Abstract
Background. Noroviruses pose a significant public health risk, particularly in very young individuals, older adults, and individuals with underlying conditions. We assessed 2 bivalent norovirus virus-like particle (VLP) vaccine candidate formulations in healthy adults aged 18–49 years.
Methods. Enrolled subjects (n = 454) randomly assigned among 3 groups received intramuscular placebo (saline) or vaccines containing either 15 µg or 50 µg of GI.1 VLP and 50 µg GII.4 VLP (15/50 and 50/50 formulations) adjuvanted with monophosphoryl lipid A and Al(OH)3. We present safety and immunogenicity assessments up to 28 days after vaccination.
Results. No vaccine-related serious adverse events or adverse events of special interest were reported. Reactions were mainly mild to moderate, the most frequent being transient pain, in 8%, 64%, and 73% of placebo, 15/50, and 50/50 groups, respectively; transient myalgia, headache, and fatigue were the commonest systemic adverse events. Subjects assessed per protocol (n = 442) displayed rapid immune responses to vaccination, peaking by days 7–10 and persisting through day 28. GI.1 responses were highest with the 50/50 formulation, but GII.4 responses were higher with the 15/50 formulation.
Conclusions. Both candidate VLP vaccines were well tolerated and elicited robust immune responses by 7–10 days that persisted through day 28. The 15/50 formulation displayed the best balance of tolerability and immunogenicity.
Clinical Trials Registration. NCT02142504.
Keywords: norovirus, vaccine, immunogenicity, tolerability, safety
Noroviruses (NoVs) are well known as the cause of so-called winter vomiting disease and are the leading cause of acute gastroenteritis (AGE) around the world, including foodborne AGE, with high morbidity in all age groups [1–3]. In countries where rotavirus vaccination has been successfully introduced, NoVs are now the leading cause of pediatric AGE [4]. NoV are endemic and highly infectious, commonly causing sporadic cases and gastroenteritis outbreaks in people living in confined quarters. Although NoV AGE may be generally regarded as relatively benign or short-lived, NoV has been associated with a higher risk of severe or fatal consequences in vulnerable age groups, particularly very young individuals in low-income countries, elderly individuals globally, and individuals with underlying conditions, such as those who are immunocompromised or have chronic renal or cardiac chronic disease [5, 6].
NoV infection outbreaks are sporadic and difficult to avoid. Transmission is mainly from person to person where people gather, by the fecal-oral route or by the aerosol route, with the latter often following a projectile vomiting event; or due to environmental exposure, through consumption of or contact with contaminated food, fomites, or water. The unpredictable nature of NoV transmission may make vaccination the best prophylactic measure for avoiding clinical disease, particularly for those living in close quarters, such as military personnel in barracks, travelers exposed in confined quarters (cruise ships), infants in child-care facilities, individuals in hospitals, individuals in schools, and elderly persons in institutionalized care.
Seven NoV genogroups (GI–VII) have been described and contain >35 genotypes. Most human disease is due to strains belonging to GI or GII. Currently, GII.4 NoVs are responsible for the majority of human disease [7–9]. Takeda Vaccines is developing a candidate bivalent NoV vaccine that uses virus-like particles (VLPs) of a consensus GII.4 strain from 3 GII.4 variants with the aim of providing broad coverage against GII strains and, in combination with the Norwalk GI.1 strain VLP, against GI strains [10–12]. It is hoped that including a range of epitopes will result in broad protection. A bivalent formulation containing 50 µg of each VLP was selected for further evaluation [12], although in that study and in a subsequent clinical trial [11, 13] the GII.4 seroresponse was lower than the GI.1 response at the same VLP antigen dose. A high GI.1 response was also achieved with 15 µg of GI.1 VLP [12]. Therefore, in this study we tested 2 different formulations, containing either 15 µg or 50 µg GI.1 VLP combined with 50 µg of the GII.4 VLP (15/50 and 50/50 antigen-formulations) and adjuvanted with 50 µg monophosphoryl lipid A (MPL) and 0.5 mg Al(OH)3.
METHODS
Study Design
We conducted a randomized, double-blinded, placebo-controlled phase 2 study with a first phase performed at 10 centers in the United States from 15 May to 2 July 2014. The study was approved by the institutional review board of each participating institution and was performed according to the prevailing Declaration of Helsinki and good clinical practice guidelines. The study protocol was registered on ClinicalTrials.gov (NCT02142504).
The primary objective was to assess the safety and tolerability of 2 formulations of an adjuvanted bivalent NoV VLP vaccine candidate, using defined safety parameters. A secondary objective was to assess the immunogenicity of these 2 formulations. Safety and immunogenicity data up to 28 days after vaccination are presented herein. There will be further evaluations of the long-term safety and immunogenicity up to 18 months from study start in an extension phase of this study, including the effect of a second immunization with a lower dose of vaccine (15/15 μg without MPL adjuvant) 1 year after the initial vaccinations, that will be reported separately.
Study Participants
Eligible participants were men or women 18–49 years of age who were in good health at the time of enrollment, based on medical history and physical examination, and were able to comply with trial procedures and be available for the entire duration of the trial. Subjects with a body mass index (calculated as the weight in kilograms divided by the height in meters squared) of ≥35 were excluded. Other exclusion criteria included any history of gastroenteritis within 14 days prior to enrollment; any known current or chronic medical conditions, particularly those likely to affect immune function; any history of allergic reaction to vaccination or vaccine components; or any other recent vaccinations or participation in another clinical trial within 30 days of study start. Female volunteers who were breastfeeding were excluded, and females who were sexually active were required to have a negative pregnancy test result and had to agree to use an acceptable form of contraception until 6 months after the final vaccination. All volunteers provided written informed consent before enrollment.
