Abstract
Background.
The majority of studies on the effects of adiposity on mortality in the elderly have been conducted in developed countries with mixed results. We investigated the association between adiposity and mortality in a cohort of community-dwelling elderly in Sao Paulo, Brazil.
Methods.
Body mass index (BMI), waist circumference, waist-to-hip ratio, and type 2 diabetes were evaluated in 1,882 participants (mean age 71.0 ± 8.3 years old, 61% female). Mortality was confirmed by national vital statistics records during a maximum of 10 years of follow-up. Proportional hazards models were used to estimate hazard ratios (HRs) for mortality after adjusting for sociodemographics and comorbidities. In a subsample of 897 participants, the effects of changes in measures of adiposity on mortality were investigated during a median follow-up of 4.6 years.
Results.
Having type-2 diabetes at baseline was associated with increased mortality (HR = 1.44, 95% CI: 1.17–1.77), with a higher HR among men. When compared with normal weight participants (BMI = 20–<25kg/m2), overweight and obesity were not associated with mortality (overweight: HR = 0.84 [0.70, 1.02]; obesity: HR = 0.82 [0.64, 1.06]), whereas participants with low-normal weight (BMI = 18.5<20 kg/m2) had increased risk of death (HR = 1.51 [1.08–2.10]). Higher waist circumference and waist-to-hip ratio were not associated with increased mortality. Weight gain was protective against mortality in all BMI categories, except in obese participants, and weight loss increased the risk of death in all BMI categories by 42–63%.
Conclusions.
In community-dwelling elderly in Sao Paulo, overweight and obesity were not associated with a higher risk of death, and weight gain seemed to reduce mortality, except in the obese.
Keywords: Body mass index, Waist circumference, Obesity, Overweight, Diabetes, Mortality, Aged.
An alarming rise in the prevalence of obesity has been observed worldwide. Between 1980 and 2008, the prevalence of obesity almost doubled, with an estimated 1.46 billion adults being overweight or obese (1). Similar rates are found among the elderly in most developed countries, where, for example, 72% of Americans aged ≥60 years are overweight or obese (2). In Brazil, the prevalence of overweight or obesity among the elderly was 44% in a recent national survey (3).
Harmful effects of overweight and obesity on overall and specific-cause mortality have been observed in large meta-analyses of observational studies (4–7). However, their effects on mortality among the elderly are less clear. A 2007 meta-analysis found a higher risk of mortality, particularly in the obese (8), whereas others found no association (9,10). Controversies also remain regarding the best measure of adiposity in the elderly (body mass index [BMI] vs waist circumference [WC] and waist-to-hip ratio [WHR]), because changes in body composition occur during the aging process (ie, adipose, muscle, and water redistribution) (11,12). In addition, few studies of adiposity in the elderly have been conducted in developing countries, where obesity is a more recent phenomenon (13–15). To fill this gap, we investigated the association between adiposity and mortality in a community-dwelling elderly cohort from Sao Paulo, Brazil. We also examined the association between type 2 diabetes and mortality because diabetes is a common consequence of obesity and may mediate the possible harmful effects of weight gain on mortality.
Methods
Study Population
The Health, Well-Being and Ageing (SABE) study in Sao Paulo (Brazil) enrolled 2,441 community-dwelling individuals aged ≥60 years in 2000 (n = 2,143) and 2006 (n = 298) using a stratified sampling design to represent the elderly population of the city. Men aged ≥75 years were oversampled to compensate for increased mortality. A detailed description of the methodology of SABE can be found elsewhere (16,17).
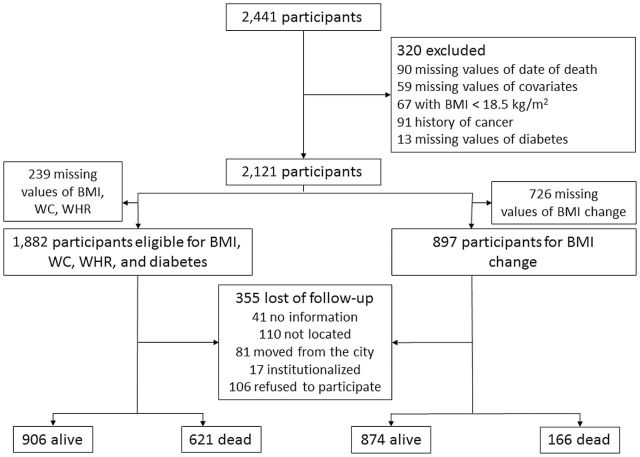
We excluded 320 participants for whom there were no data on the date of death and diabetes or who had a history of cancer or BMI < 18.5kg/m2 at baseline. Participants with history of cancer and BMI < 18.5kg/m2 at baseline were excluded to avoid unmeasured confounding associated with baseline severe disease that could affect mortality. An additional 239 participants were excluded because of missing values for BMI, WC, and WHR (Figure 1). If fewer than 10 observations had a missing value for a particular variable, we excluded those observations from the analysis. We used a missing indicator method for variables that had more than 10 observations with missing values to preserve statistical power (childhood socioeconomic status [SES], occupation, income, depressive symptoms, frailty, and nutritional status). The study was approved by the Office of Human Research Administration at the Harvard School of Public Health and by the Ethics Committee in Research at the University of Sao Paulo.
