SUMMARY
The intersection of tuberculosis (TB) with non-communicable disease, including diabetes mellitus, chronic pulmonary disease, and cardiovascular disease has emerged as a critical clinical and public health challenge. Rapidly expanding non-communicable disease epidemics threaten TB control in low- and middle-income countries, where the prevention and treatment of TB disease remains a great burden. However, to date, the notion that TB may adversely impact non-communicable disease risk and severity has not been well explored. This review summarizes biomedical hypotheses, findings from animal models, and emerging epidemiologic data related to the progression of diabetes mellitus, chronic lung disease and cardiovascular disease during and after active TB disease. We conclude that there is sufficient empirical evidence to justify a greater research emphasis on the syndemic interaction between TB and non-communicable disease.
Keywords: diabetes, cardiovascular disease, chronic pulmonary disease
INTRODUCTION
The intersection of tuberculosis (TB) and non-communicable diseases (NCDs) threatens the capacity for low- and middle-income countries (LMIC) to achieve global public health targets.1,2 Of the 10.4 million global incident TB cases and 1.7 million TB deaths in 2016, more than 85% occurred in LMIC.3 None of the LMIC are projected to meet the TB-related 2030 Sustainable Development Goals of reducing TB deaths by 90% and TB incidence by 80%, as compared to 2015 rates.2 Population-level data suggest that progress toward meeting TB control targets, including scale-up of interventions to reduce TB burdens, will be complicated by the synergistic relationship between TB and NCDs.4,5 Common NCDs and NCD risk factors—diabetes, chronic pulmonary disease, smoking and alcohol abuse—are also strong individual-level risk factors for TB disease and TB-related mortality,6–8 impact TB epidemics at the population level,4,9–11 and are increasing at an alarming rate in LMIC.4,12 Addressing non-communicable and infectious disease comorbidity has been put forth as a global research priority,13 yet the preponderance of research related to the intersection of TB and NCDs has focused on one dimension of the relationship—the impact of NCDs on TB risk and response to TB treatment.14
Active TB disease can have a prolonged clinical course, spanning years from initial infection until diagnosis and successful treatment completion. Through the course of this chronic infection, patients with TB often experience systemic inflammation, pulmonary impairment, social isolation, and reduced quality of life (Figure 1).15–18 Many of the direct and indirect effects of the biologic and socio-behavioral consequences of chronic illness from TB are recognized as risk factors for NCDs, and yet, to date, empirical evidence related to post-TB risk of NCDs is critically limited.19
Figure 1. Conceptual framework for increased risk of non-communicable disease after active tuberculosis.
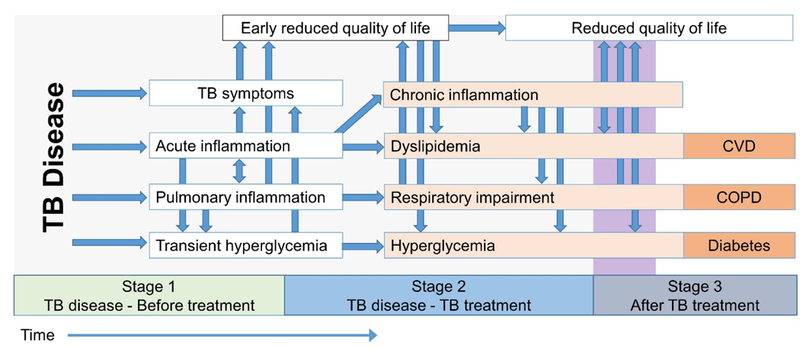
Before treatment (Stage 1), TB disease results in symptoms, acute inflammation, and may result in transient hyperglycemia. During later phases of TB disease and during TB treatment (Stage 2), symptoms experienced during early stages may result in dyslipidemia, respiratory impairment, and hyperglycemia. In some patients, the Stage 2 symptoms may result in increased risk of chronic non-communicable disease after TB treatment (Stage 3). All arrows represent non-deterministic pathways; the likelihood of variables at the heads of arrows are hypothesized to be probabilistically increased by variables at the arrow’s tails.
In contrast to much of the published literature addressing NCDs and subsequent TB comorbidity, we aim to summarize the current understanding of the relationship between TB and post-TB NCDs and pose hypotheses as to how TB may actually increase the risk and severity of NCDs. We focus on the potential impact of active TB on the pathogenesis of three of the most common NCDs: type 2 diabetes mellitus (referred to henceforth as diabetes), chronic pulmonary disease, and cardiovascular disease. We also examine the potential relationship between latent TB infection and risk of diabetes. By summarizing existing biomedical theories, putative mechanisms, and experimental and epidemiologic data, we hope to highlight current gaps in knowledge and stimulate novel lines of inquiry about the trajectory of patient health after TB disease and TB treatment. The literature for this hypothesis posing review came from existing meta-analyses, references searches of seminal articles, and targeted key-word searches in PubMed, and was not designed as a systematic review.
HYPERGLYCEMIA AND DIABETES
Evidence from animal models and human clinical studies indicates that blood glucose levels are increased, at least temporarily, in response to TB disease.20–22 Using the guinea pig model of aerosol TB disease, Podell et al. reported that post-infection blood glucose levels were significantly higher compared to pre-infection levels, indicating that TB disease is sufficient to induce hyperglycemia.20,23 Observational studies conducted in geographically diverse settings have reported a high prevalence of pre-diabetic range hyperglycemia (i.e., HbA1c 5.7–6.5% or blood glucose level 140–200 mg/dl two hours after a 75 gm oral carbohydrate challenge) among patients at the time of TB treatment initiation.24–29 While TB disease may induce hyperglycemia, TB treatment may subsequently serve to lower blood glucose levels. A few studies report a signifcant decline in HbA1c after the intensive phase of TB treatment, even among patients who were euglycemic at baseline (Figure 2). A 2017 study of patients with smear positive TB from Pakistan reported a median decrease in fasting blood glucose after 6 months of TB treatment among patients with newly screened diabetes (median decrease 38 mg/dl) and known diabetes (median decrease 31 mg/dl).25 In the Effects of Diabetes on Tuberculosis Severity (EDOTS) cohort, one third of particpants with diabetic range hyperglycemia at baseline were unaware of having diabetes prior to incident TB.21 This “new-DM” group had lower median HbA1c at baseline than those with diabetes diagnosed prior to incident TB and many reverted to pre-diabetic range hyperglycemia after the intensive phase of TB treatment. While anti-diabetic treatment might be a contributing factor to reversion in some cases, these results are consistent with infection-induced stress dysglycemia. Yet, critical gaps in knowledge remain, including the risk for sustained conversion to pre-diabetes or diabetes in patients without those disorders prior to incident TB disease, and the subsequent lifetime risk for incident diabetes in patients with transient hyperglycemia that normalizes after TB treatment (Figure 3). Notably, a study using primary care data from the UK reported an association between previous diagnosis of TB and risk of diabetes during a four-year period.30,31
Figure 2.
