Abstract
Background. Immune reconstitution inflammatory syndrome (IRIS) in human immunodeficiency virus (HIV)–infected persons beginning antiretroviral therapy (ART) has been incompletely characterized for herpes simplex virus type 2 (HSV-2).
Methods. We evaluated genital ulcer disease (GUD) and HSV-2–associated GUD at quarterly visits or when spontaneously reported at monthly visits in 3381 HIV/HSV-2–coinfected individuals in a placebo-controlled trial of suppressive acyclovir therapy to prevent HIV transmission, 349 of whom initiated ART during the study. Incidence was calculated for months before and after ART initiation, and incidence rate ratios (IRRs) were calculated.
Results. GUD incidence increased from 15.0 episodes per 100 person-years before ART to 26.9 episodes per 100 person-years in the first full quarter after ART initiation (IRR, 1.83; P = .03), and the incidence of HSV-2–associated GUD increased from 8.1 to 19.0 episodes per 100 person-years (IRR, 2.20; P = .02). Subsequently, the incidence of GUD was similar to that before ART, although the numbers were small. Persons receiving suppressive acyclovir had fewer GUD episodes, but the IRR after beginning ART was similar in the acyclovir and placebo groups.
Conclusions. Initiation of ART in HIV/HSV-2–coinfected persons is associated with a transient increase in GUD and HSV-2 GUD. Acyclovir reduces the incidence of GUD but does not prevent an increase in GUD incidence during the first quarter following initiation of ART.
Keywords: human immunodeficiency virus, herpes simplex virus, antiretroviral therapy, acyclovir
Immune reconstitution inflammatory syndrome (IRIS) was recognized soon after the first potent antiretroviral regimens were introduced [1, 2]. Among the first pathogens associated with IRIS was cytomegalovirus (CMV), manifested as “immune recovery vitritis” in patients who started combination antiretroviral therapy (ART) after recovery from CMV retinitis [3, 4]. Since then, most opportunistic pathogens have been associated with an IRIS syndrome.
Coinfection with herpes simplex virus type 2 (HSV-2) and human immunodeficiency virus type 1 (HIV) is common, owing in part to shared risk factors for infection. A variety of mechanisms (eg, disruption of mucosal barriers, recruitment of activated immune cells to genital tissue, and immunosuppression) allow each virus to facilitate the acquisition and transmission of the other, creating a viral synergy that helps to maintain both pathogens in the human population [5–9]. Because of this well-established interaction, it is logical to speculate that treatment of infection due to one of these viruses might influence the natural history of the other. This viral synergy led to evaluations of acyclovir as an agent to reduce HIV risk, but randomized, placebo-controlled trials of suppression of HSV-2 with acyclovir did not reduce HIV transmission [10] or acquisition [11, 12]. Similarly, studies have been conducted to determine the effect of antiretroviral therapy on HSV-2 recurrences. While some studies suggest chronic ART has little or no impact on HSV-2 shedding or recurrences [13–15], others have reported that genital herpes recurrences may increase with immune reconstitution following initiation of ART [16–19]. A recent study showed both an increase in clinical recurrences and an increase in HSV-2 shedding from the genital tract of persons initiating ART [20]. To strengthen this evidence in a larger population and with data on the cause of incident genital ulcers, we examined the incidence of genital ulcer disease (GUD) and HSV-2–associated GUD in the presence or absence of acyclovir among HIV/HSV-2–coinfected persons who initiated ART during a randomized, placebo-controlled study of suppressive acyclovir therapy.