Figure 1.
Flow chart of participant selection: Left: Body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR), and diabetes at baseline (2000). Right: BMI change between first and second visits (2000 and 2006), SABE cohort.
Exposure Measurements
Participants were interviewed in their households using a standardized questionnaire, and anthropometric and physical performance tests were performed. Weight was measured using a portable scale (Seca, Germany), and height was measured with an anthropometer (Harpenden, England). Eighty-nine participants who were unable to stand up were not weighted and were excluded from this analysis. Body mass index was analyzed as a categorical variable with four categories following the WHO definition of overweight and obesity: 18.5<20, 20<25, 25<30, and ≥30kg/m2 (18). Waist circumference was measured with an inelastic measurement tape placed on the midpoint between the lower margin of last palpable rib and the top of the iliac crest. Hip circumference was measured around the widest portion of the buttocks. High WC was defined by WC > 102cm in men or WC > 88cm in women; and a high WHR was defined as WHR > 0.90 for men and >0.85 for women (19). Type 2 diabetes was self-reported by responding to the question “has a doctor or nurse ever told you that you have diabetes or high blood sugar levels?”
Follow-up and Study Outcome
In 2006 and 2010, participants were interviewed and examined using the same protocol used at the baseline. If a participant could not be located or his/her relatives informed researchers that he/she had died, study personnel confirmed the reported deaths, including the date of death, using data from the national vital statistics records.
Covariates
Possible confounders in the relationship between BMI, WC, WHR, and diabetes with mortality include age, sex, race, education, childhood SES, occupation, income, marital status, depressive symptoms, lung disease, osteoarthritis, alcohol use, smoking, physical activity, activities of daily living (ADL), frailty, nutritional status, and cognitive function. Diabetes was considered a confounder in the relationship between BMI and WC with mortality. Body mass index was a confounder in the association between diabetes and mortality. All covariates were measured at baseline. Chronic diseases and lifestyle behaviors were self-reported.
Participants were asked to classify their SES in the first 15 years of life as good, regular, or poor. They were also asked about their current income, and it was classified as <1, 1–2, or ≥3× the minimum wage. Main occupation was classified into two categories according to the complexity of the work: skilled and semiskilled versus nonskilled. Nonskilled occupation included agricultural and household work. Depressive symptoms were evaluated using the shorter version of the Geriatric Depression Scale, and a score of ≥5 was considered an indicator of the presence of depressive symptoms (20). Consumption of alcohol per week in the last 3 months prior to the interview was self-reported. Physical activity was defined by engagement in moderate activities (fast walking, dancing, sports, or heavy labor work) at least three times per week. Activities of daily living included bathing, dressing, toileting, transferring, continence, and feeding (21). Participants were independent if they were able to perform all ADLs or dependent if they were unable to perform at least one of them. Hand grip measures were obtained in the sitting position by pressing a dynamometer with the dominant hand. The average of two measures was used. Lower extremity strength was evaluated by the time the participant took to sit and stand five times in a chair. Both hand grip and lower extremity strength were stratified in quartiles, and participants with any measures in the lower quartiles were classified as frail. Nutrition status and cognitive function were evaluated using the Mini-Nutritional Assessment and the Mini-Mental State Examination, and the cutoff values were 11 and 17, respectively (22,23).
Statistical Analysis
We describe the distribution of exposures and covariates separately for participants who survived until the end of follow-up, those who died and those who were lost to follow-up and compared them using the chi-square test for categorical variables and the unpaired t test or Kruskal–Wallis test for continuous variables. We used Cox proportional hazards (PH) models to investigate the association between BMI, WC, WHR, and diabetes with mortality using follow-up time in years as the time scale. In a separate analysis, we investigated the association between changes in BMI, WC, and WHR from 2000 till 2006 and mortality from 2006 till 2010. The association between changes in the measures of adiposity and mortality was estimated separately for each baseline level of adiposity using product terms. Moreover, we included an interaction term between exposures and sex to reflect prior evidence on the differential effects on mortality by gender (4,24).
