Abstract
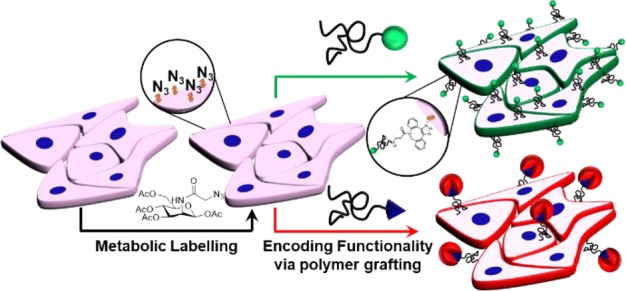
Re-engineering mammalian cell surfaces enables modulation of their phenotype, function, and interactions with external markers and may find application in cell-based therapies. Here we use metabolic glycan labeling to install azido groups onto the cell surface, which can act as anchor points to enable rapid, simple, and robust “click” functionalization by the addition of a polymer bearing orthogonally reactive functionality. Using this strategy, new cell surface functionality was introduced by using telechelic polymers with fluorescence or biotin termini, demonstrating that recruitment of biomacromolecules is possible. This approach may enable the attachment of payloads and modulation of cell function and fate, as well as providing a tool to interface synthetic polymers with biological systems.
Cell-based therapies are potent tools in modern medicine, from blood transfusions and bone marrow transplants, to rapidly emerging treatments such as stem cell and CAR-T therapy.1−4 However, these cells are limited in their native functionality and phenotype. In contrast, synthetic polymer–protein conjugates have shown significant success in improving therapeutic efficacy by increasing stability, circulation half-lives and storage.5 Such benefits have made PEGylated (poly(ethyene glycol)-grafted) proteins the gold standard in the pharmaceutical industry.6−10 Covalent polymer reformulation of cell-based therapies is the next frontier to aid translation, add non-natural functionality, such as imaging agents and/or loading of additional cargo (e.g., therapeutic drugs), and to improve logistics.11−13 Therefore, re-engineering of mammalian cell surfaces with synthetic (or natural) polymers is a valuable tool for biomedicine and biotechnology.
Polymer-coated islet cells have been the subject of study to mask cell-surface antigens, minimizing graft rejection in xenogeneic and allogenic transplants, while retaining biological function.14−19 Cell PEGylation methods used to achieve this include N-hydroxy succinimide and biotin.20−23 Similarly, PEGylated erythrocytes can improve blood transfusion compatibility by blocking AB antigens, while also reducing malaria parasite binding and preventing diseases characterized by impaired blood flow or vaso-occlusion.24−28 Despite cell-surface engineering holding great promise, challenges remain in the design of clinical-translatable and effective methods. A successful polymer-remodelling approach should be simple, versatile (i.e., applicable to multiple cell types), bioorthogonal,29 and provide additional functionality, while retaining normal membrane function.
Polyelectrolytes have been investigated for noncovalent electrostatic deposition onto cell membranes for translational applications due to their widespread use as polymer coatings.30,31 However, polycations can disrupt the (anionic) cell membrane and rapidly reduce cell viability. Cell viability is reduced even with the incorporation of PEG chains to minimize contact of polyelectrolytes with the lipid bilayer.30−33 The susceptibility of nucleated mammalian cells to mechanical and chemical stress also limits the covalent polymer conjugation methods that may be employed. Hawker and co-workers used a “grafting from” approach where ATRP initiators were immobilized onto yeast cells, followed by “grafting from” polymerization.34 This approach was found to exert stress on mammalian cells, leading to significant cytotoxicity and hence was not broadly useful for biomedical application.34
Genetic tools allow modulation of cell function by introducing or knocking out/silencing genes but, in most cases, they are not adaptable to accept synthetic components. Non-natural amino acids (with bioorthogonal functionality) are challenging to install at specific sites in whole cells.35 Therapeutic value is also limited due to tedious and costly transfection procedures along with safety and ethical concerns.36,37 As such, exploring novel cytocompatible methods of polymer conjugation to cell surfaces remains a challenging area of biomaterial science.
