Abstract
Background. The return of chloroquine-sensitive Plasmodium falciparum to the limited area of Blantyre, Malawi, has been well demonstrated in several studies.
Methods. To characterize chloroquine susceptibility over a wide geographic area, infants and children aged 6–59 months were selected using 2-stage cluster sampling in 8 Malawian districts. Pyrosequencing of the pfcrt gene codon 76 region was performed for children with asexual parasitemia.
Results. Of 7145 children, 1150 had microscopic asexual parasitemia, and sequencing was performed in 685, of whom 1 had a chloroquine-resistant genotype.
Conclusions. Systematic countrywide sampling demonstrates that the chloroquine pfcrt genotype has reached near-fixation, raising the possibility of reintroducing chloroquine for malaria prevention and treatment.
Keywords: malaria, Plasmodium falciparum, chloroquine, resistance, PfCRT, Malawi, Africa
The emergence and spread of drug-resistant Plasmodium falciparum is a major obstacle to effective malaria control and eradication, but the return of chloroquine-sensitive malaria to Malawi offers the possibility that therapies once rendered ineffective by drug resistance may become useful again [1, 2]. Molecular markers of resistance permit characterization of the dynamics of resistance over time and across geographic regions. For chloroquine, resistance has been studied using detection of a single nucleotide polymorphism encoding an amino acid change at the 76th position of the P. falciparum chloroquine resistance transporter (PfCRT) from a lysine in sensitive parasites (K76) to a threonine in resistant parasites (76T). The 76T genotype has been highly correlated with chloroquine resistance in vitro and clinical chloroquine treatment failure [3]. Malawi was the first African country to change its treatment policy from resistance-compromised chloroquine to more efficacious treatment with sulfadoxine-pyrimethamine in 1993. Since then, the prevalence of PfCRT 76T has declined, reaching undetectable levels in 2004 around Blantyre, a major Malawian urban center [1]. In 2005, the return of susceptibility in Blantyre was confirmed by in vitro experiments and a clinical trial of chloroquine demonstrating 99% efficacy [2]. A subsequent trial demonstrated that chloroquine as monotherapy and in combination with other antimalarials was highly efficacious in the treatment of uncomplicated malaria in children when used repeatedly during the course of the year. Chloroquine used alone or in combination did not lead to the reemergence of chloroquine resistance during the course of the trial [4].
Based on these findings, there is renewed interest in chloroquine as an antimalarial on the continent of Africa. However, research is needed to demonstrate that chloroquine-based treatments will be effective over a wide geographic area, including in areas bordering with countries that continued using chloroquine for several years after Malawi stopped using it. Chloroquine-resistant parasites have remained prevalent in the countries surrounding Malawi [5–7]. Within Malawi, some small studies of chloroquine resistance in regions outside Blantyre have been reported. Surveys done at several sites between 2000 and 2005 reported prevalences of PfCRT 76T infections between 0% and 2% [8, 9]. In 2007, a study using site-specific heteroduplex tracking assays, a highly sensitive new genotyping method, detected minority variant PfCRT 76T mutation in approximately 35% of samples from 2 rural villages near Blantyre [10]. Also in 2007, 9% of infections were found to have a chloroquine-resistant genotype in Karonga district, which borders Tanzania [11]. The limited data showing varying levels of resistance called for a comprehensive evaluation of resistance levels nationwide. To assess the extent and distribution of chloroquine-sensitive P. falciparum malaria throughout Malawi, a cross-sectional study of children in 8 sentinel districts across Malawi was conducted using a systematic household sampling strategy, in which the PfCRT 76 genotype was determined for all children with microscopic parasitemia.
METHODS
Study Site and Population
We conducted this study within a cross-sectional survey designed to evaluate the prevalence of anemia and malaria in Lilongwe, Blantyre, Mwanza, Chiradzulu, Phalombe, Nkhotakota, Rumphi, and Karonga districts in Malawi during the peak malaria transmission season (April through May 2009). These districts contain approximately one-third of the Malawian population, evenly divided between the northern, central and southern regions. We employed a 2-stage cluster-sampling model, using enumeration areas from the 2000 census data. A detailed description of the sampling strategy has been described elsewhere [12]. Within selected study areas, households were mapped and among those, 12 were randomly chosen for survey eligibility. Infants and children between aged 6–59 months of age in these households were eligible for the study. For those whose guardian provided informed consent, global positioning system coordinates of the household location were recorded, and thick blood film and dried blood spot specimens were collected on 3MM filter paper (Whatman).
