusion criteria (five reviews [47–51]; four technology appraisals of the National Institute for Health and Care Excellence [33–36]; one proof-of-concept-analysis [52]; and one article that reported the same results already found in another study [53]). An update of the systematic literature search identified two [54, 55] additional articles. Finally, 38 articles were included in the review. As one of the articles [56] described both, a MEE and a BIA, this review is based on 39 analyses/evaluations: four CEA [57–60], 15 MEE [54, 56, 61–73], 15 COIA [54, 74–87] and five BIA [56, 88–91]. A flow chart of the selection process is presented in Fig 1.
Fig 1. Flow chart of the selection process based on the PRISMA statement [92].
Study characteristics
The general characteristics of included CEA/MEE and COIA are presented in Tables 1 and 2, respectively. The general characteristics of included BIA are presented in S1 Table. The majority of the included CEA/MEE originated from the United States (US; n = 12) [62–73], three originated from the United Kingdom (UK) [56, 59, 75], two were multi-country [60, 61], one originated from Canada (CAN) [58] and one from the Netherlands [54], respectively. The included COIA originated majorly from the US (n = 6) [74, 75, 78, 80, 84, 87] and CAN (n = 5) [77, 79, 81, 85, 86] as well as from Japan (n = 2) [55, 82], Ireland (n = 1) [76] or Sweden (n = 1) [83], respectively. The included BIA originated from the US (n = 4) [88–91] and the UK (n = 1) [56], respectively. The earliest study year was 1993 and the most recent study year was 2018.
Table 1. General characteristics of included cost-effectiveness analyses and model-based economic evaluations.
| Reference | Country | Patients | Sample size (IG,CG) | Mean/median age (IG,CG) | Time horizon | Model type | Data source | Perspective | Year of pricing |
|---|---|---|---|---|---|---|---|---|---|
| CEA | |||||||||
| Andronis et al. [57] | UK | mCRPC | 707 (350,357) | 69a | Lifetime | − | RCT | PAY | 2012 |
| Bloomfield et al. [58] | CAN | CRCP | 114 | n.a. | Lifetime | − | RCT | PAY | 1996 |
| James et al. [59] | UK | mCRPC | 707 (350,357) | 69a | Lifetime | − | RCT | SOC | 2012 |
| Reed et al. [60] | AT, AU, BE, CAN, FR, DE, IT, NZ, SE, CH, UK, US | mCRPC | 360 (181,179) | 73 | 15 months | − | RCT | PAY | 2000 |
| MEE | |||||||||
| Carter et al. [61] | FR, DE, PT, NL | mCRPC | − | 72 | n.a. | Decision model | Saad et al. [93] | PAY | 2007 |
| Collins et al. [56] | UK | mCRPC | − | − | 180 months | Markov model | Tannok et al. [19] | PAY | 2003 |
| Gong & Hay [62] | US | mCRPC | − | 70 | Lifetime | Markov model | De Bono et al. [14], Kantoff et al. [16] | SOC | 2013 |
| Holko & Kawalec [63] | US | CRPC | − | − | Lifetime | Markov model | Kantoff et al. [16] | PAY | 2012 |
| Konski [64] | US | mCRPC with bone metastases | − | − | 24 months | Markov model | Various studies [94–100] | PAY | 2004b |
| Massoudi et al. [65] | US | mCRPC | − | − | 12 months | Statistical analysis | Beer et al. [13], Rathkopf et al. [101] | PAY | 2015 |
| Peters et al. [55] | NL | mCRPC | − | − | Lifetime | Markov model | Various studies [15, 17, 18, 102] | SOC | 2017 |
| Pilon et al. [66] | US | mCRPC | − | − | n.a. | Statistical analysis | Various studies [13, 25, 101, 103] | PAY | 2015 |
| Pollard et al. [67] | US | mCRPC | − | − | Lifetime | Decision-tree model | Various studies [14–19, 104] | PAY | 2017b |
| Snedecor et al. [68] | US | mCRPC with bone metastases | − | − | 27 months | Markov model | Fizazi et al. [102] | PAY | 2010 |
| Stopeck et al. [69] | US | mCRPC with bone metastases | − | − | Lifetime | Markov model | Fizazi et al. [102], Stopeck et al. [105], Henry et al. [106] | PAY | 2011 |
| Wilson et al. [70] | US | DX-refractory mCRPC | − | − | 18 months | Decision-tree model | De Bono et al. [14], De Bono et al. [15], Scher et al. [18] | PAY | 2012 |
| Xie et al. [71] | US | mCRPC with bone metastases | − | − | 12 months, 36 months | Markov model | Fizazi et al. [102] | PAY | 2010 |
| Zhong et al. [72] | US | DX-refractory mCRPC | − | − | 18 months | Decision-tree model | De Bono et al. [14], De Bono et al. [15] | SOC | 2010 |
| Zubek & Konski [73] | US | CRPC | − | − | 120 months | Markov model | Cordon-Cardo et al. [107] | PAY | 2006 |
AT: Austria, AU: Australia, BE: Belgium, CAN: Canada, CEA: cost-effectiveness analysis, CG: control group, CH: Switzerland, CRPC: castration-resistant prostate cancer, DE: Germany, DX: docetaxel, FR: France, IG: intervention group, IT: Italy, mCRPC: metastatic castration-resistant prostate cancer, MEE: model-based economic evaluation, n.a.: not available, NL: the Netherlands, NZ: New Zealand, PAY: costs are reported from the perspective of a third-party payer, PT: Portugal, SE: Sweden, SOC: costs are reported from the perspective of the society, UK: United Kingdom, US: United States.
a based on a larger data set
b The submission year/study year was assumed as base year.
Table 2. General characteristics of included cost-of-illness analyses.
| Reference | Country | Patients | Diagnostic criteria/inclusion criteria | Study type | Sample size | Mean age | Data source | Perspective | Year of pricing |
|---|---|---|---|---|---|---|---|---|---|
| Alemayehu et al. [74] | US | CRPC | ICD-9-CM, PSA-level | RCS | 349 | 68 | Claims data (commercial, Medicare Advantage) | PAY | 2007 |
| Likely CRPC* | Logistic regression | 2,391 | 74 | ||||||
| Armstrong et al. [75] | US | mCRPC (Medicare 5% sample) | ICD-9 | RCS | 281 | − | Claims data (Medicare, MarketScan commercial) | PAY | 2015 |
| mCRPC (MarketScan dataset) | 155 | ||||||||
| Bourke et al. [76] | IE | CRPC | − | MA | − | − | Medical literature, study data [108, 109], expert opinion | PAY | 2010 |
| Bryant-Lukosius [77] | CAN | CRPC with mental disorder** | TNM, PSA-level, UM-CIDI-SF | PCS | 19 | 69 | Self-report (HSUI) | PAY | 2001 |
| CRPC without mental disorder** | 80 | 72 | |||||||
| Bui et al. [78] | US | CRPC with CSS*** | ICD-9-CM, PSA-level | RCS | 822 | 74a | Claims data (VHA) | PAY | 2012 |
| CRPC without CSS*** | 177 | 75a | |||||||
| Dragomir et al. [79] | CAN | mCRPC | − | MA | − | − | Study data [14, 15, 19, 25, 110, 111] | PAY | 2013 |
| Engel-Nitz et al. [80] | US | CRPC (oncology cohort) | ICD-9-CM, PSA-level | RCS | 1,590 | 71 | Claims data (commercial, Medicare Advantage) | PAY | 2008 |
| CRPC (urology cohort) | 995 | 76 | |||||||
| Krahn et al. [81] | CAN | CRPC | Gleason score, TNM, PSA-level | RCS | 46 | 67a | Claims data (Ontario HIP), health care databasesb | PAY | 2008 |
| mCRPC | 46 | 67a | |||||||
| Kunisawa et al. [82] | JP | Likely CRPC† | Recorded PC diagnoses, docetaxel administration | RCS | 13 | 62a | Claims data (Japanese HMO) | PAY | 2013 |
| Malmberg et al. [83] | SE | mCRPC‡ (within county) | External radiotherapy use | RCS | 46 | 69 | Study data [112], hospital database (UHL) | SOC | 1993 |
| mCRPC‡ (out of county) | 33 | ||||||||
| Mehra et al. [84] | US | mCRPC | ICD-9, docetaxel administration | RCS | 3,642 | 70 | Claims data (IMS LifeLink) | PAY | 2012c |
| Organ et al. [85] | CAN | CRPC (intermittent LHRHa) | PSA-level, increase in number or size of metastasis, clinical progression | RCT | 18 | 73 | Health administrative databases (Dalhousie University) | PAY | 2009 |
| CRPC (continuous LHRHa) | 13 | 79 | |||||||
| Sanyal et al. [86] | CAN | mCRPC | − | MA | − | − | Study data [113] | PAY | 2014 |
| Satoh et al. [55] | JP | mCRPC (ICD-10 sample) | ICD-10, ADT treatment or CRPC-targeted treatment, Japanese MEDIS-DC system | RCS | 4,001 | 72 | Claims data (CISA database) | PAY | 2016c |
| mCRPC (MEDIC-DC sample) | 276 | 71 | |||||||
| Sherman et al. [87] | US | mCRPC (strontium) | PSA-level, clinical progression | RCT | 7 | 73 | Self-report (COIN form), hospital billing department (MSKCC) | SOC | 1997 |
| mCRPC (CT) | 6 | 66 | |||||||
| mCRPC (strontium+CT) | 7 | 65 |
ADT: androgen deprivation therapy, CAN: Canada, CG: control group, CISA: Clinical Information and Statistical Analysis, COIN: Collection of Indirect and Nonmedical Direct Costs, CRPC: castration-resistant prostate cancer, CSS: corticosteroid-sensitive comorbidities, CT: chemotherapy, HIP: health insurance plan, HMO: health maintenance organization, HSUI: Health Service Utilization Inventory, IE: Ireland, IG: intervention group, JP: Japan, LHRHa: luteinizing hormone-releasing hormone agonists, MA: model approach, mCRPC: metastatic castration-resistant prostate cancer, MEDIS-DC: Medical Information System Development Center, MSKCC: Memorial Sloan-Kettering Cancer Center, PAY: costs are reported from the perspective of a third-party payer, PC: prostate cancer, PCS: prospective cohort study, PSA: prostate-specific antigen, RCS: retrospective cohort study, RCT: randomized controlled trial, SE: Sweden, SOC: costs are reported from the perspective of the society, TNM: TNM Classification of Malignant Tumors, UHL: University Hospital Lund, UM-CIDI-SF: University of Michigan Composite Diagnostic Interview-Short Form, US: United States, VHA: Veterans Health Administration.