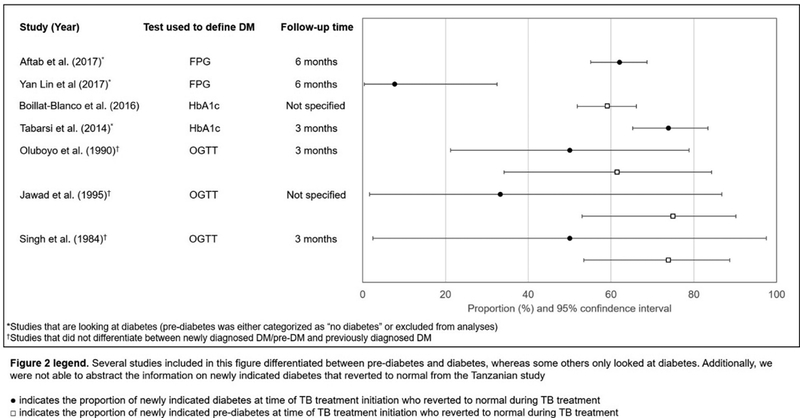
Patients with TB and newly indicated pre-diabetes or diabetes at time of TB treatment initiation who reverted to normal blood glucose levels during TB treatment
Figure 3. Hypothetical trajectories of blood glucose levels over the natural history of TB.
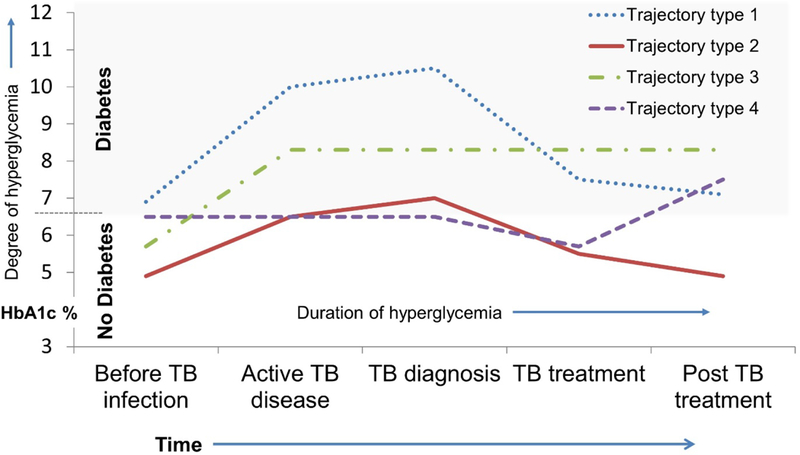
Among patients that experience TB-induced transient hyperglycemia, it is unknown what factors facilitate reversion to pre-TB blood glucose levels after TB treatment completion (Trajectory type 2) and what factors increase the likelihood of remaining elevated (Trajectory type 3) or later developing incident diabetes (Trajectory type 4).
Potential mechanisms of TB-induced hyperglycemia
One plausible mechanism by which active TB may induce hyperglycemia or diabetes is via an acute stress response resulting from an interaction between pro-inflammatory cytokines and regulatory hormones, which may be sufficient to disrupt insulin signaling and increase insulin resistance.25,32–34 Active TB results in an increased production of reactive oxidative species and increased expression of pro-inflammatory cytokines which may lead to excess production of glucose in the liver, which in turn can result in stress hyperglycemia.25 Consequently, ongoing inflammation and stress responses35 during the acute phase of TB likely results in increased blood glucose levels and hyperglycemia. If a patient was hyperglycemic prior to developing active TB, the TB-related stress response may lead to exacerbated hyperglycemia or overt diabetes.32 The phenomena of acute infections leading to stress hyperglycemia has been described; for example, Helicobacter pylori infection and subsequent induction of insulin resistance in patients is well-established.36 But the extent to which similar mechanisms of stress hyperglycemia may result from Mycobacterium tuberculosis has not been carefully evaluated.
Second, M. tuberculosis (Mtb) bacilli may disseminate to adipose tissue where activated immune cells can promote pro-inflammatory responses, impact insulin sensitivity, and contribute to diabetes risk. Using a mouse TB model, Beigier-Bompadre et al reported that Mtb persists in murine adipose tissue and that Mtb antigen-specific CD8 cells produced IFN-α in the infected tissue.37 Exploratory evidence suggests TB may also persist in human adipose tissue.38 Similarly, TB is associated with elevated heme oxygenase-1, a host antioxidant expressed in lung tissue that is correlated with inflammation, increased in the presence of diabetes, and critical for the development of metaflammation.39,40 If active TB induces persistent proinflammatory responses in adipose tissue and contributes to metaflammation, insulin sensitivity will decrease which would, in turn, increase the risk of diabetes progression.