DNA Extraction and Polymerase Chain Reaction Amplification
Thick blood films were examined for P. falciparum asexual parasitemia by 2 independent microscopists. A third microscopist read discrepant results. Detailed protocols for DNA extraction, polymerase chain reaction (PCR) amplification, pyrosequencing, and allele-specific restriction enzyme analysis are available at the Web site (http://medschool.umaryland.edu/malaria/protocols.asp). Briefly, DNA was extracted from filter papers of participants with microscopic evidence of parasitemia, using the QIAamp Investigator BioRobot Kit (Qiagen). Amplification was initially done using a single-step amplification (with the internal PCR primers described online), designed to amplify the region containing codons 72–76 of the pfcrt gene. For each plate, laboratory P. falciparum strains HB3 (chloroquine-sensitive strain) and Dd2 (chloroquine-resistant strain) were used for positive controls, and PCR reagents without DNA was used for a negative control.
Genotyping
Pyrosequencing was performed on specimens with detectable DNA by capillary electrophoresis using the QIAxcel DNA Screening Kit (Qiagen). Briefly, streptavidin-labeled sepharose beads were bound to biotinylated PCR amplicons and then sequentially washed with 70% ethanol, denaturation solution, and washing buffer. The PCR product was then incubated with the pyrosequencing primers and annealing buffer. Plates were read and analyzed using PSQ HS 96A single-nucleotide polymorphism reagents and analysis software (Biotage version 1.2, Westborough, MA). A threshold for pyrosequencing quality was set at 50 relative luminescence units for a single peak in a conserved nucleotide of the sequence of interest.
Samples with evidence of PCR product on electrophoresis but pyrosequencing output of insufficient quality underwent a nested PCR reaction and repeat pyrosequencing. Infections were classified as chloroquine sensitive, chloroquine resistant, or mixed based on the relative luminescence unit peaks corresponding to the polymorphic nucleotide position for codon 76. Samples with a threonine at position 76 have luminescence when the first guanine deoxynucleoside triphosphate is added, whereas samples with a lysine at this position have their first luminescent peak when the first thymidine deoxynucleoside triphosphate is added. Samples with evidence of resistance using pyrosequencing were confirmed using repeat pyrosequencing and allele-specific restriction enzyme analysis.
Ethics Statement
Ethical review and approval were obtained from the University of Malawi College of Medicine Research (Blantyre) and Ethics Committee, and the University of Maryland, Baltimore, institutional review board.
RESULTS
A total of 7145 filter paper samples was collected. Of these, 1150 had asexual parasitemia on thick blood smears and global positioning system coordinates that permitted mapping their location. DNA extraction and a single PCR reaction were conducted on these samples, yielding PCR product in 713 samples. Of these, 611 yielded pyrograms of sufficient quality for sequence interpretation. Of the 102 pyrograms of insufficient quality, 74 yielded pyrograms of sufficient quality after nested PCR amplication. The geographic locations of the 685 samples are shown in Figure 1.
Figure 1.