* CRPC status was modeled as a function, inter alia, of age, comorbidity, prostate cancer-related costs and docetaxel administration
** outpatient population
*** veteran population
† patients with prostate cancer who had been administered docetaxel were assumed to be CRPC patients
‡ patients with bone pain.
a based on a larger data set
b Canadian Institute for Health Information-Discharge Abstract Database, Ontario Drug Benefit Plan database, Complex Continuing Care database, Ontario Home Care Administrative System database, Queen’s University Radiation Oncology Research Unit database
c The submission year/study year was assumed as base year.
The sample size of the CEA and COIA varied from 114 to 707 patients and 13 to 4,001 patients, respectively. The mean/median age varied from 68 to 73 years and 62 to 79 years, respectively. A payer’s perspective was used by three CEA [57, 58, 60], 11 MEE [56, 61, 63–69, 71, 73] and 13 COIA [55, 74–82, 84–86]. A societal perspective was used by one CEA [59], four MEE [54, 62, 70, 72] and two COIA [83, 87]. Ten CEA/MEE reported a lifetime follow-up [54, 56–59, 62, 63, 66, 67, 69]. Nine CEA/MEE reported varying lengths of follow-up from 12 to 120 months. The COIA were based majorly on retrospective cohort studies (n = 9) [55, 74, 75, 78, 80–84]. Three COIA used a model approach [76, 79, 86], two were based on randomized controlled trials [85, 87], and one was based on a prospective cohort study [77]. A length of follow-up varying per patient was reported by seven COIA [74, 78, 80–84], whereas the remaining COIA reported a length of follow-up from six to 24 months [55, 75–77, 85, 87] or a lifetime follow-up [79, 86].
Methodological quality and risk of bias
The CEA/MEE fulfilled 43% to 95% of the CHEERS-criteria [40] (S2 and S3 Tables). Only 26% of the CEA/MEE stated relevant aspects of the study setting [56, 58, 61, 62, 71] and only 47% of the MEE described fully the methods used for identification of included studies and synthesis of clinical effectiveness data [54, 56, 61–64, 72]. The structure of the decision analytic model used for analysis and its appropriateness for use in the study was adequately described by 40% of all MEE [54, 62, 68, 69, 71, 73].
The COIA fulfilled 61% to 90% of the criteria methodological quality checklist for COIA [44] (S4 Table). Only 47% of the studies identified eligible patients based on objective diagnostic criteria [55, 74, 75, 78, 80, 81, 84] and only 27% of the studies included the costs restricted to those definitely attributable to CRPC [79, 83, 86]. Furthermore, only 17% of the applicable studies discounted future costs [83, 86] and only 20% conducted univariate and/or probabilistic sensitivity analyses [79, 82, 86].
The CEA/MEE fulfilled 19% to 100% of the ECOBIAS-criteria [45] (S5 and S6 Tables). Only one CEA considered uncertainty in sufficient detail in a sensitivity analysis, disclosed sponsorships, listed the study in a trial register and reported results according to a freely accessible study protocol [59].
Cost-effectiveness analyses
One analysis calculated the cost-effectiveness of mitoxantrone compared with prednisone alone for patients with CRPC based on a randomized controlled trial [58]. Mitoxantrone was dominant (i.e. less costly and more effective) compared with prednisone alone [58].
Five analyses calculated the cost-effectiveness of the bone-targeted therapies zoledronic acid compared with no zoledronic acid [57, 59] and compared with placebo [60], as well as strontium-89 compared with no strontium-89 [57, 59] for patients with mCRPC. The ICER of zoledronic acid compared with no zoledronic ranged between $11,468 and $42,047 per QALY gained [57, 59]. The ICERs of zoledronic acid compared with placebo was $213,513 per QALY gained or $16,496 per skeletal-related event avoided, respectively [60]. The ICER of strontium-89 compared with no strontium-89 ranged between $16,590 and $24,187 per QALY gained [57, 59].
Model-based economic evaluations
The cost-effectiveness of abiraterone compared with placebo/prednisone alone for patients with mCRPC was modeled by three analyses with ICERs of $112,100 per life-month saved [66] and of $128,895 [70] to $399,525 [62] per QALY gained. One further analysis indicated an ICER of abiraterone compared with mitoxantrone of $98,939 per QALY gained [72]. The health effects, costs, ICERs and probabilities of cost-effectiveness of all MEE are presented in Table 3.
Table 3. Cost-effectiveness analyses and model economic evaluations–health effects, costs, cost-effectiveness ratios and probabilities of cost-effectiveness.
| Reference | Comparator | Cost categoriesa | Incremental health effects | Incremental costs (in $-PPP) | ICER (in $-PPP per additional health effect) | Probability of cost-effectiveness (per additional health effect) |
|---|---|---|---|---|---|---|
| CEA | ||||||
| Andronis et al. [57] | With ZA vs. without ZA | A, B, C | 0.03 QALYs gained | 360 | 11,468 | 64% for a WTP of $42,976 |
| With S89 vs. without S89 | 0.08 QALYs gained | 1,955 | 24,187 | 60% for a WTP of $42,976 | ||
| Bloomfield et al. [58] | M+P vs. P | A, B, C | 0.26 QALYs gained | −2,051 | Dominant | − |
| James et al. [59] | With ZA vs. without ZA | A, B, C | 0.03 QALYs gained | 1,319 | 42,047 | 40% for a WTP of $42,976 |
| With S89 vs. without S89 | 0.08 QALYs gained | 1,341 | 16,590 | 76% for a WTP of $42,976 | ||
| Reed et al. [60] | ZA vs. placebo | A, B, C, D | 0.46 SRE avoided | −435b | 16,496c | − |
| 0.04 QALYs gained | 213,513c | |||||
| MEE | ||||||
| Carter et al. [61] | ZA vs. placebo | A | 0.04 QALYs gained | 1,741 (FR) | 48,833 | − |
| 1,182 (DE) | 31,136 | |||||
| 377 (PT) | 15,605 | |||||
| 116 (NL) | 3,283 | |||||
| Collins et al. [56]– 1st analysis | P vs. M+P | A | −0.00 QALYs gained | 737 | Dominated | 26% for a WTP of $74,965 |
| DX+P vs. M+P | 0.15 QALYs gained | 9,462 | 70,666 | 53% for a WTP of $74,965 | ||
| Collins et al. [56]– 2nd analysis | M+P+C vs. M+P | A | −0.02 QALYs gained | 326 | Dominated | 12% for a WTP of $74,965 |
| P vs. M+P | −0.00 QALYs gained | 737 | Dominated | 16% for a WTP of $74,965 | ||
| DX+P(weekly) vs. DX+P | −0.12 QALYs gained | 28,925 | Dominated | 0% for a WTP of $74,965 | ||
| DX70*+ES+P vs. DX+P | −0.10 QALYs gained | 10,169 | Dominated | 16% for a WTP of $74,965 | ||
| DX35**+ES+P vs. DX+P | −0.07 QALYs gained | 14,292 | Dominated | 4% for a WTP of $74,965 | ||
| DX+E vs. DX+P | 0.13 QALYs gained | 7,875 | ED | 25% for a WTP of $74,965 | ||
| DX+P(3-weekly) vs. M+P | 0.15 QALYs gained | 9,462 | 61,295 | 20% for a WTP of $74,965 | ||
| Gong & Hay [62] | A vs. P | A, B, C | 0.43 QALYs gained | 174,670 | 399,525 | 50% for a WTP of $410,985 |
| ST vs. P | 0.16 QALYs gained | 93,921 | 562,328 | 50% for a WTP of $277,415 | ||
