Abstract
Coxiella burnetii is a gram-negative bacterium that causes acute and chronic Q fever. Because of the severe adverse effect of whole-cell vaccination, identification of immunodominant antigens of C. burnetii has become a major focus of Q fever vaccine development. We hypothesized that secreted C. burnetii type IV secretion system (T4SS) effectors may represent a major class of CD8+ T-cell antigens, owing to their cytosolic localization. Twenty-nine peptides were identified that elicited robust CD8+ T-cell interferon γ (IFN-γ) recall responses from mice infected with C. burnetii. Interestingly, 22 of 29 epitopes were derived from 17 T4SS-related proteins, none of which were identified as immunodominant antigens by using previous antibody-guided approaches. These epitopes were expressed in an attenuated Listeria monocytogenes vaccine strain. Immunization with recombinant L. monocytogenes vaccines induced a robust CD8+ T-cell response and conferred measurable protection against C. burnetii infection in mice. These data suggested that T4SS effectors represent an important class of C. burnetii antigens that can induce CD8+ T-cell responses. We also showed that attenuated L. monocytogenes vaccine vectors are an efficient antigen-delivery platform that can be used to induce robust protective CD8+ T-cell immune responses against C. burnetii infection.
Keywords: type IV secretion system effector, Q fever, CD8+ T-cell epitopes, antigen presentation, protective immunity
Coxiella burnetii is a gram-negative pathogen responsible for the worldwide zoonotic disease, Q fever [1, 2]. Human Q fever usually manifests as an influenza-like, self-limiting or treatable acute illness, whereas some cases may develop into severe diseases, such as hepatitis or endocarditis [1–3]. The Netherlands had a large human Q fever outbreak between 2007 and 2010, which caused thousands of infections, including several associated deaths [4]. Thus, the prevention of Q fever remains an important goal for public health [5]. In Australia, a formalin-killed whole-cell vaccine (Q-Vax) is available to those in direct contact with infected animals and considered most at risk [6]. However, vaccination can result in severe local or systemic adverse reactions, particularly when administered to those with prior infection [7, 8]. This has led to studies aimed at identifying immunodominant antigens or peptides to produce a safe and effective vaccine that will not cause adverse reactions [5, 9, 10]. Significant efforts have gone into identifying C. burnetii immunodominant antigens by using an antibody-guided approach. Many C. burnetii antigens have been identified as strong stimulators of antibody responses during infection. However, none of the identified antigens conferred protection comparable to that of Q-Vax, suggesting that current approaches for identifying immunodominant antigens need to be improved and that other antigen-delivery systems need to be considered [9, 10].
Previous studies suggested that T cells play a critical role for protective adaptive immunity against C. burnetii [3, 10]. The role of antigen-specific CD4+ T-cell responses in protective immunity has been well characterized [7, 11–13]. Antigen-specific CD4+ T cells can secrete cytokines such as interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) to activate monocytes/macrophages and facilitate the clearance of intracellular C. burnetii [14, 15]. However, owing to the lack of an efficient high-throughput assay for identification of CD8+ T cells antigens, there are few studies on the role of antigen-specific CD8+ T cells in C. burnetii protective immunity. Read et al demonstrated that CD8+ T cells may play an important role in innate immunity against C. burnetii infection, since adoptive transfer of naive CD8+ T cells into SCID mice mitigated disease after C. burnetii Nine Mile phase I challenge, including reduced inflammation in the lungs and fewer bacteria in spleens [16]. No study has been reported that characterizes the role of CD8+ T cells in adaptive immunity against C. burnetii infection.
In this study, we hypothesized that secreted C. burnetii type IV effector proteins may represent an important class of CD8+ T-cell antigens due to their cytosolic localization during infection. Once these antigens are secreted into the cytosol, they can be further processed by the proteasome degradation pathway and presented by the major histocompatibility complex (MHC) class I pathway, which also serves as a surface signature of infected cells. We used bioinformatics predictions to identify a subset of potential CD8+ T-cell epitopes from highly translocated Coxiella T4SS substrates [17]. Surprisingly, 29 peptides derived from 22 proteins elicited a high level of CD8+ T-cell IFN-γ recall responses after infection, with only a few of these antigens having been identified as immunodominant antigens by previous antibody-guided approaches. The protective efficacy of these CD8+ T-cell epitopes was evaluated by exploiting a live, recombinant, attenuated Listeria monocytogenes ΔactA/ΔinlB strain [18] to deliver these C. burnetii CD8+ epitopes (Lm-Cb) into the cytosol of infect cells and induce robust antigen-specific CD8+ T-cell responses.
MATERIALS AND METHODS
C. burnetii Strain
C. burnetii (RSA 493/Nine Mile phase I) was grown in embryonated eggs and purified by Renografin density centrifugation as described previously [19]. The purified C. burnetii organisms were inactivated with formalin and extracted 3 times with chloroform:methanol (4:1) to obtain the C. burnetii chloroform:methanol residue fraction for use in the whole-cell vaccine (WCV), as described previously [20].
Mice and Ethics Statement
Female C57BL/6J (B6) mice (6 weeks old) were purchased from Vital River Laboratories (Beijing, China) and Jackson Laboratory (Bar Harbor, Maine). All mice were maintained under biosafety level 3 conditions. The Laboratory Animal Administration Committee of Beijing preapproved all animal experimental protocols. Animal research protocols at Texas A&M University were reviewed by the University Laboratory Animal Care and Use Committee to ensure compliance with Public Health Service standards. Experiments were performed in Association for Assessment and Accreditation of Laboratory Animal Care–approved facilities in accordance with university and federal regulations.
Epitope Prediction of CD8+ T Cells
Twenty-four T4SS substrates [21] and 6 immunodominant proteins [22] (Table 1) of C. burnetii were scanned for 9-mer peptides predicted to have a high-affinity binding capacity for the MHC class I molecules H2 Db and Kb (Supplementary Table 1), using a consensus approach on the Immune Epitope Database and Analysis Resource Web site, as described previously [23]. A set of 157 predicted peptides was synthesized by SBS Genetech (Beijing, China) as high-quality pure materials (>90% purity).
Table 1.