Cell-surface glycans play major metabolic, structural, and recognition roles in biology. Glycan metabolic labeling, a technique pioneered by Bertozzi et al.,29,38 allows azido groups (or other bioorthogonal handles)39 to be incorporated into specific cell-surface glycans by “hijacking” oligosaccharide biosynthesis pathways, such as the sialic acid biosynthetic pathway. This tool provides an extremely versatile approach to reprogram cell surfaces using only chemical, rather than genetic, methods. Shi et al. tagged azido-labeled cells with alkynyl-PEG-β-cyclodextrin and photoswitchable azobenzene-MUC1 aptamer to controllably target epithelial cancer cells (MCF-7) and, thus, promote the formation of T-cell cancer cell assembly.40 Furthermore, metabolic labeling has been used to selectively label cancer cells in vivo through the installation of “caged” azido sugars, which are cleaved by cancer-overexpressed enzymes.41 Liposomal delivery of azido-glycans can also be used for selective cell labeling, targeting overexpressed receptors on cells.42 This presents a unique opportunity in biomaterials science to take advantage of controlled polymerization techniques43 to enable selective introduction of biorthogonal “click” functionality,44 allowing cell surfaces to be re-engineered through purely chemical means.
Here we covalently graft well-defined RAFTed (reversible addition–fragmentation transfer) polymers onto live cells, which have been metabolically labeled using azido-glycans. Telechelic hydrophilic polymers are subsequently “clicked” onto the modified glycans using strained alkynes, enabling rapid, specific, and biocompatible reprogramming of the cell surface and the introduction of non-native functionality.
Poly(hydroxylethyl acrylamide) (pHEA) was selected for cell-surface remodelling due to its versatility, water solubility, and surface passivation capabilities.45 A pentafluorophenyl (PFP) ester functionalized RAFT agent was employed to synthesize telechelic polymers (PFP-pHEA), Figure 1. Polymers were characterized by SEC, 1H, 13C, and 19F NMR, and infrared spectroscopy (IR), Table 1 and Supporting Information. Two different chain lengths (DP50 and 100) were prepared with low dispersities, <1.2. Azide-reactive functionality was installed using dibenzocycloctyne-amine (DBCO-NH2) to displace the PFP ester, confirmed by the removal of PFP peaks in 19F NMR and IR spectra (Supporting Information). During functionalization, the trithiocarbonate was cleaved revealing a thiol, which was coupled to either fluorescein or biotin maleimide. Conjugation was confirmed via fluorescence spectroscopy after exhaustive dialysis (Supporting Information).
Figure 1.
Telechelic polymer synthesis. (A) RAFT polymerization with PFP-RAFT agent; (B) α,ω-functionalization with DBCO-NH2, followed by (i) fluorescein maleimide or (ii) biotin maleimide. ACVA = 4,4 Azobis(4-cyanovaleric acid).
Table 1. pHEA Precursor Polymers.
| code | [M]:[CTA] (−) | conv.a (%) | Mn(theo)b (g/mol) | Mn(SEC)c (g/mol) | Đc |
|---|---|---|---|---|---|
| PFP-pHEA100 | 100 | 91.3 | 13000 | 15000 | 1.15 |
| PFP-pHEA50 | 50 | 98.2 | 8300 | 9700 | 1.17 |
Determined by 1H NMR against an internal mesitylene standard.
Determined by the [M]:[CTA] ratio and conversion, assuming 100% CTA efficiency.
Determined by SEC in DMF against PMMA standards.
Following polymer synthesis, cell-surface glycans were metabolically labeled.38 Adenocarcinomic human alveolar basal epithelial cells (A549 cells), a stable model cell line, were incubated with ManNAz (tetraacylated N-azidoacetylmannosamine), which enters the cell and “hijacks” the sialic acid biosynthetic pathway presenting azido groups on the cell surface.46 The presence of cell-surface azides was confirmed by the SPAAC (strain-promoted azide/alkyne click) reagent DBCO-Cy5 (Supporting Information). Confocal microscopy displayed strong localized fluorescence at the cell membrane of ManNAz treated cells, along with cytosolic staining due to nonspecific uptake of the dye (see below). Control cells presented no staining, demonstrating selectivity even in the presence of the myriad of intracellular and extracellular components.
A549 cells were cultured in the presence and absence (negative control) of ManNAz and were subsequently incubated with DBCO-pHEAn-FL at 1–10 mg/mL for 1 h (initial screening showed this was sufficient for labeling), Figure 2. The highly specific nature, and rapid kinetics, of azide–alkyne reactions allowed the conjugation process to be undertaken in complete cell media, removing risks of starvation and thus exertion of unnecessary cellular stress. Membrane-associated (green) fluorescence was observed in cells treated with ManNAz at all polymer concentrations used, in a dose-dependent manner. The absence of polymer-associated fluorescence in control cells (no ManNAz) confirmed that “click” conjugation occurred, rather than nonspecific mechanisms (such as membrane insertion)47 or cellular uptake (endocytosis).