| Holko et al. [63] | ST vs. SC | A, B, C | 0.37 QALYs gained | 109,164 | 295,529 | 4% for a WTP of $155,597 |
| Konski [64] | SFX RT vs. Rx | A | 0.03 QALYs gained | 247 | 8,454 | − |
| MFX RT vs. Rx | 0.04 QALYs gained | 1,849 | 44,386 | |||
| M+P vs. Rx | −0.07 QALYs gained | 4,439 | Dominated | |||
| Massoudi et al. [65] | EZ vs. A+P | A, B, D | NNT 14 (free of progression or death) | −2,666 | Dominant | − |
| NNT 26 (CT delayed) | Dominant | |||||
| NNT 91 (death avoided) | Dominant | |||||
| Peters et al. [55] | R223 vs. A | A, B, C, D, E | 0.02 QALYs gained | −7,475d | Dominant | 61% for a WTP of 98,160d |
| R223 vs. CX | 0.01 QALYs gained | −5,479d | Dominant | 54% for a WTP of 98,160d | ||
| R223 vs. EX | −0.06 QALYs gained | −9,067d | Less costly/effective | 61% for a WTP of 98,160d | ||
| Pilon et al. [66] | A+P vs. placebo+P | A | 4.40 LMS | 112,100d | 3,231d | − |
| EZ vs. placebo | 4.00 LMS | 159,264d | 4,512d | |||
| Pollard et al. [67]– 1st analysis | ST vs. SC | A | 0.34 LYS | 106,117d | 312,109d (ED) | − |
| ST+EZe | 0.31 LYS | 68,384d | 220,594d (ED) | |||
| ST+EZ+Ae | 0.33 LYS | 50,119d | 151,876d (ED) | |||
| ST+EZ+A+DX vs. SC | 1.18 LYS | 245,103d | 207,714d | |||
| ST+EZ+A+DX+R223e | 0.30 LYS | 80,072d | 266,907d | |||
| ST+EZ+A+DX+R223+CXe | 0.20 LYS | 54,287d | 271,435d | |||
| Pollard et al. [67]– 2nd analysis | EZ vs. SC | A | 0.31 LYS | 68,384 | 220,594 (ED) | − |
| EZ+Ae | 0.33 LYS | 50,119 | 151,876 (ED) | |||
| EZ+A+DX vs. SC | 0.84 LYS | 138,986 | 165,460 | |||
| EZ+A+DX+R223e | 0.30 LYS | 80,072 | 266,907 | |||
| EZ+A+DX+R223+CXe | 0.20 LYS | 54,287 | 271,435 | |||
| Snedecor et al. [68] | D vs. ZA | A,B,C | 0.01 QALYs gained | 8,507 | 1,148,734 | 0% for a WTP of $108,500 |
| Stopeck et al. [69] | D vs. ZA | A,B,C | 0.81 SRE avoided | 7,343 | 9,104 | − |
| 0.14 QALYs gained | 52,502 | 83% for a WTP of $106,268 | ||||
| Wilson et al. [70] | A+P vs. placebo (P) | A+B | 0.27 QALYs gained | 35,265 | 128,895 | 29% for a WTP of $104,427 |
| EZ+P vs. A+P | 0.03 QALYs gained | 13,648 | 456,998 | 21% for a WTP of $104,427 | ||
| CX+P vs. EZ+P | 0.06 QALYs gained | 21,177 | 367,443 | 16% for a WTP of $104,427 | ||
| Xie et al. [71] | D vs. ZA (12 months) | A | 0.11 SRE avoided | 8,477 | 77,064 | 17.5% for a WTP of $54,250 |
| D vs. ZA (36 months) | 0.27 SRE avoided | 15,034 | 55,681 | 49.8% for a WTP of $54,250 | ||
| Zhong et al. [72] | M vs. placebo | A+B | 0.08 QALYs gained | 8,468 | 109,232 | − |
| A vs. M | 0.20 QALYs gained | 19,400 | 98,939 | 42% for a WTP of 108,500$ | ||
| CX vs. A | 0.06 QALYs gained | 59,772 | 1,037,111 | 5% for a WTP of 108,500$ | ||
| Zubek & Konski [73] | PPT vs. SC | A | 1.64 QALYs gained | 3,989 | 2,432 | 100% for a WTP of $57,898 |
| KN vs. SC | 0.92 QALYs gained | 67 | 73 | 90% for a WTP of $57,898 | ||
| PPT vs. KN | 0.72 QALYs gained | 3,922 | 5,447 | 100% for a WTP of $57,898 |
A: abiraterone, C: clondrate, CT: chemotherapy, CX: cabazitaxel, D: denosumab, DE: Germany, DX: docetaxel, ES: estramustine, ED: extended dominance, EZ: enzalutamide, FR: France, FU: follow-up, KN: Kattan nomogram, LMS: life-months saved, LYS: live-years saved, M: mitoxantrone, MFX: multiple fractions of external beam radiotherapy, NL: the Netherlands, NNT: number needed to treat, P: prednisone/prednisolone, PPT: prostate Px test, PT: Portugal, QALY: quality-adjusted life year, R223: radium-223, Rx: pain medication, S89: strontium-89, SC: standard care, SFX: single fraction of external beam radiotherapy, SRE: skeletal-related events, ST: sipuleucel-T, WTP: willingness to pay, ZA: zoledronic acid.
* 75mg/m2
** 30mg/m2
a Costs reported in the studies were assigned to the following categories: (A) treatment, (B) hospital care, (C) physician and non-physician outpatient care, (D) nursing care, (E) productivity loss
b Excluding treatment costs
c Including only treatment costs
d In $-PPP-2017 without inflation
e versus prior analyzed intervention.
The cost-effectiveness of docetaxel for patients with mCRPC was modeled by two analyses of Collins et al. [56] with an ICER of $70,666 per QALY gained for 75 mg/m2 docetaxel every 3 weeks compared with mitoxantrone. In the second analysis, the treatment regimen containing 60–70 mg/m2 docetaxel every 3 weeks was extendedly dominant, i.e. an ICER higher than that of the next most effective regimen, compared with all other treatment regimen (i.e. combinations containing mitoxantrone, docetaxel or estramustine) [56].
The cost-effectiveness of Sipuleucel-T for patients with CRPC [63] and mCRPC [62, 67] was modeled in three analyses. Two analyses indicated ICERs of $295,538 [63] to $562,328 [62] per QALY gained for Sipuleucel-T compared with standard care/prednisone alone. The third analysis indicated extended dominance of drug regimens containing Sipuleucel-T, enzalutamide and abiraterone as well as an ICER of $207,714 per life-year saved for a drug regimen containing additionally docetaxel [67].
Extended dominance was also indicated for drug regimens containing enzalutamide and abiraterone and the ICER for a drug regimen containing additionally docetaxel was $165,460 per life-year saved [67]. Three other analyses modeled the cost-effectiveness of enzalutamide for patients with mCRPC [65, 66, 70]. Two analyses reported an ICER of $456,998 per QALY gained compared with abiraterone [70] as well as dominance of enzalutamide [65], respectively. The third analysis indicated an ICER of $4,512 per life-month saved compared with placebo [66].
One analysis modeled the cost-effectiveness of single and multiple fraction external beam radiotherapy for patients with mCRPC with bone metastases compared with supportive care with ICERs of $8,454 and $44,386 per QALY gained, respectively [64]. The models of cost-effectiveness analyses of mitoxantrone [56, 64, 72], cabazitaxel [70, 72], radium-223 [54] and denosumab [68, 69, 71] for patients with mCRPC are presented exclusively in Table 3. Furthermore, the model of a cost-effectiveness analysis of risk-prediction tools in selecting patients for immediate post-prostatectomy treatment [73] are also presented exclusively in Table 3.
Cost-of-illness analyses
The overall annual direct costs of patients with CRPC and mCRPC ranged from $2,474 [85] to $50,537 [74] and from $26,707 [87] to $67,957 [75], respectively. The annual cancer-specific costs of patients with CRPC and mCRPC ranged from $14,335 [80] to $77,725 [76] and from $3,067 [86] to $73,270 [84], respectively. The costs for hospital care and outpatient care ranged from $611 [55] to $23,308 [84] per year and $507 [83] to $41,170 [80] per year, respectively. The medication costs and the costs for nursing care ranged from $3,425 [77] to $36,864 [75] per year and $490 [75] to $1,475 [81] per year, respectively. No COIA included indirect costs (Table 4).