Summary of Proteins and Peptides Selection
| No. | Locus Tag | Product | Function | Translocation Efficiency in Legionella, %a | Protein Length, Amino Acids, No. | Predicted Peptides, No. |
|---|---|---|---|---|---|---|
| 1 | CBUA0015 | Hypothetical protein | T4SS substrates | 90 | 145 | 5 |
| 2 | CBU0041 (cirA) | Hypothetical protein | T4SS substrates | 70 | 710 | 6 |
| 3 | CBU0129 | Hypothetical protein | T4SS substrates | 70 | 115 | 5 |
| 4 | CBU0410 | Hypothetical protein | T4SS substrates | 45 | 578 | 6 |
| 5 | CBU0414 | Hypothetical protein | T4SS substrates | 45 | 262 | 5 |
| 6 | CBU0425 (cirB) | Hypothetical protein | T4SS substrates | 90 | 455 | 6 |
| 7 | CBU0794 | Hypothetical protein | T4SS substrates | 80 | 464 | 4 |
| 8 | CBU0881 | Hypothetical protein | T4SS substrates | 80 | 221 | 6 |
| 9 | CBU1045 | Hypothetical protein | T4SS substrates | 40 | 331 | 6 |
| 10 | CBU1198 | Hypothetical protein | T4SS substrates | 75 | 178 | 4 |
| 11 | CBU1406 | Hypothetical protein | T4SS substrates | 40 | 144 | 5 |
| 12 | CBU1460 | Hypothetical protein | T4SS substrates | 40 | 261 | 5 |
| 13 | CBU1556 | Hypothetical protein | T4SS substrates | 50 | 567 | 6 |
| 14 | CBU1569 | Hypothetical protein | T4SS substrates | 90 | 547 | 5 |
| 15 | CBU1620 | Hypothetical protein | T4SS substrates | 90 | 76 | 4 |
| 16 | CBU1751 | Hypothetical protein | T4SS substrates | 50 | 420 | 5 |
| 17 | CBU1823 | Hypothetical protein | T4SS substrates | 50 | 349 | 5 |
| 18 | CBU1825 | Hypothetical protein | T4SS substrates | 40 | 115 | 5 |
| 19 | CBU2007 | Hypothetical protein | T4SS substrates | 90 | 395 | 6 |
| 20 | CBU2052 (cirD) | Hypothetical protein | T4SS substrates | 90 | 300 | 6 |
| 21 | CBU2059 (cirE) | Hypothetical protein | T4SS substrates | 40 | 400 | 4 |
| 22 | CBU1626 (icmG/dotF) | IcmG protein | T4SS component protein | … | 244 | 5 |
| 23 | CBU1628 (icmK/dotH) | IcmK protein | T4SS component protein | … | 344 | 5 |
| 24 | CBU1648 (dotA) | DotA protein | T4SS component protein | … | 806 | 5 |
| 25 | CBU0092 (ybgF) | tol-pal system protein YbgF | Immunodominant protein | … | 285 | 5 |
| 26 | CBU0311 | Outer membrane protein P1 | Immunodominant protein | … | 223 | 5 |
| 27 | CBU0388 | Hypothetical protein | Immunodominant protein | … | 1392 | 6 |
| 28 | CBU0630 (mip) | Peptidyl-prolyl cis-trans isomerase Mip | Immunodominant protein | … | 230 | 6 |
| 29 | CBU1718 (groEL) | Chaperonin, 60 kDa, HspB | Immunodominant protein | … | 552 | 6 |
| 30 | CBU1910 (com1) | Outer membrane protein Com1 | Immunodominant protein | … | 252 | 5 |
a The translocation efficiency of Coxiella burnetii T4SS substrates by Legionella pneumophila is reported by Weber et al [21].
Screening of CD8+ T Cells Epitopes by an Enzyme-Linked Immunospot (ELISPOT) Assay
Five mice per group were challenged with 1 × 106 genome equivalents (GEs) of C. burnetii by intraperitoneal injection or were subcutaneously vaccinated with 20 µg of WCV, and then they were euthanized 10 days after challenge or vaccination, respectively. Spleens from infected or vaccinated mice were collected and homogenized into cell suspensions, from which CD8+ T cells were isolated using microbeads (Miltenyi, Auburn, CA). Antigen-specific IFN-γ recall responses were measured in the purified CD8+ T cells by ELISPOT, as described previously [12]. Briefly, 2 × 105 purified CD8+ T cells were incubated with 1 × 105 APC (mononuclear cells from naive mice) in 100 µL of 1640 medium (Hyclone, Beijing, China) containing 10% (vol/vol) fetal bovine serum (Hyclone) in each well of a 96-well ELISPOT plate (Mabtech, Nacka Strand, Sweden). A total of 2 μg of each peptide was then added to duplicate wells and incubated for 20 hours at 37°C. The number of spot-forming cells (SFCs) after peptide stimulation was counted by an ELISPOT reader [12]. For each peptide, the stimulation index (SI) was calculated by dividing the number of SFCs in peptide-stimulated cells by the number of SFCs in medium-stimulated cells, and a SI of > 2 was considered positive [7].
Intracellular Cytokine Staining and Flow Cytometry
Approximately 1 × 106 splenocytes (pooled from 5 mice infected with C. burnetii) were incubated with 20 µg of each positive peptide in a total volume of 2 mL of complete 1640 medium containing 10% fetal bovine serum in each well for 18 hours at 37°C. Next, 2 µL of Golgistop was added to each well, and plates were incubated for another 6 hours. Then splenocytes were harvested and stained with anti–CD3e-peridinin chlorophyll protein, anti–CD8-allophycocyanin, and anti–IFN-γ-phycoerythrin antibodies as previous described [12]. For the positive control, 1 × 106 splenocytes were cultured in medium for 18 hours, and then 2 µL of a leukocyte-activation cocktail was added to each well. After incubation for 6 hours, splenocytes were harvested and stained as described above. For negative control, splenocytes were incubated with peptide LLO91–99 (GYKDGNEYI) and stained as described above. All reagents used in this assay were purchased from BD Pharmingen (San Jose, California).
Construction of an Lm-Cb Vaccine
The L. monocytogenes strain used in this study was L. monocytogenes 10403S ΔactA/ΔinlB (obtained from the laboratory of Daniel Portnoy, Department of Molecular and Cell Biology, University of California, Berkeley). The genes of the selected 29 peptides were synthesized and ligated into the pCC103 vector under the actA promoter and actA N-terminal secretion signal. The pPL2-pactA-Cb epitopes plasmid was then transformed into Escherichia coli SM10 strains and conjugated into the L. monocytogenes ΔactA/ΔinlB strain. Lm-Cb clones with genomic integration of the plasmid were screened and picked from brain heart infusion agar plates supplemented with 10 µg/mL chloramphenicol and 200 µg/mL streptomycin to generate the Lm-Cb vaccine strain DP-L-6525. For a negative control, the pPL2-pactA plasmid without Cb epitope genes was integrated into L. monocytogenes ΔactA/ΔinlB to generate vector control strain DP-L-6526 described above.
