Abstract
Quality problem
Patients recently discharged from the intensive care unit (ICU) are at high risk for clinical deterioration.
Initial assessment
Unreliable and incomplete handoffs of complex patients contributed to preventable ICU readmissions. Respiratory decompensation was responsible for four times as many readmissions as other causes.
Choice of solution
Form a multidisciplinary team to address care coordination surrounding the transfer of patients from the ICU to the surgical ward.
Implementation
A quality improvement intervention incorporating verbal handoffs, time-sensitive patient evaluations and visual cues was piloted over a 1-year period in consecutive high-risk surgical patients discharged from the ICU. Process metrics and clinical outcomes were compared to historical controls.
Evaluation
The intervention brought the primary team and respiratory therapists to the bedside for a baseline examination within 60 min of ward arrival. Stakeholders viewed the intervention as such a valuable adjunct to patient care that the intervention has become a standard of care. While not significant, in a comparatively older and sicker intervention population, the rate of readmissions due to respiratory decompensation was 12.5%, while 35.0% in the control group (P = 0.28).
Lessons learned
The implementation of this ICU transition protocol is feasible and internationally applicable, and results in improved care coordination and communication for a high-risk group of patients.
Keywords: critical care, transitions in care, handoff
Background
Patients recently discharged from the intensive care unit (ICU) are a particularly vulnerable group, at risk for clinical deterioration due to the sharp decline in monitoring and resources that occurs during the transition to the surgical ward. Respiratory, cardiovascular and infectious complications are consistently cited as the most common reasons for deterioration, resulting in unplanned readmission rates to the ICU of between 3.9% and 13.4% [1–7].
Readmissions to the ICU have been shown to result in significantly increased lengths of stay, and therefore overall hospital costs, as well as a greater than 2-fold increased risk of mortality [2, 4, 8]. Given these significantly poorer outcomes, much research over the past two decades has focused on the identification of risk factors for readmission [9]. The Acute Physiology And Chronic Health Evaluation (APACHE) II score upon initial ICU admission, increasing age, some co-morbid conditions, some common laboratory markers and respiratory or heart rate abnormalities, while in the ICU, have all been implicated as predictors [10–12]. However, these predictors of readmission are inconsistent and therefore not reproducible, making the utility of predictive nomograms questionable [13].
More recent interest in readmissions as a quality metric has shifted the focus of ICU readmissions literature onto the identification of modifiable patient-centered processes of care associated with the transition out of the ICU [14–16]. Increased hospital occupancy at the time of discharge, discharge at night and inadequate continuity of care on the ward are all examples of systems factors associated with readmission [17, 18].
The lack of coordinated and effective handoff practices for patients transitioning out of the ICU leads to discontinuities in care and exposes an already vulnerable population to adverse patient safety events. In an effort to improve the transition of once critically ill patients to the ward, the concept of critical care outreach programs has emerged [19]. However, the structure of these teams and the availability of their resources alone fail to address the multidisciplinary coordination effort that is necessary to facilitate this tenuous transition.
Local problem
As part of the quality improvement (QI) structure at our institution, unit-based leadership teams meet regularly to discuss ward-specific issues. During a mortality review, it was noted that three patients who had recently been discharged from the ICU were readmitted and died. The preventability of each death was discussed, which prompted a more in-depth investigation into the supportive care provided to recently discharged ICU patients.
Despite having an established critical care outreach program at our institution, we noted a continued gap in care coordination for surgical patients recently transferred to the ward. During the mortality review, it was also noted that two safety reports were filed after the primary team residents unknowingly assumed care of newly transferred, tenuous patients overnight and were not notified. From that issue, we noted a high variability in the patient handoff practices between the critical care team (physicians, advanced practitioners and nurses) and the ward providers. Consistent sign out of patients pending transition out of the ICU was difficult given overall hospital bed flow. Therefore, handoff was frequently incomplete, and may have happened hours to days prior to a patient's physical transition to the ward. In addition, this sign out rarely included the ICU team's subjective intuitions for possible readmission, although these were often discussed amongst the ICU team. On the ward side, there was confusion from nursing regarding how physicians are notified of patient arrival. Hence, it rarely happened. It was also recognized that although respiratory therapy was an essential part of a patient's ICU care, they were not routinely involved in the post-ICU care. No handoffs routinely occurred between therapists, and often, ward nurses had difficulty locating the correct respiratory therapist in the event of an issue.
Given the tremendous potential for QI around the ICU transfer process, we sought to develop an intervention focused on the care coordination processes necessary to safely transfer patients at high risk for readmission to lower acuity care. By way of improving communication and support, a secondary aim was to decrease respiratory readmissions to the SICU. We herein describe the development and implementation of a time-sensitive, multidisciplinary transfer protocol. Success was measured by provider feedback and compliance with process metrics. Readmission to the ICU was analyzed as a secondary outcome.
Initial assessment
Setting and context
The intervention was implemented between a 24-bed surgical intensive care unit (SICU) and 2 surgical wards at a large, urban, Level I trauma, academic medical center located in the Northeastern USA. The hospital serves a population of more than 200,000 people consisting of predominantly Non-Hispanic Black/African-Americans (60.0–76.2% in the direct and surrounding neighborhoods, respectively). Between 17% and 36% of the population is Non-Hispanic White, and between 2% and 4% are Asian [19, 20].