Table 4. Cost-of-illness analyses–Costs in categories and total costs per patient per year (in 2015 US$-PPP).
| Reference | Time horizon | Hospital care costa | Outpatient care cost | Medication costs | Nursing care costs | Overall direct costsb | CRCP-specific costs |
|---|---|---|---|---|---|---|---|
| Alemayehu et al. [74] | Varying1 | 17,121 | 25,052 | 3,993 | − | 47,465 | 24,348 |
| Known CRPC | 15,835 | 28,598 | 4,683 | − | 50,537 | 30,412 | |
| Likely CRPC | 17,324 | 24,538 | 3,898 | − | 47,018 | 23,468 | |
| Armstrong et al. [75] | 24 months | ||||||
| Medicare | 7,362 | 6,927 | 12,802 | 1,248 | 28,792 | − | |
| MarketScan | 10,109 | 19,827 | 36,864 | 490 | 67,957 | − | |
| Bourke et al. [76] | 12 months | − | − | − | − | − | 77,725 |
| Bryant-Lukosius [77] | 12 months | 1,698 | 1,840 | 3,425 | 550 | 7,514 | − |
| Bui et al. [78] | Varying1 | ||||||
| With CSS | 4,286 | 29,323 | 24,962 | − | 59,799 | − | |
| W/o CSS | 14,499 | 31,604 | 24,148 | − | 71,742 | − | |
| Dragomir et al. [79] | Lifetime | ||||||
| ADT+DX+A treatment | − | − | − | − | − | 17,441 | |
| ADT+DX+CX treatment | − | − | − | − | − | 28,242 | |
| Engel-Nitz et al. [80] | Varying2 | ||||||
| Oncologist patient | 22,364 | 41,170 | 5,314 | − | 70,627 | 47,647 | |
| Urologist patient | 17,670 | 11,240 | 3,578 | − | 33,918 | 14,335 | |
| Krahn et al. [81] | Varying3 | ||||||
| CRPC | 14,543 | 4,491 | 6,022 | 433 | 25,490 | − | |
| mCRPC | 19,243 | 5,482 | 9,810 | 1,475 | 36,010 | − | |
| Kunisawa et al. [82] | Varying1 | − | − | − | − | 17,921 | − |
| Malmberg et al. [83] | Varying4 | ||||||
| Within country | 3,743 | 520 | − | − | − | 4,996 | |
| Out of country | 6,725 | 507 | − | − | − | 7,827 | |
| Mehra et al. [84] | Varying1 | ||||||
| Pre-DX period | 8,960 | 18,408 | 5,125 | − | − | 32,494 | |
| Post-DX period | 23,308 | 25,614 | 24,323 | − | − | 73,270 | |
| Organ et al. [85] | 24 months | ||||||
| Intermittent LHRHa | − | − | − | − | 2,474 | − | |
| Continuous LHRHa | − | − | − | − | 6,514 | − | |
| Sanyal et al. [86] | Lifetime | − | − | − | − | − | 3,067 |
| Satoh et al. [55] | Varying5 | ||||||
| ICD-10 | 611c | − | 6,183c | − | − | 6,794c | |
| MEDIC-DC | 1,183c | − | 19,935c | − | − | 21,118c | |
| Sherman et al. [87] | 6 months | − | − | − | − | 31,683 | − |
| S89 treatment | − | − | − | − | 26,707 | − | |
| V+E treatment | − | − | − | − | 28,216 | − | |
| V+E+S89 treatment | − | − | − | − | 51,162 | − |
ADT: androgen deprivation therapy, CRCP: castration-resistant prostate cancer, CSS: corticosteroid-sensitive comorbidities, CX: cabazitaxel, DX: docetaxel, E: estramustine LHRHa: luteinizing hormone-releasing hormone agonist, mCRPC: metastatic castration-resistant prostate cancer, S89: strontium-89, V: vinblastine, w/o: without.
1 Costs per patient per month were reported
2 costs per patient per 6 months were reported
3 costs per patient per 100 days were reported
4 costs per patient per relapse were reported
5 costs per patient per 12 months were reported
a including costs for emergency department visits
b may include other medical, non-medical or indirect costs not previously listed
c in $-PPP-2016 without inflation.
Budget-impact analyses
The yearly budget-impact of adopting docetaxel for patients with mCRPC on the UK healthcare system was estimated to be $356,580 to $386,986 per million population [56]. The yearly budget-impact of adopting enzalutamide for patients with mCRPC on the US healthcare system was indicated to be $515,871 per million population [88]. The yearly budget-impact of adopting cabazitaxel and abiraterone for docetaxel-refractory patients with mCRPC on the US healthcare system was indicated to be $6,331,704 [91] and $36,035 to $105,982 [90] per million population, respectively. The adoption of a test for the prediction of non-response to the hormonal therapies abiraterone and enzalutamide for patients with mCRPC (AR-V7 testing) in the US healthcare system was associated with yearly cost savings of $468,854 per million population [89] (S1 Table).
Discussion
The aim of this study was to systematically review studies on the cost-effectiveness of treatments and costs of CRPC and mCRPC on behalf of their methodological quality and the risk of bias. In total, 19 CEA/MEE and 20 COIA/BIA were identified and included.
It is noteworthy that no article reported a COIA investigating the excess costs of CRPC and mCRPC using an econometric approach for cost estimation [114]. Furthermore, COIA that were included in this systematic review only reported either treatment related costs or partial costs from a narrow perspective. Notably, only COIA investigating the excess costs of a disease from a societal perspective should be the basis for health care prioritization discussions. For the allocation of health care resources, economic evaluations play an important role [39]. MEE bring together the evidence of a range of real-life sources, such as CEA based on randomized controlled trials, and provide a framework for decision-making. However, if the evidence for MEE to be based on is scarce or even inaccurate, bias might be induced to the ICER and, as a consequence, lead to invalid health care resource allocation-decisions [45]. Therefore, it is pivotal to determine uncertainty of the cost-effectiveness of treatments by means of sensitivity analyses and to conduct CEA based on randomized controlled trials as basis for future MEE.
Cost-of-illness analyses and budget-impact analyses
Annual direct healthcare costs of patients with mCRPC were relatively high (up to $68,000) and budget-impacts of adopting new treatments for patients with CRPC and mCRPC to healthcare systems (e.g. up to $387,000 per million population per year for the adoption of docetaxel) appear to be relevant. Therefore, conducting CEA and MEE with a high methodological quality and a low risk of bias are advisable for adequacy of reimbursement decisions. Furthermore, costs for outpatient care and nursing care should be regularly included to the calculation of cost in CEA and MEE of treatments of CRPC and mCRPC. The current review showed that annual costs of care were as high as costs for medication or hospital care. However, it has to be acknowledged that costs of cancer medication still have the greatest impact on the ICER of CRPC and mCRPC treatment [37].
Cost-effectiveness analyses and model-based economic evaluations
Currently, there is no generally accepted willingness to pay (WTP) threshold per QALY gained. In the UK, a cost-effectiveness threshold ranging between £20,000 ($30,000) and £30,000 ($40,000) per QALY gained has been defined [115, 116]. For treatments extending life at the end of life, a threshold of £50,000 ($70,000) per QALY gained has been defined [117, 118]. In the US, cost-effectiveness thresholds usually range between $50,000 and $100,000 per QALY gained [119–121]. Notwithstanding, a review on WTP thresholds for oncology drugs reported that those were regularly in the range of $100,000 to $150,000 per QALY gained [122].
For abiraterone and enzalutamide, CEA/MEE showed unfavorable ICERs above or around a regularly used WTP threshold for oncology drugs of $100,000 per QALY gained with a low probability of cost-effectiveness [122]. In the UK and the US, both, abiraterone and enzalutamide are recommended for treatment of mCRPC for patients with no prior docetaxel treatment [31, 32]. Sipuleucel-T is only recommended in the US for treatment of asymptomatic or minimally symptomatic mCRPC for patients with no prior docetaxel treatment [123, 124]. The current systematic review yielded ICERs way above a WTP threshold of $100,000 per QALY gained for sipuleucel-T compared with standard care with low probabilities of cost-effectiveness.
Docetaxel showed an ICER below a WTP threshold of $100,000 per QALY gained in one MEE. However, the probability of cost-effectiveness was only around 50% for a WTP of around $75,000 per QALY gained. In the UK and the US, docetaxel is recommended for treatment of mCRPC [33, 125]. For patients with docetaxel-refractory mCRPC, cabazitaxel, enzalutamide and abiraterone plus prednisone can be considered for treatment, yet in the UK only under certain circumstances (i.e. manufacturer discount) [34–36, 124, 126, 127]. All three treatments showed unfavorable ICERs above or around a WTP of $100,000 per QALY gained with low probabilities of cost-effectiveness for patients with docetaxel-refractory mCRPC in the current review.
One comparative MEE showed that radium-223 was dominant compared with abiraterone and cabazitaxel for patients with mCRPC [55]. Yet, the probabilities of cost-effectiveness were only 61% and 54% for a WTP of around $100,000 per QALY gained. Two further MEE showed unfavorable ICERs even above a WTP of $100,000 per life-year saved for treatment regimen containing radium-223 compared with treatment regimen without radium-223 [67]. Nevertheless, it must be mentioned that the latter MEE fulfilled only 43% of the CHEERS-criteria, indicating a lower methodological quality. However, radium-223 is recommended for treatment of docetaxel-refractory mCRPC with bone metastases in both, the UK and US [30, 128]. Denosumab is only recommended in the US for the prevention of skeletal-related events in patients with bone metastases from solid tumors [129, 130]. The current review yielded divergent ICERs comparing denosumab with zoledronic acid that were either way above or even around $50,000 per QALY gained with probabilities of cost-effectiveness ranging from 0% to 83% for a WTP of around $100,000 per QALY gained.
Two recently published phase 3 studies have shown favorable results of enzalutamide and apalutamide for patients with non-metastatic CRPC concerning metastasis-free survival and time to symptomatic progression compared with placebo [23, 24]. However, the cost-effectiveness as well as the budget-impact of both enzalutamide and apalutamide for patients with non-metastatic CRPC has not yet been evaluated.
