Abstract
Background. Immune activation predicts morbidity during hepatitis C virus (HCV) infection and human immunodeficiency virus (HIV) infection, although mechanisms underlying immune activation are unclear. Plasma levels of autotaxin and its enzymatic product, lysophosphatidic acid (LPA), are elevated during HCV infection, and LPA activates immunocytes, but whether this contributes to immune activation is unknown.
Methods. We evaluated plasma levels of autotaxin, interleukin 6 (IL-6), soluble CD14 (sCD14), soluble CD163 (sCD163), and Mac2 binding protein (Mac2BP) during HCV infection, HIV infection, and HCV-HIV coinfection, as well as in uninfected controls, before and after HIV antiretroviral therapy (ART) initiation and during interferon-free HCV therapy.
Results. We observed greater plasma autotaxin levels in HCV-infected and HCV-HIV–coinfected participants, compared with uninfected participants, primarily those with a higher ratio of aspartate aminotransferase level to platelet count. Autotaxin levels correlated with IL-6, sCD14, sCD163, Mac2BP, and LPA levels in HCV-infected participants and with Mac2BP levels in HCV-HIV–coinfected participants, while in HIV-infected individuals, sCD14 levels correlated with Mac2BP levels. Autotaxin, LPA, and sCD14 levels normalized, while sCD163 and Mac2BP levels partially normalized within 6 months of starting interferon-free HCV therapy. sCD163 and IL-6 levels normalized within 6 months of starting ART for HIV infection. In vitro, LPA activated monocytes.
Conclusions. These data indicate that elevated levels of autotaxin and soluble markers of immune activation during HCV infection are partially reversible within 6 months of initiating interferon-free HCV treatment and that autotaxin may be causally linked to immune activation during HCV infection and HCV-HIV coinfection.
Keywords: human, immunity, hepatitis C, monocyte, T cell, ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2), lysophospholipase D
Hepatitis C virus (HCV) is the most common cause of chronic viral hepatitis in the United States [1], and morbid outcomes, including cirrhosis and hepatocellular carcinoma (HCC), often occur within 20 years of acute infection [1, 2]. Because of overlapping modes of transmission, human immunodeficiency virus (HIV)–infected individuals are commonly (in up to 30% of cases) coinfected with HCV [3]. During coinfection, morbidity is attributable to both HCV and HIV infection [4–10]. One possible mechanism underlying interactions between these 2 infections may be immune dysfunction resulting from chronic immune activation. Immune activation is characterized by soluble markers such as interleukin 6 (IL-6) and soluble CD14 (sCD14) [11, 12]. These markers of immune activation predict disease progression during HIV infection, HCV infection, and HCV-HIV coinfection [11–18]. While levels of soluble inflammatory mediators are associated with each other and with disease activity, little is known about specific mechanisms underlying immune activation in the setting of HCV infection and HCV-HIV coinfection or about the degree of normalization after removal of HCV from the infected host.
Autotaxin and Mac2 binding protein (Mac2BP; also known as galectin-3-binding protein) were identified by our group as plasma proteins that, during HCV infection, correlate with markers of immune activation via an unbiased proteome screen [19]. Expression of autotaxin is ubiquitous, with relatively high levels expressed in brain, kidney, and lymphoid organs [20], including the high endothelial venule, where a role in lymphocyte traffic has been proposed [21]. Serum autotaxin concentration and activity are elevated in patients with chronic liver diseases, and this positively correlates with the stage of liver disease [22, 23]. Autotaxin has lysophospholipase D activity, and it is the major enzyme catalyzing the formation of lysophosphatidic acid (LPA) in the blood [24, 25]. LPA elicits a variety of biological responses, such as neurogenesis, angiogenesis, smooth-muscle contraction, platelet aggregation, and wound healing [26, 27]. LPA can modulate immune responses by attracting and directly activating T cells, B cells, and macrophages or by influencing their interaction with other cell types [28]. Many LPA subtypes have been found in plasma [29, 30], and different LPA subtypes may differentially activate different LPA receptors, resulting in activation of distinct pathways in different cell types [31, 32].
Mac2BP is a member of the scavenger receptor cysteine-rich domain family and is the dominant ligand for the macrophage-associated S-type lectin Mac-2 (also known as galectin-3) [33]. Mac2BP is expressed in activated macrophages and is thought to be involved in events as diverse as cell migration, immune response, and metastasis [34, 35]. High levels of serum Mac2BP have been found during HIV infection, hepatitis B virus (HBV) infection, and HCV infection [36, 37]. In chronic HCV infection, serum Mac2BP levels are related to both disease severity and duration of infection [37].
Here, we investigated the relationship between autotaxin, LPA, Mac2BP, and soluble markers of immune activation within HCV infection, HIV infection, and HCV-HIV coinfection before and during interferon (IFN)–free, direct-acting antiviral (DAA) therapy for HCV and antiretroviral therapy (ART) for HIV, to investigate potential causal relationships. Our data suggest that autotaxin and LPA may contribute to immune activation during HCV infection and HCV-HIV coinfection.
METHODS
Study Participants
Study participants provided written informed consent under protocols approved by the institutional review boards for human studies at University Hospitals of Cleveland and Cleveland Veterans Affairs Medical Center. Study groups included age-range–matched uninfected control (n = 28), HCV-infected (n = 29), HIV-infected (n = 30), and HCV-HIV–coinfected participants (n = 28). Individuals with chronic HCV infection were positive for HCV antibody (for >6 months) and HCV RNA, were negative for HIV (by enzyme-linked immunosorbent assay [ELISA]), and underwent IFN-free DAA therapy (with sofosbuvir/ledipasvir/ribavirin [n = 19] or sofosbuvir/ledipasvir [n = 9]), for 8–12 weeks as per standard care. HIV-infected participants were positive for HIV antibody (by ELISA and Western blot), were positive for HIV RNA by polymerase chain reaction (PCR), and had undetectable HCV antibody and RNA. HCV-HIV–coinfected participants had a combination of these criteria. HIV-infected and HCV-HIV–coinfected participants underwent ART for HIV as standard care. HCV-HIV–coinfected participants did not receive therapy for HCV. HCV-infected and HCV-HIV–coinfected participants were stratified on the basis of the aspartate aminotransferase (AST) to platelet (PLT) ratio index (APRI), calculated as follows, into low and high subgroups on the basis of the relationship to the median APRI (0.66 and 0.56, respectively): [(AST level in U/L) / (upper limit of normal AST level, defined as 45 U/L}] / [(platelet count × 109 platelets/L) × 100] (Table 1).