Vaccine
The 2 candidate vaccine formulations contained, respectively, in each 0.5-mL dose 15 µg of GI.1 VLP and 50 µg of GII.4 VLP (15/50 antigen formulation) or 50 µg of GI.1 VLP and 50 µg of GII.4 VLP (50/50 antigen formulation). Both formulations also contained 50 µg of MPL (3-O-desacyl-4′-monophosphoryl lipid A; GlaxoSmithKline Vaccines, Belgium) and 0.5 mg of aluminum as Al(OH)3 (Brenntag Biosector, Denmark) per dose as adjuvants.
At enrollment, subjects were randomly assigned at a ratio of 1:1:1 into 3 equal groups. After collection of an initial blood specimen on day 1, participants received a single dose of one of the 2 candidate vaccine formulations or placebo (physiological saline) by intramuscular injection in the deltoid muscle. Because the vaccines and saline are visually distinguishable, the administrator was not blinded to the nature of each injection. All subsequent interventions and laboratory and safety assessments were performed by study personnel blinded to the study groups. Safety evaluations were performed 30 minutes after injection and on days 8 and 29. To assess early kinetics of the immune response as an exploratory objective, participants provided 3 further blood samples, on day 3, day 5, and once between days 7 and 10. An additional blood sample was collected on day 28.
Safety and Reactogenicity
All subjects were monitored for 30 minutes after vaccination for any immediate reactions. Each participant then completed a 7-day diary card that solicited local injection site (pain, swelling, induration, and erythema) and systemic (temperature, headache, fatigue, myalgia, arthralgia, vomiting, and diarrhea) adverse events and their severity. Maximum diameters of any swelling, induration, and erythema were measured, and any reaction with a diameter of >10 cm was considered severe. Pain and solicited systemic adverse events were considered severe if, with or without treatment, they prevented normal daily activity. Unsolicited adverse events, serious adverse events (SAEs), and adverse events of special interest were recorded throughout the study duration.
Immunogenicity
Immune responses were assessed in sera as total serum antibody (pan-immunoglobulin [Pan-Ig]) and immunoglobulin A (IgA) antibodies against the GI.1 and GII.4 antigens, measured by enzyme-linked immunosorbent assay (ELISA) as previously reported [12]. Histo-blood group antigen (HBGA)–blocking antibodies have been associated with protection against NoV infection and suggested to be a serologic correlate of protection following vaccination [13, 14]. HBGA-blocking antibodies were measured as described previously [13]. Briefly, this is a semiquantitative assay to determine the extent to which human serum inhibits NoV VLP binding to porcine gastric mucin (PGM; Sigma-Aldrich, product no. M1778). PGM is coated on microtiter plates, and samples diluted in assay buffer are combined with GI.1 or GII.4 VLP ligand solution. Anti-VLP IgG antibodies are added to the plate, and an anti-rabbit IgG horseradish peroxidase conjugate is used to detect anti-VLP antibodies bound to the VLP immobilized on the PGM coated on the plate, using ABTS peroxidase as a substrate for colorimetric readout. All immune responses are expressed as geometric mean titers (GMTs) of antibodies, geometric mean-fold rises in antibody titers (GMFR) compared with baseline, and seroresponse rates (calculated as the percentages of each group displaying ≥4-fold increases in titers from baseline) for each study group.
Statistics
All safety, reactogenicity, and immunogenicity results are descriptive. No formal sample size calculations were performed; instead, the sample size was selected to provide a reasonable clinical database to support initiation of larger phase 2 and 3 studies.
RESULTS
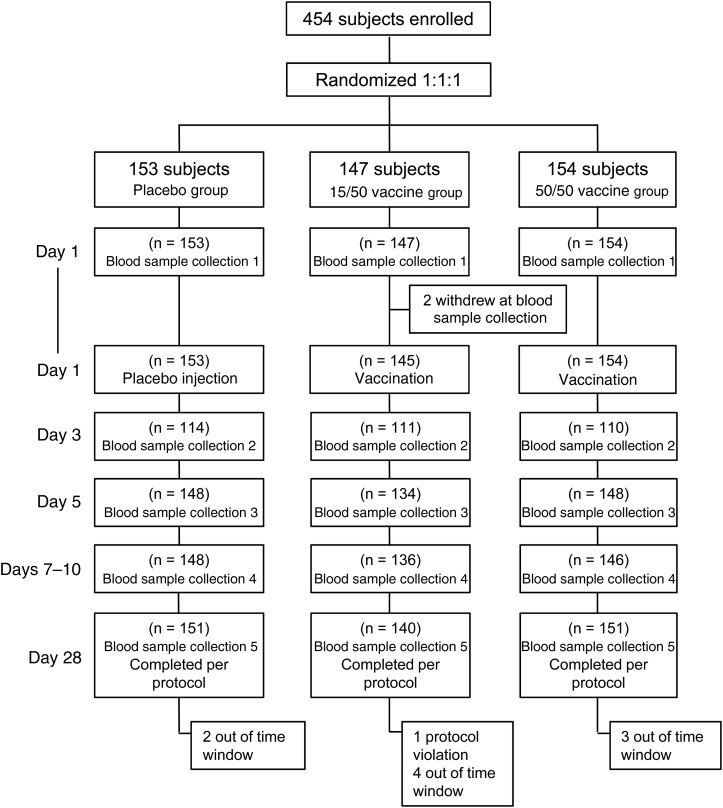
A total of 454 volunteers were enrolled and randomly assigned among the 3 groups, with similar demographic characteristics in each group (Table 1). Prior to vaccination, 2 subjects withdrew consent: one withdrew after collection of the first blood specimen, and the other had a vasovagal reaction to the phlebotomy. Of the 452 subjects vaccinated, 335 (74.1%), 430 (95.1%), 430 (95.1%), and 442 (97.8%) provided the day 3, day 5, day 7–10, and day 28 serum samples, respectively, for analysis of immune responses according to protocol. All vaccinated participants were included in the safety analyses. Ten participants were excluded from the per protocol immunogenicity analyses, mainly for being outside of the stipulated time windows (Figure 1); specifically, 2, 5, and 3 participants were excluded from the placebo, 15/50, and 50/50 groups, respectively.