Figure 2.
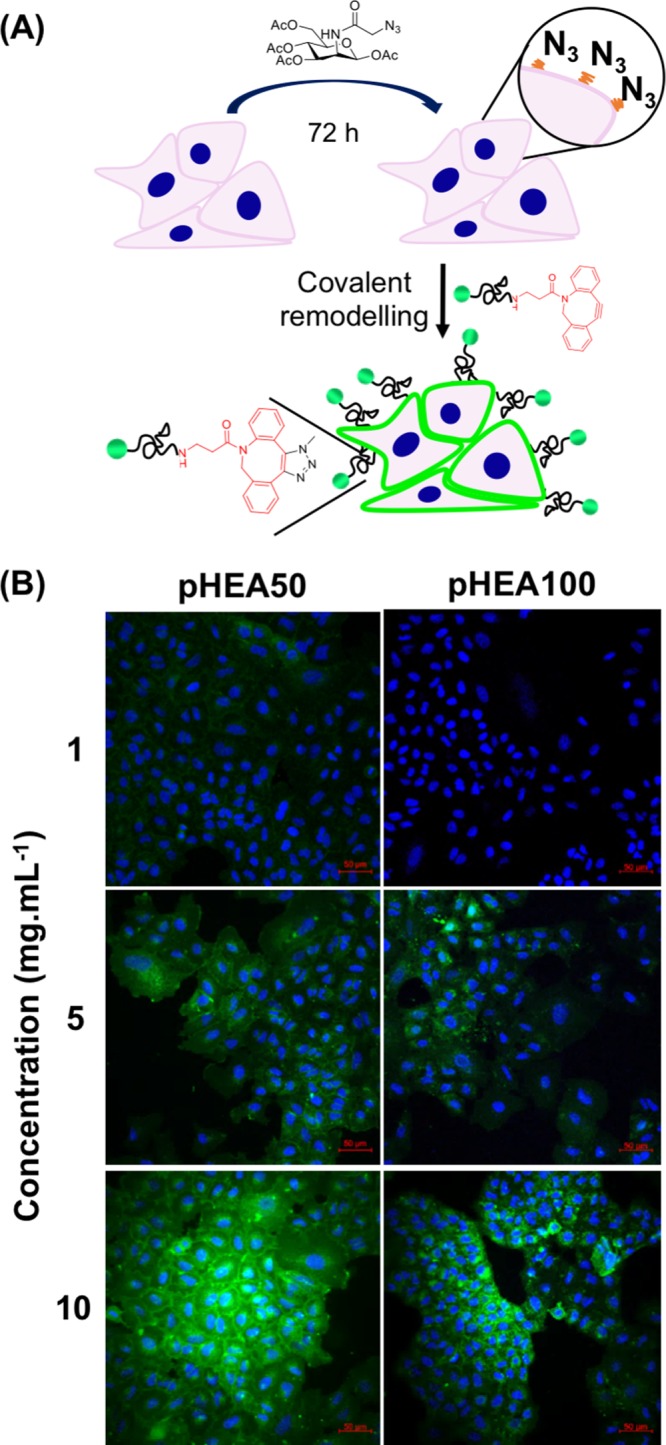
Covalent grafting of polymers to azido-labeled cells. (A) Incubation of A549 cells with ManNAz, followed by SPAAC at the cell surface; (B) Confocal images of ManNAz treated cells exposed to indicated concentrations of DBCO-pHEAn-FL (green) for 1 h (n = 3); Blue: DAPI (nuclear) stain. Scale bar = 50 μm.
Cell viability was assessed after 24 h of polymer exposure by the resazurin reduction assay (Supporting Information). At all concentrations there was no reduction in viability and the cell morphology was also unaffected by polymer incubation.