Table 1.
Characteristics of Study Participants
| Variable | No Infection (n=28) | HCV Infection |
HCV-HIV Coinfection |
HIV Infection (n=30) | P valueb | ||
|---|---|---|---|---|---|---|---|
| Low APRIa (n=15) | High APRIa (n=14) | Low APRIa (n=16) | High APRIa (n=12) | ||||
| Age, y | 52 (43–59) | 63 (58–65) | 64 (62–65) | 48 (46–53) | 46.5 (38–52) | 40 (33–48) | <.001 |
| Race | |||||||
| White | 12 (42.8) | 5 (33.3) | 5 (35.7) | 1 (6.25) | 3 (25) | 10 (33.3) | |
| African American | 15 (53.6) | 10 (66.7) | 9 (64.3) | 15 (93.75) | 9 (75) | 17 (56.7) | |
| Hispanic | 1 (3.6) | … | … | … | … | 3 (10) | |
| Male sex | … | 14 (93.3) | 14 (100) | 9 (56.2) | 10 (83.3) | 23 (76.6) | |
| HCV level, IU/mL | … | 1 112 210 (538 810–2 220 740) | 1 436 340 (920 655–2 289 650) | 1 302 957 (600 000–7 017 127) | 1 000 000 (600 000–5 592 500) | … | .040 |
| HCV genotype | |||||||
| 1 | … | 15 (100) | 13 (92.8) | 15 (93.75) | 8 (66.7) | … | |
| 2/3 | … | … | 1 (7.2) | … | … | … | |
| Unknown | … | … | … | 1 (6.25) | 4 (33.3) | … | |
| HIV RNA load, copies/mL | … | … | … | 54 100 (29 844–299 868) | 4425 (1524–28 713) | 36 491 (6 795–124 622) | <.0001 |
| CD4+ T-cell count, cells/μL | … | … | … | 285 (196–405) | 278 (156–495) | 256 (114–395) | |
| AST level, IU/mL | 23 (21–24)c | 34 (28–41) | 81 (61–104) | 36 (27–47) | 83 (60–139) | 24 (19–29) | <.0001 |
| ALT level, IU/mL | 23 (20–35)c | 41 (31–44) | 99 (59–137) | 34 (26–42) | 77 (59–128) | 35 (27–56) | <.0001 |
| Platelet count, ×103 platelets/mm3 | 214 (167–249)c | 240 (206–263) | 140 (119–155) | 241 (216–218) | 172 (116–191.2) | 212 (162–257) | .001 |
| APRIa | 0.2 (0.18–0.3)c | 0.3 (0.25–0.4) | 1.6 (0.94–1.94) | 0.4 (0.2–0.47) | 1.13 (0.89–2.34) | 0.2 (0.1–0.3) | <.0001 |
| Fibrosis 4 indexd | 1.3 (0.87–2.0)c | 1.5 (1.26–1.80) | 4.06 (3.03–5.64) | 1.3 (0.9–1.7) | 3.05 (1.8–4.14) | 0.6 (0.5–1.2) | <.0001 |
Data are median (interquartile range) or no. (%) of participants.
Abbreviations: ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
a Calculated as [AST level / 45] / [platelet count × 100] and divided into high and low APRI on the basis of the relationship to the median APRI (0.66 for HCV-infected participants and 0.56 for HCV-HIV–coinfected participants).
b Based on nonparametric analysis of variance for the comparison across uninfected participants, HCV-infected participants, HCV-HIV–coinfected participants, and HIV-infected participants.
c Data are for 15 participants.
d Calculated as [age × AST level]/[platelet count × √ALT level].
Soluble Markers of Immune Activation
Blood was collected into tubes lined with K2–ethylenediaminetetraacetic acid (Franklin Lakes, BD, NJ). Within 4 hours after collection, plasma samples were stored at −20°C, until analysis for autotaxin (Quantikine-human ATX-immunoassay; R&D Systems, Minneapolis, MN) [22]. Plasma levels of sCD14, IL-6, soluble CD163 (sCD163; R&D Systems), and Mac2BP (Affymetrix-eBioscience, Vienna, Austria) were measured by ELISA. Additionally, culture supernatants were measured for sCD14, IL-6, and sCD163 by ELISA (R&D Systems) and for interleukin 10 (IL-10), interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and granulocyte macrophage colony-stimulating factor (GM-CSF) by Luminex (EMD Millipore, Billerica, MA).
Liquid Chromatography (LC) and Selected Reaction Monitoring (SRM) Mass Spectrometry Analysis of Plasma LPA
LPA was measured in 10 µL of plasma. Protein precipitation was performed using 150 µL of methanol containing an internal standard (LPA 17:0). After centrifugation, the supernatant was collected and dried using a SpeedVac. The residue was reconstituted in 100 µL of methanol/50 mM ammonium acetate (1:1; pH 8). A total of 10 µL was injected onto an LC column. The plasma samples and calibration standards were analyzed by LC and SRM mass spectrometry. The LC–mass spectrometry system included an Agilent 1200-HPLC system (Santa Clara, CA), interfaced to a Thermo Scientific TSQ Quantum Ultra Mass Spectrometer equipped with heated electrospray ionization (HESI-II) probe, operated under negative ionization mode. Plasma LPA species 16:0, 18:0, 18:1, 18:2, and 20:4 were quantified. Supplementary Table 2 includes the SRM transitions used. The signal intensity ratios of endogenous compound to spiked-in LPA 17:0 in plasma samples were plotted against the calibration curve to determine LPA concentrations in plasma. The data were processed using Thermo-Pinpoint software.