Methodological quality and risk of bias
Although the ICER has a high priority in health economics and the decision making in the context of technology appraisals, methodological quality and risk of bias of cost-effectiveness analyses play an important role in the credibility of this outcome. The methodological quality of the included CEA and MEE in this systematic review ranged widely from 43% to 95% of CHEERS-criteria fulfilled. Particularly the choice of model in MEE was accountable for a reduced methodological quality. Markov chain models should be preferred for modelling the cost-effectiveness of mCRPC, as it is possible to model longer periods with uncertain timing of death [39, 131, 132]. Current treatment options for mCRPC have shown to prolong survival in patients, whereby the risk of adverse events related to their treatment is high [133, 134]. Consequently, decision tree models are inadequate to capture complex scenarios, as in current treatment options for mCRPC [131, 132]. Nevertheless, 40% of all MEE of the current review did actually not use adequate models, such as Markov chain models, for modelling the cost-effectiveness of mCRPC and therefore, presumably introduced bias to the evaluation [45]. The ECOBIAS-criteria for risk of bias fulfilled by CEA/MEE ranged widely between 19% and 100%. It is worth mentioning that uncertainty of the cost-effectiveness of treatments was considered in sufficient detail in sensitivity analyses by merely one CEA. Moreover, more than two thirds of MEE did not make the methods used to identify data transparent and therefore might have induced bias to the results [45].
The methodological quality of the included COIA was relatively high, as 61% to 90% of the criteria from the checklist explicitly developed for the evaluation of COIA from Stuhldreher et al. [44] were fulfilled. However, as already mentioned, no study included a non-diseased comparison group to the analysis, yet about one third of the COIA reported only disease-specific costs. Furthermore, methodological quality was impaired by misleading or missing information about discounting of costs. Moreover, relevant parameters were not varied in sensitivity analyses in order to test the robustness of the results in the vast majority of COIA in the current review [44].
Strengths and limitations
To our knowledge, this is the first systematic review of costs and cost-effectiveness in CRPC and mCRPC. Compared with another systematic review that examined cost-effectiveness studies in the field of metastatic prostate cancer [37], we additionally included studies in the field of CRPC, where effective treatment options have impressively evolved in recent years. Moreover, the focus of the current systematic review was on economic aspects of CRPC and mCRPC. Therefore, we structured the systematic review by type of economic analysis rather than by treatment option alone to facilitate comparability across treatment options. Furthermore, we additionally analyzed the risk of bias of the included studies. This review was the first to bring together the evidence of MEE, CEA, COIA and BIA in CRPC and mCRPC comprehensively and systematically. The methodological quality and risk of bias of included studies were analyzed and described using the current checklists CHEERS, ECOBIAS and the COIA checklist from Stuhldreher et al. [40, 44, 45]. Furthermore, we were able to improve comparability and interpretability of the studies, as we inflated cost data to the year 2015 and converted cost data to international dollars. However, comparability and interpretability of the studies is still limited, as they differed in characteristics of included patients (e.g. diagnostic criteria/inclusion criteria and age), study type, sample size or study perspective and methodological quality and risk of bias across studies was heterogeneous.
As we did not include grey literature in this systematic review, publications were probably omitted and, therefore, a potential publication bias was introduced. Subsequently, we also decided against inclusion of technology appraisals for the government or public health services (e.g. [33–36]), as the analysis of the methodological quality and risk of bias as well as the data extraction and adjustment would have been limited to the published information, as the corresponding manufacturer submissions were not available. Generalizability of the results of the systematic review was limited to the health care system of the respective study, as health care utilization and the resulting costs across health care systems are difficult to compare. Furthermore, external validity of the results of the systematic review was limited due to heterogeneous characteristics of the included studies (i.e. varying time horizons, sample sizes, study perspectives, included cost categories). Due do heterogeneity of the included studies, a formal meta-analysis was not performed, rendering the systematic review narrative in nature. However, accuracy and usability of the findings of this review were enhanced by inflation of cost data and conversion to international dollars. Lastly, studies with insignificant results might have been omitted due to publication bias.
Conclusions
Existing CEA/MEE included in this systematic review produced a very heterogeneous picture regarding the value for money for different recommended chemotherapies, androgen receptor targeting therapies, immunotherapeutic agents and radionuclides for the treatments of CRPC and mCRPC. Furthermore, the improvable methodological quality and a relatively high risk of bias of CEA/MEE introduce great uncertainty to the overall interpretation of the study results. There is a great need for high-quality CEA based on randomized controlled trials in order to determine cost-effectiveness of CRPC and mCRPC treatments, to bring together this evidence in MEE and finally to protect patients from unfavorable health outcomes and the society from unjustified healthcare system costs.
Supporting information
A: abiraterone, AR-V7: Androgen receptor variant-7, CX: cabazitaxel, DX: docetaxel, EZ: enzalutamide, mCRPC: metastatic castration-resistant prostate cancer, P: prednisone/prednisolone, PAY: payer’s perspective UK: United Kingdom, US: United States. * Based on United Nations estimates of the UK population in 2015 (65,397,080) [133], ** based on United Nations estimates of the US population in 2015 (319,929,162) [133], *** the submission year/study year was assumed as base year.
(PDF)
✓: Criterion fulfilled; CHEERS: consolidated health economic evaluation reporting standards, n.a.: not applicable.
(PDF)
✓: Criterion fulfilled, CHEERS: consolidated health economic evaluation reporting standards, n.a.: not applicable.
(PDF)
✓: Criterion fulfilled, n.a.: not applicable.
(PDF)
✓: Criterion fulfilled, (✓): criterion partially fulfilled, ECOBIAS: Bias in Economic Evaluation, n.a.: not applicable.
(PDF)
✓: Criterion fulfilled, (✓): criterion partially fulfilled, ECOBIAS: Bias in Economic Evaluation, n.a.: not applicable. * These biases are overlapping regarding their content, ** these biases are overlapping regarding their content.
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3: 524–548. 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14: 1165–1174. 10.1016/S1470-2045(13)70442-X [DOI] [PubMed] [Google Scholar]
- 3.Savage P. Development and economic trends in cancer therapeutic drugs in the UK from 1955 to 2009. J Oncol Pharm Pract. 2012;18: 52–56. 10.1177/1078155210389218 [DOI] [PubMed] [Google Scholar]
- 4.Savage P, Mahmoud S. Development and economic trends in cancer therapeutic drugs: a 5-year update 2010–2014. Br J Cancer. 2015;112: 1037–1041. 10.1038/bjc.2015.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karikios DJ, Schofield D, Salkeld G, Mann KP, Trotman J, Stockler MR. Rising cost of anticancer drugs in Australia. Intern Med J. 2014;44: 458–463. 10.1111/imj.12399 [DOI] [PubMed] [Google Scholar]
- 6.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71: 618–629. 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 7.Graham J, Kirkbride P, Cann K, Hasler E, Prettyjohns M. Prostate cancer: summary of updated NICE guidance. BMJ. 2014;348: f7524 10.1136/bmj.f7524 [DOI] [PubMed] [Google Scholar]
- 8.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199: 990–997. 10.1016/j.juro.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines). Prostate cancer. Version 3.2018; 2018. National Comprehensive Cancer Network. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Cited 09 July 2018.