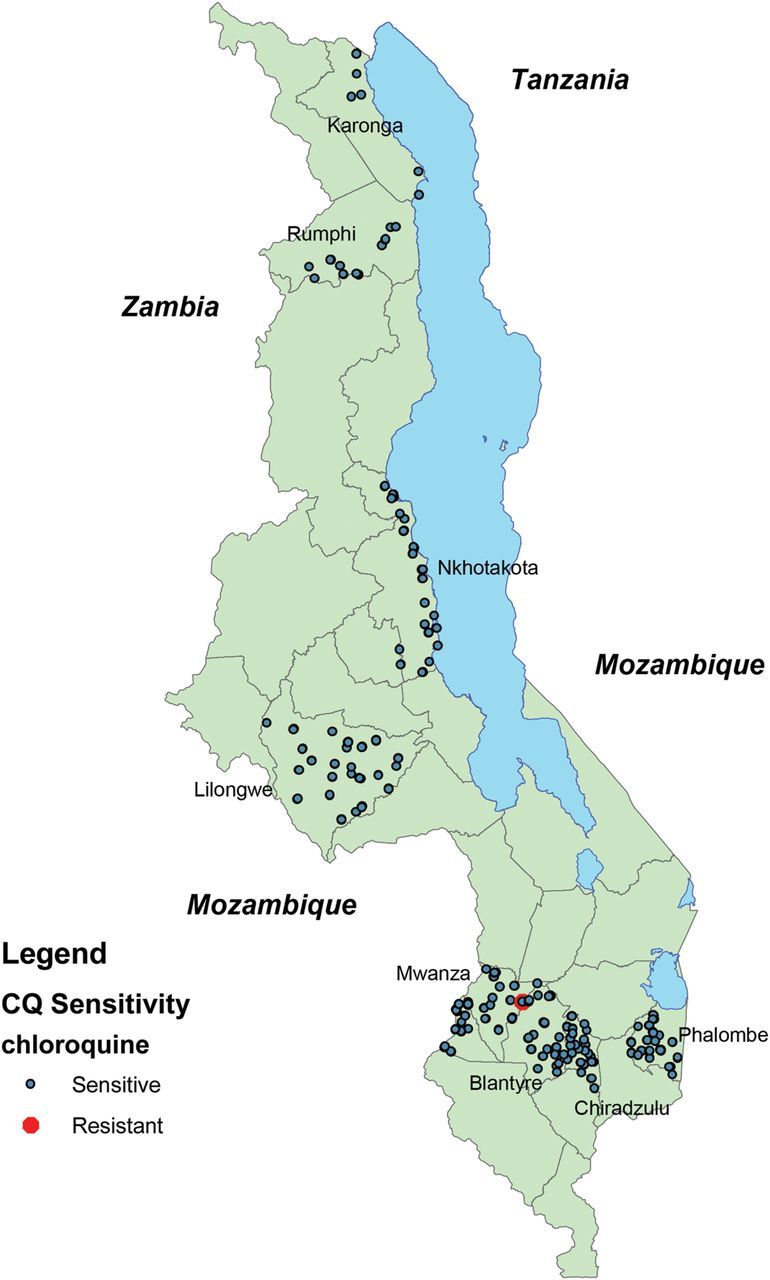
Map of sample distribution. Dots indicating chloroquine-sensitive samples represent multiple geographically proximal samples.
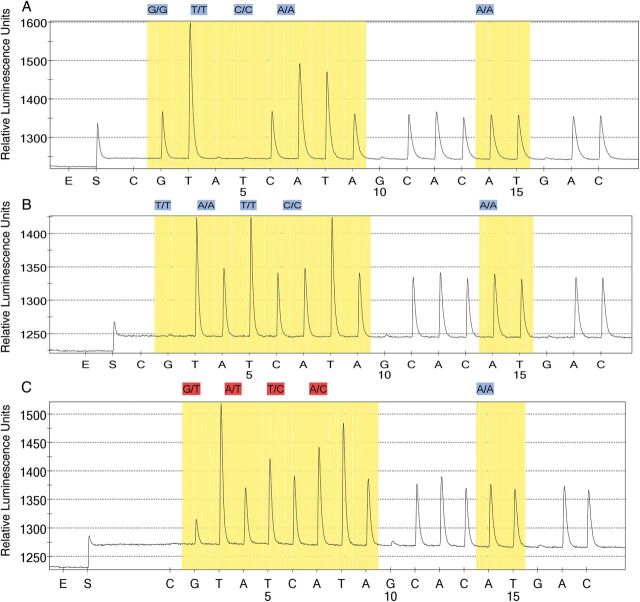
Among all the samples with evaluable genotypes using pyrosequencing with allele-specific restriction enzyme analysis confirmation, only 1 had evidence of resistance. Pyrosequencing output shown in Figure 2 suggested that this was a minority resistant genotype. This sample was taken from a child living in Mwanza district, which borders both the Blantyre district and Mozambique. Based on these results, the proportion of infections with detectable resistance in Malawi was 0.1% (95% confidence interval, <0% to .4%), and the proportion of infections with detectable resistance in Mwanza district was 0.7% (<0% to 2.0%).