Table 1.
Demographic Characteristics of All Enrolled Study Participants Who Underwent Random Assignment to Study Agent, by Study Group
| Enrolled | Placebo Group (n = 153) | 15/50 Vaccine Group (n = 147) | 50/50 Vaccine Group (n = 154) |
|---|---|---|---|
| Age, y | |||
| Mean ± SD | 32.9 ± 8.9 | 33.4 ± 9.3 | 34.0 ± 8.8 |
| Range | 18–49 | 18–49 | 18–49 |
| Sex, % | |||
| Male | 64 (42) | 58 (39) | 79 (51) |
| Female | 89 (58) | 89 (61) | 75 (49) |
| Ethnicity, % | |||
| Hispanic/Latino | 34 (22) | 38 (26) | 46 (30) |
| Non-Hispanic/Latino | 117 (76) | 109 (74) | 107 (69) |
| Not reported | 1 (1) | 0 | 1 (1) |
| Unknown | 1 (1) | 0 | 0 |
| Race, % | |||
| American Indian or Alaskan native | 0 | 0 | 2 (1) |
| Asian | 5 (3) | 3 (2) | 2 (1) |
| Black or African American | 41 (27) | 49 (33) | 47 (31) |
| Native Hawaiian | 3 (2) | 2 (1) | 2 (1) |
| White | 100 (65) | 91 (62) | 94 (61) |
| Other | 1 (1) | 0 | 0 |
| Multiracial | 3 (2) | 1 (1) | 5 (3) |
| BMIa | |||
| Mean ± SD | 26.4 ± 4.1 | 26.4 ± 4.4 | 26.1 ± 4.3 |
| Range | 15.9–34.8 | 17.6–34.9 | 15.5–34.3 |
Abbreviation: SD, standard deviation.
a Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
Figure 1.
Study flow chart.
Safety and Tolerability
No deaths or any SAEs related to vaccination were reported during the 28-day reporting period. One subject in the 15/50 group reported 3 SAEs (migraines, depression, and intentional drug overdose), each considered to be unrelated to vaccination. Subsequent investigation revealed that each event was present before enrollment and had not been disclosed. The subject was withdrawn from the study because of protocol violation but continued to be monitored for safety.
The vaccine formulations elicited more local reactions than placebo. Injection site pain was reported by 7.8% of placebo recipients, compared with 64.1% and 72.7% of the 15/50 and 50/50 vaccine recipients, respectively (Table 2). The majority of these reactions were described as mild to moderate, with 2 cases of injection site pain in each vaccine group being described as interfering with daily function (severe). Other local reactions were infrequent, reported by 1–3 subjects in the 2 vaccine groups.
Table 2.
Solicited Local Reactions and Systemic Adverse Events Within 7 Days of Vaccination in the Safety Analysis Set, by Study Group
| Variable | Placebo Group (n = 153) | 15/50 Vaccine Group (n = 145) | 50/50 Vaccine (n = 154) |
|---|---|---|---|
| Injection site reaction | |||
| Any | 12 (7.8) | 93 (64.1) | 112 (72.7) |
| Pain | |||
| Any | 12 (7.8) | 93 (64.1) | 112 (72.7) |
| Severe | 0 | 2 (1.4) | 2 (1.3) |
| Erythema | 0 | 0 | 1 (0.6) |
| Induration | 0 | 2 (1.4) | 2 (1.3) |
| Swelling | 0 | 3 (2.1) | 2 (1.3) |
| Systemic adverse event | |||
| Any | 41 (26.8) | 48 (33.1) | 70 (45.5) |
| Fever, °C | |||
| ≥38.0 | 0 | 1 (0.7) | 0 |
| ≥38.5 | 0 | 0 | 0 |
| Headache | |||
| Any | 25 (16.3) | 23 (15.9) | 28 (18.2) |
| Severe | 0 | 1 (0.7) | 0 |
| Fatigue | |||
| Any | 26 (17.0) | 23 (15.9) | 25 (16.2) |
| Severe | 1 (0.7) | 3 (2.1) | 1 (0.6) |
| Myalgia | |||
| Any | 11 (7.2) | 28 (19.3) | 39 (25.3) |
| Severe | 0 | 3 (2.1) | 1 (0.6) |
| Arthralgia | |||
| Any | 5 (3.3) | 7 (4.8) | 12 (7.8) |
| Severe | 0 | 0 | 0 |
| Vomiting | |||
| Any | 2 (1.3) | 2 (1.4) | 3 (1.9) |
| Severe | 0 | 0 | 0 |
| Diarrhea | |||
| Any | 12 (7.8) | 11 (7.6) | 16 (10.4) |
| Severe | 1 (0.7) | 0 | 0 |
Data are no. (%) of subjects.