- 10.Hirst CJ, Cabrera C, Kirby M. Epidemiology of castration resistant prostate cancer: a longitudinal analysis using a UK primary care database. Cancer Epidemiol. 2012;36: e349–353. 10.1016/j.canep.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 11.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65: 1180–1192. 10.1111/j.1742-1241.2011.02799.x [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23: 2918–2925. 10.1200/JCO.2005.01.529 [DOI] [PubMed] [Google Scholar]
- 13.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371: 424–433. 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364: 1995–2005. 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376: 1147–1154. 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 16.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363: 411–422. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 17.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369: 213–223. 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367: 1187–1197. 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 19.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351: 1502–1512. 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 20.Cookson MS, Lowrance WT, Murad MH, Kibel AS, American Urological A. Castration-resistant prostate cancer: AUA guideline amendment. J Urol. 2015;193: 491–499. 10.1016/j.juro.2014.10.104 [DOI] [PubMed] [Google Scholar]
- 21.Cookson MS, Roth BJ, Dahm P, Engstrom C, Freedland SJ, Hussain M, et al. Castration-resistant prostate cancer: AUA Guideline. J Urol. 2013;190: 429–438. 10.1016/j.juro.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 22.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71: 630–642. 10.1016/j.eururo.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 23.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378: 2465–2474. 10.1056/NEJMoa1800536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378: 1408–1418. 10.1056/NEJMoa1715546 [DOI] [PubMed] [Google Scholar]
- 25.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368: 138–148. 10.1056/NEJMoa1209096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komura K, Sweeney CJ, Inamoto T, Ibuki N, Azuma H, Kantoff PW. Current treatment strategies for advanced prostate cancer. Int J Urol. 2018;25: 220–231. 10.1111/iju.13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teply BA, Hauke RJ. Chemotherapy options in castration-resistant prostate cancer. Indian J Urol. 2016;32: 262–270. 10.4103/0970-1591.191239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saad F, Chi KN, Finelli A, Hotte SJ, Izawa J, Kapoor A, et al. The 2015 CUA-CUOG Guidelines for the management of castration-resistant prostate cancer (CRPC). Can Urol Assoc J. 2015;9: 90–96. 10.5489/cuaj.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perletti G, Monti E, Marras E, Cleves A, Magri V, Trinchieri A, et al. Efficacy and safety of second-line agents for treatment of metastatic castration-resistant prostate cancer progressing after docetaxel. A systematic review and meta-analysis. Arch Ital Urol Androl. 2015;87: 121–129. 10.4081/aiua.2015.2.121 [DOI] [PubMed] [Google Scholar]
- 30.National Institute for Health and Care Excellence. NICE technology appraisal guidance TA412. Radium-223 dichloride for treating hormone-relapsed prostate cancer with bone metastases; 2016. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/ta412. Cited 27 February 2018. [Google Scholar]
- 31.National Institute for Health and Care Excellence. NICE technology appraisal guidance TA387 Abiraterone for treating metastatic hormone-relapsed prostate cancer before chemotherapy is indicated; 2016. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/ta387. Cited 27 February 2018. [Google Scholar]
- 32.National Institute for Health and Care Excellence. NICE technology appraisal guidance TA377 Enzalutamide for treating metastatic hormone-relapsed prostate cancer before chemotherapy is indicated; 2016. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/ta377. Cited 27 February 2018. [Google Scholar]
- 33.National Institute for Health and Care Excellence. NICE technology appraisal guidance TA101 Docetaxel for the treatment of hormone-refractory metastatic prostate cancer; 2006. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/ta101. Cited 27 November 2017. [Google Scholar]
- 34.National Institute for Health and Care Excellence. NICE technology appraisal guidance TA259 Abiraterone for castration-resistant metastatic prostate cancer previously treated with a docetaxel-containing regimen.; 2012. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/ta259. Cited 27 November 2017. [Google Scholar]
- 35.National Institute for Health and Care Excellence. NICE technology appraisal guidance TA316 Enzalutamide for metastatic hormone-relapsed prostate cancer previously treated with a docetaxel-containing regimen; 2014. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/ta316. Cited 05 January 2018. [Google Scholar]
- 36.National Institute for Health and Care Excellence. NICE technology appraisal guidance TA391 Cabazitaxel for hormone-relapsed metastatic prostate cancer treated with docetaxel.; 2016. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/ta391. Cited 20 October 2017. [Google Scholar]
- 37.Norum J, Nieder C. Treatments for metastatic prostate cancer (mPC): a review of costing evidence. Pharmacoeconomics. 2017;35: 1223–1236. 10.1007/s40273-017-0555-8 [DOI] [PubMed] [Google Scholar]
- 38.Sampson M, Shojania KG, Garritty C, Horsley T, Ocampo M, Moher D. Systematic reviews can be produced and published faster. J Clin Epidemiol. 2008;61: 531–536. 10.1016/j.jclinepi.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 39.Drummond M, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddard GL. Methods for the economic evaluation of health care programmes Oxford, New York: Oxford University Press; 2005. [Google Scholar]
- 40.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16: e1–5. 10.1016/j.jval.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 41.Organisation for Economic Co-operation and Development. Economic References; 2018. Available from: http://stats.oecd.org/Index.aspx?DataSetCode=HEALTH_ECOR. Cited 02 February 2018.
- 42.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10: 779–787. 10.1002/hec.635 [DOI] [PubMed] [Google Scholar]
- 43.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16: 231–250. 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 44.Stuhldreher N, Konnopka A, Wild B, Herzog W, Zipfel S, Löwe B, et al. Cost-of-illness studies and cost-effectiveness analyses in eating disorders: a systematic review. Int J Eat Disord. 2012;45: 476–491. 10.1002/eat.20977 [DOI] [PubMed] [Google Scholar]
- 45.Adarkwah CC, van Gils PF, Hiligsmann M, Evers SM. Risk of bias in model-based economic evaluations: the ECOBIAS checklist. Expert Rev Pharmacoecon Outcomes Res. 2016;16: 513–523. 10.1586/14737167.2015.1103185 [DOI] [PubMed] [Google Scholar]
- 46.Higgins JPT, Green S, Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions Chichester, Hoboken: Wiley-Blackwell; 2008. [Google Scholar]
- 47.Anassi E, Ndefo UA. Drug forecast. Sipuleucel-T (Provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P T. 2011;36: 197–202. [PMC free article] [PubMed] [Google Scholar]
- 48.Kearns B, Lloyd Jones M, Stevenson M, Littlewood C. Cabazitaxel for the second-line treatment of metastatic hormone-refractory prostate cancer: a NICE single technology appraisal. Pharmacoeconomics. 2013;31: 479–488. 10.1007/s40273-013-0050-9 [DOI] [PubMed] [Google Scholar]
- 49.Ramaekers BLT, Riemsma R, Tomini F, van Asselt T, Deshpande S, Duffy S, et al. Abiraterone acetate for the treatment of chemotherapy-naïve metastatic castration-resistant prostate cancer: an evidence review group perspective of an NICE single technology appraisal. Pharmacoeconomics. 2017;35: 191–202. 10.1007/s40273-016-0445-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson EL, Davis S, Thokala P, Breeze PR, Bryden P, Wong R. Sipuleucel-T for the treatment of metastatic hormone-relapsed prostate cancer: a NICE single technology appraisal; an evidence review group perspective. Pharmacoeconomics. 2015;33: 1187–1194. 10.1007/s40273-015-0296-5 [DOI] [PubMed] [Google Scholar]
- 51.Dellis A, Papatsoris AG. The economics of abiraterone acetate for castration-resistant prostate cancer. Expert Rev Pharmacoecon Outcomes Res. 2014;14: 175–179. 10.1586/14737167.2014.891444 [DOI] [PubMed] [Google Scholar]
- 52.van Hasselt JGC, Gupta A, Hussein Z, Beijnen JH, Schellens JHM, Huitema ADR. Integrated simulation framework for toxicity, dose intensity, disease progression, and cost effectiveness for castration-resistant prostate cancer treatment with eribulin. CPT Pharmacometrics Syst Pharmacol. 2015;4: 374–385. 10.1002/psp4.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoomans T, Fenwick EAL, Palmer S, Claxton K. Value of information and value of implementation: application of an analytic framework to inform resource allocation decisions in metastatic hormone-refractory prostate cancer. Value Health. 2009;12: 315–324. 10.1111/j.1524-4733.2008.00431.x [DOI] [PubMed] [Google Scholar]
- 54.Peters ML, de Meijer C, Wyndaele D, Noordzij W, Leliveld-Kors AM, van den Bosch J, et al. Dutch economic value of radium-223 in metastatic castration-resistant prostate cancer. Appl Health Econ Health Policy. 2018;16: 133–143. 10.1007/s40258-017-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satoh T, Ledesma D, Yoshihara N. The economic burden of metastatic castration resistant prostate cancer and skeletal related events in Japanese university hospitals. Asian Pac J Cancer Prev. 2018;19: 21–26. doi: 10.22034/APJCP.2018.19.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al. A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer. Health Technol Assess. 2007;11: 1–198. 10.3310/hta11020 [DOI] [PubMed] [Google Scholar]