Detection of IFN-γ Recall Responses in Lm-Cb Immunized CD8+ T Cells
Five mice per group were intravenously injected with 5 × 106 colony-forming units (CFU; 0.1 median lethal doses) of Lm-Cb. As a control, 5 mice were immunized with 5 × 106 CFU of L. monocytogenes. On the tenth day after immunization, mice were euthanized, and the spleens were harvested. CD8+ T cells were isolated from splenocytes and incubated with each of the 29 positive peptides at the dose described above. Peptide LLO91–99 (GYKDGNEYI) was added as a positive control. Antigen-specific IFN-γ recall responses were measured by ELISPOT, and the SI was calculated as described above.
Adoptive Transfer of CD8+ T Cells
Six mice per group were challenged with 1 × 106 GEs of C. burnetii by intraperitoneal injection. Beginning 7 days later, they were treated with doxycycline for 2 weeks. A total of 28 days after challenge, the recovered mice were euthanized, and their spleens were collected. CD8+ T cells were isolated from the spleens, using microbeads, as described above. Six mice per group received an adoptive transfer of 1 × 107 CD8+ T cells from C. burnetii–infected mice or from naive mice via tail vein injection. Twenty-four hours later, each mouse was challenged with 1 × 106 GEs of C. burnetii by intraperitoneal injection. On day 14 after challenge, the mice were euthanized, and their spleens were harvested and weighed. C. burnetii loads were evaluated using quantitative real-time PCR (qPCR) specific for C. burnetii [24].
Mouse Immunization With Lm-Cb and Challenge
Six mice per group were immunized by intravenous injection with 5 × 106 CFU of Lm-Cb or L. monocytogenes in 100 µL of PBS. As a control, 100 µL of PBS was also intravenously injected. One boost immunization was performed 2 weeks after the initial immunization. Four weeks later, mice were challenged intraperitoneally with 1 × 106 GE of C. burnetii. Fourteen days after challenge, mice were euthanized, and their spleens were harvested for measurement of splenomegaly and quantification of C. burnetii DNA using qPCR [24].
Statistical Analysis
Intracellular cytokines, spleen weights, and C. burnetii loads were compared among groups, using 1-way analysis of variance followed by the Student-Newman-Keuls test, by means of SAS 9.1 software (SAS Institute, Cary, North Carolina).
RESULTS
CD8+ T Cells Contribute to Infection-Derived Protective Immunity Against C. burnetii
To evaluate the contribution of infection-derived CD8+ T cells on C. burnetii protective immunity, CD8+ T cells isolated from C. burnetii–infected mice were adoptively transferred into naive mice. Fourteen days after challenge with C. burnetii, the spleen weights of mice that received CD8+ T cells from C. burnetii–infected mice were significantly less than those of mice that received CD8+ T cells from naive mice (Figure 1A). The bacterial loads in the spleens of mice that received CD8+ T cells from C. burnetii–infected mice were also significantly lower than those for mice that received CD8+ T cells from naive mice (Figure 1B). These results indicated that infection-derived antigen-specific CD8+ T cells contribute to protective adaptive immunity against C. burnetii infection.
Figure 1.
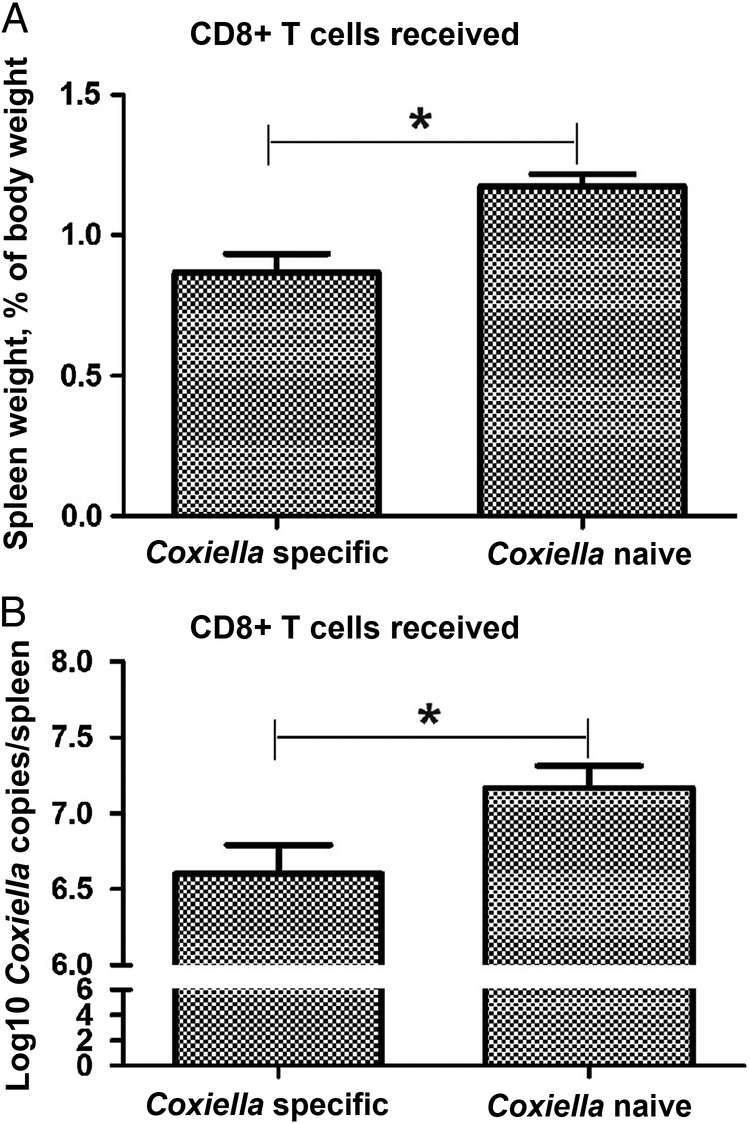
Protective adaptive immunity against Coxiella burnetii induced by the adoptive transfer of infection-derived Coxiella-specific CD8+ T cells. CD8+ T cells from C. burnetii–infected mice were transferred to groups of 6 naive mice 28 days after infection, and each recipient mouse was challenged with 1 × 106 genome equivalents of C. burnetii 24 hours after transfer. On day 14 after challenge, the mice were euthanized, and their spleens were harvested for measurement of spleen weights (A) and the detection of C. burnetii DNA by quantitative polymerase chain reaction (B). Data are expressed as the mean values (+SD) for 6 mice. *P < .05, compared with mice in which CD8+ T cells from Coxiella-naive mice.