Higher rates of solicited systemic adverse events were reported for both vaccine formulations, compared with placebo, with values of 26.8%, 33.1%, and 45.5% for the placebo, 15/50, and 50/50 groups, respectively (Table 2). The most frequent systemic adverse events were myalgia, headache, and fatigue. Myalgia was reported more frequently in both vaccine groups (19.3% and 25.3%) than in placebo recipients (7.2%), but headache and fatigue occurred at similar rates in all groups (15.9%–18.2%). There were few systemic adverse reactions described as severe (0%–2.1%). These occurred in all groups, and there was no specific association with either of the vaccine candidates or the placebo. The majority of all solicited reactions occurred within 3 days of vaccination and resolved within 7 days.
Unsolicited AEs, occurring 8–28 days after vaccination, were more frequent in the placebo group (23.5%) than in the 15/50 (15.2%) or 50/50 (21.4%) vaccine groups. No single adverse event was reported in >4% of subjects in any group, and there was no clear trend to link any adverse event to either vaccine dose or placebo injection (Table 3). The most frequent unsolicited adverse events were cases of headache, myalgia, and nausea that occurred after the 7-day postvaccination period.
Table 3.
Unsolicited Adverse Events Occurring in ≥2 Participants From Days 8–28, by Study Group
| Adverse Event | Placebo Group (n = 153) | 15/50 Vaccine Group (n = 145) | 50/50 Vaccine (n = 154) |
|---|---|---|---|
| Any | 36 (23.5) | 22 (15.2) | 33 (21.4) |
| Headache | 4 (2.6) | 4 (2.8) | 6 (3.9) |
| Nausea | 4 (2.6) | 1 (0.7) | 3 (1.9) |
| Myalgia | 1 (0.7) | 4 (2.8) | 2 (1.3) |
| Upper abdominal pain | 2 (1.3) | 2 (1.4) | 1 (0.6) |
| Back pain | 2 (1.3) | 2 (1.4) | 1 (0.6) |
| Diarrhea | 2 (1.3) | 1 (0.7) | 2 (1.3) |
| Oropharyngeal pain | 2 (1.3) | 1 (0.7) | 2 (1.3) |
| Pain in extremities | 3 (2.0) | 0 | 2 (1.3) |
| Cough | 1 (0.7) | 1 (0.7) | 2 (1.3) |
| Dizziness | 1 (0.7) | 2 (1.4) | 1 (0.6) |
| Bruising (injection site) | 3 (2.0) | 1 (0.7) | 0 |
| Pain (injection site) | 0 | 3 (2.1) | 1 (0.6) |
| Nasopharyngitis | 1 (0.7) | 2 (1.4) | 1 (0.6) |
| Sinus headache | 2 (1.3) | 0 | 2 (1.3) |
| Fatigue | 1 (0.7) | 0 | 2 (1.3) |
| Arthropod bite | 1 (0.7) | 0 | 1 (0.6) |
| Dysmenorrhea | 1 (0.7) | 1 (0.7) | 0 |
| Dyspepsia | 1 (0.7) | 0 | 1 (0.6) |
| Ligament sprain | 0 | 1 (0.7) | 1 (0.6) |
| Migraine | 0 | 1 (0.7) | 1 (0.6) |
| Neck pain | 0 | 2 (1.4) | 0 |
| Rash | 2 (1.3) | 0 | 0 |
| Allergic rhinitis | 1 (0.7) | 1 (0.7) | 0 |
| Tonsillitis | 0 | 0 | 2 (1.3) |
| Urticaria | 0 | 0 | 2 (1.3) |
Data are no. (%) of subjects.
Immunogenicity
ELISA-Determined Pan-Ig and IgA Antibody Levels
At baseline, all 3 treatment arms had similar levels of Pan-Ig and IgA antibodies reactive with the VLPs of the 2 genotypes; titers were higher against GII.4 than those against GI.1. GMTs of Pan-Ig ELISA antibodies against GI.1 were 287, 346, and 335 in the placebo, 15/50 vaccine, and 50/50 vaccine groups, respectively. For GII.4, baseline GMTs were 880, 1055, and 1164 in the placebo, 15/50 vaccine, and 50/50 vaccine groups, respectively.
Baseline IgA titers against GI.1 were also lower than those against GII.4. In the placebo, 15/50 vaccine, and 50/50 vaccine groups, baseline GI.1 IgA GMTs were 20.7, 21.1, and 24.5, respectively, and baseline GII.4 IgA GMTs were 75.3, 91.6, and 97.9, respectively.