Epigenetic reprogramming is a third mechanism by which active TB may increase the risk of hyperglycemia or diabetes. Epigenetic changes refer to mechanisms that alter gene expression without changing the underlying DNA sequence (e.g., DNA methylation and histone modification).41–43 Chronic hyperglycemia has been linked to increased acetylation of histone lysine residues, which may result in persistently increased proatherogenic gene expression even during subsequent periods of normoglycemia.44,45 A number of clinical trials among patients with diabetes have found that patients who achieve tight glycemic control after a period of sustained hyperglycemia may nonetheless experience progression to diabetic complications. This phenomenon, called metabolic memory, is thought to result from epigenetic reprogramming caused by glucotoxicity.46–49 In patients with active TB, it is plausible that an early hyperglycemic environment could be epigenetically imprinted on vascular and immune cells and alter the gene expression during the subsequent normoglycemic periods.44,45 This hypothesized epigenetic change due to TB-induced transient hyperglycemia is similar to the concept of metabolic memory in gestational diabetes, where a hyperglycemic environment during pregnancy increases the risk of diabetes incidence among mother and the offspring later in life,50,51 even after returning to euglycemia. Collectively, findings from several studies support the hypothesis that active TB can induce acute stress-related hyperglycemia, which may lead to an epigenetically determined metabolic memory that increases the risk for progression of pre-diabetes and diabetes, and that exacerbates diabetic complications even after blood glucose levels have returned to normal.
Although the association between TB and diabetes is speculated to be bi-directional, progress in understanding how active TB increases the risk of subsequent hyperglycemia and diabetes is limited by the complexity of diabetes pathogenesis. There are population-based differences in the pathogenesis of diabetes which further complicate the relationship between TB and diabetes. For example, environmental and genetic risk factors for diabetes in the South Asian phenotype is different than other regions of the world.52 Moreover, while clinical diagnostic criteria for diabetes are well established,53,54 the progression from normal glycemic to diabetes includes stages of pre-diabetic metabolic dysregulation,55–57 which are difficult to measure and observe epidemiologically. Given the complexity and long duration of incident diabetes pathophysiology, determining whether active TB and transient increases in metabolic risk factors increase post-TB diabetes risk will require long-term cohort studies of patients after TB treatment completion. Prospective cohorts of patients who have completed TB treatment and that include frequent measurement of metabolic parameters are needed to determine if incidence rates of diabetes and other metabolic disorders are different compared to those without a history of TB disease. In addition, because incident TB might unmask indiviudals who were predisposed to diabetes, post-TB diabetes incidence rates may be higher, not due to TB but rather pre-existing risk factors. In studies that aim to determine the effect of TB on post-TB diabetes risk, indentifying appropriate control groups, and ruling out reverse causality will pose a substantial analytic challenge. Ideally prospective cohorts are need that include regular follow up and rigorous measures of metabolic function, but given the resources needed for prospective designs existing data sources may be leveraged to answer these questions. Large health insurance databases, national primary care data, or other data that can link history of TB disease with valid measures of diabetes incidence will also provide informative observational data on the TB-NCD association.
Latent TB infection and hyperglycemia and diabetes risk
A basic, yet essentially unexplored area of epidemiologic research is whether latent TB infection may increase the risk of diabetes. While substantial observational data indicate that diabetes increases the risk of active TB, current understanding of the biologic pathways between diabetes and risk of TB is greatly limited by the inability to characterize the direction of the relationship between latent TB infection and diabetes. For example, the extent to which the increased risk of active TB in patients with diabetes is due to 1) increased risk of TB infection, 2) increased risk of reactivation from latent TB infection to active TB disease, or 3) primary progression from exposure to active TB disease have not been characterized. Meanwhile, if latent TB infection increases the risk of diabetes, each of the three pathways described previously could be impacted.
The current conventional thinking is that diabetes increases the risk of active TB, but there is increasing evidence that there is also an association between latent TB infection and prevalence of diabetes. A meta-analysis of 12 cross-sectional studies reported the pooled odds of latent TB infection among patients with diabetes was estimated to be 1.2 to 2.0 times the odds of latent TB in those without diabetes even after adjusting for multiple potential confounders such as age, sex, and smoking status.58 Two cross-sectional studies using data from the nationally representative 2011–2012 National Health and Nutrition Examination Survey (NHANES) reported the prevalence of latent TB infection among those with diabetes to be more than twice the prevalence among those without diabetes.59,60 A multisite cross-sectional analysis of data from the UK also reported an adjusted prevalence ratio for the association between latent TB and diabetes to be 1.15 (95% CI 1.02–1.30).61 Similar to the bidirectional interaction between active TB disease and diabetes, the association of diabetes with latent TB infection could reflect diabetes-induced susceptibility to become infected with Mtb as well as an adverse effect of latent TB infection on glucose metabolism in the host. Therefore, many of the challenges related to determining causality between active TB and diabetes will apply to studies that focus on the relationship between latent TB and risk of diabetes.
Potential mechanisms of latent TB-induced hyperglycemia and diabetes
A paradigm shift in the characterization of TB infection now conceptualizes latent TB infection to have a spectrum of host-pathogen interactions spanning sterilizing immunity, dormant infection, and subclinical but biologically active site of infection. Thus, a subpopulation of individuals with clinically latent TB infection mount an immune response that maintains a balance between Mtb replication and elimination. Animal models and advanced 18F-fluorodeoxyglucose imaging techniques in humans demonstrate that immunological activity within granulomas is heterogeneous.62,63 This heterogeneity reflects a spectrum of metabolic and responses, ranging from dormant hypoxia, to pro-inflammatory cell proliferation and angiogenesis.64–66
Latent TB infection can impact organs beyond the lungs, in sites such as the spleen, kidney and liver.67 DNA from Mtb has also been detected in human fat tissue surrounding the kidneys, stomach, lymph nodes, heart, and skin.67 Another study found that Mtb bacilli can be directly isolated from visceral, subcutaneous, peri-renal, and mesenteric adipose tissue of immunocompetent mice.68 Moreover, Neyrolles et al. demonstrated that Mtb can enter primary human adipocytes and survive in a non-replicating state.38 Given that metabolically active Mtb appears to persist in fat, lung and liver tissue of individuals with latent TB infection, it is plausible that the immune activity required to maintain the subclinical state could exert a diabetogenic effect.