PARTICIPANTS AND METHODS
Participants
All of the individuals in this report were enrolled in the Partners in Prevention HSV/HIV Transmission Study [10, 21]. Briefly, this study was a multicenter, randomized, placebo-controlled trial that examined the ability of acyclovir (400 mg twice daily) given to an HSV-2/HIV-coinfected individual to prevent transmission of HIV to their heterosexual partner who was not infected with HIV at enrollment. The study was conducted between November 2004 and April 2007 at 14 sites in 7 countries in Eastern and Southern Africa. The HIV-infected partner had a CD4+ T-cell count of >250 cells/µL at enrollment and was not receiving ART. If the HIV-infected partner met local guidelines for antiretroviral treatment during the 2-year study period, they were referred for treatment, but they were permitted to remain in the study. The study randomized 3408 couples, among which 3381 HIV-infected partners were confirmed as coinfected with HIV/HSV-2 and were eligible for this analysis; follow-up data were available for 3324. HIV-infected participants were seen monthly for up to 24 months for study drug refills, adherence counseling, and transmission risk reduction counseling. At quarterly visits, interviews were conducted to obtain information on symptoms of GUD and on ART initiation in the last 3 months. Participants underwent a genital examination at each quarterly visit and at any visit if the participant reported the presence of a genital ulcer. If a genital ulcer was present, the lesion was swabbed and subsequently tested for HSV-2 by polymerase chain reaction (PCR) analysis [22, 23]; samples with ≥150 copies/mL in swab eluate were considered positive [24]. Episodic treatment (acyclovir 800 mg twice daily for 5 days) was provided to treat recurrent episodes of genital herpes lesions, based on clinical appearance. CD4+ T cells were counted at enrollment and every 6 months thereafter during the study. All participants provided written informed consent, using documents approved by local ethics committees, the University of Washington Institutional Review Board (the coordinating center), and US partner institutions.
Statistical Analysis
The primary outcome for this analysis was the number of GUD episodes observed in a calendar quarter. The primary exposure was ART use by HIV-infected participants, analyzed as a time-dependent variable and categorized as (1) prior to initiation of ART, (2) in the first full quarter after initiating ART, or (3) >1 full quarter after initiating ART; the quarter in which ART initiation was reported was excluded from the primary analysis, as it could not be classified as entirely before or after ART initiation. After starting ART, participants were conservatively assumed to be continuing treatment, and adherence to therapy was not measured. Participants who never started ART during the study contributed to the analysis with quarters categorized as “prior to initiation of ART.” An alternative analysis was performed using only data from individuals who eventually started ART for the “prior to initiation of ART” calculations. Incidence rate ratios (IRRs) for the effect of initiating ART on the frequency of GUD were estimated using negative binomial regression with generalized estimating equations, to account for correlation in multiple outcomes assessed on the same participant over time. IRRs were estimated for each randomization group in the clinical trial (ie, acyclovir or placebo) and overall. IRRs were adjusted for sex, duration of study participation, CD4+ T-cell count as a time-dependent covariate, and randomization group. The secondary outcome of HSV-2–positive GUD was analyzed in the same manner. Episodes of GUD for which swabs were either not collected or not suitable for PCR testing were conservatively treated as negative for HSV-2; a sensitivity analysis treated them as missing. Additionally, we examined the incidence of GUD before and after ART initiation in a subset of individuals who responded to ART, defined as an increase in CD4+ T-cell count by at least 50 cells/µL or as a viral load decrease of at least 2 log10 following ART initiation. Data were analyzed with SAS, version 9.3).
RESULTS
The baseline characteristics of the study population divided by study arm and ART initiation are shown in Table 1. About two thirds of the study participants were women, and the overall median CD4+ T-cell count was 462 cells/mm3 at enrollment. Participants who started ART during study follow-up had a lower median baseline CD4+ T-cell count (325 cells/mm3 vs 480 cells/mm3). Almost a quarter had a history of a genital ulcer in the last 3 months, and 2.9% had a genital ulcer present upon entry into the study.
Table 1.