The SICU is a 24-bed semi-open unit, primarily staffed by 2 critical care teams who communicate with the primary surgical services. About half of patients cared for in this SICU are trauma and emergency surgery service patients; however, patients from all other surgical specialties (except cardiac and neurosurgery) are also represented. The SICU team holds all ordering privileges, and when patients are tagged for transfer, are responsible for handing off clinical details to the co-managing team (which can be a junior resident or nurse practitioner). Patients transition primarily to two surgical wards, where the nurse:patient ratio is 1:4–5.
Planning the intervention
A multidisciplinary team with physician, advanced practitioner, nursing, respiratory therapy and resident representation from both the ICU and both surgical wards was formed to address care coordination surrounding the safe transfer of patients. In order to be comprehensive in our approach to an intervention, the team wanted to address both hospital-specific systems factors as well as clinical factors associated with readmission. To be more focused, the team distributed a one question survey to key stakeholder groups asking for identification of the three most common reasons for readmission to the ICU based on an Ishikawa diagram created by the team. Delayed recognition of acute patient issues (67.6% of respondents), bed allocation/overnight transfers (51.4%) and poor communication/handoffs (43.2%) were the most frequently chosen. Using data from our SICU outreach program to identify clinical factors related to readmission, we identified respiratory decompensation as the primary cause of readmission in the 2 years prior to this pilot, responsible for more than four times that of any other reason (i.e. sepsis, arrhythmia) and constituting 42% of all cases.
Choice of solution and implementation
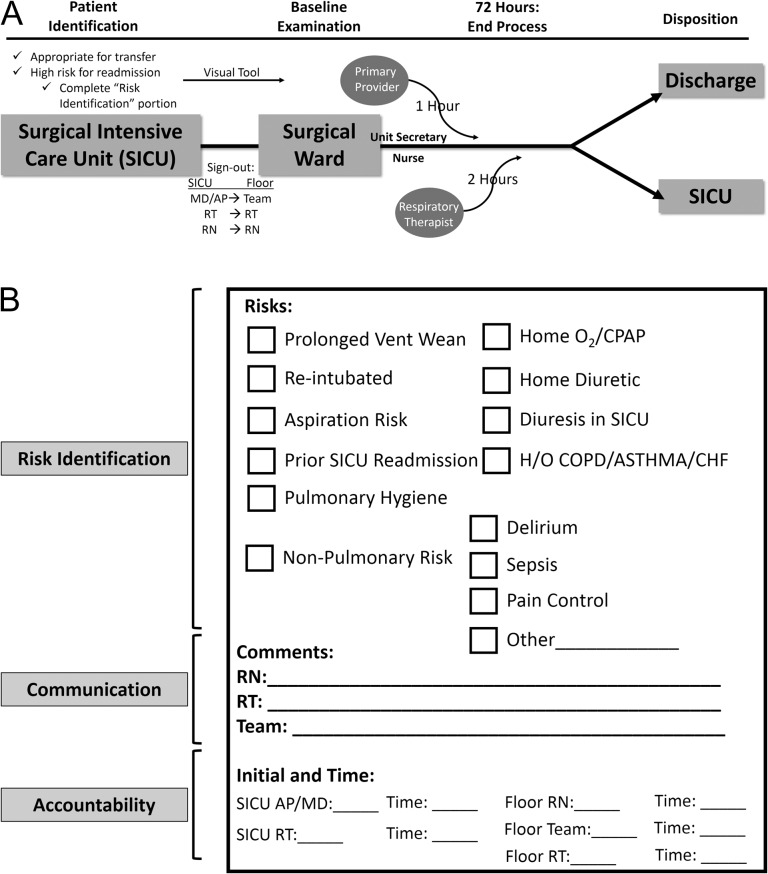
The final re-designed transfer process incorporated two elements: a highly coordinated, yet intuitive patient care communication algorithm and a tangible, interactive visual aid that literally brought providers to the bedside after ICU transfer (Fig. 1). The protocol is initiated in the SICU, where providers collectively identify patients eligible for SICU discharge, but who are deemed high risk for readmission. While readmission risk prediction tools for ICU patients are reported in the literature, their standard utility in clinical practice is less well accepted [21–24]. These tools are often complex and time consuming to use, and tend to vary widely in their performance characteristics and are therefore rarely reproducible. Fernandez et al. demonstrated that a subjective evaluation of mortality risk after ICU discharge is as important to consider as other objective physiologic predictors, as it accounts for unmeasurable dimensions of patient disease and care [25]. It is for these reasons that the designation of ‘high risk’ was assigned based largely on an overall subjective SICU team assessment. The specific reason for concern is then recorded on a fluorescent green sheet of paper (Fig. 1B), which is not a prediction tool, but a risk identifier and mechanism for accountability.
Figure 1.