Figure 2.
Pyrosequencing results. Pyrograms from a positive control, chloroquine-resistant strain Dd2 (A); a negative control, chloroquine-sensitive strain HB3 (B); and a field sample from Mwanza district with evidence of mixed chloroquine-resistant and chloroquine-sensitive infections (C). Note presence of guanine in the 76th codon of the field sample, which is the second nucleotide added in pyrosequencing, indicating the presence of chloroquine-resistant parasites. This field sample also contains adenosine in the 75th codon, which is the fourth nucleotide added, indicating the presence of chloroquine-sensitive parasites.
DISCUSSION
This study demonstrates near fixation of chloroquine-sensitive P. falciparum genotypes over a broad geographic range in Malawi in 2009, including rural areas and areas bordering Zambia, Mozambique, and Tanzania. Further sampling would be valuable from the northern region given the comparatively small number of samples from that area and the previously reported levels of resistance [11]. Known high-transmission regions for malaria, including the Nsanje and Chichwawa river valleys, should also be evaluated in future studies. With this caveat, these data suggest that the phenomenon of return of chloroquine susceptibility after discontinuation of widespread chloroquine use is not isolated to Blantyre but has taken place throughout Malawi.
Significant reservations attend the reintroduction of chloroquine for malaria treatment or prevention in Africa because of continued high-level resistance in many countries and the potential for rapid expansion of resistant parasites under renewed drug pressure. Evidence from Malawi suggests that chloroquine-susceptible malaria is a reexpansion of susceptible parasites that survived, probably in semi-immune hosts during periods of high chloroquine drug pressure [13]. This suggests that this same reversion to the chloroquine susceptibility is possible throughout Africa. Of note, the PfCRT allele that predominates Africa (CVIET), as was seen in our single resistant sample, has been proposed as a less-fit chloroquine resistance allele [14]. The SVMNT chloroquine resistance allele, which is also associated with amodiaquine resistance, is widely prevalent in South America and Asia but has been rarely observed in Africa. This may help explain why a return of chloroquine sensitivity has been seen after chloroquine withdrawal in Africa but not in Southeast Asia. Particular attention must be paid to regions in Africa where the SVMNT allele has been found when considering future chloroquine use.
Conversely, chloroquine susceptibility may come to dominate the parasite population more quickly in other areas of Africa than occurred in Malawi with current malaria treatment strategies. It has been suggested that the use of lumefantrine has promoted the rapid decline of chloroquine resistance by selective pressure on PfCRT favoring the wild-type chloroquine-sensitive K76 [15], and pooled analyses of molecular and clinical data from artemether-lumefantrine trials in Africa are being done to assess this possibility. The trend toward chloroquine susceptibility is now also being seen in other regions of Africa, where the use of chloroquine persisted much longer. With the emerging threat of artemisinin resistance and continuing use of sulfadoxine-pyrimethamine for intermittent preventive therapy in pregnant women, infants, and children despite high levels of antifolate resistance, all available tools should be considered to continue to provide efficacious, safe, and inexpensive antimalarials.
Although the vast majority of samples in this study had chloroquine-sensitive genotypes, the 1 resistant sample and low levels of resistance reported in other studies suggest that a small reservoir of these parasites persists. Because of the danger of rapid expansion of this reservoir under drug pressure, any reintroduction of chloroquine should be with efficacious partner drugs and monitored with continued resistance surveillance. Overall, the data presented here support the consideration of chloroquine as a safe and effective drug that should be considered as a component of new regimens for malaria treatment and prevention in Malawi and other settings where chloroquine-susceptible P. falciparum predominates. The use of chloroquine as a component of mass drug administration for malaria elimination or prevention in vulnerable populations (e.g., pregnant women and persons with human immunodeficiency virus infection) may be particularly attractive because any selection of resistance would not compromise the efficacy of current first-line antimalarial drugs.
Notes
Acknowledgments. We acknowledge Adam Wolkon for his help with preparing the figures. We also thank the participants and their parents for participation in this study as well as the staff and field teams from the Malaria Alert Center and Blantyre Malaria Project for their technical assistance.