Enrollment Characteristics of Participants Coinfected With Human Immunodeficiency Virus Type 1 (HIV) and Herpes Simplex Virus Type 2, by Antiretroviral Therapy (ART) Status
| Characteristic | Placebo (n=1688) |
Acyclovir (n=1693) |
||
|---|---|---|---|---|
| Not Started ART (n = 1498) | Started ART (n = 190) | Not Started ART (n = 1534) | Started ART (n = 159) | |
| Age, y | 31.8 (26.4–38.0) | 34.0 (28.5–42.3) | 32.0 (26.9–37.9) | 33.7 (29.0–41.0) |
| Female sexa | 69 | 61 | 67 | 62 |
| Plasma HIV load, log10 copies/mL | 3.99 (3.30–4.58) | 4.65 (3.91–5.12) | 4.04 (3.33–4.61) | 4.68 (4.01–5.18) |
| CD4+ T-cell count, cells/mm3 | 478 (361–647) | 324 (286–402) | 486 (367–660) | 325 (280–416) |
| History of GUD in last 3 months | 328 (21.9) | 48 (25.3) | 347 (22.6) | 40 (25.2) |
| GUD present on entry examination | 37 (2.5) | 10 (5.3) | 46 (3.0) | 4 (2.5) |
| Pathogen | ||||
| T. pallidumb | 94 (6.3) | 10 (5.3) | 92 (6.0) | 4 (2.5) |
| C. trachomatisc | 33 (2.4) | 2 (1.1) | 33 (2.3) | 0 |
| N. gonorrhoeaec | 18 (1.3) | 1 (0.6) | 29 (2.0) | 1 (0.7) |
| T. vaginalisc | 191 (13.7) | 15 (8.2) | 193 (13.3) | 17 (11.0) |
| Any of the aboved | 296 (21.3) | 24 (13.1) | 300 (20.6) | 19 (12.3) |
Data are median value (interquartile range) or no. (%) of subjects.
Abbreviation: GUD, genital ulcer disease.
a The numbers in this row are percentages.
b Serological testing for Treponema pallidum was performed at all sites, using rapid plasma reagin, with confirmation by the microhemagglutination test for T. pallidum at sites with that capacity.
c Batch testing for Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis was performed on archived cervical and urine samples at the University of Washington.
d Denominator for this calculation excludes 195 participants who had no laboratory results available.
Over 5005 person-years of follow-up during the study, with a median follow-up duration of 20 months (interquartile range [IQR], 15–24 months), 752 genital ulcers were reported (213 in the acyclovir arm and 539 in the placebo arm). Of those lesions, swab specimens were collected from 622 (83%) and tested for HSV-2 by PCR; 415 (67%) were positive, including 89 of 180 (49%) in the acyclovir arm and 326 of 442 (74%) in the placebo arm.
During the follow up period, 349 HIV-infected study participants initiated ART, with a median duration of follow-up of 10 months (IQR, 5–13 months). The incidence of genital ulcers increased from 15.0 per 100 person-years prior to initiation of ART to 26.9 per 100 person-years in the first full quarter after initiation of ART (adjusted IRR, 1.83 [95% confidence interval {CI}, 1.06–3.16]; P = .03; Table 2). For HSV-2–positive genital ulcers, the rate increased from 8.1 per 100 person-years to 19.0 per 100 person-years (IRR, 2.20 [95% CI, 1.12–4.33]; P = .02). Sensitivity analysis treating this outcome as missing for episodes of GUD without HSV-2 PCR testing results yielded similar rate ratio estimates (Supplementary Table 1). After the first full quarter, the incidence of GUD and HSV-2–positive ulcers returned to values closer to pre-ART levels, although the numbers were small. Sensitivity analyses that included the quarter in which ART was initially reported, categorized as the first quarter, yielded results that were not as strong, likely reflecting increased misclassification of ART status for the additional included quarters (IRR, 1.37 [95% CI, .89–2.10; P = .16] for GUD and 1.65 [95% CI, .95–2.87; P = .08] for HSV-2–positive GUD). The effect of acyclovir on HSV-2–positive genital ulcers is shown graphically in Figure 1. We observed lower HSV-2–associated GUD incidence in the acyclovir group, compared with the placebo group, during each period; however, the pronounced increase in GUD and HSV-2–positive GUD during the first full quarter after the start of ART was noted whether acyclovir or placebo was received (P = .25). An alternative analysis limited to only those individuals who ultimately started ART for the “prior to ART initiation” calculation had wider CIs but showed a similar increase in adjusted IRR (Supplementary Table 2).