Flow Cytometry of LPA-Induced Monocyte Activation
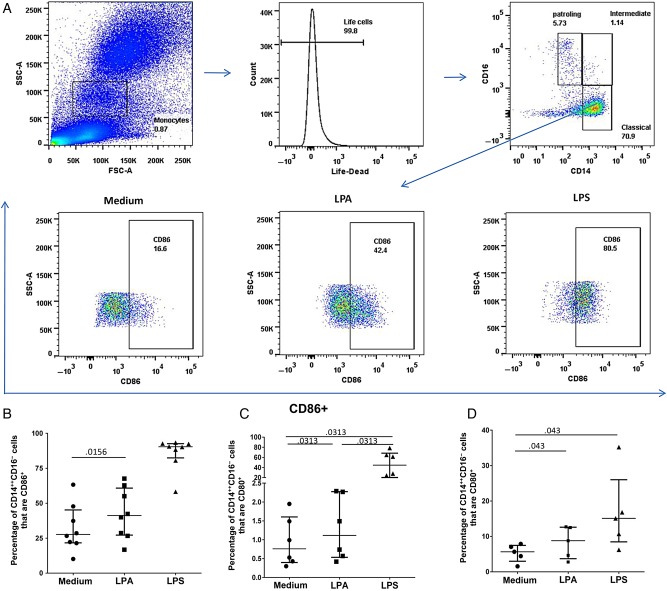
Whole blood (2 mL) was incubated at 37°C for 3 hours with 10 µM LPA 20:4 (Echelon, solvated in chloroform 98.2%, methanol 1.2%) or lipopolysaccharide (LPS; 10 ng/mL; Sigma-Aldrich). A total of 200 µL of whole blood was stained with Yellow Live/Dead stain (Invitrogen, Grand Island, NY), anti-CD14-FITC (clone M5E2), anti-CD16-APC-H7 (clone 3G8), anti-CD80-PE-Cy5 (clone L307), and anti-CD86-PE (clone IT2.2; BD, San Jose, CA) for 10 minutes, lysed after 10 minutes at room temperature, washed, and fixed with stabilizing fixative (BD, San Jose). Flow cytometry was performed on a LSRII cytometer with FACSDiva (version 6.2). Analysis of the acquired data was performed using FlowJo software, version 10. Monocytes were identified by forward scatter/side scatter and by CD14 and CD16 expression into classic (CD14++CD16−), intermediate (CD14++CD16+), and nonclassic (CD14+CD16++) subsets [38]. CD80 and CD86 expression were quantified for each subset.
Purified monocytes were isolated using the RosetteSep human monocyte enrichment kit (Stemcell Technologies, Vancouver, Canada). A total of 1.5–2 million cells were incubated at 37°C 24 hour with media (Roswell Park Memorial Institute 1640 medium, L-glut, pen/strep, and 5% human-AB serum), LPA (20:4; 10 µM), or LPS (10 ng/mL). After incubation, the supernatants were collected and stored at −20°C, and the cells were stained with anti-CD14-BV510 (clone M5E2), anti-CD16-APC-H7 (clone 3G8), anti-CD80-FITC (clone L307), and anti-CD86-PE (clone IT2.2) for 20 minutes. Flow cytometry was performed as described above.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows v. 22.0 (IBM, Armonk, NY). Associations between continuous variables were evaluated using the Spearman rank correlation coefficient. Group comparisons were analyzed by the Mann–Whitney U test. All tests of significance were 2-sided, and P values of ≤.05 were considered statistically significant.
RESULTS
Study Subject Characteristics
HCV infected and HCV-HIV–coinfected study subjects were predominantly male and had genotype 1 HCV infection (Table 1). HIV-infected participants were younger than HCV-infected participants. Median CD4+ T-cell counts were 285, 278, and 256 cells/μL in the HCV-HIV–coinfected group with a low APRI, the HCV-HIV–coinfected group with a high APRI, and the HIV-infected group, respectively. The APRI, AST level, and alanine aminotransferase (ALT) level were higher in HCV-infected groups than in uninfected donors or HIV-infected participants.
Among HCV-Infected Individuals, Levels of Autotaxin and Soluble Markers of Immune Activation Are Elevated, With Higher Levels Among Those With High APRIs
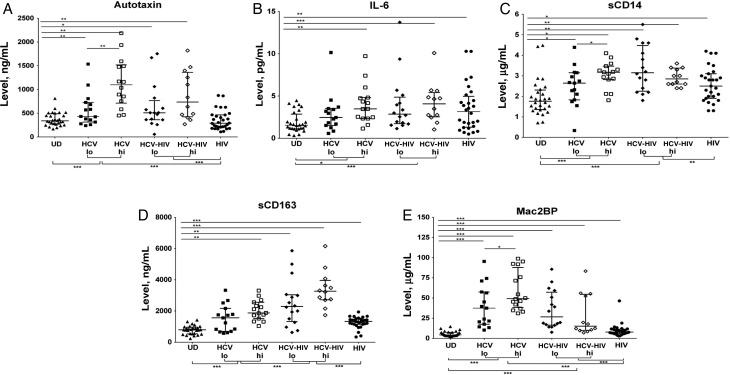
Elevated plasma levels of autotaxin have been described during chronic HCV infection [22, 23]. In a discovery data set, we found that autotaxin and Mac2BP levels positively correlate with markers of immune activation during HCV infection [19]. Autotaxin activity is the main source of circulating LPA [24, 25], and LPA is known to be capable of activating immunocytes [28]. Therefore, we first investigated whether elevated autotaxin levels are related to markers of immune activation during chronic HCV infection and HCV-HIV coinfection. We observed elevated plasma levels of autotaxin, IL-6, sCD14, sCD163, and Mac2BP in HCV infection and/or HIV infection when compared to uninfected donors (Figure 1). Autotaxin plasma levels observed here were similar to those previously reported [22]. In the case of sCD163 and sCD14, plasma levels were greater during HCV-HIV coinfection than during HIV monoinfection (P < .001 and P = .025, respectively) and HCV monoinfection (P < .001 and P < .001, respectively). These results corroborate prior data sets evaluating markers of immune activation in HIV monoinfection [19] and HCV monoinfection [19], extending here to HCV-HIV coinfection. The data are also consistent with an interactive or additive effect of each infection upon systemic immune activation.
Figure 1.
Autotaxin levels and soluble markers of immune activation are elevated during hepatitis C virus (HCV) infection and more so in those with advanced liver disease. Shown are week 0 plasma levels of autotaxin (A), interleukin 6 (IL-6; B), soluble CD 14 (sCD14; C), sCD163 (D), and Mac2 binding protein (Mac2BP; E) in 29 HCV-infected, 28 HCV and human immunodeficiency virus (HIV)–coinfected, 30 HIV-infected, and 28 uninfected participants. HCV-infected and HCV-HIV–coinfected participants are divided into low (lo) and high (hi) groups on the basis of aspartate aminotransferase to platelet ratio indexes (APRIs), using median APRIs. Median APRIs for HCV-infected and HCV-HIV–coinfected groups were 0.66 and 0.56, respectively. *P < .05, **P < .01, and ***P < .001. Abbreviation: UD, donor.
