Abstract
Organisms must distribute sufficient energy among different and often competing physiological systems. This task can become challenging, however, as resources are often limiting, resulting in energetic trade-offs. For example, energetically based trade-offs between the reproductive and immune systems are common across taxa, yet the regulatory mechanisms underlying these trade-offs remain unclear. The adipose tissue hormone leptin is an ideal candidate for the modulation of energetic trade-offs between different physiological systems as this hormone serves as a gage of fat reserves and also modulates a range of physiological activities including the reproductive and immune processes. This article presents a review of the evidence for the role of leptin as a modulator of energetic trade-offs with the immune system and suggests its importance in disease ecology. In addition, we provide a case study of the ornate tree lizard (Urosaurus ornatus), testing whether leptin is involved in mediating a well-documented influence of energy state on the trade-off between reproductive activity and immune function. Overall, the combined results suggest that leptin serves as a proximate endocrine signal of available energy to the immune system, and therefore likely to affect susceptibility to diseases.
Introduction
Energetic costs of immunity
Balancing the distribution of energy resources among different systems, such as the immune and reproductive systems, is critical for survival in all organisms. However, because resources in the environment are often limiting, adequate allocation of these resources to multiple systems can be challenging. A more precise quantification of the energetic costs of mounting an immune response has been demonstrated recently by Lochmiller and Deerenberg (2000) and Nelson et al. (2002). Although some debate exists as to the exact cost of maintaining immune defenses (Klasing 1998), mounting an immune response unquestionably incurs an energetic cost (Nelson and Demas 1996; Sheldon and Verhulst 1996; Lochmiller and Deerenberg 2000).
For example, mice immunized with the antigen keyhole limpet hemocyanin (KLH) display ∼25% increases in oxygen consumption and metabolic heat production compared to pre-immunization baseline values (Demas et al. 1997). Similar increases in resting metabolic rate have been observed in great tits (∼5%; Parus major) and zebra finches (∼16%; Taeniopygia guttata), along with decrease in body mass in response to lipopolysaccharide (LPS) (Burness et al. 2010) and phytohemagglutinin (PHA) challenges (Nilsson et al. 2007) and sheep-red-blood-cell challenge (∼9%) (Ots et al. 2001), suggesting that these responses have an energetic cost. Similarly, indirect evidence of behavioral fever in ectothermic animals demonstrates the significant energetic demands of mounting an immune response (Sherman et al. 1991; Deen and Hutchison 2001).
Consistent with this idea, reductions in total body fat are correlated with impaired immunity in a wide range of species, including humans (Norgan 1997; Spurlock et al. 1997; Klasing 1998; Lin and Shiau 2003), and experimental reductions in body fat can impair the formation of antibodies (Demas et al. 2003). In contrast, natural increases in body fat can restore previous impairments in immunity (Demas et al. 2003). Furthermore, immunological disorders (e.g., AIDS) trigger marked changes in whole-body lipid metabolism, suggesting an important role of adipose tissue in immunity (Pond 1996).
It should be clear from the studies reviewed above that immune function is costly in which there is a quantifiable metabolic cost to mounting an immune response. The idea that there exists adaptive resource (energy) re-allocation or energetic trade-offs between immunity and other costly responses, however, remains an open question. While some studies have found support for trade-offs with immune responses (French et al. 2007b), others have not (Svensson et al. 1998; Burness et al. 2010). It is important to note some of these studies were conducted under conditions of ad libitum food availability; thus it is possible that potential energetic trade-offs were masked under conditions where resources were not limiting. Ongoing studies are aimed at determining whether the energetic costs of immunity have any functional significance under free-living conditions where resources are more limiting (Demas et al. In Press).
Leptin as a mediator of energetic trade-offs
The precise neuroendocrine mechanisms whereby availability of energy or fat is translated into a signal indicating the current energy balance are not well understood. In the past few years alone, however, a variety of endocrine factors have been identified as potential candidates for providing signals of current availability of energy (Woods et al. 1998). Recent work on mammals demonstrates that leptin, a protein hormone first identified in 1994 and which is secreted predominantly by adipose tissue and likely to serve as an indicator of fat reserves, is also involved in immunoregulation (Zhang et al. 1994; Drazen et al. 2000; Drazen et al. 2001; Demas and Sakaria 2005; Fantuzzi 2006). Moreover, circulating concentrations of leptin are directly proportional to the mass of adipose tissue. High levels of leptin indicate adequate energy stores, whereas low circulating levels of leptin are consistent with an energy deficit and likely to “decide” on energetic investment into different systems (Fig. 1).