Table 2.
Associations Between Observed Genital Ulcer Disease (GUD), With or Without Herpes Simplex Virus (HSV), and Initiation of Antiretroviral Therapy (ART)
| All GUD |
HSV2-positive GUD |
|||||
|---|---|---|---|---|---|---|
| Placebo Arm | Acyclovir Arm | Overall: Both Treatment Arms Combined | Placebo Arm | Acyclovir Arm | Overall: Both Treatment Arms Combined | |
| Before ART start | ||||||
| Events, no. | 517 | 204 | 721 | 308 | 82 | 390 |
| Person-years, no. | 2374.3 | 2443.5 | 4817.8 | 2374.3 | 2443.5 | 4817.8 |
| Incidencea (95% CI) | 21.8 (19.9, 23.7) | 8.3 (7.2, 9.6) | 15.0 (13.9, 16.1) | 13.0 (11.6, 14.5) | 3.4 (2.7, 4.2) | 8.1 (7.3, 8. 9) |
| First full quarter after ART start | ||||||
| Events, no. | 12 | 5 | 17 | 8 | 4 | 12 |
| Person-years, no. | 35.0 | 28.3 | 63.3 | 35.0 | 28.3 | 63.3 |
| Incidencea (95% CI) | 34.3 (17.7, 59.9) | 17.7 (5.7, 41.2) | 26.9 (1.5, 42.7) | 22.9 (9.9, 45.0) | 14.1 (3.9, 36.2) | 19.0 (9.7, 32.9) |
| bAdjusted IRRc (95% CI) | 1.73 (.89, 3.26); P = .11 | 2.17 (.86, 5.46); P = .09 | 1.83 (1.06, 3.16); P = .03 | 1.81 (.78, 4.21); P = .17 | 3.92 (1.37, 11.15; P = .01 | 2.20 (1.12, 4.33); P = .02 |
| Second full quarter and later after ART start | ||||||
| Events, no. | 10 | 4 | 14 | 10 | 3 | 13 |
| Person-years, no. | 73.0 | 51.0 | 124.0 | 73.0 | 51.0 | 124.0 |
| Incidencea (95% CI) | 13.7 (6.6, 25.2) | 7.8 (2.1, 20.1) | 11.3 (6.2, 18.9) | 13.7 (6.6, 25.2) | 5.9 (12.1, 17.2) | 10.5 (5.6, 17.9) |
| bAdjusted IRRc (95% CI) | 0.85 (.46, 1.57), P = .60 | 0.81 (.20, 3.17), P = .76 | 0.83 (.47, 1.49), P = .55 | 1.21 (.64, 2.27), P = .56 | 1.37 (.33, 5.78), P = .67 | 1.23 (.69, 2.22), P = .48 |
Abbreviations: CI, confidence interval; IRR, incident rate ratio.
a Incidence rates per 100 person-years.
b Adjusted for sex, CD4+ T-cell count, time on study, and acyclovir arm (for the overall analysis).
c The incidence before ART initiation was used as a reference.
Figure 1.