At the same time, inflammation in adipose tissue, principally due to loss of immunologic adipocyte homeostasis, is instrumental in the development of diabetes. In adipose tissue, insulin sensitivity and immune cell homeostasis are maintained by a balance between B-cells, monocytes and T cells.69 Obesity induces increased free-fatty acids which stimulate monocytes and subsequent increases in both pro-inflammatory cytokines and adipokine secretion. Adipocyte hypertrophy increases cell stress and apoptosis, which promote monocyte migration and macrophage differentiation. The resulting adipose tissue inflammation is thought to initiate the progression from non-diabetic obesity to diabetes. While metaflammation is generally thought to result from lipotoxicity, it is plausible that the inflammatory response that develops in adipose tissue during TB would contribute to the loss of adipocyte homeostasis and metabolic perturbation. An inflammatory impact of granulomas on immunopathology has been observed in multiple other infectious and non-infectious disease states.70–73
CHRONIC PULMONARY DISEASE
By 2030, it is projected that chronic obstructive pulmonary disease (COPD)-related deaths will eclipse lower respiratory tract infections to become the third commonest cause of death globally.74 While this upsurge is due in part to rising levels of air pollution and tobacco use, TB is increasingly recognized as a substantial contributor to the global burden of chronic lung disease.75,76 Among those who survive their initial TB episode and achieve microbiologic cure, it is estimated that 50–70% will develop chronic lung disease with either obstructive, restrictive, or mixed obstructive and restrictive limitations.77–82 With an estimated 9 million TB survivors annually,83 up to 6 million individuals might develop post-TB pulmonary impairment every year.
In a nationally-representative survey from South Africa, a history of pulmonary TB was associated with a fivefold increase in the odds of post-TB chronic bronchitis, an association that was nearly three-times greater than the association between smoking and chronic bronchitis.84 Similarly, South African gold miners with a history of pulmonary TB had an accelerated loss of lung function, even after accounting for baseline characteristics and mining history. Furthermore, the extent of airflow limitation increased with successive TB episodes.80,85 In another study conducted in Indonesia, nearly 60% of patients treated for pulmonary TB had persistent respiratory symptoms at 6 months after treatment completion, one third of whom had moderately-severe impairment in pulmonary function.81 On an individual patient level, the extent of disease on chest radiography at the time of initial diagnosis has been shown to be inversely associated with long-term pulmonary impairment.82 However, in a population-based study from Korea, even minimal scarring on chest radiography was associated with chronic airflow limitation.86
Although post-TB obstructive lung disease was described in the mid-20th century, our understanding of the mechanisms responsible for chronic lung injury following pulmonary TB remains limited.87–90 The inflammatory response in active TB disease promotes lung matrix destruction by hydrolytic enzymes including matrix metalloproteinases.18,91,92 As such, lung damage in the wake of active pulmonary TB disease can include any number of changes to the lung architecture—from residual cavitation and bronchiectasis to fibrosis or scarring with resultant volume loss.93–95 Furthermore, lung remodeling likely occurs following resolution of the active infection, although the nature and time course of such remodeling is not known.
There are many aspects of post-TB lung disease that warrant further investigation. First and foremost, at this time there is little understanding of the risk factors for the development of post-TB pulmonary impairment. Given that smoking is overwhelmingly the most important risk factor for COPD, it raises the question of whether smoking also raises the risk of post-TB pulmonary impairment. As compared to non-smokers, cigarette smokers are more likely to be infected with TB, more likely to progress to active disease, and more likely to die of their TB.7,76 It is less clear if smoking is an independent risk factor for post-TB lung injury, with several studies finding no relationship between smoking and pulmonary impairment after TB.79,81 It is also unclear whether other factors, including bacillary burden, delayed culture conversion, drug-resistant TB or coinfection with HIV, might increase the risk of post-TB lung disease.96 Finally, patients cured of TB may have ongoing pulmonary inflammation, as evidenced by persistent positron emission tomography (PET) avidity at the end of treatment. This raises the possibility that lung injury could progress after treatment completion.97 To date, studies of lung function among patients with TB have largely been limited to the time period during and shortly after completion of TB treatment. A more comprehensive understanding of the natural history of post-TB pulmonary impairment will require longitudinal follow-up of large cohorts or registries with serial pulmonary function tests conducted for several years following TB treatment completion.
The observation that pulmonary impairment after TB correlates with the extent of disease at diagnosis presents a challenge for efforts to limit lung injury and improve lung function after TB. It is, however, reasonable to predict that interventions which accelerate the control of inflammation faster than antimicrobial treatment alone could be beneficial, particularly if they target pathways responsible for irreversible tissue injury. Fibrotic resolution of pulmonary TB was a host-protective response in the pre-antibiotic era but may be maladaptive in the setting of effective anti-TB treatment. In the modern era, novel therapies to shift the resolution of inflammation away from fibrogenesis might limit chronic pulmonary impairment after TB. Drugs currently approved to treat reversible airflow limitation in COPD might improve respiratory quality of life after TB. Promising recent studies conducted in Korea using the long acting anti-muscarinic antagonist tiotropium or the long acting beta-adrenoceptor agonist indacaterol demonstrated moderate improvement in lung function and symptoms.98,99 These studies need to be replicated in other settings and extended (e.g. with combination therapies) in order to build a firm evidence base for managing and treating this increasingly recognized chronic lung disease.
With an estimated 10.4 million incident cases of TB worldwide in 2016, post-TB lung disease is likely to have a substantial impact on individuals infected with TB, their families and their communities.83 Post-TB lung disease was estimated to be the largest contributor to the loss of disability adjusted life-years (DALYs) among survivors of TB in the United States.78 Such analyses ought to be replicated in high TB burden settings in order to provide a more comprehensive understanding of the global burden of this relatively under-recognized epidemic. A greater understanding of the biological drivers behind these colliding epidemics of infectious and pulmonary diseases is urgently needed in order to design public health and clinical interventions to decrease TB-related pulmonary morbidity.
CARDIOVASCULAR DISEASE AND DYSLIPIDEMIA
TB related inflammation may also increase the risk of adverse cardiovascular outcomes including stroke and acute coronary syndrome (ACS). However, to date, few studies have examined the relationship between TB and cardiovascular disease (CVD) risk. A nationwide retrospective cohort study from Taiwan aimed to evaluate the risk of subsequent ACS in patients with newly diagnosed pulmonary TB. They found that patients with pulmonary TB who had received outpatient or inpatient medical care at least three times for a principal diagnosis of TB, had a 1.4-fold increased risk of ACS as compared to uninfected controls from the general population.100 Another study from Taiwan estimated the 3-year risk of ischemic stroke in patients who had received anti-tuberculosis medication for more than 6 months after first TB diagnosis, compared to randomly selected controls without TB from a nationwide population based cohort. Study results indicated that after adjustment for relevant covariates, the risk of ischemic stroke in TB patients was 50% greater than that of controls.101 Similarly, recent work from a large de-identified insurance claims database in the United States compared the occurrence of acute myocardial infarction claims between individuals who had filed previous claims for TB and those who did not—a history of active TB was associated with a two-fold increased risk of acute myocardial infarction.102 Evidence is therefore emerging that patients with TB are at increased risk for cardiovascular outcomes compared to the general population, and care for TB should include both anti-tuberculous treatment, and also active risk reduction for cardiovascular disease.