(A) Conceptual model of the multidisciplinary intervention. SICU, surgical intensive care unit; RT, respiratory therapist; RN, registered nurse. (B) The main visual aid which accompanies the patient to the ward and hangs on the patient's door. The key areas addressed by the tool are noted: (i) risk identification, (ii) communication and (3) accountability.
All ICU practitioners, including respiratory therapists, are required to give a handoff to their respective ward counterpart and include explicit mention of the patient's readmission risk factors and their designation as part of the intervention (Colloquially, ‘the patient is a green sheet because …’). The fluorescent green sheet of paper (Fig. 1B) accompanies the patient to the ward and is displayed on the patient's door.
Once at their ward destination, the accepting nurse and/or ward clerk activates a ‘bounce-back risk’ flag on the hospital's existing patient navigation software system. This activation automatically text pages the receiving respiratory therapist. In addition, the accepting provider is alerted of the patient's arrival by the unit clerk or covering nurse. The expectation is that the primary team will see the patient within 1 h of the patient's ward arrival, and respiratory therapy will see the patient within 2 h, for baseline examination and care coordination. To document real-time completion, all providers write in their initials and timestamp the form. The fluorescent green sheet remains active on the patient's door for 72 h, or longer if desired, and care continues as necessary per patient needs.
Evaluation of the intervention
Stakeholder feedback
Process metrics were analyzed and a stakeholder-specific feedback survey was administered after 3 months of the intervention to gauge compliance and satisfaction with the process. The survey consisted of four common core yes/no response questions about the utility of the intervention as well as yes/no/multiple choice stakeholder-specific questions regarding perceptions of the process and implementation strategy. Survey data were collected and managed using REDCap electronic data capture tools hosted at the University of Pennsylvania. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies [26].
Clinical outcome assessment
After 1 year, process metrics were tabulated and patients’ clinical outcomes determined from chart review. Patients receiving the intervention over the course of 1 year were compared to a historical control group. The control group was drawn from a pre-existing ICU database that contained all patients transferred out of the SICU in the 8 months prior to the intervention. Those who were deemed high risk (using the same subjective scale that was available to the intervention patients), and who were also transferred to the same surgical wards as the intervention group were included as controls.
Demographic data, comorbidities, primary procedure/indication for ICU stay were obtained from the patient's history and physical examination note at the time of admission. A Charlson comorbidity index was calculated for each patient [27]. In addition, an APACHE II score based on physiologic measurements for the patient was also calculated for each patient following the first 24 h of the ICU stay [28]. Clinical outcomes, including SICU readmission, hospital and SICU length of stay, rate of re-intubation and mortality were obtained from medical records. Planned readmission to the SICU after an operative procedure was not counted as a readmission. For patients who were readmitted, each subsequent discharge from the SICU was counted as an independent encounter.
One possible unintended consequence of the intervention was diffusion of the key concepts of the intervention (communication and accountability) to patients who either had never been in the SICU but were perceived as high risk for SICU admission, or who were in the SICU but were not tagged to receive the intervention. In order to test this, we analyzed the number and time of day of rapid response calls (regardless of participation in the pilot) from the main surgical ward piloting the intervention.
Analysis
Descriptive statistics were used to summarize process measures and stakeholder feedback, and to compare patient characteristics and clinical outcomes between the control and intervention groups. Frequencies within the cohort are given for categorical data and measures of central tendency are given for continuous data as appropriate. Outcome variables were analyzed using the Chi-square, Student's t-test or Mann–Whitney tests as appropriate. A P value less than or equal to 0.05 was considered statistically significant. All analyses were performed using STATA, Version 12.1 (STATA Corp, College Station, TX). Statistical process control charts were generated using Minitab 17.2.1 (Minitab Inc., State College, PA).
This QI study was approved by the University of Pennsylvania Institutional Review Board.
Results
Patient characteristics
There were 335 (61.8%) patients identified within the 8-month control period and 207 (38.2%) high-risk patients who received the intervention in the subsequent 1-year period. Of the intervention patients, 171 (82.6%) were able to be identified and outcomes determined. A comparison of the patients in the control and intervention groups is presented in Table 1. Patients receiving the intervention were significantly older (60.5 ± 16.9 years vs. 55.9 ± 20.0 years; P = 0.01) and sicker on their initial ICU admission (APACHE score 20.8 ± 8.2 vs. 17.8 ± 7.7; P < 0.001). Notably, the groups did not differ by primary diagnosis/mechanism of injury (P = 0.13) or primary procedure (P = 0.16). Because the SICU cares for a relatively high number of trauma patients, the primary diagnoses at admission included a disproportionate number of falls, motor vehicle collisions and gunshot wounds.
Table 1.