Peptide Prediction and CD8+ T Cell IFN-γ Recall Responses in Coxiella-Infected CD8+ T Cells
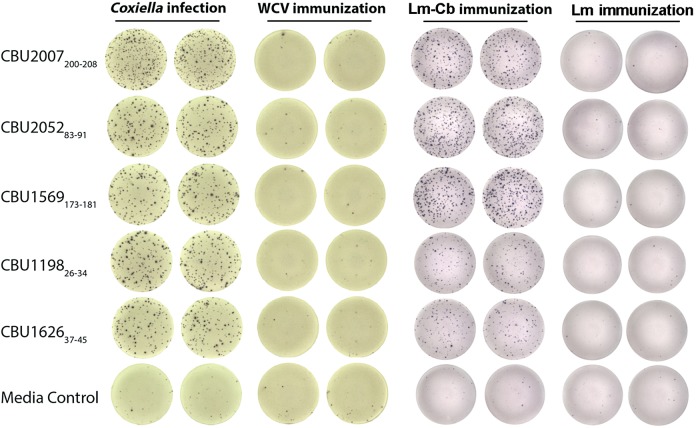
To identify antigens that induced protective CD8+ T-cell populations after infection, we hypothesized that T4SS effector proteins secreted by C. burnetii during infection would be strong candidates because of their cytosolic localization. Using bioinformatics analysis, we identified 157 peptides from 24 T4SS components of highly translocated substrates [21] and 6 previously identified immunodominant C. burnetii antigens [22] as having the high-affinity binding capacity to H2 Db or Kb. The synthesized peptides were incubated with naive APC and CD8+ T cells isolated from C. burnetii–infected mice to determine IFN-γ recall responses by ELISPOT assay. As a result, 29 of 157 candidate peptides elicited significantly elevated IFN-γ secretion in infection-derived CD8+ T cells, compared with medium stimulation (SI > 2; Table 2). None of the 29 peptides elicited a positive response in CD8+ T cells isolated from WCV recipients or naive mice (Figure 2). Interestingly, 22 positive peptides were derived from T4SS proteins of C. burnetii, and none of these proteins have been identified as immunodominant antigens in previous screens. Seven of 8 peptides that elicited higher IFN-γ secretion with an approximate SI of > 4 were derived from C. burnetii T4SS–related proteins (SI > 4; Table 2).
Table 2.
Summary of Distinct CD8+ T-Cell Epitopes Recognized After Infection With Coxiella burnetii and Immunization With a Live, Recombinant, Attenuated Listeria monocytogenes Vaccine Expressing These Epitopes (Lm-Cb)
| Identified Peptide ID | Function | Translocation Efficiency in Legionella,%a | MHC Allele | Sequence | Stimulation Indexb |
|
|---|---|---|---|---|---|---|
| After C. burnetii Infectionc | After Lm-Cb Receiptd | |||||
| CBU2007200–208 | T4SS substrates | 90 | H-2-Db | FCYQNHTYF | 5.11 ± 0.11 | 5.32 ± 0.28 |
| CBU205283–91 | T4SS substrates | 90 | H-2-Db | RASKNLLNY | 4.99 ± 0.24 | 7.99 ± 0.52 |
| CBU1569173–181 | T4SS substrates | 90 | H-2-Db | FQISNPPYL | 4.78 ± 0.13 | 6.93 ± 1.46 |
| CBU119827–35 | T4SS substrates | 75 | H-2-Kb | SVVNYATLF | 4.64 ± 0.29 | 2.34 ± 0.29 |
| CBU1751360–368 | T4SS substrates | 50 | H-2-Db | VAIKSYRKF | 3.96 ± 0.15 | 7.59 ± 0.46 |
| CBU2007292–300 | T4SS substrates | 90 | H-2-Kb | FVPKFFLTF | 3.73 ± 0.44 | 2.8 ± 0.52 |
| CBU0414137–145 | T4SS substrates | 45 | H-2-Db | AQILNGDKL | 3.45 ± 0.46 | 1.28 ± 0.57 |
| CBU1823136–144 | T4SS substrates | 50 | H-2-Db | SGICSVATY | 3.34 ± 0.04 | 4.59 ± 0.21 |
| CBU0794327–335 | T4SS substrates | 80 | H-2-Db | SVNSNSGSF | 3.24 ± 0.2 | 4.84 ± 0.71 |
| CBU2052236–244 | T4SS substrates | 90 | H-2-Kb | TVLLRTPLF | 3.13 ± 0.48 | 1.83 ± 0.32 |
| CBU1460114–122 | T4SS substrates | 40 | H-2-Db | NALLDEETI | 3.11 ± 0.39 | 6.63 ± 0.48 |
| CBU0881129–137 | T4SS substrates | 80 | H-2-Kb | VALFILTHL | 2.76 ± 0.44 | 1.94 ± 0.35 |
| CBU0425320–328 | T4SS substrates | 90 | H-2-Kb | RVKVFLTHF | 2.72 ± 0.35 | 2.44 ± 0.04 |
| CBU041026–34 | T4SS substrates | 45 | H-2-Db | NYATHKASY | 2.44 ± 0.13 | 2.48 ± 0.10 |
| CBU042573–81 | T4SS substrates | 90 | H-2-Kb | ISLLRTLRY | 2.25 ± 0.06 | 1.91 ± 0.35 |
| CBU1626(IcmG/DotF)37–45 | T4SS component | … | H-2-Kb | KPANFFVRF | 4.44 ± 0.2 | 2.47 ± 0.18 |
| CBU1628(IcmK/DotH)20–28 | T4SS component | … | H-2-Kb | ASIIPVLAL | 3.93 ± 0.26 | 2.39 ± 0.18 |
| CBU1648(DotA)5–13 | T4SS component | … | H-2-Db | SSLLASISL | 3.83 ± 0.22 | 7.61 ± 0.04 |
| CBU1648(DotA)668–676 | T4SS component | … | H-2-Kb | IVVYTFSGL | 3.48 ± 0.2 | 2.87 ± 0.04 |
| CBU1626(IcmG/DotF)52–60 | T4SS component | … | H-2-Kb | ITAVIAVVL | 2.97 ± 0.18 | 1.94 ± 0.09 |
| CBU1628(IcmK/DotH)106–114 | T4SS component | … | H-2-Db | NTIRNMMPL | 2.94 ± 0.33 | 4.24 ± 0.10 |
| CBU1628(IcmK/DotH)165–173 | T4SS component | … | H-2-Kb | SGFVTSLVF | 2.32 ± 0.26 | 2.42 ± 0.23 |
| CBU_0311119–127 | Immunodominant | … | H-2-Kb | SNYSYRTRL | 4 ± 0.57 | 8.37 ± 0.67 |
| CBU_009272–80 | Immunodominant | … | H-2-Kb | TVTHRLARL | 3.74 ± 0.57 | 8.28 ± 0.16 |
| CBU_0092194–202 | Immunodominant | … | H-2-Kb | AQASFQNYL | 3.54 ± 0.15 | 9.04 ± 0.23 |
| CBU_0311186–194 | Immunodominant | … | H-2-Kb | RHYFMNNVF | 3.49 ± 0.37 | 7.95 ± 0 |
| CBU1910(Com1)213–221 | Immunodominant | … | H-2-Db | LQLAGTPTF | 3.45 ± 0.31 | 6.53 ± 0.32 |
| CBU_1718181–189 | Immunodominant | … | H-2-Db | SGLENALEV | 2.84 ± 0.22 | 1.92 ± 0.40 |
| CBU_0388770–778 | Immunodominant | … | H-2-Db | YSAQNEISF | 2.76 ± 0.15 | 2.34 ± 0.15 |
Abbreviations: ID, identifier; MHC, major histocompatibility complex; SD, standard deviation.