Since autotaxin has been described to correlate with liver fibrosis and inflammation during HCV infection [22, 39], we next evaluated whether autotaxin levels are associated with liver inflammation or liver damage in both HCV infection and HCV-HIV coinfection. Autotaxin positively correlated with AST levels and APRIs and negatively correlated with platelet counts in HCV-infected and HCV-HIV–coinfected participant groups (Supplementary Figure 1).
Our previous data set indicated correlations between autotaxin, Mac2BP, and markers of immune activation during HCV infection [19], but we were unable to examine HCV-HIV coinfection in that data set. We next evaluated here whether autotaxin levels are associated with soluble markers of immune activation during both HCV infection and HCV-HIV coinfection. We found that plasma levels of autotaxin correlated with plasma levels of IL-6, sCD14, sCD163, and Mac2BP during HCV monoinfection and with Mac2BP during HCV-HIV coinfection (Table 2). Additionally, levels of IL-6 correlated with those of sCD14 and Mac2BP (P = .036 and P = .018, respectively) and levels of sCD14 correlated with levels of sCD163 (P = .021) during HCV monoinfection, while levels of IL-6 correlated with those of sCD163 and Mac2BP (P = .015 and P = .023) and levels of sCD14 correlated with levels of Mac2BP (P = .007) during HCV-HIV coinfection. During HIV monoinfection, there were no correlations between autotaxin and markers of immune activation, while sCD14 levels correlated with Mac2BP levels (P = .039).
Table 2.
Correlations Between Immune Activation Parameters Before and During Therapy for Hepatitis C Virus (HCV) and Human Immunodeficiency Virus (HIV) Infection
| Time, Parameter | HCV-Infected Subjects |
HCV-HIV Coinfected Subjects |
HIV-Infected Subjects |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | Autotaxin | sCD14 | sCD163 | Mac2BP | IL-6 | Autotaxin | sCD14 | sCD163 | Mac2BP | IL-6 | Autotaxin | sCD14 | sCD163 | Mac2BP | |
| Week 0 | |||||||||||||||
| IL-6 | … | r = 0.602 P = .000 | r = 0.391 P = .036 | … | r = 0.437 P = .018 | … | … | … | r = 0.441 P = .015 | r = 0.415 P = .023 | … | … | … | … | … |
| Autotaxin | r = 0.602 P = .000 | … | r = 0.513 P = .006 | r = 0.489 P = .033 | r = 0.645 P = .000 | … | … | … | … | r = 0.400 P = .028 | … | … | … | … | … |
| sCD14 | r = 0.391 P = .036 | r = 0.513 P = .006 | … | r = 0.525 P = .021 | … | … | … | … | … | r = 0.479 P = .007 | … | … | … | … | r = 0.392 P = .039 |
| sCD163 | … | r = 0.489 P = .033 | r = 0.525 P = .021 | … | … | r = 0.441 P = .015 | … | … | … | … | … | … | … | … | … |
| Mac2BP | r = 0.43 P = .018 | r = 0.645 P = .000 | … | … | … | r = 0.415 P = .023 | r = 0.400 P = .028 | r = 0.479 P = .007 | … | … | … | … | r = 0.392 P = .039 | … | … |
| Weeks 20–24 | |||||||||||||||
| IL-6 | … | … | … | … | … | … | … | … | r = 0.453 P = .010 | … | … | … | … | … | |
| Autotaxin | … | … | … | … | … | … | … | … | r = 0.382 P = .037 | r = 0.434 P = .017 | … | … | … | … | … |
| sCD14 | … | … | … | r = 0.483 P = .020 | … | … | … | … | … | r = 0.459 P = .012 | … | … | … | … | … |
| sCD163 | … | … | r = 0.483 P = .020 | … | … | … | r = 0.382 P = .037 | … | … | r = 0.550 P = .002 | … | … | … | … | r = 0.405 P = .026 |
| Mac2BP | … | … | … | … | … | … | r = 0.434 P = .017 | r = 0.459 P = .012 | r = 0.550 P = .002 | … | … | … | … | r = 0.405 P = .026 | … |
Correlations were determined between levels at week 0 (baseline) and/or week 20–24 of interferon-free direct-acting antiviral therapy for HCV infection, among HCV-infected participants, and at 6 months of antiretroviral therapy for HIV, among HCV-HIV–coinfected and HIV-infected participants.
Abbreviations: IL-6, interleukin 6; Mac2BP, Mac2 binding protein; sCD14, soluble CD14; sCD163; soluble CD163.
Levels of Autotaxin and Soluble Markers of Immune Activation Normalize or Partially Normalize During IFN-Free DAA Therapy for HCV, While Levels of Different Markers of Immune Activation Normalize or Partially Normalize After Institution of ART During HIV Infection
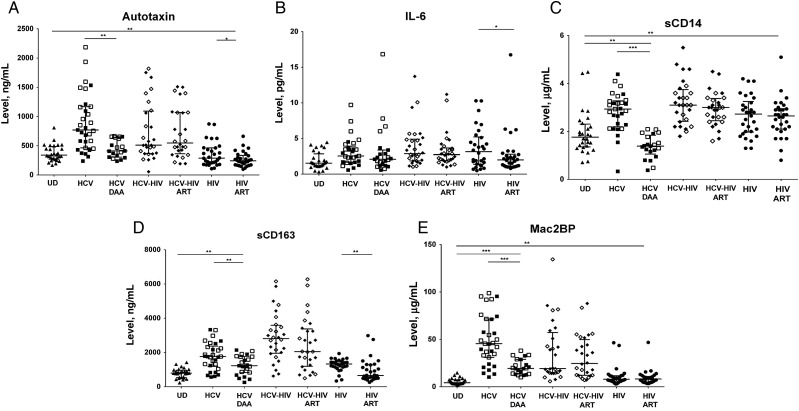
To begin to understand the dependence of markers of immune activation upon active viremia and the degree of reversibility of immune activation, we evaluated autotaxin and markers of immune activation upon induction of viral suppression with HCV IFN-free DAA therapy in HCV monoinfected participants and ART in HIV-infected and HCV-HIV–coinfected participants. Within HCV-monoinfected participants, we observed a decline in autotaxin and sCD14 levels (P = .012 and P < .001, respectively, compared with levels at baseline) to levels more similar to those of uninfected participants (P = .07 and P = .018, respectively, compared with those in uninfected donors) and a decline in sCD163 and Mac2BP levels (P = .0001 and P = .0003, respectively, compared with levels at baseline) to levels greater than those of uninfected participants (P = .0011 and P < .0001, respectively, compared with those in uninfected donors) within 24 weeks of IFN-free DAA therapy (Figure 2). Notably, for sCD163 this decline was observed in the high APRI subgroup (P < .0001) but not the low APRI subgroup (P = .2). For Mac2BP the decline was observed for both high (P < .0001) and low (P < .0001) APRI subgroups. Furthermore, IL-6 and sCD163 levels in HIV-monoinfected participants declined after ART initiation (P = .05 and P = .009, respectively) to levels similar to those in uninfected donors (Figure 2). Although levels of autotaxin were not significantly different between HIV-infected and uninfected donors, after ART initiation levels of autotaxin modestly declined (P = .021). No significant difference was noticed in plasma levels of any soluble immune activation marker in the HCV-HIV–coinfected group after 6 months of ART, consistent with the continued presence of HCV driving these elevated plasma immune activation markers.