Patient characteristics of the control and intervention groups
| Total (n = 506) | Control (n = 335) | Intervention (n = 171) | P value | |
|---|---|---|---|---|
| Male sex | 287 (56.7) | 199 (59.4) | 88 (51.5) | 0.09 |
| Age, mean (SD) | 57.4 (19.1) | 55.9 (20.0) | 60.5 (16.9) | 0.01 |
| Age group | ||||
| ≤40 years | 108 (21.3) | 84 (25.1) | 24 (14.0) | 0.03 |
| 41–60 years | 136 (26.9) | 89 (26.6) | 47 (27.5) | |
| 61–80 years | 202 (39.9) | 125 (37.3) | 77 (45.0) | |
| 80+ years | 60 (11.9) | 37 (11.0) | 23 (13.5) | |
| Race | ||||
| White | 276 (54.6) | 184 (54.9) | 92 (53.8) | 0.99 |
| Black | 161 (31.8) | 106 (31.6) | 55 (32.2) | |
| Asian | 8 (1.6) | 5 (1.5) | 3 (1.8) | |
| Other | 61 (12.1) | 40 (11.9) | 21 (12.3) | |
| Charlson Index | ||||
| 0 | 119 (23.5) | 87 (26.0) | 32 (18.7) | 0.19 |
| 1–2 | 120 (23.7) | 76 (22.7) | 44 (25.7) | |
| ≥3 | 267 (52.8) | 172 (51.3) | 95 (55.6) | |
| Apache score (mean, SD) | 18.6 (8.0) | 17.8 (7.7) | 20.8 (8.2) | <0.001 |
| Service | ||||
| Gastrointestinal surgery | 90 (17.8) | 55 (16.4) | 35 (20.5) | <0.001 |
| Emergency surgery | 135 (26.7) | 68 (20.3) | 67 (39.2) | |
| Trauma | 189 (37.4) | 138 (41.2) | 51 (29.8) | |
| Orthopedics | 10 (2.0) | 8 (2.4) | 2 (1.2) | |
| Other | 82 (16.2) | 66 (19.7) | 16 (9.4) | |
| Procedure | ||||
| Non-operative | 93 (18.4) | 70 (20.9) | 23 (13.5) | 0.16 |
| Exploratory laparotomy | 82 (16.2) | 49 (14.6) | 33 (19.3) | |
| Internal fixation/pinning | 58 (11.5) | 37 (11.0) | 21 (12.3) | |
| Bowel resection | 67 (13.2) | 40 (11.9) | 27 (15.8) | |
| Other | 206 (40.7) | 139 (41.5) | 67 (39.2) | |
| Primary diagnosis | ||||
| Fall | 73 (14.4) | 53 (15.8) | 20 (11.7) | 0.13 |
| Malignancy/mass | 52 (10.3) | 35 (10.5) | 17 (9.9) | |
| Motor vehicle collision | 41 (8.1) | 27 (8.1) | 14 (8.2) | |
| Gunshot wound | 38 (7.5) | 31 (9.3) | 7 (4.1) | |
| Othera | 302 (59.7) | 189 (56.4) | 113 (66.1) | |
a‘Other’ includes a diverse number of diagnoses which are too varied to categorize, including but not limited to: abdominal abscesses, aneurysms, ulcer disease, pancreatitis, diverticulitis, gastrointestinal hemorrhage, bowel obstruction, necrotizing fasciitis, sepsis and bowel ischemia.
Process measures
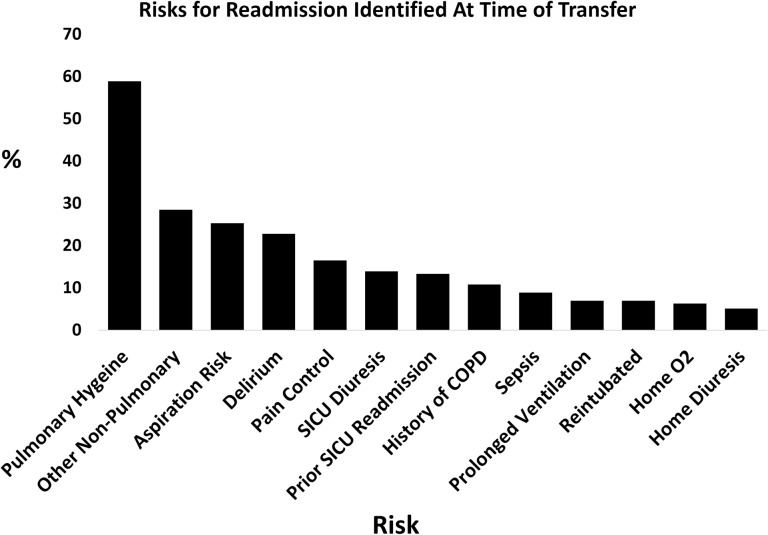
During year-long intervention period, the process completion rate was at least 82%. The most commonly cited risks for readmission were a concern for pulmonary hygiene (58.9%), aspiration (25.3%) and delirium (22.8%). Non-pulmonary risks such as cardiac arrhythmias, gastrointestinal bleeding and electrolyte imbalances were also common (Fig. 2). The ‘comment’ section of the visual aid was utilized 83.0% of the time, and at least one member of the ward team initialed 91.2% of the time. Respiratory therapists commented and initialed most frequently (65.5% and 74.9%, respectively). Many comments addressed the patient's current state on arrival or gave special recommendations (aspiration precautions, the use of positive pressure therapy, etc.). Respiratory therapy was able to see the patient within the designated time frame 74.7% of the time, and the primary team 50.0% of the time. Median times for baseline provider examination 55 min (IQR 30120) for respiratory therapy and 30 min (IQR 16.5–89.5) for the primary team.