Compared to small molecule probes, which require DMSO solubilization, p(HEA)-DBCO polymers offered several advantages due to their high water solubility. DMSO promotes cell uptake, hence, small molecule probes lead to off-target cytosolic labeling, whereas the low cell permeability of water-soluble polymers ensures selective extracellular interactions. The approach presented here provides benefits compared to the “grafting from” approach used by Hawker et al., which required fine-tuning to avoid damage to cells, or the use of robust yeast cells.34 Confocal images of labeled cells (Figure 2) showed that DP50 polymers gave increased membrane labeling at all concentrations compared to DP100, potentially due to a combination of lower molar concentrations and increased steric hindrance. Hence, the macromolecular engineering of the grafted layer was crucial to the success of this methodology.
Having demonstrated successful cell labeling, the robustness of the polymer coating was evaluated, which is crucial due to the potential recycling of glycan anchors.48 Established methods of coating cells, such as lipid anchors,47 suffer from lack of robustness due to membrane recycling and polymer dissociation due to weak hydrophobic interactions.11 Polymer-labeled cells were imaged over 24 h by monitoring fluorescein (green) fluorescence, Figure 3. Image cytometry showed a relatively even distribution of labeling and degrafting across the population (Supporting Information). Total average fluorescence suggested that DP50 polymers retained over 2× greater surface coverage compared to DP100 polymers, even 24 h postconjugation. However, initial degrafting of pHEA50 occurred within 8 h whereas loss of pHEA100 occurred later, between 8 and 16 h following conjugation. Thus, this may present opportunities for temporal control over conjugation using polymer length. No evidence was found of increased intracellular green fluorescence, suggesting that the fate of cell surface polymers was degrafting rather than uptake and processing.
Figure 3.
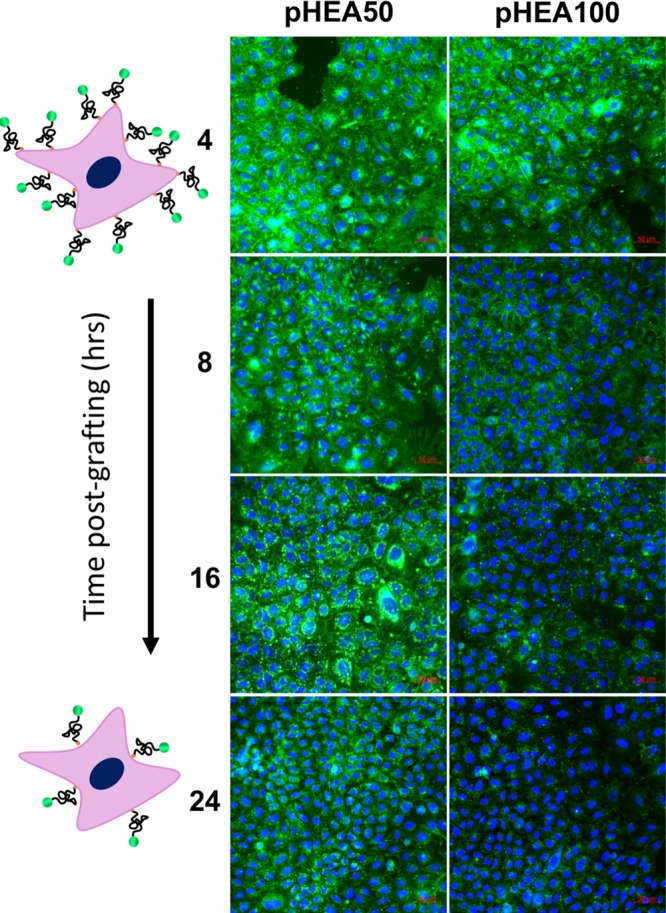
Grafting lifetime of glycan-immobilized polymers. Timelapse confocal images over 24 h are shown (n = 3); Blue: DAPI (nuclear) stain; Green: DBCO-pHEAn-FL polymer (10 mg/mL). Scale bar = 50 μm.
Following the above success, we wanted to demonstrate that additional functionality can be brought to the cell surface using these polymers while retaining availability of the nongrafted chain end (and not sterically limited by, for example, the glycocalyx). This approach enables encoding of additional information to the cell surface without using genetic engineering. Biotin-maleimide was introduced to the thiol chain end of polymers (Figure 1). Cells treated with azido sugars were subsequently functionalized with the biotinylated polymers, using the optimized procedures described above. Cy3-labeled (red) streptavidin was introduced either immediately, or 24 h after, incubation with polymers. Confocal images of pHEA-biotinylated cells after incubation with streptavidin demonstrated clear membrane associated red fluorescence consistent with recruitment of streptavidin to the cell surface, Figure 4. Due to the increased loading of the DP50 polymers, there was approximately double (values in Supporting Information) the recruitment extent of streptavidin compared to DP100. This model system demonstrates the versatility of this method to re-engineer cells with non-native functionality using accessible and versatile tools.