a The translocation efficiency of C. burnetii T4SS substrates by Legionella pneumophila is reported by Weber et al [21].
b The stimulation index was determined by an interferon γ enzyme-linked immunospot assay and was calculated using the number of spot-forming cells (SFCs) in peptide-stimulated cells divided by the number of SFCs in medium-stimulated cells. A stimulation index of > 2 was considered to be positive.
c A total of 2 × 105 CD8+ T cells were isolated on day 10 after infection from 5 mice infected with 1 × 106C. burnetii. Each peptide was added at a concentration of 2 µg/mL for 20 hours. The data presented are the mean (±SD) of 3 independent experiments.
d A total of 2 × 105 CD8+ T cells were isolated on day 10 after infection from 5 mice immunized with 5 × 106 Lm-Cb. Each peptide was added at a concentration of 2 µg/mL for 20 hours. The data presented are the mean (±SD) of 3 independent experiments.
Figure 2.
Interferon γ (IFN-γ) enzyme-linked immunospot–based quantification of peptide-specific IFN-γ–producing CD8+ T cells in mice infected with Coxiella burnetii, mice that received whole-cell vaccine (WCV), mice that received live, recombinant, attenuated Listeria monocytogenes vaccine expressing Coxiella-specific CD8+ T-cell epitopes (Lm-Cb), and mice that received L. monocytogenes control vaccine (Lm).
Frequency of Infection-Derived Peptide-Specific IFN-γ–Producing CD8+ T Cells
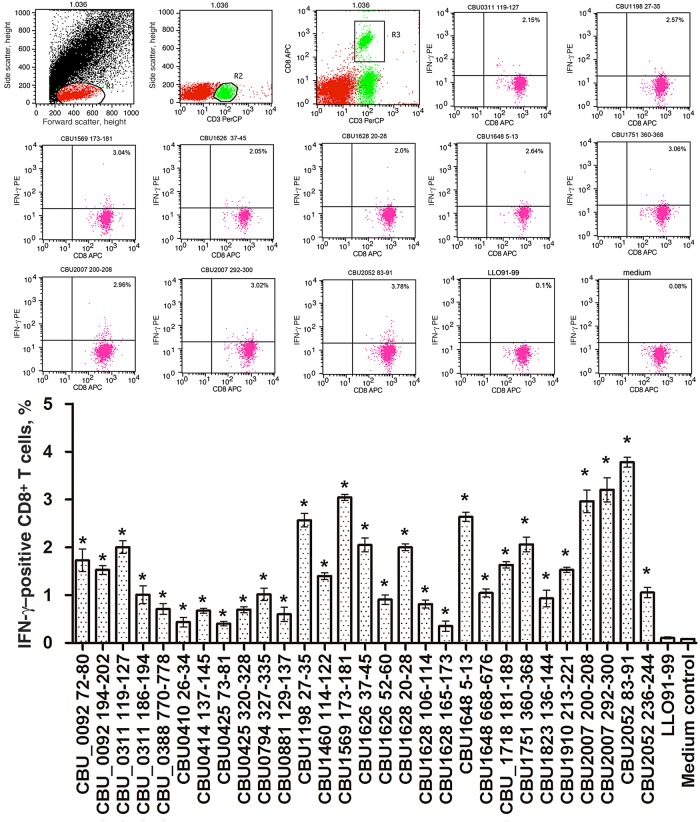
Twenty-nine positive peptides were used individually to stimulate CD8+ T cells from C. burnetii–infected mice. Then, the frequency of peptide-specific IFN-γ–producing CD8+ T cells was quantified by flow cytometry. As shown in Figure 3, the frequency of responding cells ranged from 0.44% to 3.78% of the total number of CD8+ T cells. Compared with negative peptide stimulation, all peptides induced detectable IFN-γ responses, and 10 of 29 peptides induced an IFN-γ response by >2% of total CD8+ T cells. Among those, 9 were derived from T4SS-related proteins, and 1 was from an immunodominant protein, CBU_0311(P1) [25].
Figure 3.
Quantification of peptide-specific interferon γ (IFN-γ)–producing CD8+ T cells by intracellular cytokine staining and flow cytometry. Twenty micrograms of each peptide was used to stimulate 1 × 106 lymphocytes obtained from 5 mice 10 days after challenge with Coxiella burnetii. After stimulation of lymphocytes for 24 hours with peptides, the expression of IFN-γ in CD8+ T cells was measured by flow cytometry. T cells cultured with Listeriolysin O peptide (LLO91–99) were used as a negative control. The data are representative of 3 independent experiments, and the average percentages of double-positive cells among the T cells are indicated in the top right corners. The mean values (±SDs) from the results of 3 independent experiments are also shown. *P < .05 for comparison of T cells stimulated with epitope peptides to T cells cultured with LLO91–99. Abbreviations: APC, allophycocyanin; PE, phycoerythrin; PerCP, peridinin chlorophyll protein.