Figure 2.
Frequency of risks for readmission identified by ICU providers and denoted on the visual aid. Percentages add to >100% because multiple risk factors can be checked on each tool.
Stakeholder feedback
There were 52 respondents to the stakeholder survey; 19 ward nurses, 8 residents and 3 advanced practitioners from the primary team, 2 SICU residents, 4 SICU advanced practitioners, 5 ward respiratory therapists, 10 SICU therapists and 1 unknown. Not all respondents answered every question. Overall, 60% (30 of 50) felt that the intervention improved the care of their patient, and 71% (36 of 51) wanted to see the intervention become standard of care. Of the targeted modalities on the green sheet visual aid (risk identification, communication and accountability), 50% (24/48) felt that communication was most improved, followed by risk identification (27%; n = 13) and accountability (23%; n = 11). The majority of nurses, 67% (12/18) felt more supported as a result of the GS, and 85% (16/19) felt that communication was improved with the team, respiratory therapy, or both. The most frequent action taken by the primary provider team was more frequent communication with nursing and respiratory therapy. Eighty-five percent (28/33) felt that the 72 h lifetime of the green sheet was ‘just right’.
Patient outcomes
The overall 72-h readmission rate for high-risk patients discharged from the SICU to the two surgical wards was 5.5% (28/506). There were 20 readmissions in the control group (6.0%) and 8 (4.7%) in the intervention group (P = 0.68). The rate of readmissions due to respiratory decompensation was 35.0% (7/20) in the control group and 12.5% (1/8) in the intervention group (P = 0.28) (Appendix A). Of all those readmitted, the rate of re-intubation was 28.6% (8/28); 30.0% (6/20) in the control and 25.0% (2/8) in the intervention (1.00). The rate of death in those readmitted was 30.0% (6/20) in the controls and 37.5% (3/8) in the intervention group (P = 0.70). Of the deaths, 1 patient was readmitted for respiratory distress (intervention group). The average age of those who died was 68.5 years in the control group and 83.0 years in the intervention group.
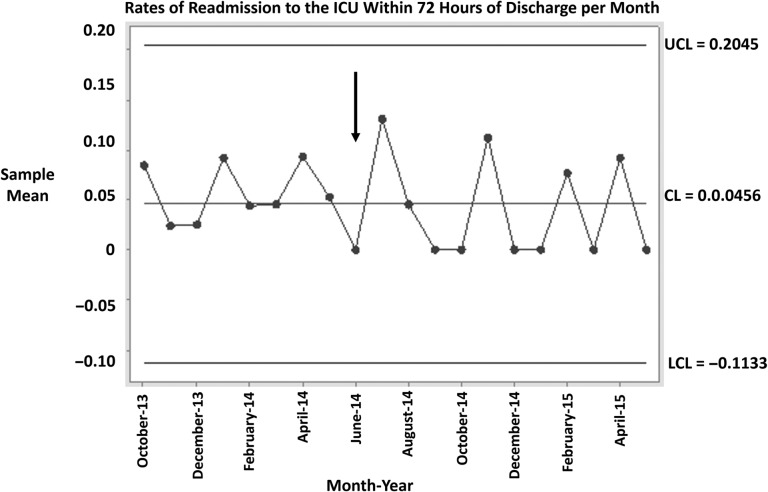
Figure 3 shows a statistical process control chart representing the rates of readmission within 72 h of SICU discharge per month both before and after the intervention (denoted with an arrow). Control charts are commonly used in QI to tease out the difference between variation that has occurred as a result of an intervention and the occurrence of natural variation [29]. A commonly accepted dictum is that seven consecutive points above or below the central mean represents a significant change in an outcome of interest. While Figure 3 does not demonstrate a significant change, there is a visible trend in readmission rates, with more points falling below the line post-intervention.
Figure 3.
Process control chart depicting rates of readmission to the SICU within 72 h of transfer to ward. The vertical arrow denotes the start of the intervention. UCL, upper control limit; LCL, lower control limit; CL, center line or mean.
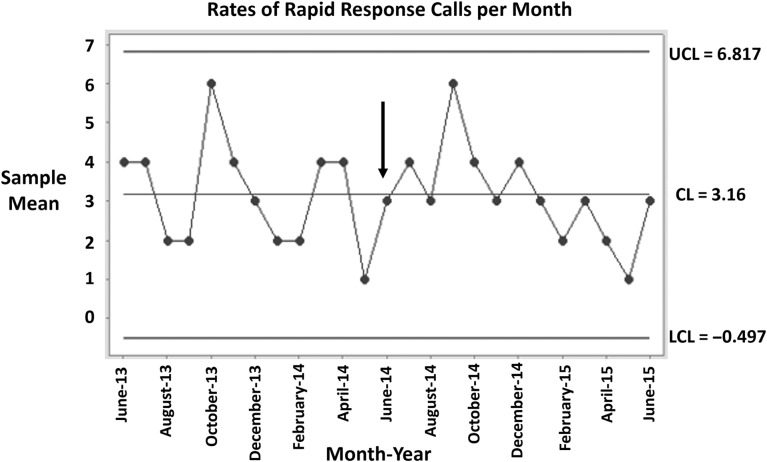
There were 41 rapid response calls in the year preceding the intervention and 38 in the year during the intervention. Pre-intervention, 58.5% of calls happened between the hours of 6 pm and 6 am, while in the intervention year, the majority (57.9%) occurred during the daytime (6 am–6 pm). Figure 4 depicts a process control chart for the number of rapid response calls per month on the wards participating in the intervention. Notably, the last 6 months (points) shown in the chart are all below the central mean, demonstrating a nearly significant change in the number of rapid response calls in the months following the intervention.