Fig. 1.
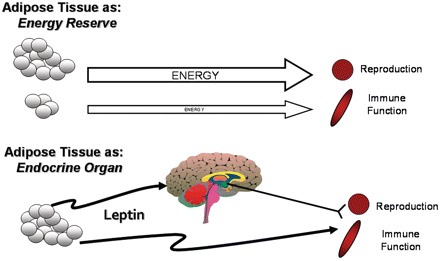
Proposed mechanisms for energetic trade-offs between reproduction and immune function. In the top panel, energy is invested in physiological functions, including reproduction (represented by gonad), and immune function (represented by spleen). The degree of energetic investment is directly dependent on the amount of available energy. The bottom panel represents an alternative mechanism by which trade-offs are coordinated via the use of an endocrine signal, in this case leptin. In this scenario, rather than energy directly affecting reproduction or immune function, leptin provides a signal of available energy; specific physiological functions are then modified via the relative presence or absence of the leptin signal. Further, leptin can act directly on reproductive and lymphoid tissues or indirectly via the central nervous system (brain) to regulate physiological trade-offs.
Initial studies of leptin suggested that the primary function of this hormone was that of a satiety factor, as treatment of mice with physiological levels of leptin triggered marked reductions in food intake and in body fat (Zhang et al. 1994; Drazen et al. 2000). Interestingly, however, decreases in body fat were still evident, even when food intake was kept constant; suggesting that leptin also exerted a direct effect on energy metabolism, independent of food intake (Elmquist 2001; Rayner and Trayhurn 2001). Since these initial findings, it has become increasingly clear that leptin is a pleiotropic molecule involved in a wide-range of physiological functions, including reproduction, energy balance, and immune function (Baldelli et al. 2002; Fantuzzi 2006).
A wide variety of actions within the immune system are influenced by leptin. For example, specific immune responses are disrupted in mice with impaired leptin signaling due to genetic defects (e.g., ob/ob mice, db/db mice) (Lord et al. 1998). Specifically, ob/ob mice that are unable to produce leptin experience atrophy of lymphoid tissues (e.g., spleen, thymus), and decrease in the number of circulating lymphocytes (Lord et al. 1998). Exogenous leptin added to T-cell cultures from mice enhances proliferation in response to allogenic stimulator cells in both naïve and memory T-cell types (Lord et al. 1998). Leptin also appears to mediate seasonal changes in immune function. Studies show that leptin fluctuates according to photoperiod and season (Rousseau et al. 2002; Gaspar-López et al. 2009). For example, Siberian hamsters (Phodopus sungorus) exposed to short, winter-like days typically exhibit decreased body fat and immunity; however, treatment with leptin attenuates this seasonal immunosuppression (Drazen et al. 2001; Gaspar-López et al. 2009). Similarly, treatment with leptin reverses the immunosuppressive effects of experimental lipectomy (surgical fat removal) on humoral immune function (Demas and Sakaria 2005).
Resource-based trade-offs between the reproductive and immune systems have been repeatedly demonstrated (Norris and Evans 2000; French et al. 2007a, 2007c), but these studies do not provide a regulatory mechanism for the detected trade-off. Leptin represents a likely mediator for the observed resource-based trade-offs that naturally occur between the immune system and other physiological systems, specifically reproduction. Leptin is a permissive regulator of reproduction in mammals; it allows reproduction to occur when resources are limiting but does not necessarily enhance it above normal function (Casanueva and Dieguez 1999; Norris and Evans 2000; Baldelli et al. 2002; Margetic et al. 2002; Zieba et al. 2005). Pregnant female hamsters treated with leptin retain more embryos through parturition, but also rear more offsprings through weaning, via reduced maternal infanticide (French et al. 2009). However, innate immune response is suppressed, seemingly as a result of the enlarged litters, suggesting that the observed fitness increase is not without costs to the mother (French et al. 2009). This well-established link between leptin, fat stores, and organismal immunocompetence (Lord et al. 1998), makes leptin a likely candidate for mediating physiological trade-offs between the immune and other systems, especially reproduction (Fig. 1).