Figure 2.
Autotaxin, soluble CD14 (sCD14), sCD163, and Mac2 binding protein (Mac2BP) levels normalize or partially normalize during interferon (IFN)–free direct-acting antiviral (DAA) therapy for hepatitis C virus (HCV) infection, while autotaxin, interleukin 6 (IL-6), and sCD163 levels normalize after institution of antiretroviral therapy (ART) for human immunodeficiency virus (HIV) infection. Shown is a comparison of plasma levels of autotaxin (A), IL-6 (B), sCD14 (C), sCD163 (D), and Mac2BP (E) for HCV-monoinfected participants at week 0 (HCV) and week 20–24 (HCV DAA) in participants receiving IFN-free DAA therapy and for HIV-infected or HCV-HIV–coinfected participants at week 0 (HCV-HIV and HIV) and after 6 months of ART (HCV-HIV ART and HIV ART), as well as UD participants. *P < .05, **P < .01, and ***P < .001. Abbreviation: UD, uninfected donor.
Correlations Between Immune Activation Parameters Before and During Therapy for HCV Infection and HIV Infection
To understand how suppression of HCV or HIV affects the relationship between autotaxin and parameters of immune activation, we evaluated relationships between soluble markers of immune activation before and during virally suppressed HCV infection (suppressed with IFN-free DAA therapy), HCV-HIV coinfection (suppressed with ART), and HIV infection (suppressed with ART). We found that baseline correlations between autotaxin levels and IL-6, sCD14, sCD163, and Mac2BP levels no longer remained after treatment-induced HCV suppression (Table 2). Additionally, we observed that the correlations between IL-6 and Mac2BP levels during coinfection were no longer present after suppression of HIV (Table 2), suggesting that HIV drives these relationships. Correlations between IL-6 and sCD163 levels, autotaxin and Mac2BP levels, and sCD14 and Mac2BP levels persisted after suppression of HIV in the HCV-HIV–coinfected group, consistent with HCV infection driving at least a component of these relationships (Table 2).
Autotaxin Levels Correlate With LPA Levels, and, Like Autotaxin Levels, LPA Levels Normalize During IFN-Free DAA Therapy
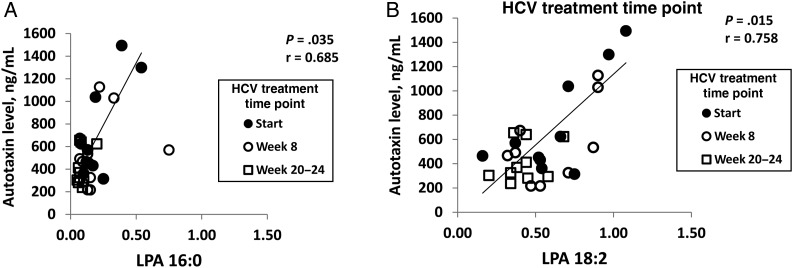
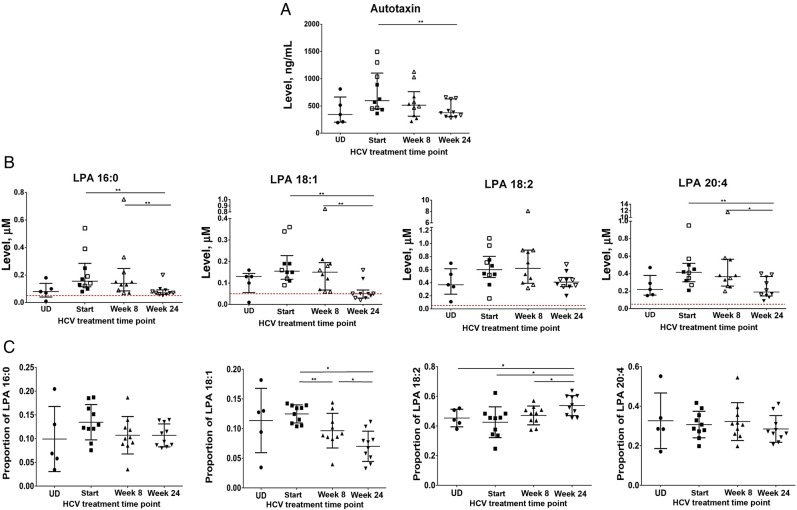
Because autotaxin is known to be the primary source of plasma LPA [24, 25], we next evaluated whether autotaxin levels observed here were associated with elevated LPA levels during chronic HCV or HIV infection. Previously it has been shown that 16:0, 18:2, 20:4, 18:1, and 18:0 LPA forms are particularly abundant in plasma [29, 40]. We measured 5 different LPA subtypes: LPA 16:0, 18:2, 20:4, 18:1, and 18:0 in plasma samples of 10 HCV-infected and 5 uninfected participants. We observed that autotaxin levels positively correlated with LPA 16:0 and 18:2 levels (P = .035 and .015, respectively) in HCV-infected participants (Figure 3). Autotaxin levels also tended to correlate with plasma levels of LPA 18:1 at the start of HCV therapy (P = .104; data not shown), while there were no significant associations between LPA 20:4 and autotaxin at any time point of HCV therapy (data not shown). LPA 18:0 levels were below the limit of detection (data not shown). We observed LPA 16:0 and LPA 20:4 levels to be elevated or nearly significantly elevated in HCV-infected participants as compared to uninfected donors (P = .036 and P = .07, respectively; Figure 4B). We next evaluated the effect of IFN-free DAA HCV therapy on LPA levels. We observed that, like autotaxin, LPA levels normalized during IFN-free DAA therapy (Figure 4B). Additionally, we evaluated the proportion of the total LPA level composed by each measured LPA subtype (Figure 4C). Notably, LPA subtype distribution changed over the course of HCV DAA therapy, with an increasing proportion of the 18:2 subtype over the course of therapy and a decreasing proportion of the 18:1 subtype, indicating that the dynamics of LPA production and/or substrate composition are altered over the course of HCV DAA therapy. Finally, LPA subtypes were measured in 5 HIV-infected participants at the start and at week 24 of ART and compared to those in uninfected participants (Supplementary Figure 2B). Even though autotaxin levels did not differ in HIV-infected participants as compared to those in uninfected donors, we observed that LPA 18:2 levels were elevated in HIV-infected donors as compared to those in uninfected participants and that this persisted at 24 weeks of ART, indicating that factors other than autotaxin level may contribute to LPA levels. At the same time, LPA 18:1, 18:2, and 16:0 levels correlated with autotaxin levels before therapy in HIV-infected donors (Supplementary Figure 3). Together, these data are consistent with elevated autotaxin levels during HCV infection driving elevated LPA levels for 4 of 5 LPA subtypes measured here and the finding that both autotaxin and LPA levels normalize soon after initiation of IFN-free DAA HCV therapy.