Figure 4.

Recruitment of streptavidin to polymer-remodelled cell surfaces. (A) Immobilization of DBCO-pHEAn-biotin to azido-functionalized A549 cells, followed by recruitment of Strepavidin-Cy3 (red); (B) Time lapse confocal images of cells after streptavidin recruitment (n = 3); Blue: DAPI (nuclear) stain. Scale bar = 50 μm.
In summary, we have covalently grafted synthetic polymers onto the surface of living cells. The tethering point is introduced to A549 cells by metabolic labeling of cell surface glycans by acetylated N-azidoacetylmannosamine (ManNAz). This azide was then used to anchor telechelic polymers via strain-promoted azide/alkyne “click” reaction. The optimized polymers remain on the cell surface for over 24 h, which is longer than reported for lipid-remodelling methods. Furthermore, the extent and durability of the grafting was found to be linked to the molecular weight of the polymer chain used. Additional functionality was introduced through biotin-terminated polymers, which were immobilized onto the cell surface and subsequently used to recruit fluorophore labeled streptavidin to the surface. Hence, demonstrating applicability in biomedical engineering and offering several advantages over genetic encoding methods. In this study, we have devised a versatile yet simple approach to remodel cell surfaces that will find application in cell-based therapies and for fundamental studies on cell surface interactions.
Acknowledgments
M.I.G. holds an ERC Starter Grant (CRYOMAT 638661). R.T. thanks EPSRC for a Ph.D. studentship through the EPSRC Centre for Doctoral Training in Molecular Analytical Science, (EP/L015307/1RT), and to B.M (EP/M506679/1). UoW Advanced BioImaging RTP BBSRC ALERT14 Award BB/M01228X/1 and polymer characterization RTP are thanked.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmacrolett.8b00675.
Experimental procedures and characterization data, plus additional binding experiments (PDF).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version. The research data supporting this publication can be accessed at http://wrap.warwick.ac.uk.
The authors declare no competing financial interest.
Supplementary Material
References
- Park J. H.; Rivière I.; Gonen M.; Wang X.; Sénéchal B.; Curran K. J.; Sauter C.; Wang Y.; Santomasso B.; Mead E.; Roshal M.; Maslak P.; Davila M.; Brentjens R. J.; Sadelain M. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378 (5), 449–459. 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J.; Oertle J.; Warren D.; Prato D. Chimeric Antigen Receptor (CAR) T Cell Therapy for Malignant Cancers: Summary and Perspective. J. Cell. Immunother. 2016, 2 (2), 59–68. 10.1016/j.jocit.2016.08.001. [DOI] [Google Scholar]
- Fraietta J. A.; Lacey S. F.; Orlando E. J.; Pruteanu-Malinici I.; Gohil M.; Lundh S.; Boesteanu A. C.; Wang Y.; O’connor R. S.; Hwang W. T.; Pequignot E.; Ambrose D. E.; Zhang C.; Wilcox N.; Bedoya F.; Dorfmeier C.; Chen F.; Tian L.; Parakandi H.; Gupta M.; Young R. M.; Johnson F. B.; Kulikovskaya I.; Liu L.; Xu J.; Kassim S. H.; Davis M. M.; Levine B. L.; Frey N. V.; Siegel D. L.; Huang A. C.; Wherry E. J.; Bitter H.; Brogdon J. L.; Porter D. L.; June C. H.; Melenhorst J. J. Determinants of Response and Resistance to CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy of Chronic Lymphocytic Leukemia. Nat. Med. 2018, 24 (5), 563–571. 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude S. L.; Laetsch T. W.; Buechner J.; Rives S.; Boyer M.; Bittencourt H.; Bader P.; Verneris M. R.; Stefanski H. E.; Myers G. D.; Qayed M.; De Moerloose B.; Hiramatsu H.; Schlis K.; Davis K. L.; Martin P. L.; Nemecek E. R.; Yanik G. A.; Peters C.; Baruchel A.; Boissel N.; Mechinaud F.; Balduzzi A.; Krueger J.; June C. H.; Levine B. L.; Wood P.; Taran T.; Leung M.; Mueller K. T.; Zhang Y.; Sen K.; Lebwohl D.; Pulsipher M. A.; Grupp S. A. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378 (5), 439–448. 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini R. J.; Lee J.; Maynard H. D. Trehalose Glycopolymers for Stabilization of Protein Conjugates to Environmental Stressors. J. Am. Chem. Soc. 2012, 134 (20), 8474–8479. 10.1021/ja2120234. [DOI] [PubMed] [Google Scholar]