Leptin in nonmammalian systems
Do nonmammalian vertebrates have leptin? Although a gene sequence homologous to mouse leptin has been reported in avian species (Taouis et al. 1998) and fishes (Johnson et al. 2000; Kurokawa et al. 2005) the likelihood that this gene represents an avian or fish leptin gene is still debated (Volkoff et al. 2005; Sharp et al. 2008; Simon et al. 2009). Interestingly, a report details that turkeys (Meleagris gallopavo), provided with the protein generated by the leptin-like chicken gene had enhanced T-cell proliferation in response to concavalin A (Lõhmus et al. 2004), and zebra finches receiving recombinant murine leptin had an enhanced wing-web swelling in response to a PHA injection (Alonso-Alvarez et al. 2007).
Similarly in reptiles, although putative leptin has only been localized in a few lizard species, the physiological effects (e.g., temperature regulation, food intake) are robust, rendering it a likely mediator of energetic trade-offs (Sciarrillo et al. 2005; Niewiarowski et al. 2000; Putti et al. 2009). A recent study by Boorse and Libbon (2010) localized lizard leptin from the Anolis genome and found that it has a similar size, structure and location within the genome as mammalian leptin.
There is also evidence that leptin-like molecules can influence reproduction in nonmammalian vertebrates. Treatment with murine leptin alters fat metabolism in green sunfish (Lepomis cyanellus) (Londraville and Duvall 2002), and inhibits food intake in goldfish (Carassius auratus) (Volkoff et al. 2003). In great tits (P.major), females treated with leptin were more likely to have a second clutch in the breeding season than were those treated with vehicle alone (Lõhmus and Björklund 2009). A recent study describes changes in plasma leptin-like immunoreactivity, whereby it increases during breeding in free-living female European starlings (Sturnus vulgaris) (Kordonowy et al. 2010). A study of the Italian wall lizard Podarcis sicula, demonstrated that putative leptin delays testicular regression, thereby regulating reproduction (Putti et al. 2009). These results all suggest that a leptin-like signal is a likely mediator for resource-based trade-offs in nonmammalian vertebrates. These results would be predicted, were the effects of leptin similar in birds, reptiles, and mammals, and they suggest that even if birds and reptiles do not generate the leptin protein, they are likely to have a functional receptor, able to respond to a leptin-like ligand.
Leptin in lizards: a case study
Given the existing evidence for a role of leptin in regulating reproduction in lizards (see above), we examined whether leptin is involved in the resource-based regulation of immune function during reproduction in the tree lizard (Urosaurus ornatus). Putative leptin has been previously studied in the fence lizard (Sceloporus undulates) a closely related species to the tree lizard (Niewiarowski et al. 2000). Niewiarowski et al. isolated putative endogenous leptin and found that exogenous treatment with it was physiologically active, inducing increased body temperature and metabolic rate and decreased food intake, phenotypic effects similar to those seen in mammals. Additionally, a study in the Italian wall lizard, Podarcis sicula, demonstrated that treatment with leptin could delay testicular regression, thereby playing a regulatory role in reproduction, as has been established for numerous mammalian species (Putti et al. 2009). Furthermore, a recent study localized lizard leptin from the Anolis genome, identifying that it has a similar size, structure and location within the genome as does mammalian leptin (Boorse and Libbon 2010), further demonstrating its physiological action in reptilian species.
In a recent study, we experimentally delivered murine leptin to individually-housed vitellogenic female tree lizards with either unlimited (ad libitum) or restricted access to food, and measured the effect on immune function as measured by cutaneous wound-healing. Limiting food intake reduces fat stores and suppresses immune function in reproductive (vitellogenic) females, but not in nonreproductive females (French et al. 2007a, 2007c). The hypothesis that leptin mediates immune-reproduction trade-offs led us to predict that leptin would prevent the decreased wound-healing typically associated with restriction of food.