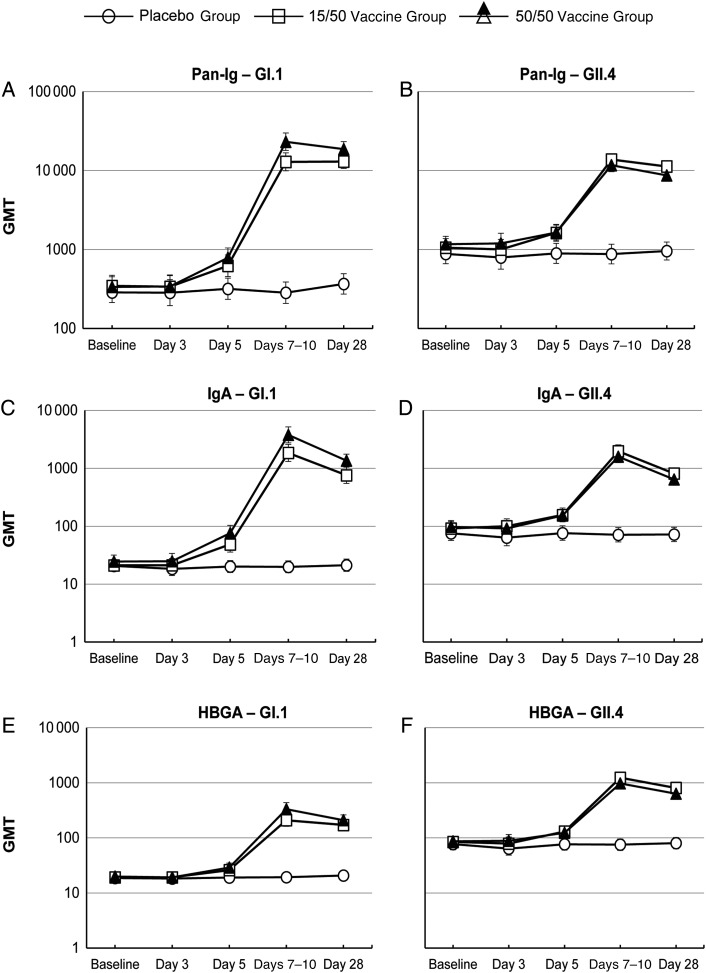
Following placebo injection, Pan-Ig and IgA levels remained constant over the 28-day follow-up period, while immunization with both vaccine formulations elicited rapid and persisting increases in both Pan-Ig and IgA ELISA antibody titers against the GI.1 and GII.4 VLPs (Figure 2A–2D). Increases in Pan-Ig and IgA GMTs against both GI.1 and GII.4 VLPs first became apparent at day 5, and the antibody levels peaked at the day 7–10 postvaccination study visit.
Figure 2.
Geometric mean titers (GMTs) (with 95% confidence intervals) of pan-immunoglobulin (A and B), immunoglobulin A (IgA; C and D), and histo-blood group antigen (HBGA)–blocking (E and F) antibodies against norovirus GI.1 and GII.4 virus-like particles at each of the sampling time points.
The 50/50 vaccine formulation elicited higher Pan-Ig responses to GI.1 VLPs; GMTs at day 7–10 were 12 893 and 23 349 for GI.1 VLPs in the 15/50 and 50/50 vaccine groups, respectively, and these differences were also associated with higher seroresponse and GMFR rates in the 50/50 vaccine group (Table 4). Corresponding GMTs against GII.4 VLP were 13 819 and 11 728. Pan-Ig GMTs then persisted at similar levels to day 28, when 91.4% and 90.1% of subjects in the 15/50 and 50/50 vaccine groups, respectively, displayed seroresponses against GI.1, and 75.0% and 69.5%, respectively, had responses against GII.4 VLPs, relative to the baseline serum antibody levels (Table 4). The higher response to GI.1 VLPs in the 50/50 group was also evident with a higher GMFR (55.9 vs 37.6) at day 28.
Table 4.
Pan-Immunoglobulin (Pan-Ig), Immunoglobulin A (IgA), and Histo-Blood Group Antigen (HBGA)–Blocking Antibody (Ab) Responses Against Norovirus GI.1 and GII.4 at the Indicated Time Points in the Per-Protocol Set, by Study Group
| Pan-Ig Ab, Titer (95% CI) |
IgA Ab, Titer (95% CI) |
HBGA-Blocking Ab, Titer (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype, Time, Variable | Placebo (n = 151) | 15/50 Vaccine Group (n=140) | 50/50 Vaccine Group (n = 151) | Placebo (n = 151) | 15/50 Vaccine Group (n=140) | 50/50 Vaccine Group (n = 151) | Placebo (n = 151) | 15/50 Vaccine Group (n=140) | 50/50 Vaccine Group (n = 151) |
| GI.1 | |||||||||
| Baseline | |||||||||
| GMT | 287 (213–386) | 346 (253–473) | 335 (248–452) | 21 (17–26) | 21 (17–27) | 25 (19–32) | 19 (17–21) | 19 (17–21) | 20 (18–23) |
| Day 3 | |||||||||
| GMT | 284 (195–415) | 338 (244–467) | 340 (243–475) | 18 (14–24) | 21 (16–28) | 25 (18–34) | 18 (16–20) | 19 (17–22) | 19 (17–22) |
| SR, %a | 3.5 (1.0–8.7) | 1.8 (.2–6.4) | 1.8 (.2–6.4) | 0.9 (0–4.8) | 1.8 (0.2–6.4) | 0 (0–3.3) | 0.9 (0–4.8) | 0 (0–3.3) | 0 (0–3.3) |
| Day 5 | |||||||||
| GMT | 318 (234–433) | 622 (454–852) | 784 (588–1045) | 20 (16–26) | 49 (36–66) | 76 (56–104) | 19 (17–21) | 26 (22–31) | 29 (24–52) |
| SR, %a | 4.7 (1.9–9.5) | 19.4 (13.1–27.1) | 27.0 (20.1–34.9) | 0.7 (0–3.7) | 25.4 (18.3–33.6) | 34.5 (26.8–42.7) | 0.7 (0–3.7) | 8.2 (4.2–14.2) | 11.0 (6.4–17.2) |
| Day 7–10 | |||||||||
| GMT | 284 (208–386) | 12 893 (9912–16 770) | 23 150 (17 939–29 875) | 20 (16–26) | 1852 (1319–2601) | 3783 (2755–5196) | 19 (17–22) | 209 (162–270) | 332 (253–436) |