The mechanisms by which TB may lead to increased CVD risk are currently not well known. However, various infectious agents have been previously associated with an increased risk of CVD, including influenza, HIV, and Chlamydia pneumoniae.103–107 Possible pathophysiological mechanisms by which infections may lead to CVD include systemic inflammation leading to atherosclerotic plaque formation and/or plaque rupture.108 In particular, inflammatory processes increase the secretion of leukocyte soluble adhesion molecules which aid in the attachment of monocytes to endothelial cells.109 The subsequent transformation of monocytes into macrophages, coupled with the uptake of cholesterol lipoproteins, are thought to both initiate and accelerate growth of the atherosclerotic fatty streak.109 Furthermore, as arterial plaques progress, they become covered by a ‘cap’ of smooth muscle cells and collagen-rich matrix, which may rupture when triggered by a variety of inflammatory stressors.108 In the case of TB, individuals with active disease have increased levels of pro-inflammatory cytokines,108 which are associated with increased risk of cardiovascular disease.110–112 Specifically, IL-6 is associated with insulin resistance, dyslipidemia, and endothelial dysfunction,113 and is found to inhibit lipoprotein lipase and stimulate lipolysis.110 In addition, IL-6 is expressed in fatty streaks and the atheromatous ‘cap’ suggesting a role of this pro-inflammatory cytokine in the progression of atherosclerosis.110 It is therefore possible that the activation of a cell-mediated immune response triggered by TB may play a role in promoting atherosclerosis and increasing cardiovascular risk in individuals with active TB.108 This hypothesis is supported a recent systems immunology study that identified upregulation of CVD risk biomarkers (neutrophil:lymphocyte and monocyte:HDL ratios) and pathways (e.g. complement and platelet activation) in pulmonary TB patients.114
Active TB and increased dyslipidemia
Active TB in itself may also directly or indirectly impact lipid levels and dyslipidemia. Studies have reported that levels of serum total cholesterol, HDL-cholesterol, and LDL cholesterol are generally lower in individuals with pulmonary TB than in healthy controls.115,116 However, it is unclear whether lower lipid levels in individuals with TB disease are the cause of or the result of TB. It is possible that TB-related inflammation triggers a host response that causes LDL oxidation, thereby leading to lower levels of LDL in the serum.115 It is also possible that individuals with low levels of cholesterol are at greater risk for active TB or TB infection, which could be due to the role of cholesterol in cellular immunity. Specifically, low cholesterol may impair lymphocyte and macrophage function, thereby increasing the likelihood of TB infection and progression.116 A recent study also noted an association of low HDL with increased molecular degree of perturbation—a gene transcription marker reflecting immunological activation—in individuals with co-morbid TB and diabetes.114 HDL cholesterol has been shown to modulate innate and adaptive immunity, and so decreased levels of HDL may have negative impacts on the immune response.114
There is little information as to how TB treatment may alter serum lipid levels. A study from West Africa found that before treatment, mean levels of total cholesterol, HDL cholesterol, and LDL cholesterol were significantly lower in individuals with TB compared to controls. However, after six months of TB treatment, mean total cholesterol and HDL cholesterol levels were significantly increased, while the atherogenic index of TC/HDL-C was significantly reduced.117 These findings suggest that TB treatment may normalize lipid parameters in individuals with low lipid levels prior to treatment initiation. Additional research is warranted to further assess the relationship between TB treatment, lipid levels and risk of future cardiovascular outcomes. However, similar to the methodologic difficulties of studies to gauge the impact of TB on hyperglycemia and diabetes incidence, study designs to determine the causal effect of TB on risk of cardiovascular disease or dyslipidemia will need to account for the possibility that underlying cardiovascular disease risks may predispose patients to developing TB. Therefore, separating the direct effects of TB from indirect effects of preexisting cardiovascular risk factors will be a major design challenge.
CONCLUSION
Although the knowledge base for determining the burden of post-TB NCDs is limited by a critical lack of definitive studies, this review suggests there is sufficient epidemiologic and biologic evidence to justify greater research emphasis on the potential negative impact of TB on subsequent pathogenesis of diabetes, chronic pulmonary disease and cardiovascular disease. Current study design approaches to build an improved understanding of long-term health outcomes among patients post-TB are hindered by imprecise tools to measure NCD incidence. Research quality on post-TB NCDs will be improved by studies that incorporate prospective cohort designs with long follow-up time after TB to compare incidence rates of NCD. Research related to the TB-NCD nexus are also limited by important theoretical challenges in estimating causal effects and identifying appropriate counterfactual controls of persons without TB. Nonetheless, the convergence of TB with NCD comorbidity, including TB-attributable disease, will continue to burden individuals, healthcare systems, and economic growth in low- and middle-income countries.118 Characterizing the extent to which TB contributes to NCD incidence, and determining TB host and pathogen factors that modify this risk, will be necessary to identify existing clinical interventions and develop new therapies to reduce post-TB morbidity and mortality.