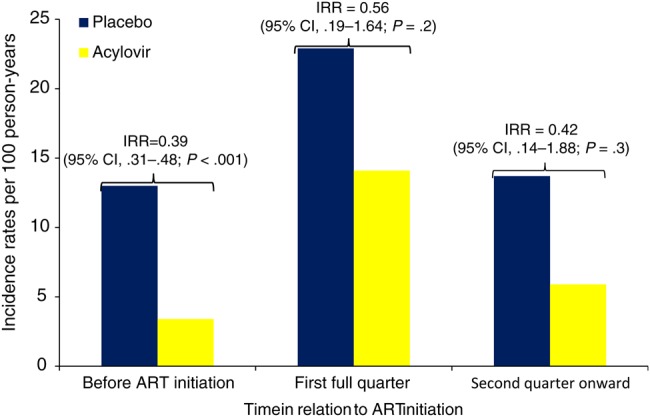
Incidence of herpes simplex virus type 2 (HSV-2)–specific ulcers, stratified by randomization treatment group and time since antiretroviral therapy (ART) initiation. The incidence rate ratio (IRR) for HSV-2 ulcers was calculated by comparing acyclovir versus placebo in the respective strata of time from ART initiation. The pre-ART group is based on the entire study population, whereas the 2 subsequent groups are based only on the 349 individuals who initiated ART. Incidence rates in the figure are as reported for HSV-2–positive genital ulcer disease in Table 2. Abbreviation: CI, confidence interval.
To understand better whether the increased risk was associated with a brisk response to ART, we identified 252 persons who had a documented increase in CD4+ T-cell count by at least 50 cells/mm3 or a viral load decrease of at least 2 log10, compared with the baseline measurement. In this group, the median increase in CD4+ T-cell count was from 192 to 319 cells/mm3 and the decrease in plasma HIV RNA load was from 5.01 to 2.08 log10 copies/mm3 after a median of 6 months. Their adjusted IRRs for GUD and HSV-2–positive GUD were qualitatively similar to those from analysis of the overall population (Supplementary Table 3).
DISCUSSION
We showed here that initiation of ART in HIV/HSV-2–coinfected persons is associated with a transient increase in the incidence of genital ulcers and specifically genital ulcers caused by HSV-2. This increase in GUD is the primary clinical manifestation of HSV-2–associated IRIS. We also showed that acyclovir 400 mg twice daily reduces the incidence of genital ulcers overall but does not prevent an increase in GUD incidence following initiation of ART. Although HSV is mentioned in several reports about IRIS, most referred to GUD or clinically diagnosed genital herpes [16–19], and many had variable follow-up. With the exception of an early small case series [16], ours is the first to document that most of the GUD observed after initiation of ART was caused by HSV-2. It is likely that some of the HSV-2–negative genital ulcers in our study were caused by HSV-2, but viral shedding ceased by the time the ulcer was swabbed, especially among acyclovir recipients.
The recent report by Tobian et al [20] is the best-documented study to date of increased reactivation of HSV-2 after initiation of ART. Their report was a secondary analysis from a study to evaluate the impact of suppressive acyclovir therapy on progression of HIV disease [25]. In that study, 132 of 440 enrollees initiated ART and were followed for development of GUD. These authors did not test the genital ulcers for HSV-2. This study found that the adjusted prevalence rate ratio of GUD was 1.94 in the 3 months following ART initiation. The magnitude of this change is similar to the IRR that we found for GUD after initiating ART (1.83) in our larger cohort, although the results cannot be directly compared. The 96 female subjects in the study of Tobian et al [20] who initiated ART provided monthly vaginal swabs that were tested for HSV-2 DNA, enabling evaluation of genital HSV-2 shedding before and after ART. The impact on viral shedding was also significant, with an odds ratio of 2.83 at month 1, 2.84 at month 2, and 3.15 at month 3 (all calculated using the 6 months prior to ART initiation as a reference). Viral shedding returned to baseline at month 4 and beyond. Our study design did not allow us to measure viral shedding in this same way, so we were unable to confirm this observation.
Acyclovir remains a useful drug, but its efficacy may be blunted in persons coinfected with HIV and HSV-2. Although it significantly reduced the incidence of GUD in our study, it did not improve the IRR following initiation of ART. The report by Tobian et al had comparable rates in their acyclovir and placebo arms [20]. In a separate analysis from this same cohort, we also showed that acyclovir suppression did not reduce transmission of HSV-2 to susceptible partners [26]. However, another analysis from this cohort showed that acyclovir suppression reduced the incidence of herpes zoster [27]. These observations confirm acyclovir's usefulness but suggest that more-potent antiherpes drugs are needed.