Figure 3.
Autotaxin levels correlate with lysophosphatidic acid (LPA) levels. Correlations between autotaxin at baseline/start, week 8, and week 20–24 of interferon-free therapy combined and for LPA 16:0 (A) and LPA 18:2 (B) subtypes. Analyses were performed to evaluate correlations between autotaxin at each time point and for each LPA subtype. Significant correlations between LPA 16:0 and autotaxin levels at week 0/baseline/start (P = .035) and LPA 18:2 at week 0/baseline/start (P = .015) were observed (trend line shown), while there were no significant relationships between LPA 16:0 and autotaxin levels for week 8 and week 20–24 time points (P = .407 and P = .395, respectively), between LPA 18:2 and autotaxin levels at other time points (P = .156 and P = .993, respectively), or at any time point for other LPA subtypes (LPA 18:1, P = .104, P = .980, and P = .994, respectively; LPA 20:4, P = .144, P = .740, and P = .657, respectively).
Figure 4.
In addition to autotaxin, lysophosphatidic acid (LPA) levels normalize during interferon (IFN)–free direct-acting antiviral (DAA) therapy for hepatitis C virus (HCV) infection. A and B, Levels of autotaxin (A) and LPA subtypes 16:0, 18:1, 18:2, and 20:4 (B), with the lower level of detection of 0.05 shown in dotted line. C, Percentage of total LPA that is LPA 16:0, 18:1, 18:2, or 20:4 in 5 UD participants and 10 HCV-infected participants at baseline, week 8, and week 24 of IFN-free DAA therapy for HCV. *P < .05, **P < .01, and ***P < .001. Abbreviation: UD, uninfected donor.
LPA Induces Monocyte Activation
The correlations observed here suggested that autotaxin and its enzymatic product, LPA, may be activating monocytes to induce expression of sCD14, IL6, and sCD163. To evaluate whether LPA can activate monocytes in vitro, we examined CD80 and CD86 expression on monocytes after incubation with LPA or LPS for either 3 hours with whole blood or 24 hours with purified/enriched monocytes. We observed that the proportion of classic CD14++CD16− monocytes that express CD86 and CD80 was higher after stimulation with LPA as compared to medium after 3 hours (P = .0156 and P = .013, respectively; Figure 5B and 5C). In purified monocyte cultures, LPA also induced CD80 expression (P = .013; Figure 5D). When we evaluated cell culture supernatants for soluble factors associated with immune activation, we observed that LPA tended to induce IL-6 in PBMC cultures (P = .08; data not shown). We additionally evaluated sCD14, sCD163, IL-10, IL-1β, TNF-α, and GM-CSF in these cultures and found no significant LPA-associated induced expression of these factors in our summative data set, while in a select case induced expression of IL-10 and TNF-α was observed (in a sample where IL-6 was also induced). LPS treatment reproducibly induced all of these factors (data not shown). These data provide proof of concept that elevated LPA levels may contribute to monocyte activation and systemic immune activation.
Figure 5.
Gating strategy for monocytes and lysophosphatidic acid (LPA)–induced monocyte activation. A, Whole blood sample from uninfected participant demonstrating monocyte gating strategy. On the basis of forward scatter (FSC)/side scatter (SSC) and CD14 and CD16 expression, monocytes were gated into classic CD14++CD16−, intermediate CD14++CD16+, and nonclassic CD14+CD16++ subsets. CD86 expression on classic CD14++CD16− monocytes is shown. CD86 gating was based on isotype. B, Percentage of the classic monocyte population that expressed CD86. C, Percentage of the classic monocyte population that expressed CD80 after 3 hours of whole blood incubation with medium alone, LPA 20:4 (10 µM), or lipopolysaccharide (LPS; 10 ng/mL). D, Percentage of the classic monocyte population that expressed CD80 after 24-hour purified/enriched monocyte culture with medium alone, LPA 20:4 (10 µM), or LPS (10 ng/mL).
DISCUSSION
Data here provide evidence of elevated levels of autotaxin in HCV-HIV coinfection, attributable to HCV infection, and demonstrate that autotaxin levels are associated with levels of soluble markers of immune activation in both HCV infection and HCV-HIV coinfection. These data extend previous observations of elevated autotaxin and LPA levels during HCV monoinfection [19, 22, 39], to the setting of HCV-HIV infection. They also extend our prior observation of associations between autotaxin and immune activation during HCV monoinfection [19] to the setting of HCV-HIV coinfection. We further observed normalization of autotaxin and sCD14 levels and a significant decline in levels of a number of soluble markers of immune activation (sCD163 and Mac2BP) during IFN-free DAA HCV therapy. While we observed normalization of autotaxin and sCD14 plasma levels and partial normalization of sCD163 and Mac2BP levels 6 months after institution of IFN-free DAA HCV therapy, there were no changes in levels of any of the soluble markers in HCV-HIV–coinfected participants after ART therapy. This indicates a continued influence of chronic HCV infection after removal of HIV. Whether the same changes occur after IFN-free DAA therapy in HCV-HIV–coinfected subjects during ART is yet to be determined.