Proportional hazards assumption was tested using the method described by Lin and coworkers (25) and was not rejected for BMI, WC, WHR and diabetes, but the test was rejected for age groups. Therefore, we used stratified Cox’s PH models by age groups: 60<65, 65<70, 70<75, 75<80, and ≥80 years.
In sensitivity analyses, we used BMI, WC, and WHR as continuous variables with and without the inclusion of quadratic terms. In a sensitivity analysis for missing data, we conducted a complete case approach and excluded 439 participants with missing values for any covariate (n = 1,443). In addition, a multivariate logistic regression model that included additional 90 deaths without information on time of death was performed (n = 1,617). We also applied inverse probability (IP) weighting to adjust for differential loss to follow-up (26). In sensitivity analyses for exposure changes, we estimated a different set of IP weights to adjust for determinants of having measures of adiposity in 2006. Finally, we restricted the analysis of exposure changes to 510 healthy participants who did not have diabetes, smoking, frailty, stroke, or heart and lung diseases at baseline.
All analyses were performed using SAS 9.3 (Cary, North Carolina). All p values were two-sided, and statistical significance was evaluated at a .05 level.
Results
A total of 1,882 participants were eligible for the analysis of the association between baseline BMI, WC, WHR, and diabetes with mortality (Figure 1). The mean age at baseline was 71 years, 39% were male, 68% were white, the mean BMI was 27kg/m2, the mean WC was 95cm, and 17% had diabetes. The distribution of exposures and covariates regarding the vital status at the end of follow-up or censoring can be found in Table 1. During 12,684 person-years of follow-up (median of 6.5 years), 355 participants were lost to follow-up and 621 died. Participants who remained in the study were older, included more men and diabetic patients, had a larger WC, and had worse cognition and functionality than those who were lost to follow-up (Supplementary Table 1).
Table 1.
Baseline Characteristics of the Eligible Study Population according to Vital Status (SABE cohort, n = 1,882)
| Variable | Alive (n = 906) | Dead (n = 621) | Lost to Follow-up (n = 355) | p |
|---|---|---|---|---|
| Body mass index (kg/m2), n (%)* | <.0001 | |||
| 18.5–<20 | 22 (2) | 49 (8) | 19 (5) | |
| 20–<25 | 273 (30) | 233 (38) | 119 (34) | |
| 25–<30 | 368 (41) | 233 (38) | 137 (39) | |
| ≥30 | 243 (27) | 106 (17) | 80 (22) | |
| High waist circumference, n (%)*,† | 462 (51) | 304 (49) | 167 (47) | .42 |
| High waist-to-hip ratio, n (%)*,‡ | 696 (77) | 517 (83) | 259 (73) | .0003 |
| Diabetes, n (%)* | 139 (15) | 134 (22) | 39 (11) | <.0001 |
| Age (years), mean (SD)§ | 67.6 (6.6) | 76.8 (7.8) | 69.5 (7.3) | <.0001 |
| Male, n (%)* | 312 (34) | 306 (49) | 120 (34) | <.0001 |
| Race, n (%)*,|| | <.0001 | |||
| White | 570 (63) | 453 (73) | 251 (71) | |
| Black | 116 (13) | 81 (13) | 52 (15) | |
| Other | 220 (24) | 87 (14) | 52 (15) | |
| Marital status, n (%)* | .0004 | |||
| Married | 547 (60) | 311 (50) | 198 (56) | |
| Nonmarried | 359 (40) | 310 (50) | 157 (44) | |
| Education (years), mean (SD)¶ | 4.2 (3.9) | 3.1 (3.5) | 3.9 (3.6) | <.0001 |
| Childhood socioeconomic status, n (%)* | .09 | |||
| Good | 278 (31) | 185 (30) | 89 (25) | |
| Poor | 358 (40) | 254 (41) | 159 (45) | |
| Regular | 267 (29) | 174 (28) | 106 (30) | |
| Missing | 3 (0) | 8 (1) | 1 (0) | |
| Occupation, n (%)*,# | .44 | |||
| Skilled or semiskilled | 507 (56) | 372 (60) | 196 (55) | |