- 57.Andronis L, Goranitis I, Pirrie S, Pope A, Barton D, Collins S, et al. Cost-effectiveness of zoledronic acid and strontium-89 as bone protecting treatments in addition to chemotherapy in patients with metastatic castrate-refractory prostate cancer: results from the TRAPEZE trial (ISRCTN 12808747). BJU Int. 2017;119: 522–529. 10.1111/bju.13549 [DOI] [PubMed] [Google Scholar]
- 58.Bloomfield DJ, Krahn MD, Neogi T, Panzarella T, Smith TJ, Warde P, et al. Economic evaluation of chemotherapy with mitoxantrone plus prednisone for symptomatic hormone-resistant prostate cancer: based on a Canadian randomized trial with palliative end points. J Clin Oncol. 1998;16: 2272–2279. 10.1200/JCO.1998.16.6.2272 [DOI] [PubMed] [Google Scholar]
- 59.James N, Pirrie S, Pope A, Barton D, Andronis L, Goranitis I, et al. TRAPEZE: a randomised controlled trial of the clinical effectiveness and cost-effectiveness of chemotherapy with zoledronic acid, strontium-89, or both, in men with bony metastatic castration-refractory prostate cancer. Health Technol Assess. 2016;20: 1–127. 10.3310/hta20530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reed SD, Radeva JI, Glendenning GA, Saad F, Schulman KA. Cost-effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer. J Urol. 2004;171: 1537–1542. 10.1097/01.ju.0000116777.94426.60 [DOI] [PubMed] [Google Scholar]
- 61.Carter JA, Joshi A, Kaura S, Botteman MF. Cost effectiveness of zoledronic acid in the management of skeletal metastases in hormone-refractory prostate cancer patients in France, Germany, Portugal, and the Netherlands. J Med Econ. 2011;14: 288–298. 10.3111/13696998.2011.570170 [DOI] [PubMed] [Google Scholar]
- 62.Gong CL, Hay JW. Cost-effectiveness analysis of abiraterone and sipuleucel-T in asymptomatic metastatic castration-resistant prostate cancer. J Natl Compr Canc Netw. 2014;12: 1417–1425. 10.6004/jnccn.2014.0139 [DOI] [PubMed] [Google Scholar]
- 63.Holko P, Kawalec P. Economic evaluation of sipuleucel-T immunotherapy in castration-resistant prostate cancer. Expert Rev Anticancer Ther. 2014;14: 63–73. 10.1586/14737140.2014.856270 [DOI] [PubMed] [Google Scholar]
- 64.Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60: 1373–1378. 10.1016/j.ijrobp.2004.05.053 [DOI] [PubMed] [Google Scholar]
- 65.Massoudi M, Balk M, Yang H, Bui CN, Pandya BJ, Guo J, et al. Number needed to treat and associated incremental costs of treatment with enzalutamide versus abiraterone acetate plus prednisone in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer. J Med Econ. 2017;20: 121–128. 10.1080/13696998.2016.1229670 [DOI] [PubMed] [Google Scholar]
- 66.Pilon D, Queener M, Lefebvre P, Ellis LA. Cost per median overall survival month associated with abiraterone acetate and enzalutamide for treatment of patients with metastatic castration-resistant prostate cancer. J Med Econ. 2016;19: 777–784. 10.3111/13696998.2016.1173042 [DOI] [PubMed] [Google Scholar]
- 67.Pollard ME, Moskowitz AJ, Diefenbach MA, Hall SJ. Cost-effectiveness analysis of treatments for metastatic castration resistant prostate cancer. Asian J Urol. 2017;4: 37–43. 10.1016/j.ajur.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snedecor SJ, Carter JA, Kaura S, Botteman MF. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J Med Econ. 2013;16: 19–29. 10.3111/13696998.2012.719054 [DOI] [PubMed] [Google Scholar]
- 69.Stopeck A, Rader M, Henry D, Danese M, Halperin M, Cong Z, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ. 2012;15: 712–723. 10.3111/13696998.2012.675380 [DOI] [PubMed] [Google Scholar]
- 70.Wilson L, Tang J, Zhong L, Balani G, Gipson G, Xiang P, et al. New therapeutic options in metastatic castration-resistant prostate cancer: can cost-effectiveness analysis help in treatment decisions? J Oncol Pharm Pract. 2014;20: 417–425. 10.1177/1078155213509505 [DOI] [PubMed] [Google Scholar]
- 71.Xie J, Namjoshi M, Wu EQ, Parikh K, Diener M, Yu AP, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm. 2011;17: 621–643. doi: 10.18553/jmcp.2011.17.8.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong L, Pon V, Srinivas S, Nguyen N, Frear M, Kwon S, et al. Therapeutic options in docetaxel-refractory metastatic castration-resistant prostate cancer: a cost-effectiveness analysis. PLoS One. 2013;8: e64275 10.1371/journal.pone.0064275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zubek V, Konski A. Cost effectiveness of risk-prediction tools in selecting patients for immediate post-prostatectomy treatment. Mol Diagn Ther. 2009;13: 31–47. 10.2165/01250444-200913010-00006 [DOI] [PubMed] [Google Scholar]
- 74.Alemayehu B, Buysman E, Parry D, Becker L, Nathan F. Economic burden and healthcare utilization associated with castration-resistant prostate cancer in a commercial and Medicare Advantage US patient population. J Med Econ. 2010;13: 351–361. 10.3111/13696998.2010.491435 [DOI] [PubMed] [Google Scholar]
- 75.Armstrong A, Bui C, Fitch K, Sawhney TG, Brown B, Flanders S, et al. Docetaxel chemotherapy in metastatic castration-resistant prostate cancer: cost of care in Medicare and commercial populations. Curr Med Res Opin. 2017;33: 1133–1139. 10.1080/03007995.2017.1308919 [DOI] [PubMed] [Google Scholar]
- 76.Bourke S, Burns RM, Gaynor C. Challenges in generating costs and utilisation rates associated with castration-resistant prostate cancer. J Mark Access Health Policy. 2014;2 10.3402/jmahp.v2.24072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bryant-Lukosius DE. Defining the advanced practice nursing role in advanced prostate cancer: application of a systematic patient-focused approach Hamilton: McMaster University; 2003. [Google Scholar]
- 78.Bui CN, Wang L, Baser O. Resource utilization and use of life-extending therapies and corticosteroids in prostate cancer patients with corticosteroid-sensitive comorbidities. Curr Med Res Opin. 2014;30: 2355–2364. 10.1185/03007995.2014.955170 [DOI] [PubMed] [Google Scholar]
- 79.Dragomir A, Dinea D, Vanhuyse M, Cury FL, Aprikian AG. Drug costs in the management of metastatic castration-resistant prostate cancer in Canada. BMC Health Serv Res. 2014;14: 252–252. 10.1186/1472-6963-14-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Engel-Nitz NM, Alemayehu B, Parry D, Nathan F. Differences in treatment patterns among patients with castration-resistant prostate cancer treated by oncologists versus urologists in a US managed care population. Cancer Manag Res. 2011;3: 233–245. 10.2147/CMR.S21033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krahn MD, Bremner KE, Zagorski B, Alibhai SMH, Chen W, Tomlinson G, et al. Health care costs for state transition models in prostate cancer. Med Decis Making. 2014;34: 366–378. 10.1177/0272989X13493970 [DOI] [PubMed] [Google Scholar]
- 82.Kunisawa S, Tange C, Shimozuma K. Realities in cost-effectiveness analyses: a study of castration-resistant prostate cancer patients using a medical claims database. Springerplus. 2015;4: 624 10.1186/s40064-015-1413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malmberg I, Persson U, Ask A, Tennvall J, Abrahamsson PA. Painful bone metastases in hormone-refractory prostate cancer: economic costs of strontium-89 and/or external radiotherapy. Urology. 1997;50: 747–753. 10.1016/S0090-4295(97)00326-9 [DOI] [PubMed] [Google Scholar]
- 84.Mehra M, Wu Y, Dhawan R. Healthcare resource use in advanced prostate cancer patients treated with docetaxel. J Med Econ. 2012;15: 836–843. 10.3111/13696998.2012.681718 [DOI] [PubMed] [Google Scholar]
- 85.Organ M, Wood L, Wilke D, Skedgel C, Cheng T, North S, et al. Intermittent LHRH therapy in the management of castrate-resistant prostate cancer (CRPCa): results of a multi-institutional randomized prospective clinical trial. Am J Clin Oncol. 2013;36: 601–605. 10.1097/COC.0b013e31825d5664 [DOI] [PubMed] [Google Scholar]
- 86.Sanyal C, Aprikian AG, Cury FL, Chevalier S, Dragomir A. Management of localized and advanced prostate cancer in Canada: a lifetime cost and quality-adjusted life-year analysis. Cancer. 2016;122: 1085–1096. 10.1002/cncr.29892 [DOI] [PubMed] [Google Scholar]
- 87.Sherman EJ, Pfister DG, Ruchlin HS, Rubin DM, Radzyner MH, Kelleher GH, et al. The Collection of Indirect and Nonmedical Direct Costs (COIN) form: a new tool for collecting the invisible costs of androgen independent prostate carcinoma. Cancer. 2001;91: 841–853. 10.1002/1097-0142(20010215)91:4<841::AID-CNCR1072>3.0.CO;2-B [PubMed] [Google Scholar]
- 88.Bui CN, O'Day K, Flanders S, Oestreicher N, Francis P, Posta L, et al. Budget impact of enzalutamide for chemotherapy-naïve metastatic castration-resistant prostate cancer. J Manag Care Spec Pharm. 2016;22: 163–170. doi: 10.18553/jmcp.2016.22.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Markowski MC, Frick KD, Eshleman JR, Luo J, Antonarakis ES. Cost-savings analysis of AR-V7 testing in patients with metastatic castration-resistant prostate cancer eligible for treatment with abiraterone or enzalutamide. Prostate. 2016;76: 1484–1490. 10.1002/pros.23232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sorensen S, Ellis L, Wu Y, Hutchins V, Linnehan JE, Senbetta M. Budgetary impact on a U.S. health plan adopting abiraterone acetate plus prednisone for the treatment of patients with metastatic castration-resistant prostate cancer. J Manag Care Pharm. 2013;19: 799–808. doi: 10.18553/jmcp.2013.19.9.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flannery K, Drea E, Hudspeth L, Corman S, Gao X, Xue M, et al. Budgetary impact of cabazitaxel use after docetaxel treatment for metastatic castration-resistant prostate cancer. J Manag Care Spec Pharm. 2017;23: 416–426. doi: 10.18553/jmcp.2017.23.4.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94: 1458–1468. 10.1093/jnci/94.19.1458 [DOI] [PubMed] [Google Scholar]