Lm-Cb Construction and Immune Responses Analysis
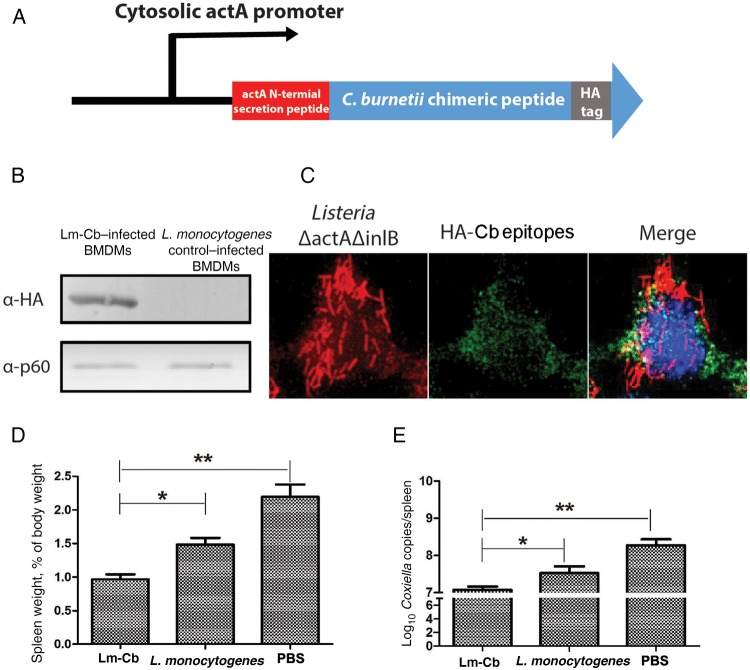
To efficiently deliver the identified CD8+ T-cell peptides into the cytosol of antigen-presenting cells and mount peptide-specific CD8+ T-cell responses, we exploited the attenuated L. monocytogenes vaccine platform. A synthetic gene was created that comprised genes encoding 29 positive peptides that were fused after codon optimization and cloned into pPL2-PactA shuttle plasmid under a strong cytosolic L. monocytogenes actA promoter and actA N-terminal secretion peptide (Figure 4A). The resulting pPL2-PactA plasmid was integrated into the genome of the live, attenuated L. monocytogenes ΔactA/ΔinlB vaccine strain by bacterial conjugation. To confirm the expression and delivery of synthetic antigens by the recombinant L. monocytogenes vaccine strain, Western blotting was used to confirm the expression of fused peptide antigen in the host cytosol (Figure 4B and 4C). To determine the CD8+ T-cell IFN-γ recall responses induced by Lm-Cb immunization, mice were intravenously injected with the Lm-Cb strains. CD8+ T cells from immunized mice were then isolated and incubated with each peptide to determine IFN-γ production. A total of 23 of 29 peptides elicited IFN-γ secretion, with a SI of > 2 (Table 2), whereas none of the peptides elicited a positive response in CD8+ T cells isolated from mice immunized with the L. monocytogenes control strain (Figure 2). These results demonstrate that the live, attenuated L. monocytogenes ΔactA/ΔinlB vaccine strain can be used as a potent antigen delivery system to induce robust CD8+ T-cell responses.
Figure 4.
Evaluation of the expression of synthetic Coxiella burnetii chimeric epitopes and the protective efficacy of the live, recombinant, attenuated Listeria monocytogenes strain expressing Coxiella-specific CD8+ T-cell epitopes (Lm-Cb). A, Diagram of the transcription and translation of synthetic C. burnetii epitopes in L. monocytogenes. C. burnetii chimeric epitopes were cloned under a strong cytosolic L. monocytogenes actA promoter and actA N-terminal secretion peptide for efficient expression and secretion of chimeric epitopes after L. monocytogenes escaped into the cytosol of infected cells. B, Lm-Cb was used to infect Raw264.7 cells for 5 hours at a multiplicity of infection of 10. One hour after infection, cells were washed with phosphate-buffered saline (PBS) and supplied with fresh medium containing gentamicin. Five hours after infection, cell lysates were analyzed by Western blot and probed with anti-HA antibody for detection of synthetic C. burnetii epitopes. C, Intracellular localization of C. burnetii CD8+ T-cell epitopes in Raw264.7 cells 5 hours after infection with L. monocytogenes. Blue, DAPI-stained nuclear and bacterial DNA; green, HA-Coxiella chimeric epitopes; red, L. monocytogenes. D and E, Spleen weight (D) and C. burnetii load (E) in the spleen of infected mice. Six mice per group were immunized with 5 × 106 colony-forming units of Lm-Cb or L. monocytogenes in 100 µL of PBS twice, with a 2-week interval between immunizations. Mice immunized with PBS were used as a negative control. Four weeks after the boost immunization, each mouse was challenged with 1 × 106 genome equivalents of C. burnetii. Fourteen days after challenge, mice were euthanized, and the spleens were collected. *P < .05 and **P < .01, compared with negative control. Abbreviation: BMDM, bone marrow–derived macrophage.
Protection Against C. burnetii Infection Induced by Lm-Cb
Mice immunized with live, attenuated Lm-Cb or the L. monocytogenes control strain were challenged with C. burnetii after 28 days post immunization. Fourteen days after challenge, the spleen weights of Lm-Cb–immunized mice were significantly lighter than those of L. monocytogenes– or PBS-immunized mice (Figure 4D). The bacterial loads in the spleens from Lm-Cb–immunized mice were also significantly reduced, compared with those from L. monocytogenes– or PBS-immunized mice (Figure 4E). This result supports the hypothesis that expression of C. burnetii chimerized epitopes in a live, attenuated L. monocytogenes vaccine strain induces a significant protective CD8+ T-cell response.
DISCUSSION
Protection against C. burnetii infection requires the generation of memory B-cell and T-cell immunity [26]. Significant efforts have gone into identifying C. burnetii–immunodominant antigens by using antibody-guided approaches. As a result, many C. burnetii antigens have been identified as strong stimulators of antibody responses during infection. However, owing to the lack of an efficient prediction algorithm and high-throughput assays to identify T-cell antigens, few T-cell antigens have been identified in C. burnetii. MHC class I–restricted CD8+ effector T cells could recognize the infected cells and are therefore capable of mediating clearance of intracellular pathogens; hence, we speculated that antigen-specific CD8+ T cells may be involved in infection-derived protection. In this study, we have shown that adoptive transfer of CD8+ T cells from mice challenged with virulent Nine Mile phase I C. burnetii conferred measurable protection. Compared with previous studies, the level of protection mediated by the adoptively transferred CD8+ T cells is similar to that of CD4+ T cells from mice that received WCV [12] but less than that of whole splenocytes from mice that received WCV [3]. This finding supports the hypothesis that CD8+ T cells play some role in protective immunity against C. burnetii.