One of the limitations of our study is the relatively short period of observation following initiation of ART. This limits the precision with which we can estimate the duration of the increased incidence of GUD following initiation of ART. However, our estimate of ≥3 months (the first full quarter) is similar to that identified in other studies [20]. Because we asked about antiretroviral initiation only at quarterly visits, the estimate of the time of initiation was imprecise. By excluding the quarter in which ART was initiated from the analysis, we may have excluded up to nearly 3 months of postinitiation observation for some individuals. Similarly, because we only asked about genital ulcers at quarterly visits, some episodes of GUD may have gone unreported. This limitation was partially abated by recording spontaneous reports of genital ulcers and swabbing them at monthly visits, but because routine examinations were not done at monthly visits, some episodes may still have been missed.
In summary, initiation of ART in HIV/HSV-2–coinfected persons doubles the incidence of GUD and HSV-2–associated GUD in the first 3 months. This impact is not eliminated by suppressive acyclovir therapy, although the incidence of GUD is reduced. Clinicians should consider acyclovir suppression around the time of ART initiation, especially in persons with a history of symptomatic GUD, in order to blunt the anticipated increase in GUD incidence. This recommendation is also consistent with the current opportunistic infection treatment guidelines [28].
STUDY GROUP MEMBERS
Members of the Partners in Prevention HSV/HIV Transmission Study Team are as follows: Connie Celum (principal investigator), Anna Wald (protocol co-chair), Jairam Lingappa (medical director), Jared M. Baeten, Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, and James I. Mullins (University of Washington Coordinating Center and Central Laboratories, Seattle). Study sites and site principal investigators are as follows: University of Cape Town, South Africa: David Coetzee; Moi University, Indiana University, Eldoret, Kenya: Kenneth Fife and Edwin Were; Botswana Harvard Partnership, Gaborone: Max Essex and Joseph Makhema; Infectious Disease Institute, Makerere University, Kampala, Uganda: Elly Katabira and Allan Ronald; Rwanda Zambia HIV Research Group and Emory University, Kigali: Susan Allen, Kayitesi Kayitenkore, and Etienne Karita; Kenya Medical Research Institute, University of California San Francisco, Kisumu: Elizabeth Bukusi and Craig Cohen; Rwanda-Zambia HIV Research Group and Emory University, Kitwe, Zambia: Susan Allen and William Kanweka; Rwanda-Zambia HIV Research Group and Emory University, Lusaka, Zambia: Susan Allen and Bellington Vwalika; Kilimanjaro Christian Medical College, Harvard University, Moshi, Tanzania: Saidi Kapiga and Rachel Manongi; University of Nairobi, University of Washington, Nairobi, Kenya: Carey Farquhar, Grace John-Stewart, and James Kiarie; Rwanda Zambia HIV Research Group and Emory University, Ndola, Zambia: Susan Allen and Mubiana Inambao; Wits Reproductive Health and HIV Institute, University of the Witwatersrand, Orange Farm, South Africa: Sinead Delany-Moretlwe and Helen Rees; Perinatal HIV Research Unit, University of the Witwatersrand, Soweto, South Africa: Guy de Bruyn, Glenda Gray, and James McIntyre; and University of Nairobi, University of Washington, Thika, Kenya: Nelly Rwamba Mugo.
Supplementary Material
Notes
Acknowledgments. We thank all of the study participants for their dedication to the study and faithful adherence to the protocol.