Disclaimers. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the United States Agency for International Development, or the United States President's Malaria Initiative.
Financial support. This work was supported by the United States President's Malaria Initiative, US Agency for International Development, under the terms of an Interagency Agreement with the Centers for Disease Control and Prevention (CDC) and through a Cooperative Agreement (No. 5 U01 CI000189) between the CDC and the Malaria Alert Centre, University of Malawi College of Medicine; and by the Doris Duke Charitable Foundation and the Howard Hughes Medical Institute to (awards to C. V. P.).
Potential conflicts of interest. All authors have no commercial or other associations that might pose a conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kublin JG, Cortese JF, Njunju EM, et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–5. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 2.Laufer MK, Thesing PC, Eddington ND, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–66. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 3.Djimde A, Doumbo OK, Cortese JF, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–63. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 4.Laufer MK, Thesing PC, Dzinjalamala FK, et al. A longitudinal trial comparing chloroquine as monotherapy or in combination with artesunate, azithromycin or atovaquone-proguanil to treat malaria. PLoS One. 2012;7:e42284. doi: 10.1371/journal.pone.0042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enosse S, Magnussen P, Abacassamo F, et al. Rapid increase of Plasmodium falciparum dhfr/dhps resistant haplotypes, after the adoption of sulphadoxine-pyrimethamine as first line treatment in 2002, in southern Mozambique. Malar J. 2008;7:115. doi: 10.1186/1475-2875-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raman J, Mauff K, Muianga P, Mussa A, Maharaj R, Barnes KI. Five years of antimalarial resistance marker surveillance in Gaza Province, Mozambique, following artemisinin-based combination therapy roll out. PLoS One. 2011;6:e25992. doi: 10.1371/journal.pone.0025992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frosch AE, Venkatesan M, Laufer MK. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar J. 2011;10:116. doi: 10.1186/1475-2875-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzinjalamala FK, Laufer MK, Connors N, et al. Surveillance to confirm the disappearance of chloroquine resistant malaria following chloroquine withdrawal in Malawi. Malawi Med J. 2009;21:34–49. [Google Scholar]
- 9.Mita T, Kaneko A, Lum JK, et al. Recovery of chloroquine sensitivity and low prevalence of the Plasmodium falciparum chloroquine resistance transporter gene mutation K76T following the discontinuance of chloroquine use in Malawi. Am J Trop Med Hyg. 2003;68:413–5. [PubMed] [Google Scholar]
- 10.Juliano JJ, Kwiek JJ, Cappell K, Mwapasa V, Meshnick SR. Minority-variant pfcrt K76T mutations and chloroquine resistance, Malawi. Emerg Infect Dis. 2007;13:872–7. doi: 10.3201/eid1306.061182. [DOI] [PubMed] [Google Scholar]
- 11.Bridges DJ, Molyneux M, Nkhoma S. Low level genotypic chloroquine resistance near Malawi's northern border with Tanzania. Trop Med Int Health. 2009;14:1093–6. doi: 10.1111/j.1365-3156.2009.02340.x. [DOI] [PubMed] [Google Scholar]
- 12.Skarbinski J, Mwandama D, Luka M, et al. Impact of health facility-based insecticide treated bednet distribution in Malawi: progress and challenges towards achieving universal coverage. PLoS One. 2011;6:e21995. doi: 10.1371/journal.pone.0021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laufer MK, Takala-Harrison S, Dzinjalamala FK, Stine OC, Taylor TE, Plowe CV. Return of chloroquine-susceptible falciparum malaria in Malawi was a reexpansion of diverse susceptible parasites. J Infect Dis. 2010;202:801–8. doi: 10.1086/655659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ecker A, Lehane AM, Clain J, Fidock DA. PfCRT and its role in antimalarial drug resistance. Trends Parasitol. 2012;28:504–14. doi: 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen TT, Madsen LB, Hansson HH, et al. Rapid selection of Plasmodium falciparum chloroquine resistance transporter gene and multidrug resistance gene-1 haplotypes associated with past chloroquine and present artemether-lumefantrine use in Inhambane District, Southern Mozambique. Am J Trop Med Hyg. 2013;88:536–41. doi: 10.4269/ajtmh.12-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]