| Nonskilled | 355 (39) | 216 (35) | 139 (39) | |
| Missing | 44 (5) | 33 (5) | 20 (6) | |
| Current income, n (%)* | .002 | |||
| < 1 Minimum wage | 115 (13) | 57 (9) | 41 (12) | |
| 1–2 Minimum wages | 367 (41) | 311 (50) | 150 (42) | |
| > 2 Minimum wages | 338 (37) | 188 (30) | 116 (33) | |
| Missing | 86 (9) | 65 (10) | 48 (14) | |
| Lung disease, n (%)* | 67 (7) | 81 (13) | 31 (9) | .0009 |
| Heart disease, n (%)* | 127 (14) | 170 (27) | 49 (14) | <.0001 |
| Stroke, n (%)* | 36 (4) | 50 (8) | 22 (6) | .003 |
| Arthritis, n (%)* | 69 (8) | 60 (10) | 14 (4) | .008 |
| Missing | 16 (2) | 6 (1) | 9 (3) | |
| Depressive symptoms, n (%)*,** | 140 (15) | 93 (15) | 64 (18) | <.0001 |
| Missing | 19 (2) | 113 (18) | 15 (4) | |
| Alcohol use/week, n (%)* | .01 | |||
| None | 602 (66) | 457 (74) | 248 (70) | |
| ≥1 drink | 304 (34) | 164 (26) | 107 (30) | |
| Smoking status, n (%)* | .02 | |||
| Never smoker | 529 (58) | 309 (50) | 199 (56) | |
| Current smoker | 103 (11) | 93 (15) | 48 (14) | |
| Former smoker | 274 (30) | 219 (35) | 108 (30) | |
| Physical activity, n (%)* | 349 (39) | 107 (17) | 124 (35) | <.0001 |
| Mini-Mental State Examination, n (%)* | <.0001 | |||
| <17 | 443 (49) | 442 (71) | 186 (52) | |
| ≥17 | 463 (51) | 179 (29) | 169 (48) | |
| ADL, mean (SD)§,†† | 5.6 (0.8) | 5.2 (1.2) | 5.6 (0.8) | <.0001 |
| Frailty, n (%)*,‡‡ | 173 (19) | 212 (34) | 84 (24) | <.0001 |
| Missing | 14 (2) | 6 (1) | 6 (2) | |
| Mininutritional assessment, n (%)*,§§ | <.0001 | |||
| Normal | 634 (70) | 315 (51) | 220 (62) | |
| At risk or malnourished | 217 (24) | 259 (42) | 101 (28) | |
| Missing | 55 (6) | 47 (7) | 34 (10) |
Notes: ADL = activities of daily living; SD = standard deviation.
*Chi-square test.
†High risk: waist circumference > 102cm for male and > 88cm for females.
‡High risk: waist circumference-hip ratio > 0.90 for males and 0.85 for females.
§Unpaired t test.
¶Kruskal–Wallis test comparing each variables among those who were alive, dead, or lost to follow-up.
||Other races include Brazilian Indian, Asian, and unknown race.
#Nonskilled occupation includes agricultural, household, and nonskilled works.
**Score ≥5 in the Geriatric Depression Scale.
††ADL, number of activities of daily living that participants were unable to perform without help (bathing, dressing, toileting, transferring, continence, and feeding).
‡‡Composite measure of hand grip and lower extremity strength. Both measures were stratified in quartiles and participants with any measures in the lower quartiles were classified as frail.
§§Normal ≥12; at risk of malnourished ≤11.
Compared with elderly with normal BMI (BMI = 20<25kg/m2), those with low-normal BMI (18.5 ≤ BMI < 20kg/m2) had a 51% higher hazard of death (HR = 1.51, 95% CI: 1.08–2.10), whereas those who were overweight or obese did not have a significantly different hazard of death: the HR for overweight was 0.80 (0.66–0.95) before adjustment for potential confounders and 0.84 (0.70–1.02) after adjustment; the HR for obese was 0.63 (0.50–0.79) before adjustment and 0.82 (0.64–1.06) after adjustment (Table 2). No association was found between WC or WHR and mortality: the fully adjusted HR for high WC was 1.07 (0.89–1.30) and for high WHR, 1.02 (0.82–1.27) (Table 3). We found no significant interaction between measures of adiposity and sex (Tables 2 and 3). The diabetic elderly had 1.44 (1.17–1.77) times the hazard of death compared to nondiabetic participants, and the association was slightly stronger among men than women (Table 4).
Table 2.