| SR, %a | 4.1 (1.5–8.6) | 84.6 (77.4–90.2) | 93.2 (87.8–96.7) | 0.7 (0–3.7) | 93.4 (87.8–96.9) | 95.2 (90.4–98.1) | 0 (0–2.5) | 78.0 (70.0–84.8) | 81.1 (73.7–87.2) |
| GMFR | 1.0 (.86–1.13) | 38.0 (27.4–52.6) | 69.8 (51.9–94.0) | 1.0 (.91–1.03) | 89.1 (65.7–121) | 148 (109–202) | 1.0 (1.00–1.06) | 10.7 (8.29–13.7) | 16.4 (12.5–21.5) |
| Day 28 | |||||||||
| GMT | 367 (273–493) | 13 023 (10 616–15 977) | 18 712 (15 070–23 233) | 21 (17–27) | 760 (551–1047) | 1363 (1055–1760) | 21 (18–24) | 172 (137–216) | 210 (168–261) |
| SR, %a | 8.6 (4.7–14.3) | 91.4 (85.5–95.5) | 90.1 (84.1–94.3) | 3.3 (1.1–7.6) | 92.1 (86.4–96.0) | 95.4 (90.7–98.1) | 3.3 (1.1–7.6) | 74.3 (66.2–81.3) | 80.8 (73.6–86.8) |
| GMFR | 1.3 (1.03–1.59) | 37.6 (27.8–50.9) | 55.9 (42.0–74.4) | 1.0 (.89–1.19) | 36.0 (27.3–47.5) | 55.6 (43.7–70.7) | 1.1 (1.02–1.21) | 8.9 (7.19–11.1) | 10.5 (8.53–12.8) |
| GII.4 | |||||||||
| Baseline | |||||||||
| GMT | 880 (660–1172) | 1055 (811–1373) | 1164 (921–1471) | 75 (57–100) | 92 (70–120) | 98 (76–125) | 77 (61–98) | 84 (67–107) | 87 (69–108) |
| Day 3 | |||||||||
| GMT | 792 (565–1109) | 1001 (748–1341) | 1197 (889–1612) | 64 (46–89) | 100 (75–135) | 92 (68–123) | 64 (49–83) | 79 (60–103) | 89 (68–115) |
| SR, %a | 1.8 (.2–6.2) | 0.9 (0–4.9) | 3.6 (1.0–9.0) | 0 (0–3.2) | 2.7 (.6–7.7) | 0 (0–3.3) | 0.9 (0–4.8) | 0 (0–3.3) | 0.9 (0–5.0) |
| Day 5 | |||||||||
| GMT | 894 (668–1195) | 1628 (1276–2076) | 1637 (1314–2039) | 76 (57–102) | 157 (119–207) | 154 (121–196) | 77 (60–98) | 130 (102–167) | 123 (98–155) |
| SR, %a | 2.0 (0.4–5.8) | 11.2 (6.4–17.8) | 5.4 (2.4–10.4) | 0.7 (0–3.7) | 12.7 (7.6–19.5) | 7.4 (3.9–12.9) | 0.7 (0–3.7) | 11.9 (7.0–18.7) | 8.1 (4.3–13.7) |
| Day 7–10 | |||||||||
| GMT | 875 (656–1167) | 13 819 (11 677–16 353) | 11 713 (9690–14 158) | 71 (54–94) | 1976 (1537–2541) | 1591 (1252–2020) | 75 (59–96) | 1234 (987–1541) | 973 (787–1204) |
| SR, %a | 1.4 (.2–4.8) | 76.5 (68.4–83.3) | 74.0 (66.1–80.9) | 0 (0–2.5) | 81.6 (74.1–87.7) | 80.8 (73.5–86.9) | 0.7 (0–3.7) | 75.0 (66.9–82.0) | 72.6 (64.6–79.7) |
| GMFR | 1.0 (.94–1.06) | 13.6 (10.4–17.9) | 10.0 (7.89–12.7) | 1.0 (.91–1.01) | 22.2 (16.7–29.4) | 16.0 (12.3–20.9) | 1.0 (.93–1.04) | 15.1 (11.4–20.0) | 11.3 (8.87–14.5) |
| Day 28 | |||||||||
| GMT | 956 (736–1241) | 11 276 (9757–13 030) | 8668 (7330–10 250) | 72 (54–96) | 816 (654–1017) | 642 (523–787) | 80 (63–101) | 804 (658–984) | 631 (528–755) |
| SR, %a | 4.6 (1.9–9.3) | 75.0 (67.0–81.9) | 69.5 (61.5–76.8) | 1.3 (0.2–4.7) | 70.0 (61.7–77.4) | 60.9 (52.7–68.8) | 2.6 (0.7–6.6) | 71.2 (62.9–78.6) | 68.2 (60.1–75.5) |
| GMFR | 1.1 (0.97–1.22) | 10.7 (8.36–13.7) | 7.4 (6.10–9.09) | 1.0 (0.87–1.06) | 8.9 (6.92–11.4) | 6.6 (5.46–7.87) | 1.0 (0.95–1.14) | 9.7 (7.65–12.2) | 7.3 (6.05–8.82) |
Abbreviations: CI, confidence interval; GMFR, geometric mean fold rise in antibody titer; GMT, geometric mean antibody titer.
a Seroresponse rates (SRs) denote the percentage showing a ≥4-fold increase in titer over baseline. The 95% confidence CIs are calculated on the basis of the Clopper-Pearson method.