- 94.Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52: 111–121. 10.1016/S0167-8140(99)00097-3 [PubMed] [Google Scholar]
- 95.Cole DJ. A randomized trial of a single treatment versus conventional fractionation in the palliative radiotherapy of painful bone metastases. Clin Oncol (R Coll Radiol). 1989;1: 59–62. 10.1016/S0936-6555(89)80035-4 [DOI] [PubMed] [Google Scholar]
- 96.Gaze MN, Kelly CG, Kerr GR, Cull A, Cowie VJ, Gregor A, et al. Pain relief and quality of life following radiotherapy for bone metastases: a randomised trial of two fractionation schedules. Radiother Oncol. 1997;45: 109–116. 10.1016/S0167-8140(97)00101-1 [DOI] [PubMed] [Google Scholar]
- 97.Nielsen OS, Bentzen SM, Sandberg E, Gadeberg CC, Timothy AR. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol. 1998;47: 233–240. 10.1016/S0167-8140(98)00011-5 [DOI] [PubMed] [Google Scholar]
- 98.Niewald M, Tkocz HJ, Abel U, Scheib T, Walter K, Nieder C, et al. Rapid course radiation therapy vs. more standard treatment: a randomized trial for bone metastases. Int J Radiat Oncol Biol Phys. 1996;36: 1085–1089. 10.1016/S0360-3016(96)00388-4 [DOI] [PubMed] [Google Scholar]
- 99.Price P, Hoskin PJ, Easton D, Austin D, Palmer SG, Yarnold JR. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol. 1986;6: 247–255. 10.1016/S0167-8140(86)80191-8 [DOI] [PubMed] [Google Scholar]
- 100.Steenland E, Leer JW, van Houwelingen H, Post WJ, van den Hout WB, Kievit J, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch bone metastasis study. Radiother Oncol. 1999;52: 101–109. 10.1016/S0167-8140(99)00110-3 [DOI] [PubMed] [Google Scholar]
- 101.Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66: 815–825. 10.1016/j.eururo.2014.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377: 813–822. 10.1016/S0140-6736(10)62344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16: 152–160. 10.1016/S1470-2045(14)71205-7 [DOI] [PubMed] [Google Scholar]
- 104.Sheikh NA, Petrylak D, Kantoff PW, dela Rosa C, Stewart FP, Kuan L-Y, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62: 137–147. 10.1007/s00262-012-1317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stopeck AT, Lipton A, Body J-J, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28: 5132–5139. 10.1200/JCO.2010.29.7101 [DOI] [PubMed] [Google Scholar]
- 106.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic Acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29: 1125–1132. 10.1200/JCO.2010.31.3304 [DOI] [PubMed] [Google Scholar]
- 107.Cordon-Cardo C, Kotsianti A, Verbel DA, Teverovskiy M, Capodieci P, Hamann S, et al. Improved prediction of prostate cancer recurrence through systems pathology. J Clin Invest. 2007;117: 1876–1883. 10.1172/JCI31399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Djavan B, Nelson K, Kazzazi A, Bruhn A, Sadri H, Gomez-Pinillos A, et al. Immunotherapy in the treatment of advanced prostate cancer. Can J Urol. 2011;18: 5865–5874. [PubMed] [Google Scholar]
- 109.Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16: 579–584. 10.1093/annonc/mdi122 [DOI] [PubMed] [Google Scholar]
- 110.Manikandan R, Srirangam SJ, Pearson E, Brown SCW, O'Reilly P, Collins GN. Diethylstilboestrol versus bicalutamide in hormone refractory prostate carcinoma: a prospective randomized trial. Urol Int. 2005;75: 217–221. 10.1159/000087797 [DOI] [PubMed] [Google Scholar]
- 111.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22: 1025–1033. 10.1200/JCO.2004.06.037 [DOI] [PubMed] [Google Scholar]
- 112.Porter AT, McEwan AJB, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25: 805–813. 10.1016/0360-3016(93)90309-J [DOI] [PubMed] [Google Scholar]
- 113.Sanyal C, Aprikian A, Cury F, Chevalier S, Dragomir A. Clinical management and burden of prostate cancer: a Markov Monte Carlo model. PLoS One. 2014;9: e113432 10.1371/journal.pone.0113432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20: 327–337. 10.3350/cmh.2014.20.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26: 733–744. 10.2165/00019053-200826090-00004 [DOI] [PubMed] [Google Scholar]
- 116.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013; 2013. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/pmg9. Cited 27 February 2018. [PubMed] [Google Scholar]
- 117.National Institute for Health and Care Excellence. Consultation Paper. Value Based Assessment of Health Technologies; 2014. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/VBA-TA-Methods-Guide-for-Consultation.pdf. Cited 27 Februrary 2018. [Google Scholar]
- 118.Paulden M, O’Mahony JF, Culyer AJ, McCabe C. Some inconsistencies in NICE’s consideration of social values. Pharmacoeconomics. 2014;32: 1043–1053. 10.1007/s40273-014-0204-4 [DOI] [PubMed] [Google Scholar]
- 119.Braithwaite RS, Meltzer DO, King JT Jr., Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46: 349–356. 10.1097/MLR.0b013e31815c31a7 [DOI] [PubMed] [Google Scholar]
- 120.Weinstein MC. How much are Americans willing to pay for a quality-adjusted life year? Med Care. 2008;46: 343–345. 10.1097/MLR.0b013e31816a7144 [DOI] [PubMed] [Google Scholar]
- 121.Shiroiwa T, Sung Y-K, Fukuda T, Lang H-C, Bae S-C, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19: 422–437. 10.1002/hec.1481 [DOI] [PubMed] [Google Scholar]
- 122.Bae YHJ, Mullins CD. Do value thresholds for oncology drugs differ from nononcology drugs? J Manag Care Spec Pharm. 2014;20: 1086–1092. doi: 10.18553/jmcp.2014.20.11.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17: 3520–3526. 10.1158/1078-0432.CCR-10-3126 [DOI] [PubMed] [Google Scholar]
- 124.Center for Drug Evaluation and Research. Application number: 203415Orig1s000 Office director memo; 2013. Silver Spring, MD: U.S. Food and Drug Administration; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203415orig1s000odmemo.pdf. Cited 16 May 2018. [Google Scholar]
- 125.Center for Drug Evaluation and Research. Approval package for: application number: 203551Orig1s000; 2010. Silver Spring, MD: U.S. Food and Drug Administration; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203551orig1s000approv.pdf. Cited 16 May 2018. [Google Scholar]
- 126.Center for Drug Evaluation and Research. Application number: 201023 Summary review; 2010. Silver Spring, MD: U.S. Food and Drug Administration; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/201023s000sumr.pdf. Cited 16 May 2018. [Google Scholar]
- 127.Center for Drug Evaluation and Research. Application number: 202379Orig1s000 Office director memo; 2013. Silver Spring, MD: U.S. Food and Drug Administration; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202379orig1s000dmemo.pdf. Cited 16 May 2018. [Google Scholar]
- 128.Center for Drug Evaluation and Research. Application number: 203971Orig1s000 Summary review; 2013. Silver Spring, MD: U.S. Food and Drug Administration; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203971Orig1s000SumR.pdf. Cited 16 May 2018. [Google Scholar]
- 129.National Institute for Health and Care Excellence. NICE technology appraisal guidance TA265 Denosumab for the prevention of skeletal-related events in adults with bone metastases from solid tumours; 2012. London: National Institute for Health and Care Excellence; Available from: https://www.nice.org.uk/guidance/ta265. Cited 27 February 2018. [Google Scholar]
- 130.Center for Drug Evaluation and Research. Approval package for: application number: BLA 125320/S-007; 2010. Silver Spring, MD: U.S. Food and Drug Administration; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/bla/2010/125320orig1s007.pdf. Cited 16 May 2018. [Google Scholar]
- 131.Karnon J, Brown J. Selecting a decision model for economic evaluation: a case study and review. Health Care Manag Sci. 1998;1: 133–140. 10.1023/A:1019090401655 [DOI] [PubMed] [Google Scholar]
- 132.Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011;342 10.1136/bmj.d1766 [DOI] [PubMed] [Google Scholar]
- 133.Markowski MC, Carducci MA. Early use of chemotherapy in metastatic prostate cancer. Cancer Treat Rev. 2017;55: 218–224. 10.1016/j.ctrv.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tonyali S, Haberal HB, Sogutdelen E. Toxicity, adverse events, and quality of life associated with the treatment of metastatic castration-resistant prostate cancer. Curr Urol. 2017;10: 169–173. 10.1159/000447176 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: abiraterone, AR-V7: Androgen receptor variant-7, CX: cabazitaxel, DX: docetaxel, EZ: enzalutamide, mCRPC: metastatic castration-resistant prostate cancer, P: prednisone/prednisolone, PAY: payer’s perspective UK: United Kingdom, US: United States. * Based on United Nations estimates of the UK population in 2015 (65,397,080) [133], ** based on United Nations estimates of the US population in 2015 (319,929,162) [133], *** the submission year/study year was assumed as base year.
(PDF)
✓: Criterion fulfilled; CHEERS: consolidated health economic evaluation reporting standards, n.a.: not applicable.
(PDF)
✓: Criterion fulfilled, CHEERS: consolidated health economic evaluation reporting standards, n.a.: not applicable.
(PDF)
✓: Criterion fulfilled, n.a.: not applicable.
(PDF)
✓: Criterion fulfilled, (✓): criterion partially fulfilled, ECOBIAS: Bias in Economic Evaluation, n.a.: not applicable.
(PDF)
✓: Criterion fulfilled, (✓): criterion partially fulfilled, ECOBIAS: Bias in Economic Evaluation, n.a.: not applicable. * These biases are overlapping regarding their content, ** these biases are overlapping regarding their content.
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.