Figure 4.
Process control chart depicting the number of rapid response calls per month in the year preceding and in the year after intervention. The vertical arrow indicates start of the intervention. UCL, upper control limit; LCL, lower control limit; CL, center line or mean.
Discussion and lessons learned
Using only the efforts of a dedicated multidisciplinary team, an improved SICU to ward transfer process was successfully implemented for patients at high risk of readmission. This process resulted in improved communication and multidisciplinary engagement, smoother transition processes and greater staff satisfaction. We have shown that the involvement of a multidisciplinary team and a visual aid that brings increased awareness to potential patient issues can alter the reasons for readmission. Furthermore, we utilized resources already in place in our hospital to optimize a highly coordinated process in order to circumvent the need for additional funding.
In a review of factors contributing to the ICU discharge process, teamwork was rarely studied [30]. In this process, we combine efforts with the SICU team in order to extend the outreach services to incorporate other providers and to further empower the ward team to take ownership of the patient's issues. To the best of our knowledge, this is the first description of a comprehensive, resource non-intensive, feasible and practical initiative to improve the transition process from the SICU to the surgical ward.
During year-long intervention period, the process completion rate was at least 82% (for those that had trackable outcomes). The uptake of other QI initiatives is cited in the literature as anywhere from 74% to 94% [31]. This demonstrates that despite the façade of a highly complex process (many moving parts), the responsibilities of each stakeholder group were small but meaningful. The workflow of each individual group did not need to change dramatically, and therefore, the process was easier for stakeholders to adopt.
This intervention has effectively brought providers to the bedside in a timely fashion by combining a physical tool with a well thought out and beneficial process. A high proportion of patients are being seen within an hour or two of arrival to the ward by both the primary team and for an initial respiratory therapy evaluation. This ensures that the primary caregivers are equipped with a baseline examination of a high-risk patient, better preparing them for future care, and for preparing the next group of providers at the time of handoffs. Furthermore, this brings the entire care team (nurses, residents/advanced practitioners and respiratory therapists) in nearly immediate communication and physical proximity with one another as soon as the patient arrives on the ward. Finally, the extremely high utilization rate of the ‘comments’ section demonstrates not only interaction with the green sheet visual aid, but an effort on the part of all stakeholders to improve communication. In addition, the results of the feedback survey were extremely positive. The fact that 60% of users felt that the process improved the care of their patient, and that nearly three quarters wanted this to be standard of care is an important statement signifying that the need for improving this particular process of care was largely met.
While the improvement in care coordination and communication among key stakeholders provides evidence of a successful QI initiative, it is important to consider the impact of the intervention on clinical outcomes. Although the event rates are small and prevent determination of statistical significance, when compared to an equivalent pre-intervention control group, the readmission rate to the ICU fell from 6.0% to 4.7%. Although the death rates in the intervention group trended up, the deaths occurred in much older and sicker patients whose pathology may or may not have been preventable by our intervention.
While the improvements in readmission may be modest, the combination of a modest improvement with an exceptional improvement in communication and care coordination together demonstrates the utility of this QI intervention for the high-risk cohort of surgical patients. Furthermore, the decreasing trend in rapid responses over the time period, as well as the shift toward recognition of serious patient issues during the daytime hours, suggests diffusion of the concepts inherent in this process to other patients.
There are several key components of this QI intervention worth noting which contributed to its success, and which would make it easily translatable to any hospital setting where patients are transferred between levels of care. First, the process involves the careful coordination of multiple provider types, which means that only small process changes are needed by any one group in order to accomplish a much larger process redesign. Second, the combination of a physical component (visual aid) with a process made the intervention strategy tangible and measureable, and served to bring providers to the bedside. Third, this QI intervention strategy was accomplished without the use of any additional funding or personnel. Finally, we cannot underestimate the role of teamwork, effective leadership and senior level support for this initiative. These themes associated with high-performing hospitals provided the contextual framework for success in this particular initiative [32]. The fact that this initiative simply improves care coordination that should already exist, and centers around a tangible checklist that can easily be reproduced anywhere, however, makes it easy to gain organizational support.
Given the overwhelming positive response, this intervention is being applied to a broader range of both high-risk surgical and medical patients at multiple institutions within our health system and has become the standard of care. The fact that we have translated this process to other institutions is telling of its ability to benefit an even larger international audience. The personnel required and the expertise necessary are universal as long as there are patients who are transferred between units and wherever handoffs occur. There is nothing particularly extraordinary or complicated about this intervention—it is simply a thoughtfully designed handoff and patient monitoring process that explicitly coordinates processes that should intuitively be happening—but that often do not.