Hazard Ratio of Death According to Baseline Body Mass Index (BMI) Categories (SABE cohort, 2000–2010, n = 1,882)
| BMI (kg/m2) | 18.5–<20 | 20–<25 | 25–<30 | ≥30 | p for Trend |
|---|---|---|---|---|---|
| Person-years | 461 | 4,126 | 5,145 | 2,953 | |
| Deaths | 49 | 233 | 233 | 106 | |
| Hazard ratio (95% CI) | |||||
| Crude | 1.98 (1.46–2.70) | 1.0 | 0.80 (0.66–0.95) | 0.63 (0.50–0.79) | <.0001 |
| Adjusted* | 1.51 (1.08–2.10) | 1.0 | 0.84 (0.70–1.02) | 0.82 (0.64–1.06) | .14 |
| Hazard ratio (95% CI) by sex† | |||||
| Female | 1.41 (0.96–2.07) | 1.0 | 0.79 (0.60–1.05) | 0.80 (0.57–1.13) | .13 |
| Male | 1.55 (1.07–2.26) | 1.10 (0.93–1.30) | 0.87 (0.71–1.08) | 0.88 (0.67–1.16) | .18 |
Notes: *Adjusted for age, sex, race, marital status, years of education, childhood socioeconomic status, occupation, income, diabetes, heart disease, lung disease, stroke, arthritis, depressive symptoms, alcohol use, smoking, physical activity, cognitive function, activities of daily living, frailty, and nutritional status, year of entry in the study.
†Adjusted for all of the variables in footnote * and adding an interaction term between BMI and sex.
Table 3.
Hazard Ratio of Death According to Waist Circumference and Waist-Hip-Ratio (SABE cohort, 2000–2010, n = 1,882)
| Waist Circumference* | Waist-Hip Ratio† | |||||
|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | |
| Person-years | 6,213 | 6,471 | 2,679 | 10,005 | ||
| Deaths | 317 | 304 | 104 | 517 | ||
| Hazard ratio (95% CI) | ||||||
| Crude | 1.0 | 0.91 (0.78–1.07) | 0.25 | 1.0 | 1.34 (1.09–1.65) | .007 |
| Adjusted‡ | 1.0 | 1.07 (0.89–1.30) | 0.47 | 1.0 | 1.02 (0.82–1.27) | .87 |
| Hazard ratio (95% CI) by sex§ | ||||||
| Female | 1.0 | 0.96 (0.72–1.26) | 0.77 | 1.0 | 0.99 (0.72–1.36) | .95 |
| Male | 1.0 | 1.28 (0.96–1.69) | 0.09 | 1.0 | 1.05 (0.75–1.46) | .08 |
Notes: *High: Waist circumference > 102cm for male and > 88cm for females.
†High: Waist-hip ratio > 0.90 for males and 0.85 for females.
‡Adjusted for age, sex, race, marital status, years of education, childhood socioeconomic status, occupation, income, diabetes, heart disease, lung disease, stroke, arthritis, depressive symptoms, alcohol use, smoking, physical activity, frailty, nutritional status, year of entry in the study.
§Adjusted for all of the variables in footnote * and adding an interaction term between waist circumference or waist-to-hip ratio and sex.
Table 4.
Mortality Hazard Ratio for Self-Reported Diabetes (SABE cohort, 2000–2010, n = 1,882)
| No Diabetes | Diabetes | p | |
|---|---|---|---|
| Person-years | 10,627 | 2,057 | |
| Deaths | 487 | 134 | |
| Hazard ratio (95% CI) | |||
| Crude | 1.0 | 1.45 (1.20–1.76) | .0001 |
| Adjusted* | 1.0 | 1.47 (1.17–1.83) | .0006 |
| Hazard ratio (95% CI) by sex† | |||
| Male | 1.0 | 1.66 (1.23–2.23) | .0009 |
| Female | 1.0 | 1.42 (1.07–1.87) | .01 |
Notes: *Adjusted for age, sex, race, marital status, years of education, childhood socioeconomic status, occupation, income, heart disease, lung disease, stroke, arthritis, depressive symptoms, alcohol use, smoking, body mass index, physical activity, frailty, nutritional status, and year of entry in the study.
†Adjusted for all of the variables in footnote * and adding an interaction term between diabetes and sex.
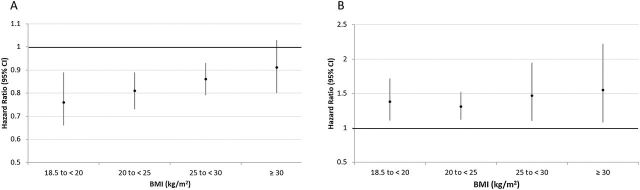
The effect of weight change on mortality was investigated in 897 participants. During 5,159 person-years of follow-up (median of 4.6 years), 121 participants were lost to follow-up and 166 died. The BMI gain was associated with decreased mortality in all BMI categories at baseline, except for those who were obese at baseline, where the association was not significant. Hazard ratios ranged from 0.76 to 0.91 for one unit larger BMI gain. Similarly, BMI loss was associated with increased mortality in all BMI categories at baseline (HRs ranging from 1.32 to 1.57 for one unit lower BMI) (Figure 2). The WC gain was protective in the low-WC group (HR = 0.90 [0.86–0.95]), but had no effect on the high-WC group (HR = 0.98 [0.94–1.03]). Loss in WC increased the risk of mortality in both groups (low-WC: HR = 1.11 [1.06–1.16] and high-WC: HR = 1.20 [1.09–1.33]). We did not find an association between WHR changes and mortality (p > .20 for all comparisons, results not shown).