Financial support. This work was supported by the Bill and Melinda Gates Foundation.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. DeSimone JA, Pomerantz RJ, Babinchak TJ. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy. Ann Intern Med 2000; 133:447–54. [DOI] [PubMed] [Google Scholar]
- 2. Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karavellas MP, Lowder CY, Macdonald C, Avila CP Jr, Freeman WR. Immune recovery vitritis associated with inactive cytomegalovirus retinitis: a new syndrome. Arch Ophthalmol 1998; 116:169–75. [DOI] [PubMed] [Google Scholar]
- 4. Zegans ME, Walton RC, Holland GN, O'Donnell JJ, Jacobson MA, Margolis TP. Transient vitreous inflammatory reactions associated with combination antiretroviral therapy in patients with AIDS and cytomegalovirus retinitis. Am J Ophthalmol 1998; 125:292–300. [DOI] [PubMed] [Google Scholar]
- 5. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20:73–83. [DOI] [PubMed] [Google Scholar]
- 6. Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 2002; 185:45–52. [DOI] [PubMed] [Google Scholar]
- 7. McClelland RS, Wang CC, Overbaugh J et al. . Association between cervical shedding of herpes simplex virus and HIV-1. AIDS 2002; 16:2425–30. [DOI] [PubMed] [Google Scholar]
- 8. Serwadda D, Gray RH, Sewankambo NK et al. . Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis 2003; 188:1492–7. [DOI] [PubMed] [Google Scholar]
- 9. Tobian AA, Quinn TC. Herpes simplex virus type 2 and syphilis infections with HIV: an evolving synergy in transmission and prevention. Curr Opin HIV AIDS 2009; 4:294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Celum C, Wald A, Lingappa JR et al. . Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Celum C, Wald A, Hughes J et al. . Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:2109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watson-Jones D, Weiss HA, Rusizoka M et al. . Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med 2008; 358:1560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ameli N, Bacchetti P, Morrow RA et al. . Herpes simplex virus infection in women in the WIHS: epidemiology and effect of antiretroviral therapy on clinical manifestations. AIDS 2006; 20:1051–8. [DOI] [PubMed] [Google Scholar]
- 14. Mayaud P, Nagot N, Konate I et al. . Effect of HIV-1 and antiretroviral therapy on herpes simplex virus type 2: a prospective study in African women. Sex Transm Infect 2008; 84:332–7. [DOI] [PubMed] [Google Scholar]
- 15. Posavad CM, Wald A, Kuntz S et al. . Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis 2004; 190:693–6. [DOI] [PubMed] [Google Scholar]
- 16. Fox PA, Barton SE, Francis N et al. . Chronic erosive herpes simplex virus infection of the penis, a possible immune reconstitution disease. HIV Med 1999; 1:10–8. [DOI] [PubMed] [Google Scholar]
- 17. Couppie P, Sarazin F, Clyti E et al. . Increased incidence of genital herpes after HAART initiation: a frequent presentation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients. AIDS Patient Care STDS 2006; 20:143–5. [DOI] [PubMed] [Google Scholar]
- 18. French MA, Lenzo N, John M et al. . Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med 2000; 1:107–15. [DOI] [PubMed] [Google Scholar]
- 19. Graham SM, Masese L, Gitau R et al. . Increased risk of genital ulcer disease in women during the first month after initiating antiretroviral therapy. J Acquir Immune Defic Syndr 2009; 52:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tobian AA, Grabowski MK, Serwadda D et al. . Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis 2013; 208:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lingappa JR, Baeten JM, Wald A et al. . Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 2010; 375:824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol 2002; 40:2609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: Comparison with HSV isolation in cell culture. J Infect Dis 2003; 188:1345–51. [DOI] [PubMed] [Google Scholar]
- 24. Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol 2007; 45:1618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reynolds SJ, Makumbi F, Newell K et al. . Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis 2012; 12:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mujugira A, Magaret AS, Celum C et al. . Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: A randomized controlled trial. J Infect Dis 2013; 208:1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barnabas RV, Baeten JM, Lingappa JR et al. . Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: Results of a randomized clinical trial. J Infect Dis 2016; 213:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf Accessed 11 Nov 2015:O1–7.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