Limitations
There are a few potential limitations to this QI study. First, and foremost, because of the small event rates, the study was underpowered to detect significant differences in the clinical outcomes. As the main purpose of the intervention was for QI, however, achieving power was not our primary objective. Second, the identification of high-risk patients is based predominantly on a subjective evaluation of the patient's condition by the ICU providers. While many studies have developed formal prediction models for ICU readmission, the data are inconsistent and many of the tools lack validation are too complex, require sophisticated software interfacing with an electronic medical record and are not reproducible [12, 13, 33, 34]. A third limitation is the inability to calculate a response rate for the feedback survey. This is a result of the dynamic workforce that is involved in the process. It is impossible to know how many end users interacted with each green sheet/patient, and to selectively administer the survey. Ongoing evaluation of the project would benefit from a more comprehensive qualitative assessment of end-user feedback. Finally, there are some missing data points that warrant mention. Some of the visual tools, from which process metrics were drawn and linkage to patients was usually made, did not contain patient labels. However, the results do not reveal a particular gap that would suggest the missing labels were biased toward a certain population or outcome.
Conclusion
This initiative demonstrates the successful use of QI methodology to develop and implement a multidisciplinary ICU transfer process that results in improved communication and care coordination. Despite an older and sicker population, we have demonstrated a trend toward decreased respiratory readmissions. Without the need for additional funding, we have shown that with a dedicated QI team, this is an easily adaptable strategy to improve communication and care coordination around the transfer of high-risk surgical patients.
Acknowledgements
The authors would like to acknowledge the contributions and support of Aliza Narva MSN, RN, JD; Robert Bayer RRT; Sebastian Ramagnano BSN RN; Juliane Jablonski DNP RN CCRN CCNS, Jose Pascual-Lopez MD, Scott Falk MD, Kirsten Smith MSN ACNP-BC ACNS-BC, Georgianna Telford CRNP, FNP-BC, AGACNP-BC, Justin Ziemba MD and Jennifer Myers, MD.
Appendix
Table A1.
Risk identification (for patients in the intervention group only) and reason for readmission in all patients who were readmitted within 72 h
| Intervention? | Prospectively identified risk(s) for ICU readmission | Reason for readmission |
|---|---|---|
| No | Sepsis | |
| No | Bleeding | |
| No | Respiratory distress | |
| No | Respiratory distress | |
| No | Sepsis | |
| No | Respiratory distress | |
| No | Respiratory distress | |
| No | Sepsis | |
| No | Sepsis | |
| No | Sepsis | |
| No | Respiratory distress | |
| No | Bleeding | |
| No | Respiratory distress | |
| No | Respiratory distress | |
| No | Cardiac/arrhythmia | |
| No | Sepsis | |
| No | Cardiac/arrhythmia | |
| No | Cardiac/arrhythmia | |
| No | Sepsis | |
| No | Cardiac/arrhythmia | |
| Yes | Aspiration, pulmonary hygiene, delirium, sepsis | Sepsis |
| Yes | Delirium | Cardiac/arrhythmia |
| Yes | Pulmonary hygiene | Volume overload |
| Yes | Reintubated, aspiration, SICU diuresis | Unresponsive |
| Yes | Pulmonary hygiene, SICU diuresis, home diuresis | Bleeding |
| Yes | Prolonged ventilation, aspiration, pulmonary hygiene, SICU diuresis, pain control | Cardiac/arrhythmia |
| Yes | Prior SICU readmission, pulmonary hygiene, SICU diuresis | Altered mental status |
| Yes | Pulmonary hygiene | Respiratory distress |
Funding
No financial support was used for this study.