- Borchmann D. E.; Carberry T. P.; Weck M. Bio”-Macromolecules: Polymer-Protein Conjugates as Emerging Scaffolds for Therapeutics. Macromol. Rapid Commun. 2014, 35 (1), 27–43. 10.1002/marc.201300792. [DOI] [PubMed] [Google Scholar]
- Larson N.; Ghandehari H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. 10.1021/cm2031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y.; Chilkoti A. Protein-Polymer Conjugation-Moving beyond PEGylation. Curr. Opin. Chem. Biol. 2015, 28, 181–193. 10.1016/j.cbpa.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. W.; Strickland R. A.; Schumacher F. F.; Caddick S.; Baker J. R.; Gibson M. I.; Haddleton D. M. Polymeric Dibromomaleimides as Extremely Efficient Disulfide Bridging Bioconjugation and Pegylation Agents. J. Am. Chem. Soc. 2012, 134 (3), 1847–1852. 10.1021/ja210335f. [DOI] [PubMed] [Google Scholar]
- Pelegri-Oday E. M.; Lin E. W.; Maynard H. D. Therapeutic Protein-Polymer Conjugates: Advancing beyond Pegylation. J. Am. Chem. Soc. 2014, 136 (41), 14323–14332. 10.1021/ja504390x. [DOI] [PubMed] [Google Scholar]
- Matthias T.; Irvine D. Enhancing Cell Therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials. Nano Today 2011, 6 (3), 309–325. 10.1016/j.nantod.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.; Bailey T. L.; Healey J. R. J.; Marcellini M.; Deville S.; Gibson M. I. Polyproline Is a Minimal Antifreeze Protein Mimetic and Enhances the Cryopreservation of Cell Monolayers. Angew. Chem., Int. Ed. 2017, 56, 15941–15944. 10.1002/anie.201706703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. E.; Lovett J. R.; Armes S. P.; Gibson M. I. Combining Biomimetic Block Copolymer Worms with an Ice-Inhibiting Polymer for the Solvent-Free Cryopreservation of Red Blood Cells. Angew. Chem., Int. Ed. 2016, 55 (8), 2801–2804. 10.1002/anie.201511454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H.; Fahmy T. M.; Metcalfe S. M.; Morton S. L.; Dong X.; Inverardi L.; Adams D. B.; Gao W.; Wang H. Immuno-Isolation of Pancreatic Islet Allografts Using Pegylated Nanotherapy Leads to Long-Term Normoglycemia in Full MHC Mismatch Recipient Mice. PLoS One 2012, 7 (12), e50265. 10.1371/journal.pone.0050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol S.; Del Guerra S.; Grupillo M.; Diaspro A.; Gliozzi A.; Marchetti P. Multilayer Nanoencapsulation. New Approach for Immune Protection of Human Pancreatic Islets. Nano Lett. 2006, 6 (9), 1933–1939. 10.1021/nl061049r. [DOI] [PubMed] [Google Scholar]
- Gattás-Asfura K. M.; Stabler C. L. Bioorthogonal Layer-by-Layer Encapsulation of Pancreatic Islets via Hyperbranched Polymers. ACS Appl. Mater. Interfaces 2013, 5 (20), 9964–9974. 10.1021/am401981g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi P.; Luca G.; Mancuso F.; Schoubben A.; Calvitti M.; Giovagnoli S.; Basta G.; Becchetti E.; Ricci M.; Calafiore R. Conformal Polymer Coatings for Pancreatic Islets Transplantation. Int. J. Pharm. 2013, 440 (2), 141–147. 10.1016/j.ijpharm.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Teramura Y.; Kaneda Y.; Iwata H. Islet-Encapsulation in Ultra-Thin Layer-by-Layer Membranes of Poly(Vinyl Alcohol) Anchored to Poly(Ethylene Glycol)-Lipids in the Cell Membrane. Biomaterials 2007, 28 (32), 4818–4825. 10.1016/j.biomaterials.2007.07.050. [DOI] [PubMed] [Google Scholar]