IgA GMTs were also increasing by day 5, peaked at day 7–10, and declined by day 28 (Figure 2C and 2D). Peak IgA GMTs at day 7–10 were 1852 and 3742 for GI.1 in the 15/50 and 50/50 vaccine groups, respectively. Corresponding IgA GMTs against GII.4 VLP were 1976 and 1576, respectively. The difference in the IgA response to GI.1 VLP was less pronounced than observed for Pan-Ig, with both groups displaying seroresponse rates of >92% at day 28 (Table 4).
HBGA-Blocking Antibody Levels
The patterns of HBGA responses were similar to those observed as IgA responses to GI.1 and GII.4 VLPs (Figure 2E and 2F); baseline titers were higher for GII.4 than for GI.1, and responses peaked at day 7–10, with waning of the response evident by day 28. The magnitude of the HBGA-blocking antibody responses were lower than that for Pan-Ig or IgA, and differences between the responses to the 2 different vaccine formulations were less evident.
DISCUSSION
The unpredictable nature of NoV transmission and infection makes vaccination an appealing approach for the control of this global public health problem if an effective vaccine is available. Previous studies with candidate bivalent NoV VLP vaccine formulations evaluated equal quantities of the GI.1 and GII.4 VLP antigens per dose [11, 12], whereas this study evaluated formulations with unequal compared with equal quantities of the 2 VLPs. The 2 candidate VLP NoV vaccine formulations studied in this trial were similarly well tolerated, with acceptable safety and reactogenicity profiles. The most frequent reaction to both formulations was transient pain at the injection site, possibly associated with the MPL or aluminum adjuvants.
Both formulations elicited robust immune responses to the 2 genotype component VLPs by day 7–10, as evidenced by Pan-Ig, IgA, and HBGA-blocking antibody responses. Proportionately, the responses against GI.1 were higher than against GII.4 in terms of GMFR, likely because of the lower baseline values for GI.1, as peak Pan-Ig and IgA GMTs to both genotypes were similar. Pan-Ig, IgA, and HBGA-blocking responses declined by day 28 but remained at levels higher than at baseline or in the placebo group. This pattern of serological response was observed in prior studies of the bivalent NoV VLP vaccine [11, 12].
No vaccine-associated correlate of protection has so far been established for NoV, but HBGA-blocking antibodies have been associated with protection against gastroenteritis in humans challenged with NoV [14]. In a human challenge study, HBGA-blocking antibody titers of ≥500 were associated with a degree of protection against moderate-to-severe vomiting or diarrheal illness due to GII.4 genotype among recipients of a similar VLP-vaccine formulation [13]. Notably, the assays used in that study and the current study are not directly comparable, as measurements were made in different laboratories, and in the present study the assay method was modified to improve sensitivity, with a lower limit of quantitation. Using this modified assay in the current study, the day 28 HBGA-blocking antibody levels were lower, compared with those in the previous report [13]. Nevertheless, it is reassuring that, in the present study, both formulations induced titers of >500. By day 7–10, anit-GII.4 HBGA-blocking antibody GMTs were 1234 and 973 for the 15/50 and 50/50 formulations, respectively. Respective levels at day 28 were 804 and 631.
Prior studies with equal quantities of the 2 NoV VLP components in the vaccine showed higher seroresponses to the GI.1 component than to the GII.4 component [12, 13]. In this study, the 15/50 formulation provided numerically higher responses to GII.4 than the 50/50 formulation, even though the concentration of GII.4 VLP antigen was identical in each formulation. The immunogenicity of the 15/50 formulation is considered to be acceptable for both GI.1 and GII.4 antigens.
The GI.1 VLP used in the present formulations is based on the original Norwalk virus responsible for the first report of winter vomiting disease [1]. The consensus sequence used in the development of the GII.4 VLP is based on 3 GII.4 viruses—2006a (Yerseke), 2006b (Den Haag), and 2002 (Houston) [15]—and is intended to afford immune responses against current and future different GII.4 strains, which are currently responsible for most human infections [7]. Proof-of-concept animal studies have shown that the consensus VLP generates immunity against different strains that have developed from antigenic drift [15]. More-recent clinical studies [11, 12, 16] have shown broad immune responses against both GI.1 and GII.4 genotypes, including the recently identified GII.4 Sydney variant that only emerged after development of the consensus VLP [16], suggesting protection is possible against newly emerging strains.
The relatively mild symptoms and short duration of NoV infection in healthy subjects often leads to underdiagnosis and underreporting [17], so the true burden in the community is unknown. In contrast, NoV outbreaks are responsible for serious economic and social disruption in the overall population. Information is available from studies of nosocomial outbreaks, in which accurate diagnosis and reporting is more likely [4, 18]. In at-risk populations such as very young individuals, dehydration due to diarrhea and vomiting can readily lead to hospitalization and may have fatal consequences [3]. Similarly, NoV illness in older adults, particularly those with comorbidities, carries a risk of fatal consequences [5, 6].
These medical factors, together with the high economic costs of NoV infections [19–21] and the sporadic and unpredictable nature of NoV transmission, make development of effective NoV vaccines a health priority. Vaccination may also be justified for healthy adults, especially those likely to play a role in viral transmission, such as food handlers, healthcare workers, parents of young children, or those at greater risk of exposure (eg, military personnel and travelers) or in communal living conditions (eg, college students living in dormitories).