Figure 2.
Hazard ratio (HR)* and 95% confidence interval (CI) for death related to one unit increase (A) or decrease (B) in body mass index (BMI) according to baseline BMI categories, SABE cohort 2000–2010. *HR were adjusted for age, sex, race, marital status, years of education, childhood socioeconomic status, occupation, income, diabetes, heart disease, lung disease, stroke, arthritis, depressive symptoms, alcohol use, smoking, physical activity, cognitive function, activities of daily living, frailty, nutritional status, and year of entry in the study.
Sensitivity Analyses
When we analyzed BMI as a continuous variable, a higher BMI was associated with a significantly lower mortality (using a model with linear and quadratic terms): the HR for comparing a BMI of 25 with 20 was 0.77 (0.64–0.92), and the HR for comparing a BMI of 30 with 20 was 0.70 (0.54; 0.90). On the other hand, we found no association between WC or WHR and mortality when we analyzed these measures as continuous variables with and without quadratic terms (results not shown). Other sensitivity analyses for baseline BMI and WC did not materially change the results (Supplementary Tables 2–Supplementary Data). The use of IP weights for potential selection bias in exposure change analyses increased the uncertainty of the results, but the qualitative results remained unchanged (Supplementary Table 5). Restricting the study population to healthy elderly did not change the results either (Supplementary Table 6).
Discussion
In this cohort of community-dwelling elderly from Brazil, type 2 diabetes and low normal BMI (18.5<20kg/m2) at baseline were associated with a higher mortality. Weight gain was protective against mortality, except in the obese elderly, and weight loss was associated with increased mortality irrespective of baseline weight. Analyses using measures of abdominal adiposity (ie, WC and WHR) showed similar results. Our results are consistent with either no harmful effect of overweight or obesity on mortality in the elderly or harmful effects that are being masked by bias due to confounding or measurement error. We found no harmful effects on mortality of higher WC and WHR, which reflect abdominal adiposity better than BMI. These findings reduce the possibility of bias due to measurement error, but confounding remains a concern. Specifically, an underlying disease that is causing both weight loss and increased mortality could create a situation where overweight and obesity would seem to be harmless, despite the presence of true harmful effects. We also observed a lower risk of mortality in elderly who gained weight and higher mortality in those who lost weight during follow-up, which further strengthens the case for lack of a true harmful effect for weight gain on mortality in the healthy elderly population.
Our result of the lack of association between higher adiposity and mortality in the elderly is consistent with the findings of several previous cohort studies, including one from a different Brazilian cohort (9,10,12,15,27–30). Pooled analysis of two European elderly cohorts found that BMI levels in the overweight range were associated with the lowest mortality risk (13). The effect of obesity on mortality in the elderly, if present, may be modest (8). Although some previous analyses found that a high WC was associated with higher mortality in the elderly (4,8), we could not replicate these findings. A potential explanation is that nonoptimal body weight may play a causal role in atherosclerosis early in life but loses its importance in the elderly, in whom the atherosclerotic process may be already established. Several pooled analyses of prospective studies have reported larger relative risks for BMI in younger ages (31,32). Unfortunately, we did not have measures of adiposity in younger ages for this cohort.
When we considered the effects of adiposity change on mortality, we observed a harmful effect of weight loss in all baseline BMI groups and a protective effect of weight gain in all groups, except obese elderly. Similar results were found for WC. An earlier study, using data from the Cardiovascular Health Study, found similar results regarding the effects of weight loss on mortality; however, no protective effect of weight gain was observed (33). A possible explanation for our findings is that weight gain may indicate the absence of underlying wasting conditions or additional energy reserves that can be helpful in acute catabolic illness. The increased mortality associated with diabetes has been consistently reported in other elderly cohort studies (24,34–37).