- Cabric S.; Sanchez J.; Lundgren T.; Foss A.; Felldin M.; Källen R.; Salmela K.; Tibell A.; Tufveson G.; Larsson R.; Korsgren O.; Nilsson B. Islet Surface Heparinization Prevents the Instant Blood-Mediated Inflammatory Reaction in Islet Transplantation. Diabetes 2007, 56 (8), 2008–2015. 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- Teramura Y.; Kaneda Y.; Totani T.; Iwata H. Behavior of Synthetic Polymers Immobilized on a Cell Membrane. Biomaterials 2008, 29 (10), 1345–1355. 10.1016/j.biomaterials.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Teramura Y.; Iwata H. Islets Surface Modification Prevents Blood-Mediated Inflammatory Responses. Bioconjugate Chem. 2008, 19 (7), 1389–1395. 10.1021/bc800064t. [DOI] [PubMed] [Google Scholar]
- Rengifo H. R.; Giraldo J. A.; Labrada I.; Stabler C. L. Long-Term Survival of Allograft Murine Islets Coated via Covalently Stabilized Polymers. Adv. Healthcare Mater. 2014, 3 (7), 1061–1070. 10.1002/adhm.201300573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gołąb K.; Kizilel S.; Bal T.; Hara M.; Zielinski M.; Marek-trzonkowska N.; Millis J. M.; Trzonkowski P. Improved Coating of Pancreatic Islets with Regulatory T Cells (Tregs) to Create Local Immunosuppression by Using Biotin- Polyethylene Glycol-Succinimidyl Valeric Acid Ester Molecule. Transplant. Proc. 2014, 46 (6), 1967–1971. 10.1016/j.transproceed.2014.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratty G. Progress in Modulating the RBC Membrane to Produce Transfusable Universal/Stealth Donor RBCs. Transfusion Medicine Reviews 2004, 18, 245–256. 10.1016/j.tmrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Garratty G. Modulating the Red Cell Membrane to Produce Universal/Stealth Donor Red Cells Suitable for Transfusion. Vox Sang. 2007, 0, 87–95. 10.1111/j.1423-0410.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- Bradley A. J.; Murad K. L.; Regan K. L.; Scott M. D. Biophysical Consequences of Linker Chemistry and Polymer Size on Stealth Erythrocytes: Size Does Matter. Biochim. Biophys. Acta, Biomembr. 2002, 1561 (2), 147–158. 10.1016/S0005-2736(02)00339-5. [DOI] [PubMed] [Google Scholar]
- Blackall D. P.; Armstrong J. K.; Meiselman H. J.; Fisher T. C. A-Specific Antibodies and Are Impervious to Invasion by the Plasmodium Falciparum Malaria Parasite Polyethylene Glycol – Coated Red Blood Cells Fail to Bind Glycophorin A – Specific Antibodies and Are Impervious to Invasion by the Plasmodium Falciparum Ma. Blood 2001, 97 (2), 551–556. 10.1182/blood.V97.2.551. [DOI] [PubMed] [Google Scholar]
- Stott M. D.; Murad K. L.; Kooumpouras F.; Talbot M.; Eaton J. W.; Scott M. D.; Koumpouras F. Chemical Camouflage of Antigenic Determinants: Stealth Erythrocytes. Proc. Natl. Acad. Sci. U. S. A. 1997, 94 (14), 7566–7571. 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescher J. A.; Bertozzi C. R. Chemistry in Living Systems. Nat. Chem. Biol. 2005, 1 (1), 13–21. 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- Lee D. Y.; Park S. J.; Lee S.; Nam J. H.; Byun Y. Highly Poly(Ethylene) Glycolylated Islets Improve Long-Term Islet Allograft Survival without Immunosuppressive Medication. Tissue Eng. 2007, 13 (8), 2133–2141. 10.1089/ten.2006.0009. [DOI] [PubMed] [Google Scholar]