ACKNOWLEDGEMENTS
MJM, ADS, UPG, and SCA, and HK drafted and conceived of the article. All authors made substantial contributions to interpretation of data, critically revised the article for intellectual content, and provided approval of the final version. The authors had no conflicts of interest to disclose. This publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R03AI133172. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Magee MJ, Narayan KM. Global confluence of infectious and non-communicable diseases - The case of type 2 diabetes. Prev Med 2013;57:149–51. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GS. Measuring progress and projecting attainment on the basis of past trends of the health-related Sustainable Development Goals in 188 countries: an analysis from the Global Burden of Disease Study 2016. Lancet 2017;390:1423–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Global tuberculosis report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 4.Pan SC, Ku CC, Kao D, Ezzati M, Fang CT, Lin HH. Effect of diabetes on tuberculosis control in 13 countries with high tuberculosis: a modelling study. The lancet Diabetes & endocrinology 2015. [DOI] [PubMed] [Google Scholar]
- 5.Houben R, Menzies NA, Sumner T, et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. The Lancet Global health 2016;4:e806–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis - a systematic review. BMC public health 2008;8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS medicine 2007;4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS medicine 2008;5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. The Lancet Infectious diseases 2009;9:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin HH, Murray M, Cohen T, Colijn C, Ezzati M. Effects of smoking and solid-fuel use on COPD, lung cancer, and tuberculosis in China: a time-based, multiple risk factor, modelling study. Lancet 2008;372:1473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu S, Stuckler D, Bitton A, Glantz SA. Projected effects of tobacco smoking on worldwide tuberculosis control: mathematical modelling analysis. Bmj 2011;343:d5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marais BJ, Lonnroth K, Lawn SD, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. The Lancet Infectious diseases 2013;13:436–48. [DOI] [PubMed] [Google Scholar]
- 13.Glass RI. HIV/AIDS and noncommunicable disease comorbidities: emerging research priorities. Journal of acquired immune deficiency syndromes 2014;67 Suppl 1:S1. [DOI] [PubMed] [Google Scholar]
- 14.Harries AD, Kumar AM, Satyanarayana S, et al. Communicable and non-communicable diseases: connections, synergies and benefits of integrating care. Public health action 2015;5:156–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang SH, Cataldo JK. A systematic review of global cultural variations in knowledge, attitudes and health responses to tuberculosis stigma. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2014;18:168-73, i–iv. [DOI] [PubMed] [Google Scholar]
- 16.Bieh KL, Weigel R, Smith H. Hospitalized care for MDR-TB in Port Harcourt, Nigeria: a qualitative study. BMC infectious diseases 2017;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst JD. The immunological life cycle of tuberculosis. Nature reviews Immunology 2012;12:581–91. [DOI] [PubMed] [Google Scholar]
- 18.Ravimohan S, Tamuhla N, Kung SJ, et al. Matrix Metalloproteinases in Tuberculosis-Immune Reconstitution Inflammatory Syndrome and Impaired Lung Function Among Advanced HIV/TB Co-infected Patients Initiating Antiretroviral Therapy. EBioMedicine 2016;3:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harries AD, Ade S, Burney P, Hoa NB, Schluger NW, Castro JL. Successfully treated but not fit for purpose: paying attention to chronic lung impairment after TB treatment. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2016;20:1010–4. [DOI] [PubMed] [Google Scholar]
- 20.Podell BK, Ackart DF, Kirk NM, Eck SP, Bell C, Basaraba RJ. Non-Diabetic Hyperglycemia Exacerbates Disease Severity in Mycobacterium tuberculosis Infected Guinea Pigs. PloS one 2012;7:e46824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornfeld H, West K, Kane K, et al. High Prevalence and Heterogeneity of Diabetes in Patients With TB in South India: A Report from the Effects of Diabetes on Tuberculosis Severity (EDOTS) Study. Chest 2016;149:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oluboyo PO, Erasmus RT. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle 1990;71:135–8. [DOI] [PubMed] [Google Scholar]
- 23.Podell BK, Ackart DF, Obregon-Henao A, et al. Increased severity of tuberculosis in Guinea pigs with type 2 diabetes: a model of diabetes-tuberculosis comorbidity. Am J Pathol 2014;184:1104–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boillat-Blanco N, Ramaiya KL, Mganga M, et al. Transient Hyperglycemia in Patients With Tuberculosis in Tanzania: Implications for Diabetes Screening Algorithms. The Journal of infectious diseases 2016;213:1163–72. [DOI] [PubMed] [Google Scholar]
- 25.Aftab H, Christensen DL, Ambreen A, et al. Tuberculosis-Related Diabetes: Is It Reversible after Complete Treatment? Am J Trop Med Hyg 2017;97:1099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basoglu OK, Bacakoglu F, Cok G, Sayiner A, Ates M. The oral glucose tolerance test in patients with respiratory infections. Monaldi Arch Chest Dis 1999;54:307–10. [PubMed] [Google Scholar]
- 27.Jawad F, Shera AS, Memon R, Ansari G. Glucose intolerance in pulmonary tuberculosis. J Pak Med Assoc 1995;45:237–8. [PubMed] [Google Scholar]
- 28.Almeida-Junior JL, Gil-Santana L, Oliveira CA, et al. Glucose Metabolism Disorder Is Associated with Pulmonary Tuberculosis in Individuals with Respiratory Symptoms from Brazil. PloS one 2016;11:e0153590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh MM, Biswas SK, Shah A. Impaired Glucose Tolerance in Active Pulmonary Tuberculosis. Ind J Tuberc 1984;31:118–21. [Google Scholar]
- 30.Critchley JA, Restrepo BI, Ronacher K, et al. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 1: Epidemiology and Clinical Management. Chest 2017;152:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson F, Huangfu P, Pearce M, McNally R, Unwin N, Critchley J. OP52 Exploring the association between tuberculosis and diabetes in a UK primary care dataset. Journal of epidemiology and community health 2016;70:A31–A2. [DOI] [PubMed] [Google Scholar]
- 32.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009;373:1798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. The New England journal of medicine 1995;332:1351–62. [DOI] [PubMed] [Google Scholar]
- 34.Barth E, Albuszies G, Baumgart K, et al. Glucose metabolism and catecholamines. Crit Care Med 2007;35:S508–18. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Chatterji D. Stress responses in mycobacteria. IUBMB Life 2005;57:149–59. [DOI] [PubMed] [Google Scholar]