In normal liver, autotaxin is taken up from the hepatic sinusoids and degraded by sinusoidal endothelial cells. In fibrotic liver tissue, capillarization of the sinusoids occurs. This is accompanied by the impairment of autotaxin uptake. In this way, liver fibrosis is thought to contribute to reduced autotaxin clearance and to elevated and increased autotaxin plasma levels [41]. LPA, the main product of autotaxin enzymatic activity [24], has a variety of effects on immunocytes, including promoting T-cell proliferation, preventing T-cell death, and inducing inflammatory cytokine expression [42, 43]. LPA modulates monocyte migration via induced endothelial cell secretion of interleukin 8 and monocyte chemoattractant protein 1 [44] and endothelial adhesion molecule expression [45]. Here, we performed a multiple LPA subtype analysis during HCV infection and observed that elevated autotaxin levels correlate with elevated levels of a number of LPA subtypes. Furthermore, LPA levels, along with autotaxin levels, normalized with IFN-free DAA-induced viral level decline during HCV infection. LPA subtype composition also changed over the course of IFN-free DAA therapy. The latter may reflect LPA substrate (LPC) composition change or LPA subtype stability change during HCV level decline. The observation that LPA can activate monocytes to express CD86 and CD80 provides proof of concept that elevated LPA levels may contribute to activation of monocytes and elaboration of soluble markers of immune activation. Finally, although autotaxin levels were not elevated during HIV infection, they modestly declined during ART. Additionally, LPA 18:2 levels were elevated and this persisted at 24 weeks of ART, indicating that factors other than autotaxin may contribute to LPA levels.
The mechanism of action of monocytes and resident liver macrophages (Kupffer cells) in inflammation and fibrogenesis during chronic viral hepatitis is not well understood. Macrophages have been observed in close proximity to cells involved in fibrosis, producing a number of proinflammatory factors involved in fibrosis [46]. Activated macrophages are described to polarize into M1 (IL-1β, TNF, IL-6, and Mac2-BP producing) and M2 (CD163 expressing and IL-10 producing) phenotypes [47]. Here, we observed elevated sCD14, IL-6, and sCD163 levels during both HCV and HIV infection, while Mac2BP and autotaxin levels were mainly elevated during HCV infection. HCV DAA treatment significantly decreased sCD163 and Mac2-BP levels but not IL-6 levels in HCV monoinfection. In vitro treatment of unfractionated PBMCs and purified monocytes induced expression of some IL-6, perhaps providing proof of concept for an M1 polarizing effect of LPA.
There are a number of limitations in this data set. First, the analysis does not include all LPA subtypes, including 22:6 and 20:5, that have been described during ovarian cancer and cardiovascular disease [48, 49]. Second, we have not used autotaxin inhibitors in our activity assays, and therefore LPA generation in vitro may add to the activity observed. However, we did evaluate the effect of exogenously added LPA in our analysis of CD80 and CD86 expression and found that there was no appreciable upregulation of CD80 or CD86 in medium culture in shortest-term whole blood cultures, while in the case of PBMC cultures there was modest upregulation of CD86 in medium cultures. Third, LPA quantification is influenced by a number of variables, including production of LPA after sample acquisition. LPA levels observed here in HCV-infected participants were similar to those observed in one prior study [50]. While our results may be influenced by ex vivo LPA production, given that the LPA subtype composition was observed to change over the course of IFN-free DAA therapy, it appears that these observations are reflective of in vivo events.
In conclusion, elevated autotaxin levels during HCV infection may contribute to immune activation in HCV infection and HCV-HIV coinfection. Whether the relationships described here are causally related in vivo merits further study. Furthermore, whether LPA-induced immune activation contributes to immune dysfunction is yet to be determined.
Supplementary Material
Notes
Acknowledgments. We thank the study participants for their time and dedication to this effort.
Financial support. This work was supported by VA Merit (1IO1CX001104-01), the Center for AIDS Research (catalytic funding), and the Clinical Translational Science Collaborative of Cleveland (UL1TR000439).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ansaldi F, Orsi A, Sticchi L, Bruzzone B, Icardi G. Hepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J Gastroenterol 2014; 20:9633–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freeman AJ, Dore GJ, Law MG et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001; 34:809–16. [DOI] [PubMed] [Google Scholar]
- 3. Zylberberg H, Pol S. Reciprocal interactions between human immunodeficiency virus and hepatitis C virus infections. Clin Infect Dis 1996; 23:1117–25. [DOI] [PubMed] [Google Scholar]
- 4. Backus LI, Phillips BR, Boothroyd DB et al. Effects of hepatitis C virus coinfection on survival in veterans with HIV treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2005; 39:613–9. [PubMed] [Google Scholar]
- 5. Cacoub P, Geffray L, Rosenthal E, Perronne C, Veyssier P, Raguin G. Mortality among human immunodeficiency virus-infected patients with cirrhosis or hepatocellular carcinoma due to hepatitis C virus in French Departments of Internal Medicine/Infectious Diseases, in 1995 and 1997. Clin Infect Dis 2001; 32:1207–14. [DOI] [PubMed] [Google Scholar]
- 6. Greub G, Ledergerber B, Battegay M et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet 2000; 356:1800–5. [DOI] [PubMed] [Google Scholar]
- 7. Limketkai BN, Mehta SH, Sutcliffe CG et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA 2012; 308:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monga HK, Rodriguez-Barradas MC, Breaux K et al. Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin Infect Dis 2001; 33:240–7. [DOI] [PubMed] [Google Scholar]
- 9. Palella FJ Jr, Delaney KM, Moorman AC et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 10. Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA 2002; 288:199–206. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez VD, Landay AL, Sandberg JK. Innate immunity and chronic immune activation in HCV/HIV-1 co-infection. Clin Immunol 2010; 135:12–25. [DOI] [PubMed] [Google Scholar]