The present study supports that the adjuvanted 15/50 formulation of the bivalent VLP candidate vaccine generates an immune response that, on the basis of current knowledge of potential correlates, may be associated with protection against NoV-related disease. Field efficacy studies will be required to confirm this finding. Participants in the present study will be followed to assess safety and the persistence of the immune response after 1 year, and at that time the immune response to a challenge with a lower vaccine dose (15 μg GI.1 and 15 μg GII.4 VLP with aluminum but no MPL adjuvant) will be assessed. The 15/50 formulation will be taken forward for further clinical development, based on the high prevalence of GII.4 viruses as a cause of human infections and the higher immune responses to GII.4 VLP, compared with those of the 50/50 formulation, in this study. Further studies are also planned to investigate the benefits of a booster dose of the bivalent VLP candidate vaccine, as well as the necessity of the MPL adjuvant component.
STUDY GROUP MEMBERS
Members of the NOR-201 Study Group are as follows: Mohamed S. Al-Ibrahim (Baltimore, Maryland), David L. Bernstein (Cincinnati, Ohio), Donald M. Brandon (San Diego, California), Laurence Chu (Austin, Texas), Matthew G. Davis (Rochester, New York), Robert J. Epstein (San Francisco, California), Sharon E. Frey (St. Louis, Missouri), Jeffrey B. Rosen (Coral Gables, Florida), and John J. Treanor (Rochester, New York).
Notes
Acknowledgments. We thank all of the volunteers who participated in the study; the staff at each of the study centers; the NOR-201 Study Group, for invaluable support and advice; Vincent Mwangi (Takeda Vaccines medical monitor), Laszlo Varga (Takeda Serology), Sue Forsythe (Takeda Vaccines Data Management), Julie Cordova, Bobbi Carlin, Michele Hurliman, and Jennifer Kilbury (Takeda clinical trial managers), and the staff at Quintiles CRO, for expert assistance; and Keith Veitch (Takeda Vaccines), for assisting in preparation of the manuscript and providing general editorial assistance.
Financial support. This work was supported by Takeda Vaccines.
Potential conflicts of interest. R. L. A. reports grants from Takeda Vaccines during the conduct of the study, being an unpaid consultant for Takeda Vaccines outside the submitted work, and having a patent for the growth of Norovirus in intestinal enteroids pending. F. B., J. P. C., and A. B. are employees of Takeda Pharmaceuticals International (Zurich, Switzerland). E. S. and P. M. M. are employees of Takeda Vaccines (Deerfield, IL). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Adler JL, Zickl R. Winter vomiting disease. J Infect Dis 1969; 119:668–73. [DOI] [PubMed] [Google Scholar]
- 2. Foodborne Disease Burden Epidemiology Reference Group 2007–2015. WHO estimates of the global burden of foodborne diseases. http://www.who.int/foodsafety/publications/foodborne_disease/fergreport/en/. Accessed 22 December 2015.
- 3. Spackova M, Altmann D, Eckmanns T, Koch J, Krause G. High level of gastrointestinal nosocomial infections in the German surveillance system, 2002–2008. Infect Control Hosp Epidemiol 2010; 31:1273–8. [DOI] [PubMed] [Google Scholar]
- 4. Payne DC, Vinjé J, Szilagyi PG et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013; 368:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattner F, Sohr D, Heim A, Gastmeier P, Vennema H, Koopmans M. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin Microbiol Infect 2006; 12:69–74. [DOI] [PubMed] [Google Scholar]
- 6. Trivedi TK, Desai R, Hall AJ, Patel M, Parashar UD, Lopman BA. Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am J Infect Control 2013; 41:654–7. [DOI] [PubMed] [Google Scholar]
- 7. Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol 2013; 56:185–93. [DOI] [PubMed] [Google Scholar]
- 8. White PA. Evolution of norovirus. Clin Microbiol Infect 2014; 20:741–5. [DOI] [PubMed] [Google Scholar]
- 9. Lu J, Sun L, Fang L et al. Gastroenteritis outbreaks caused by norovirus GII.17, Guangdong Province, China, 2014–2015. Emerg Infect Dis 2015; 21:1240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atmar RL, Bernstein DI, Harro CD et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med 2011; 365:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernstein DI, Atmar RL, Lyon GM et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 2015; 211:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Treanor JJ, Atmar RL, Frey SE et al. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate--reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis 2014; 210:1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atmar RL, Bernstein DI, Lyon GM et al. Serological correlates of protection against a GII.4 norovirus. Clin Vaccine Immunol 2015; 22:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reeck A, Kavanagh O, Estes MK et al. Serological correlate of protection against Norovirus-induced gastroenteritis. J Infect Dis 2010; 202:1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parra GI, Bok K, Taylor R et al. Immunogenicity and specificity of norovirus Consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 2012; 30:3580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindesmith LC, Ferris MT, Mullan CW et al. Broad blockade antibody responses in human volunteers after immunization with a multivalent norovirus VLP candidate vaccine: immunological analyses from a phase I clinical trial. PLoS Med 2015; 12:e1001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tam CC, Rodrigues LC, Viviani L et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 2012; 61:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wikswo ME, Kambhampati A, Shioda K, Walsh KA, Bowen A, Hall AJ. Outbreaks of acute gastroenteritis transmitted by person-to-person contact, environmental contamination, and unknown modes of transmission — United States, 2009–2013. MMWR Surveill Summ 2015; 64(SS12):1–16. [DOI] [PubMed] [Google Scholar]
- 19. Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. The potential economic value of a human norovirus vaccine for the United States. Vaccine 2012; 30:7097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffman SJ, Batz MB, Morris JG Jr. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Protect 2012; 7:1292–302. [DOI] [PubMed] [Google Scholar]
- 21. Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. Global economic burden of norovirus gastroenteritis. PLoS One 2016; 11:e0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]