- 36.Polyzos SA, Kountouras J, Zavos C, Deretzi G. The Association Between Helicobacter pylori Infection and Insulin Resistance: A Systematic Review. Helicobacter 2011;16:79–88. [DOI] [PubMed] [Google Scholar]
- 37.Beigier-Bompadre M, Montagna GN, Kuhl AA, et al. Mycobacterium tuberculosis infection modulates adipose tissue biology. PLoS pathogens 2017;13:e1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neyrolles O, Hernandez-Pando R, Pietri-Rouxel F, et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PloS one 2006;1:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrade BB, Kumar NP, Sridhar R, et al. Heightened plasma levels of heme oxygenase-1 and tissue inhibitor of metalloproteinase-4 as well as elevated peripheral neutrophil counts are associated with TB-diabetes comorbidity. Chest 2014;145:1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrade BB, Pavan Kumar N, Mayer-Barber KD, et al. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PloS one 2013;8:e62618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bird A DNA methylation patterns and epigenetic memory. Genes Dev 2002;16:6–21. [DOI] [PubMed] [Google Scholar]
- 42.Jayaraman S Epigenetic mechanisms of metabolic memory in diabetes. Circ Res 2012;110:1039–41. [DOI] [PubMed] [Google Scholar]
- 43.Miao F, Chen Z, Genuth S, et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes 2014;63:1748–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 2008;205:2409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nathan DM, Cleary PA, Backlund JYC, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New Engl J Med 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steffes MW, Chavers BM, Molitch ME, et al. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy - The Epidemiology of Diabetes Interventions and Complications (EDIC) study. Jama-J Am Med Assoc 2003;290:2159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin CL, Albers J, Herman WH, et al. Neuropathy among the Diabetes Control and Complications Trial cohort 8 years after trial completion. Diabetes care 2006;29:340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cleary PA, Orchard TJ, Genuth S, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the diabetes control and complications Trial/Epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes 2006;55:3556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goh SY, Cooper ME. The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocr Metab 2008;93:1143–52. [DOI] [PubMed] [Google Scholar]
- 50.Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory”. Exp Diabetes Res 2011;2011:218598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes care 2002;25:1862–8. [DOI] [PubMed] [Google Scholar]
- 52.Chowdhury R, Narayan KM, Zabetian A, Raj S, Tabassum R. Genetic studies of type 2 diabetes in South Asians: a systematic overview. Current diabetes reviews 2014;10:258–74. [DOI] [PubMed] [Google Scholar]
- 53.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes care 2004;27 Suppl 1:S5–S10. [DOI] [PubMed] [Google Scholar]
- 54.Kerner W, Bruckel J, German Diabetes A. Definition, classification and diagnosis of diabetes mellitus. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association 2014;122:384–6. [DOI] [PubMed] [Google Scholar]
- 55.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005;54 Suppl 2:S97–107. [DOI] [PubMed] [Google Scholar]
- 56.Saltiel AR. New Perspectives into the Molecular Pathogenesis and Treatment of Type 2 Diabetes. Cell;104:517–29. [DOI] [PubMed] [Google Scholar]
- 57.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005;365:1333–46. [DOI] [PubMed] [Google Scholar]
- 58.Hensel RL, Kempker RR, Tapia J, Oladele A, Blumberg HM, Magee MJ. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2016;20:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez L, Zhu L, Castellanos ME, et al. Glycemic Control and the Prevalence of Tuberculosis Infection: A Population-based Observational Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2017;65:2060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barron MM, Shaw KM, Bullard KM, Ali MK, Magee MJ. Diabetes is associated with increased prevalence of latent tuberculosis infection: Findings from the National Health and Nutrition Examination Survey, 2011–2012. Diabetes research and clinical practice 2018;139:366–79. [DOI] [PubMed] [Google Scholar]
- 61.Jackson C, Southern J, Lalvani A, et al. Diabetes mellitus and latent tuberculosis infection: baseline analysis of a large UK cohort. Thorax 2018. [DOI] [PubMed] [Google Scholar]
- 62.Barry CE 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature reviews Microbiology 2009;7:845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Via LE, Schimel D, Weiner DM, et al. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [(1)(8)F]2-fluoro-deoxy-D-glucose positron emission tomography and computed tomography. Antimicrobial agents and chemotherapy 2012;56:4391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matty MA, Roca FJ, Cronan MR, Tobin DM. Adventures within the speckled band: heterogeneity, angiogenesis, and balanced inflammation in the tuberculous granuloma. Immunological reviews 2015;264:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marakalala MJ, Raju RM, Sharma K, et al. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nature medicine 2016;22:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gideon HP, Phuah J, Myers AJ, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS pathogens 2015;11:e1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrios-Payan J, Saqui-Salces M, Jeyanathan M, et al. Extrapulmonary locations of mycobacterium tuberculosis DNA during latent infection. The Journal of infectious diseases 2012;206:1194–205. [DOI] [PubMed] [Google Scholar]
- 68.Agarwal P, Khan SR, Verma SC, et al. Mycobacterium tuberculosis persistence in various adipose depots of infected mice and the effect of anti-tubercular therapy. Microbes and infection / Institut Pasteur 2014;16:571–80. [DOI] [PubMed] [Google Scholar]
- 69.Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes and immunity 2011;12:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Timmermans WM, van Laar JA, van Hagen PM, van Zelm MC. Immunopathogenesis of granulomas in chronic autoinflammatory diseases. Clinical & translational immunology 2016;5:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. The New England journal of medicine 2007;357:2153–65. [DOI] [PubMed] [Google Scholar]
- 72.Geboes K, van den Oord J, De Wolf-Peeters C, et al. The cellular composition of granulomas in mesenteric lymph nodes from patients with Crohn’s disease. Virchows Archiv A, Pathological anatomy and histopathology 1986;409:679–92. [DOI] [PubMed] [Google Scholar]
- 73.Asano S Granulomatous lymphadenitis. Journal of clinical and experimental hematopathology : JCEH 2012;52:1–16. [DOI] [PubMed] [Google Scholar]
- 74.The global burden of disease: 2004 update. WHO; 2004.