The results of our analysis should be interpreted with some limitations in mind. Our main measure of adiposity, BMI, is a rather poor measure of body fat and metabolic abnormalities in the elderly. Some individuals with a normal BMI have a high-risk metabolic profile (38), while some obese participants have a healthy profile (39). However, even the use of measures of abdominal adiposity showed similar results. Another limitation is that almost one in six participants were lost to follow-up, and those who were lost were on average younger and had better overall health than those who remained in the study. We used IP weights to adjust for potential bias due to differential loss to follow-up, and the results remained the same. We did not have enough events to conduct the analysis for specific causes of death; information on nonfatal events was not available either. We could not exclude deaths that occurred early in the follow-up to limit confounding by underlying disease, as that would reduce the number of events substantially. Finally, although we found no deleterious effect of weight gain on mortality, we did not consider other health outcomes, including physical disability (40).
On the other hand, our study has several advantages. The BMI, WC, and WHR were measured using standard methods during a detailed physical examination. To avoid confounding factors, we excluded participants who were underweight at baseline or had a history of cancer. We also adjusted for a large number of potential confounders in the analysis using high-quality data. We explored the relationship between adiposity and mortality with different measures of adiposity and conducted a set of comprehensive sensitivity analyses that showed that the results were fairly robust.
In summary, we found a protective effect for weight gain and increased mortality after weight loss in a cohort of community-dwelling elderly in Brazil. Further studies should explore these associations in similar settings when various measures of adiposity are measured more frequently and starting earlier in life to evaluate the long-term impact of weight gain or weight loss on mortality in the elderly. Moreover, novel measures of adiposity may be required for the elderly population to capture the potential harmful effects of adiposity on metabolic and cardiovascular diseases.
Funding
This work was supported by the Swiss Re–HSPH collaboration fellowship (to C.K.S.); the National Institute of Health (DK090435); Sao Paulo Research Foundation (FAPESP) (1999/051257, 2005/549472, and 2009/537783 to M.L.L. and Y.A.D.).
Supplementary Material
References
- 1. Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi:10.1016/S0140-6736(10)62037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Beydoun MA. The obesity epidemic in the United States—Gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi:10.1093/epirev/mxm007 [DOI] [PubMed] [Google Scholar]
- 3. Campos MA, Pedroso ER, Lamounier JA, Colosimo EA, Abrantes MM. [Nutritional status and related factors among elderly Brazilians]. Rev Assoc Med Bras. 2006;52:214–221. doi:10.1590/S0104-42302006000400019 [DOI] [PubMed] [Google Scholar]
- 4. Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi:10.1056/NEJMoa0801891 [DOI] [PubMed] [Google Scholar]
- 5. Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi:10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi:10.1016/S0140-6736(11)60105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–729. doi:10.1056/NEJMoa1010679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59. doi:10.1111/j.1467-789X. 2006.00248.x [DOI] [PubMed] [Google Scholar]
- 9. Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the longitudinal study of aging. J AmGeriatr Soc. 2001;49:968–979. doi:10.1046/j.1532-5415.2001.49189.x [DOI] [PubMed] [Google Scholar]
- 10. Dahl AK, Fauth EB, Ernsth-Bravell M, Hassing LB, Ram N, Gerstof D. Body mass index, change in body mass index, and survival in old and very old persons. J Am Geriatr Soc. 2013;61:512–518. doi:10.1111/jgs.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wijnhoven HAH, van Bokhorst-de van der Schueren MAE, Heymans MW, et al. Low mid-upper arm circumference, calf circumference, and body mass index and mortality in older persons. JGerontol Ser A-Biol Sci Med Sci. 2010;65:1107–1114. doi:10.1093/gerona/glq10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han SS, Kim KW, Kim K-I, et al. Lean mass index: a better predictor of mortality than body mass index in elderly Asians. J Am Geriatr Soc. 2010;58:312–317. doi:10.1111/j.1532-5415.2009.02672.x [DOI] [PubMed] [Google Scholar]
- 13. Drumond Andrade FC, Mohd Nazan AI, Lebrão ML, de Oliveira Duarte YA. The impact of body mass index and weight changes on disability transitions and mortality in Brazilian older adults. J Aging Res. 2013;2013:905094. doi:10.1155/2013/905094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hwang LC, Chen SC, Chen CJ. Increased risk of mortality from overweight and obesity in middle-aged individuals from six communities in Taiwan. J Formos Med Assoc. 2011;110:290–298. doi:10.1016/S0929-6646(11)60044-2 [DOI] [PubMed] [Google Scholar]