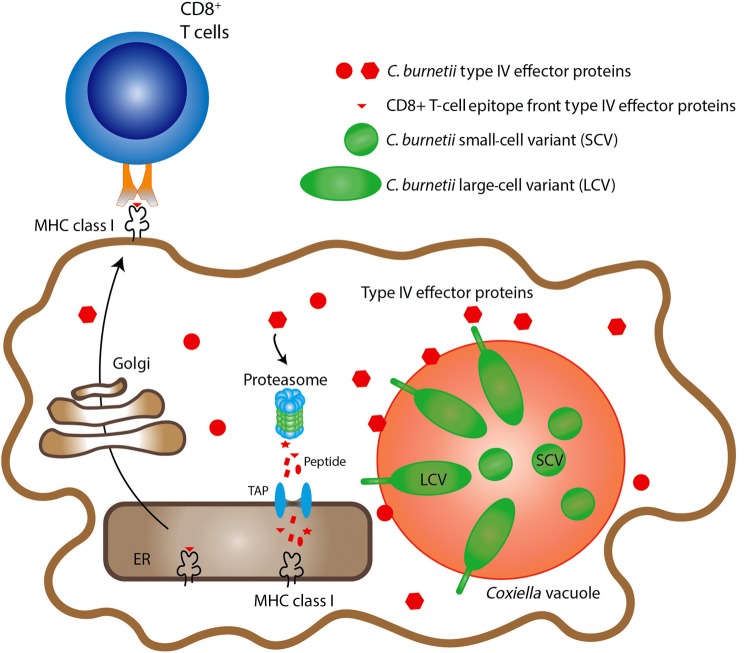
C. burnetii harbors an essential T4SS highly similar to the Dot/Icm T4SS of Legionella pneumophila that delivers effector proteins into the cytosol of infected cells to remodel the host cellular processes [17]. More than 150 C. burnetii T4SS substrates have been identified [17, 21, 27–29]. However, immune responses to these secreted effectors are largely undefined. In this study, we hypothesized that some secreted effector proteins are degraded by the proteasomal degradation pathway and presented by MHC class I molecules on the surface of infected cells, serving as a target for antigen-specific cytotoxic CD8+ T cells, as outlined in our model (Figure 5). We used bioinformatics tools to predict a subset of candidate CD8+ T-cell epitopes from 24 T4SS-related proteins, including 5 T4SS component proteins and 19 confirmed highly translocated substrates [17]. Additionally, based on our previous studies, we tested several highly abundant immunodominant proteins to compare their ability to induce CD8+ T-cell responses [13].
Figure 5.
Hypothesized model of the proteasomal processing and major histocompatibility complex (MHC) class I presentation of cytosolic secreted T4SS effector proteins. According to our hypothesized model, some of the secreted Coxiella burnetii T4SS effector proteins were degraded by the proteasomal degradation pathway and presented by MHC class I molecules on the surface of infected cells, which enable recognition by antigen-specific CD8+ T cells. These secreted antigens may provide a novel set of vaccine candidates to induce anti–C. burnetii CD8+ T-cell immunity. Abbreviations: ER, endoplasmic reticulum; TAP, transporter associated with antigen processing.
From this survey, we identified 29 epitopes that elicited detectable CD8+ T-cell IFN-γ recall responses from C. burnetii–infected mice. None of these 29 peptides elicited IFN-γ recall responses when incubated with CD8+ T cells from mice that receive WCV. Interestingly, 22 of these 29 epitopes were derived from 17 T4SS-related proteins, none of which were identified as immunodominant antigens by using previous antibody-guided approaches. It was noteworthy that many of the strongest CD8+ T-cell–responding peptides were derived from most highly translocated C. burnetii effectors (Table 2), suggesting a positive correlation between cytosolic protein secretion and antigen presentation. These results suggest that previous antibody-guided immunodominant antigen identification approaches were biased against identifying CD8+ T-cell epitopes. Moreover, these data also suggest that C. burnetii T4SS–secreted effector proteins represent important candidates as immunodominant CD8+ T cells antigens.
To test the protective efficacy of these identified CD8+ T-cell epitopes, we used the live, attenuated L. monocytogenes delivery platform that can specifically deliver peptides into the cytosol of antigen-presenting cells and induce CD8+ T-cell immune responses, similar to C. burnetii infection. The attenuated L. monocytogenes strain is designed to enable the safe administration of L. monocytogenes by deleting 2 genes critical to the bacterium's natural virulence, internalin B and act A, which control infection of hepatocytes and the spread of bacteria [30]. The attenuated strain of L. monocytogenes was engineered to encode and express identified C. burnetii epitopes as a single chimeric fusion protein. The ability of L. monocytogenes to effectively stimulate robust, multifunctional, cell-mediated immunity is based primarily on its intracellular life cycle and ability to target dendritic cells during infection [30–32]. Following internalization into phagocytic cells, bacteria are initially contained within host vacuoles but rapidly escape into the cytosol. In response to bacterial vacuolar escape, host intracellular pattern recognition receptors recognize bacterial products in the cytosol, leading to initiation of a powerful immunostimulatory cascade through Sting, nuclear factor κb, and the IRF3 pathways [33–35]. The peptide-specific CD8+ T-cell IFN-γ recall responses from Lm-Cb–immunized mice were significantly higher than that for mice immunized with the empty vector control strain. These results indicated that live, attenuated L. monocytogenes vectors can efficiently deliver C. burnetii peptides into the cytosol of antigen-presenting cells and induce robust peptide-specific CD8+ T-cell responses.
To explore whether Lm-Cb can also induce protection against C. burnetii in mice, we vaccinated C57BL/6J mice with either Lm-Cb or L. monocytogenes and then challenged them with virulent Nine Mile phase I C. burnetii. By both metrics of protection, the spleen weights and C. burnetii loads of mice immunized with Lm-Cb were significantly lower than those of mice immunized with L. monocytogenes or PBS, indicating that Lm-Cb can induce measurable protection against C. burnetii infection. However, long-term protective efficacy would need to be investigated to predict how suitable this approach will be for future vaccine development.