- 75.Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2015;32:138–46. [DOI] [PubMed] [Google Scholar]
- 76.van Zyl Smit RN, Pai M, Yew WW, et al. Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. The European respiratory journal 2010;35:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pasipanodya JG, Miller TL, Vecino M, et al. Pulmonary impairment after tuberculosis. Chest 2007;131:1817–24. [DOI] [PubMed] [Google Scholar]
- 78.Pasipanodya JG, McNabb SJ, Hilsenrath P, et al. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC public health 2010;10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vecino M, Pasipanodya JG, Slocum P, et al. Evidence for chronic lung impairment in patients treated for pulmonary tuberculosis. Journal of infection and public health 2011;4:244–52. [DOI] [PubMed] [Google Scholar]
- 80.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000;55:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ralph AP, Kenangalem E, Waramori G, et al. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena. PLoS One 2013;8:e80302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respiratory medicine 1989;83:195–8. [DOI] [PubMed] [Google Scholar]
- 83.Global Tuberculosis Report. WHO; 2017.
- 84.Ehrlich RI, White N, Norman R, et al. Predictors of chronic bronchitis in South African adults. Int J Tuberc Lung Dis 2004;8:369–76. [PubMed] [Google Scholar]
- 85.Ross J, Ehrlich RI, Hnizdo E, White N, Churchyard GJ. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax 2010;65:1010–5. [DOI] [PubMed] [Google Scholar]
- 86.Jung KH, Kim SJ, Shin C, Kim JH. The considerable, often neglected, impact of pulmonary tuberculosis on the prevalence of COPD. American journal of respiratory and critical care medicine 2008;178:431; author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 87.Hallett WY, Martin CJ. The diffuse obstructive pulmonary syndrome in a tuberculosis sanatorium. I. Etiologic factors. Ann Intern Med 1961;54:1146–55. [DOI] [PubMed] [Google Scholar]
- 88.Martin CJ, Hallett WY. The diffuse obstructive pulmonary syndrome in a tuberculosis sanatorium. II. Incidence and symptoms. Ann Intern Med 1961;54:1156–64. [DOI] [PubMed] [Google Scholar]
- 89.Anno H, Tomashefski JF. Studies on the impairment of respiratory function in pulmonary tuberculosis. Am Rev Tuberc 1955;71:333–48. [DOI] [PubMed] [Google Scholar]
- 90.Snider GL, Doctor L, Demas TA, Shaw AR. Obstructive airway disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis 1971;103:625–40. [DOI] [PubMed] [Google Scholar]
- 91.Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax 1996;51:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsu T, Hingley-Wilson SM, Chen B, et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 2003;100:12420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. J Infect Dis 2005;192:1201–9. [DOI] [PubMed] [Google Scholar]
- 94.Curtis JK. The significance of bronchiectasis associated with pulmonary tuberculosis. Am J Med 1957;22:894–903. [DOI] [PubMed] [Google Scholar]
- 95.Bobrowitz ID, Rodescu D, Marcus H, Abeles H. The destroyed tuberculous lung. Scandinavian journal of respiratory diseases 1974;55:82–8. [PubMed] [Google Scholar]
- 96.Lee CH, Lee MC, Lin HH, et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PloS one 2012;7:e37978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malherbe ST, Shenai S, Ronacher K, et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nature medicine 2016;22:1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yum HK, Park IN. Effect of inhaled tiotropium on spirometric parameters in patients with tuberculous destroyed lung. Tuberculosis and respiratory diseases 2014;77:167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim CJ, Yoon HK, Park MJ, et al. Inhaled indacaterol for the treatment of COPD patients with destroyed lung by tuberculosis and moderate-to-severe airflow limitation: results from the randomized INFINITY study. International journal of chronic obstructive pulmonary disease 2017;12:1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chung WS, Lin CL, Hung CT, et al. Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2014;18:79–83. [DOI] [PubMed] [Google Scholar]
- 101.Sheu JJ, Chiou HY, Kang JH, Chen YH, Lin HC. Tuberculosis and the risk of ischemic stroke: a 3-year follow-up study. Stroke 2010;41:244–9. [DOI] [PubMed] [Google Scholar]
- 102.Huaman MA, Kryscio RJ, Fichtenbaum CJ, et al. Tuberculosis and risk of acute myocardial infarction: a propensity score-matched analysis. Epidemiology and infection 2017;145:1363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. The New England journal of medicine 2004;351:2611–8. [DOI] [PubMed] [Google Scholar]
- 104.Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet 1998;351:1467–71. [DOI] [PubMed] [Google Scholar]
- 105.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism 2007;92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. Journal of acquired immune deficiency syndromes (1999) 2003;33:506–12. [DOI] [PubMed] [Google Scholar]
- 107.Saikku P, Leinonen M, Tenkanen L, et al. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med 1992;116:273–8. [DOI] [PubMed] [Google Scholar]
- 108.Huaman MA, Henson D, Ticona E, Sterling TR, Garvy BA. Tuberculosis and Cardiovascular Disease: Linking the Epidemics. Tropical diseases, travel medicine and vaccines 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 110.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 2000;148:209–14. [DOI] [PubMed] [Google Scholar]
- 111.Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Archivum immunologiae et therapiae experimentalis 2009;57:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). The American journal of cardiology 2003;92:522–8. [DOI] [PubMed] [Google Scholar]
- 113.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–80. [DOI] [PubMed] [Google Scholar]
- 114.Prada-Medina CA, Fukutani KF, Pavan Kumar N, et al. Systems Immunology of Diabetes-Tuberculosis Comorbidity Reveals Signatures of Disease Complications. Scientific reports 2017;7:1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deniz O, Gumus S, Yaman H, et al. Serum total cholesterol, HDL-C and LDL-C concentrations significantly correlate with the radiological extent of disease and the degree of smear positivity in patients with pulmonary tuberculosis. Clinical biochemistry 2007;40:162–6. [DOI] [PubMed] [Google Scholar]
- 116.Sahin F, Yildiz P. Distinctive biochemical changes in pulmonary tuberculosis and pneumonia. Archives of medical science : AMS 2013;9:656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Akpovi DC, Gbaguidi LHS, Anago E, et al. Tuberculosis treatment raises total cholesterol level and restores high density lipoprotein cholesterol (HDLC) in patients with pulmonary tuberculosis. African Journal of Biotechnology 2013;12. [Google Scholar]
- 118.Verguet S, Riumallo-Herl C, Gomez GB, et al. Catastrophic costs potentially averted by tuberculosis control in India and South Africa: a modelling study. The Lancet Global health 2017;5:e1123–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]