- 15. Beleigoli AM, Boersma E, Diniz Mde F, Lima-Costa MF, Ribeiro AL. Overweight and class I obesity are associated with lower 10-year risk of mortality in Brazilian older adults: the Bambuí Cohort Study of Ageing. PLoS One. 2012;7:e52111. doi:10.1371/journal.pone.0052111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Albala C, Lebrao ML, Diaz EML, et al. The health, well-being, and aging (“SABE”) survey: methodology applied and profile of the study population. Revista Panamericana De Salud Publica—Pan American Journal of Public Health. 2005;17:307–322. doi:10.1590/S1020-49892005000500003 [DOI] [PubMed] [Google Scholar]
- 17. Lebrao ML, Laurenti R. Saude, bem-estar e envelhecimento: o estudo SABE no Municipio de Sao Paulo. Revista Brasileira de Epidemiologia. 2005;8:127–141. doi:10.1590/S1415-790X2005000200005 [Google Scholar]
- 18. WHO. Obesity: Preventing and Managing the Global Epidemic. Geneva: World Health Organization; 1999. [PubMed] [Google Scholar]
- 19. WHO. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. Geneva: World Health Organization; 2008. [Google Scholar]
- 20. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi:10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 21. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi:10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 22. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 23. Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol Ser A Biol Sci Med Sci. 2001;56:M366–M72. doi:10.1093/gerona/56.6.M366 [DOI] [PubMed] [Google Scholar]
- 24. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi:10.1136/bmj.38678.389583.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. doi:10.1007/s10985-008-9082-4 [Google Scholar]
- 26. Li L, Shen C, Li X, Robins JM. On weighting approaches for missing data. Stat Methods Med Res. 2013;22:14–30. doi:10.1177/0962280211403597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Victoria Zunzunegui M, Teresa Sanchez M, Garcia A, Ribera Casado JM, Otero A. Body mass index and long-term mortality in an elderly mediterranean population. J Aging Health. 2012;24:29–47. doi:10.1177/0898264311408419 [DOI] [PubMed] [Google Scholar]
- 28. Somes GW, Kritchevsky SB, Shorr RI, Pahor M, Applegate WB. Body mass index, weight change, and death in older adults—The systolic hypertension in the elderly program. Am J Epidemiol. 2002;156:132–138. doi:10.1093/aje/kwf019 [DOI] [PubMed] [Google Scholar]
- 29. Thomas F, Pannier B, Benetos A, Vischer UM. Visceral obesity is not an independent risk factor of mortality in subjects over 65 years. Vasc Health Risk Manag. 2013;9:739–745. doi:10.2147/VHRM.S49922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clark DO, Gao S, Lane KA, et al. Obesity and 10-year mortality in very old African Americans and Yoruba-Nigerians: exploring the obesity paradox. J Gerontol A Biol Sci Med Sci. 2014;69:1162–1169. doi:10.1093/gerona/glu035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams KF, Leitzmann MF, Ballard-Barbash R, et al. Body mass and weight change in adults in relation to mortality risk. Am J Epidemiol. 2014;179:135–144. doi:10.1093/aje/kwt254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. doi:10.1371/journal.pone.0065174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65:63–70. doi:10.1093/gerona/glp050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stengard JH, Tuomilehto J, Pekkanen J, et al. Diabetes mellitus, impaired glucose tolerance and mortality among elderly men: the Finnish cohorts of the Seven Countries Study. Diabetologia. 1992;35:760–765. doi:10.1007/BF00429097 [DOI] [PubMed] [Google Scholar]
- 35. Waugh NR, Dallas JH, Jung RT, Newton RW. Mortality in a cohort of diabetic patients. Causes and relative risks. Diabetologia. 1989;32:103–104. doi:10.1007/BF00505181 [DOI] [PubMed] [Google Scholar]
- 36. Roche MM, Wang PP. Sex differences in all-cause and cardiovascular mortality, hospitalization for individuals with and without diabetes, and patients with diabetes diagnosed early and late. Diabetes Care. 2013;36:2582–2590. doi:10.2337/dc12-1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barnett KN, McMurdo ME, Ogston SA, Morris AD, Evans JM. Mortality in people diagnosed with type 2 diabetes at an older age: a systematic review. Age Ageing. 2006;35:463–468. doi:10.1093/ageing/afl019 [DOI] [PubMed] [Google Scholar]
- 38. Conus F, Rabasa-Lhoret R, Peronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab. 2007;32:4–12. doi:10.1139/H06-092 [DOI] [PubMed] [Google Scholar]
- 39. Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes. 2011;35:971–981. doi:10.1038/ijo.2010.216 [DOI] [PubMed] [Google Scholar]
- 40. Stenholm S, Sainio P, Rantanen T, et al. High body mass index and physical impairments as predictors of walking limitation 22 years later in adult Finns. J Gerontol A Biol Sci Med Sci. 2007;62:859–865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.