References
- 1. Kaben A, Correa F, Reinhart K et al. Readmission to a surgical intensive care unit: incidence, outcome and risk factors. Crit Care 2008;12:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kramer AA, Higgins TL, Zimmerman JE. Intensive care unit readmissions in U.S. hospitals: patient characteristics, risk factors, and outcomes. Crit Care Med 2012;40:3–10. [DOI] [PubMed] [Google Scholar]
- 3. Campbell AJ, Higgins TL, Zimmerman JE et al. Predicting death and readmission after intensive care discharge. Br J Anaesth 2008;100:656–62. [DOI] [PubMed] [Google Scholar]
- 4. Chan KS, Tan CK, Fang CS et al. Readmission to the intensive care unit: an indicator that reflects the potential risks of morbidity and mortality of surgical patients in the intensive care unit. Surg Today 2009;39:295–9. [DOI] [PubMed] [Google Scholar]
- 5. Metnitz PG, Fieux F, Jordan B et al. Critically ill patients readmitted to intensive care units--lessons to learn. Intensive Care Med 2003;29:241–8. [DOI] [PubMed] [Google Scholar]
- 6. Ho KM, Dobb GJ, Lee KY et al. The effect of comorbidities on risk of intensive care readmission during the same hospitalization: a linked data cohort study. J Crit Care 2009;24:101–7. [DOI] [PubMed] [Google Scholar]
- 7. Helling TS, Martin LC, Martin M et al. Failure events in transition of care for surgical patients. J Am Coll Surg 2014;218:723–31. [DOI] [PubMed] [Google Scholar]
- 8. Alban RF, Nisim AA, Ho J et al. Readmission to surgical intensive care increases severity-adjusted patient mortality. J Trauma 2006;60:1027–31. [DOI] [PubMed] [Google Scholar]
- 9. Kramer AA, Higgins TL, Zimmerman JE. The association between ICU readmission rate and patient outcomes. Crit Care Med 2013;41:24–33. [DOI] [PubMed] [Google Scholar]
- 10. Nishi GK, Suh RH, Wilson MT et al. Analysis of causes and prevention of early readmission to surgical intensive care. Am Surg 2003;69:913–7. [PubMed] [Google Scholar]
- 11. Ouanes I, Schwebel C, Francais A et al. A model to predict short-term death or readmission after intensive care unit discharge. J Crit Care 2012;27:422 e1–9. [DOI] [PubMed] [Google Scholar]
- 12. Rosenberg AL, Watts C. Patients readmitted to ICUs*: a systematic review of risk factors and outcomes. Chest 2000;118:492–502. [DOI] [PubMed] [Google Scholar]
- 13. Kastrup M, Powollik R, Balzer F et al. Predictive ability of the stability and workload index for transfer score to predict unplanned readmissions after ICU discharge. Crit Care Med 2013;41:1608–15. [DOI] [PubMed] [Google Scholar]
- 14. Brown SE, Ratcliffe SJ, Halpern SD. Assessing the utility of ICU readmissions as a quality metric: An analysis of changes mediated by residency work-hour reforms. Chest 2015;147(3):626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown SS. The epidemiology of readmissions to the intensive care unit and its use as a quality indicator. 2013: Dissertations available from ProQuest. Paper AAI3564589.
- 16. Chalmers JD, Black E. Critical care transition and prevention of ICU readmissions: a bridge over troubled waters. Crit Care Med 2014;42:216–7. [DOI] [PubMed] [Google Scholar]
- 17. Chrusch CA, Olafson KP, McMillan PM et al. High occupancy increases the risk of early death or readmission after transfer from intensive care. Crit Care Med 2009;37:2753–8. [DOI] [PubMed] [Google Scholar]
- 18. Goldfrad C, Rowan K. Consequences of discharges from intensive care at night. Lancet 2000;355:1138–42. [DOI] [PubMed] [Google Scholar]
- 19. Franklin C, Mathew J. Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med 1994;22:244–7. [PubMed] [Google Scholar]
- 20. Philadelphia Department of Public Health Community Health Assessment 2014; Available from: www.phila.gov/health/pdfs/CHAreport_52114_final.pdf
- 21. Fernandez R, Serrano JM, Umaran I et al. Ward mortality after ICU discharge: a multicenter validation of the Sabadell score. Intensive Care Med 2010;36:1196–201. [DOI] [PubMed] [Google Scholar]
- 22. Gajic O, Malinchoc M, Comfere TB et al. The Stability and Workload Index for Transfer score predicts unplanned intensive care unit patient readmission: initial development and validation. Crit Care Med 2008;36:676–82. [DOI] [PubMed] [Google Scholar]
- 23. Chandra S, Agarwal D, Hanson A et al. The use of an electronic medical record based automatic calculation tool to quantify risk of unplanned readmission to the intensive care unit: a validation study. J Crit Care 2011;26:634 e9–634 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frost SA, Tam V, Alexandrou E et al. Readmission to intensive care: development of a nomogram for individualising risk. Crit Care Resusc 2010;12:83–9. [PubMed] [Google Scholar]
- 25. Fernandez R, Baigorri F, Navarro G et al. A modified McCabe score for stratification of patients after intensive care unit discharge: the Sabadell score. Crit Care 2006;10:R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charlson M, Szatrowski TP, Peterson J et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 28. Knaus WA, Draper EA, Wagner DP et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- 29. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care 2003;12:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin F, Chaboyer W, Wallis M. A literature review of organisational, individual and teamwork factors contributing to the ICU discharge process. Aust Crit Care 2009;22:29–43. [DOI] [PubMed] [Google Scholar]
- 31. Hager DN, Dinglas VD, Subhas S et al. Reducing deep sedation and delirium in acute lung injury patients: a quality improvement project. Crit Care Med 2013;41:1435–42. [DOI] [PubMed] [Google Scholar]
- 32. Taylor N, Clay-Williams R, Hogden E et al. High performing hospitals: a qualitative systematic review of associated factors and practical strategies for improvement. BMC Health Serv Res 2015;15:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hosein FS, Bobrovitz N, Berthelot S et al. A systematic review of tools for predicting severe adverse events following patient discharge from intensive care units. Crit Care 2013;17:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piper GL, Kaplan LJ, Maung AA et al. Using the Rothman index to predict early unplanned surgical intensive care unit readmissions. J Trauma Acute Care Surg 2014;77:78–82. [DOI] [PubMed] [Google Scholar]