- Wilson J. T.; Cui W.; Kozlovskaya V.; Kharlampieva E.; Pan D.; Qu Z.; Krishnamurthy V. R.; Mets J.; Kumar V.; Wen J.; Song Y.; Tsukruk V. V.; Chaikof E. L. Cell Surface Engineering with Polyelectrolyte Multilayer Thin Films. J. Am. Chem. Soc. 2011, 133 (18), 7054–7064. 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- Wilson J. T.; Cui W.; Chaikof E. L. Supporting Information Layer-by-Layer Assembly of a Conformal Nano-Thin PEO Coating for Intraportal Islet Transplantation. Nano Lett. 2008, 8 (7), 1940–1948. 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Bookstaver M. L.; Jewell C. M. Engineering Cell Surfaces with Polyelectrolyte Materials for Translational Applications. Polymers (Basel, Switz.) 2017, 9 (2), 1–21. 10.3390/polym9020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J.; Lunn D. J.; Pusuluri A.; Yoo J. I.; O’Malley M. A.; Mitragotri S.; Soh H. T.; Hawker C. J. Engineering Live Cell Surfaces with Functional Polymers via Cytocompatible Controlled Radical Polymerization. Nat. Chem. 2017, 9 (6), 537–545. 10.1038/nchem.2713. [DOI] [PubMed] [Google Scholar]
- Chin J. W. Expanding and Reprogramming the Genetic Code. Nature 2017, 550, 53–60. 10.1038/nature24031. [DOI] [PubMed] [Google Scholar]
- Naldini L. Gene Therapy Returns to Centre Stage. Nature 2015, 526, 351–360. 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- Verma I. M.; Weitzman M. D. GENE THERAPY: Twenty-First Century Medicine. Annu. Rev. Biochem. 2005, 74 (1), 711–738. 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- Saxon E.; Bertozzi C. R. Cell Surface Engineering by a Modified Staudinger Reaction. Science 2000, 287 (5460), 2007–2010. 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- Stairs S.; Neves A. A.; Stöckmann H.; Wainman Y. A.; Ireland-Zecchini H.; Brindle K. M.; Leeper F. J. Metabolic Glycan Imaging by Isonitrile-Tetrazine Click Chemistry. ChemBioChem 2013, 14 (9), 1063–1067. 10.1002/cbic.201300130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P.; Ju E.; Yan Z.; Gao N.; Wang J.; Hou J.; Zhang Y.; Ren J.; Qu X. Spatiotemporal Control of Cell-Cell Reversible Interactions Using Molecular Engineering. Nat. Commun. 2016, 7, 1–9. 10.1038/ncomms13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Wang R.; Cai K.; He H.; Liu Y.; Yen J.; Xu M.; Yiwen Sun X. Z. Selective in Vivo Metabolic Cell-Labeling-Mediated Cancer Targeting. Nat. Chem. Biol. 2017, 13, 415–424. 10.1038/nchembio.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R.; Hong S.; Feng L.; Rong J.; Chen X. Cell-Selective Metabolic Glycan Labeling Based on Ligand-Targeted Liposomes. J. Am. Chem. Soc. 2012, 134 (24), 9914–9917. 10.1021/ja303853y. [DOI] [PubMed] [Google Scholar]
- Boyer C.; Bulmus V.; Davis T. P.; Ladmiral V.; Liu J.; Perrier S. Bioapplications of RAFT Polymerization. Chem. Rev. 2009, 109 (11), 5402–5436. 10.1021/cr9001403. [DOI] [PubMed] [Google Scholar]
- McKay C. S.; Finn M. G. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. 10.1016/j.chembiol.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S.-J.; Gibson M. I. Optimization of the Polymer Coating for Glycosylated Gold Nanoparticle Biosensors to Ensure Stability and Rapid Optical Readouts. ACS Macro Lett. 2014, 3 (10), 1004–1008. 10.1021/mz5004882. [DOI] [PubMed] [Google Scholar]
- Laughlin S. T.; Bertozzi C. R. Metabolic Labeling of Glycans with Azido Sugars and Subsequent Glycan-Profiling and Visualization via Staudinger Ligation. Nat. Protoc. 2007, 2 (11), 2930–2944. 10.1038/nprot.2007.422. [DOI] [PubMed] [Google Scholar]
- Huang M. L.; Smith R. A. A.; Trieger G. W.; Godula K. Glycocalyx Remodeling with Proteoglycan Mimetics Promotes Neural Specification in Embryonic Stem Cells. J. Am. Chem. Soc. 2014, 136 (30), 10565–10568. 10.1021/ja505012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nischan N.; Kohler J. J. Advances in Cell Surface Glycoengineering Reveal Biological Function. Glycobiology 2016, 26 (8), 1–8. 10.1093/glycob/cww045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