- 12. Sandler NG, Wand H, Roque A et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anthony DD, Conry SJ, Medvik K et al. Baseline levels of soluble CD14 and CD16+56- natural killer cells are negatively associated with response to interferon/ribavirin therapy during HCV-HIV-1 coinfection. J Infect Dis 2012; 206:969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benhamou Y, Bochet M, Di Martino V et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999; 30:1054–8. [DOI] [PubMed] [Google Scholar]
- 15. Giorgi JV, Hultin LE, McKeating JA et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 1999; 179:859–70. [DOI] [PubMed] [Google Scholar]
- 16. Graham CS, Baden LR, Yu E et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001; 33:562–9. [DOI] [PubMed] [Google Scholar]
- 17. Kovacs A, Karim R, Mack WJ et al. Activation of CD8 T cells predicts progression of HIV infection in women coinfected with hepatitis C virus. J Infect Dis 2010; 201:823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sandler NG, Koh C, Roque A et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141:1220–30, 30 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlatzer DM, Sugalski JM, Chen Y et al. Plasma proteome analysis reveals overlapping, yet distinct mechanisms of immune activation in chronic HCV and HIV infections. J Acquir Immune Defic Syndr 2013; 63:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giganti A, Rodriguez M, Fould B et al. Murine and human autotaxin alpha, beta, and gamma isoforms: gene organization, tissue distribution, and biochemical characterization. J Biol Chem 2008; 283:7776–89. [DOI] [PubMed] [Google Scholar]
- 21. Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol 2008; 9:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pleli T, Martin D, Kronenberger B et al. Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis - a prospective cohort study. PLoS One 2014; 9:e103532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakagawa H, Ikeda H, Nakamura K et al. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta 2011; 412:1201–6. [DOI] [PubMed] [Google Scholar]
- 24. Tokumura A, Majima E, Kariya Y et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 2002; 277:39436–42. [DOI] [PubMed] [Google Scholar]
- 25. Umezu-Goto M, Kishi Y, Taira A et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 2002; 158:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moolenaar WH. Lysophospholipids in the limelight: autotaxin takes center stage. J Cell Biol 2002; 158:197–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moolenaar WH, van Meeteren LA, Giepmans BNG. The ins and outs of lysophosphatidic acid signaling. Bioessays 2004; 26:870–81. [DOI] [PubMed] [Google Scholar]
- 28. Graler MH, Goetzl EJ. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim Biophys Acta 2002; 1582:168–74. [DOI] [PubMed] [Google Scholar]
- 29. Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem 2001; 292:287–95. [DOI] [PubMed] [Google Scholar]
- 30. Scherer M, Schmitz G, Liebisch G. High-throughput analysis of sphingosine 1-phosphate, sphinganine 1-phosphate, and lysophosphatidic acid in plasma samples by liquid chromatography-tandem mass spectrometry. Clin Chem 2009; 55:1218–22. [DOI] [PubMed] [Google Scholar]
- 31. Kano K, Arima N, Ohgami M, Aoki J. LPA and its analogs-attractive tools for elucidation of LPA biology and drug development. Curr Med Chem 2008; 15:2122–31. [DOI] [PubMed] [Google Scholar]
- 32. Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett 2000; 478:159–65. [DOI] [PubMed] [Google Scholar]
- 33. Ullrich A, Sures I, D'Egidio M et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J Biol Chem 1994; 269:18401–7. [PubMed] [Google Scholar]
- 34. Shaked I, Hanna DB, Gleissner C et al. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol 2014; 34:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lotz MM, Andrews CW Jr, Korzelius CA et al. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci U S A 1993; 90:3466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iacobelli S, Ullrich A, Tinari N et al. The 90k tumor-associated antigen and clinical progression in human-immunodeficiency-virus infection. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 10:450–6. [DOI] [PubMed] [Google Scholar]
- 37. Artini M, Natoli C, Tinari N et al. Elevated serum levels of 90K/MAC-2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients. J Hepatol 1996; 25:212–7. [DOI] [PubMed] [Google Scholar]
- 38. Funderburg NT, Zidar DA, Shive C et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120:4599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watanabe N, Ikeda H, Nakamura K et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol 2007; 41:616–23. [DOI] [PubMed] [Google Scholar]
- 40. Sano T, Baker D, Virag T et al. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem 2002; 277:21197–206. [DOI] [PubMed] [Google Scholar]
- 41. Ikeda H, Yatomi Y. Autotaxin in liver fibrosis. Clin Chim Acta 2012; 413:1817–21. [DOI] [PubMed] [Google Scholar]
- 42. Zheng Y, Voice JK, Kong Y, Goetzl EJ. Altered expression and functional profile of lysophosphatidic acid receptors in mitogen-activated human blood T lymphocytes. FASEB J 2000; 14:2387–9. [DOI] [PubMed] [Google Scholar]
- 43. Goetzl EJ, Kong Y, Mei B. Lysophosphatidic acid and sphingosine 1-phosphate protection of T cells from apoptosis in association with suppression of Bax. J Immunol 1999; 162:2049–56. [PubMed] [Google Scholar]
- 44. Gustin C, Van Steenbrugge M, Raes M. LPA modulates monocyte migration directly and via LPA-stimulated endothelial cells. Am J Physiol Cell Physiol 2008; 295:C905–14. [DOI] [PubMed] [Google Scholar]
- 45. Rizza C, Leitinger N, Yue J et al. Lysophosphatidic acid as a regulator of endothelial/leukocyte interaction. Lab Invest 1999; 79:1227–35. [PubMed] [Google Scholar]
- 46. Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 2010; 30:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol 2013; 120:163–84. [DOI] [PubMed] [Google Scholar]
- 48. Kurano M, Suzuki A, Inoue A et al. Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic acid in acute coronary syndrome. Arterioscler Thromb Vasc Biol 2015; 35:463–70. [DOI] [PubMed] [Google Scholar]
- 49. Shan L, Jaffe K, Li S, Davis L. Quantitative determination of lysophosphatidic acid by LC/ESI/MS/MS employing a reversed phase HPLC column. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 864:22–8. [DOI] [PubMed] [Google Scholar]
- 50. Skill NJ, Jianmin W, Yan X, Zhao Z, Tector AJ, Maluccio MA. Lysophospholipid variants in hepatocellular carcinoma. J Surg Res 2013; 182:241–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.