Our results confirmed that cytosolic secreted effector proteins represent a group of strong CD8+ T-cell antigens. In addition, we evaluated the efficiency of using attenuated L. monocytogenes to properly deliver these antigens to mount a robust CD8+ T-cell response. Both findings provide a new vaccine design strategy for C. burnetii and highlight the potential for this tool to be used for other intracellular pathogens that release cytosolic proteins for proteasomal degradation.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. We thank Dr Gabriel Mitchell and Darren Ma for suggestions and thoroughly reviewing the manuscript.
Financial support. This work was supported by the Innovation Foundation of the Chinese Academy of Military Medical Sciences (grant 2015CXJJ16), the Chinese State Key Laboratory of Pathogen and Biosecurity (grant SKLPBS1514), the National Natural Science Foundation of China (grant 31470894), and the US Department of Defense (grant DTRA1-CMB-03 to J. E. S.).
Potential conflicts of interest. D. A. P. has a consulting relationship with and a financial interest in Aduro Biotech. Both he and the company stand to benefit from the commercialization of the results of this research. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999; 12:518–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parker N, Barralet J, Bell A. Q fever. Lancet 2006; 367:679–88. [DOI] [PubMed] [Google Scholar]
- 3. Zhang G, Russell-Lodrigue KE, Andoh M, Zhang Y, Hendrix LR, Samuel JE. Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice. J Immunol 2007; 179:8372–80. [DOI] [PubMed] [Google Scholar]
- 4. Georgiev M, Afonso A, Neubauer H et al. Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveill 2013; 18:pii=20407. [PubMed] [Google Scholar]
- 5. Zhang G, Zhang Y, Samuel JE. Components of protective immunity. Adv Exp Med Biol 2012; 984:91–104. [DOI] [PubMed] [Google Scholar]
- 6. Gidding H, Wallace C, Lawrence G, McIntyre P. Australia's national Q fever vaccination program. Vaccine 2009; 27:2037–41. [DOI] [PubMed] [Google Scholar]
- 7. Chen C, Dow C, Wang P et al. Identification of CD4+ T cell epitopes in C. burnetii antigens targeted by antibody responses. PLoS One 2011; 6:e17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fries LF, Waag DM, Williams JC. Safety and immunogenicity in human volunteers of a chloroform-methanol residue vaccine for Q fever. Infect Immun 1993; 61:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hendrix L, Chen C. Antigenic analysis for vaccines and diagnostics. Adv Exp Med Biol 2012; 984:299–328. [DOI] [PubMed] [Google Scholar]
- 10. Zhang G, Samuel J. Vaccines against Coxiella infection. Expert Rev Vaccines 2004; 3:577–84. [DOI] [PubMed] [Google Scholar]
- 11. Wei Y, Wang X, Xiong X, Wen B. Coxiella burnetii Antigen-Stimulated Dendritic Cells Mediated Protection against Coxiella burnetii in BALB/c Mice. J Infect Dis 2011; 203:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiong X, Qi Y, Jiao J, Gong W, Duan C, Wen B. Exploratory study on Th1 epitope-induced protective immunity against Coxiella burnetii infection. PLoS One 2014; 9:e87206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiong X, Meng Y, Wang X et al. Mice immunized with bone marrow-derived dendritic cells stimulated with recombinant Coxiella burnetii Com1 and Mip demonstrate enhanced bacterial clearance in association with a Th1 immune response. Vaccine 2012; 30:6809–15. [DOI] [PubMed] [Google Scholar]
- 14. Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, Samuel JE. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect Immun 2007; 75:3245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brennan RE, Russell K, Zhang G, Samuel JE. Both inducible nitric oxide synthase and NADPH oxidase contribute to the control of virulent phase I Coxiella burnetii infections. Infect Immun 2004; 72:6666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Read AJ, Erickson S, Harmsen AG. Role of CD4+ and CD8+ T cells in clearance of primary pulmonary infection with Coxiella burnetii. Infect Immun 2010; 78:3019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen C, Banga S, Mertens K et al. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc Natl Acad Sci U S A 2010; 107:21755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruhn KW, Craft N, Miller JF. Listeria as a vaccine vector. Microbes Infect 2007; 9:1226–35. [DOI] [PubMed] [Google Scholar]
- 19. Williams JC, Peacock MG, McCaul TF. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun 1981; 32:840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waag D, England M, Pitt M. Comparative efficacy of a Coxiella burnetii chloroform:methanol residue (CMR) vaccine and a licensed cellular vaccine (Q-Vax) in rodents challenged by aerosol. Vaccine 1997; 15:1779–83. [DOI] [PubMed] [Google Scholar]
- 21. Weber MM, Chen C, Rowin K et al. Identification of Coxiella burnetii type IV secretion substrates required for intracellular replication and Coxiella-containing vacuole formation. J Bacteriol 2013; 195:3914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiong X, Wang X, Wen B, Graves S, Stenos J. Potential serodiagnostic markers for Q fever identified in Coxiella burnetii by immunoproteomic and protein microarray approaches. BMC Microbiol 2012; 12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moutaftsi M, Peters B, Pasquetto V et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol 2006; 24:817–9. [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Wen B, Chen M, Niu D. Balb/c mouse model and real-time quantitative polymerase chain reaction for evaluation of the immunoprotectivity against Q fever. Ann N Y Acad Sci 2005; 1063:171–5. [DOI] [PubMed] [Google Scholar]
- 25. Varghees S, Kiss K, Frans G, Braha O, Samuel JE. Cloning and porin activity of the major outer membrane protein P1 from Coxiella burnetii. Infect Immun 2002; 70:6741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang G, Peng Y, Schoenlaub L, Elliott A, Mitchell W, Zhang Y. Formalin-inactivated Coxiella burnetii phase I vaccine-induced protection depends on B cells to produce protective IgM and IgG. Infect Immun 2013; 81:2112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Schaik EJ, Chen C, Mertens K et al. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat Rev Microbiol 2013; 11:561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newton HJ, Kohler LJ, McDonough JA et al. A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog 2014; 10:e1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larson CL, Beare PA, Voth DE et al. Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication. Infect Immun 2015; 83:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brockstedt DG, Giedlin MA, Leong ML et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A 2004; 101:13832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol 2004; 4:812–23. [DOI] [PubMed] [Google Scholar]
- 32. Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect 2007; 9:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Connell RM, Saha SK, Vaidya SA et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med 2004; 200:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hansen K, Prabakaran T, Laustsen A et al. Listeria monocytogenes induces IFNbeta expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J 2014; 33:1654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 2010; 328:1703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.