Animals
All animals had ad libitum access to water throughout the study. Early vitellogenic female tree lizards were collected from April 3to 9 2007. All lizards were collected within Tonto National Forest, 16 km east of Superior, Arizona (Maricopa County), just off of highway 60 (Latitude: 33.29° N, Longitude: 111.10° W). For specific collection and housing protocols and description, see French et al. (2007b). Experimental groups (n = 10 per group) included (1) saline injections and ad libitum diet, (2) saline injections and restricted diet, (3) leptin injections and ad libitum diet, and (4) leptin injections and restricted diet. Animals on a restricted diet received one cricket two times per week. While the ad libitum diet is sufficient for maintaining body mass and fat stores, the restricted diet is energetically constraining and leads to a decrease in body mass and fat stores (French et al. 2007b).
Overview
Upon capture, all animals were placed on their respective dietary treatment for 2 days to habituate to laboratory housing and to enter the desired energy state. After 2 days, all animals received daily injections of either leptin (10 µg/g body mass dissolved in phosphate buffered saline) or saline (equivalent volume) for the duration of the study. The dosage, duration, and type (murine recombinant; NHPP-NIDDK Harbor-UCLA Medical Center, Torrance CA, 90509) of leptin treatment were based on previous work on a similar species (Niewiarowski et al. 2000) that demonstrated physiological effects but not pharmacological effects. Animals were allowed 3 days to adjust to hormone treatment. On Day 3 after treatment (Day 5 postcapture), all animals received a cutaneous biopsy that created a measurable superficial wound for methodology see: French et al. (2006). High-resolution digital images were taken of wounds on the day of biopsy and at the termination of the study (Day 10 after biopsy). Images included a scale reference in the frame, and were analyzed using Image Pro Analysis Software® (Media Cybernetics, Inc., Bethesda MD, USA) for wound area using. Wound healing was calculated as percent change in wound size over the healing period. We terminated the study 10 days postbiopsy (15 days postcapture) at which point we re-measured cutaneous wounds, recorded body mass, and performed surgical laparotomies to determine sizes of follicles and fat bodies (scored 0–3); for methodology, see: French et al. (2007a). Throughout the course of the study, we recorded food intake by measuring daily the mass of crickets placed in the cage and the mass of crickets remaining from the previous day. All handling, care, and procedures were approved by the Arizona State University Institutional Animal Care and Use Committee under protocol # 05-822R.
Results
Females did not significantly differ in initial body size (svl, 47.3 ± 0.7 mm) or mass (2.82 ± 0.11 g) across treatment groups (all F < 0.318, all P > 0.813) (Table 1). As expected, ad libitum animals ate more food (0.80 ± 0.03 g) than did restricted animals (0.16 ± 0.01 g) (Table 1). Treatment with leptin significantly altered food intake; leptin-treated animals consumed significantly more than did the controls, when given unlimited access to food (P = 0.02) (Table 1). At the end of the study ad libitum animals (2.17 ± 0.15) had larger fat bodies than did food-restricted animals (1.20 ± 0.19) (Table 1). Healing of wounds significantly differed among treatment groups (one-way ANOVA; F = 3.794, df = 3, 37, P = 0.019) (Fig. 2A) and was significantly suppressed in food-restricted animals receiving saline injections relative to all other treatments (Tukey’s HSD corrected posthoc comparisons). Treatment significantly affected follicle size (one-way ANOVA; F = 3.839, df = 3, 37, P = 0.018) (Fig. 2B), such that food-restricted saline controls had significantly smaller follicles than their ad libitum counterparts. Ad libitum and food-restricted leptin-treated animals had follicles that were not significantly different from those of either saline group (Tukey’s HSD corrected post hoc comparisons).
Table 1.
Mean snout-vent length (svl), mass change, fat store, and food intake of each treatment group ± 1 SE
| Treatment | svl (mm) | Body mass change (g) | Fat body score (0–3)a | Mass eaten (g)a |
|---|---|---|---|---|
| Ad libitum leptin | 46.39 ± 1.15 | 0.00 ± 0.06 | 1.89 ± 0.11 | 0.83 ± 0.03 |
| Ad libitum saline | 48.17 ± 1.85 | 0.02 ± 0.10 | 2.44 ± 0.18 | 0.72 ± 0.03 |
| Restricted leptin | 47.40 ± 1.17 | −0.12 ± 0.04 | 1.40 ± 0.16 | 0.14 ± 0.02 |
| Restricted saline | 47.40 ± 1.41 | −0.26 ± 0.24 | 1.00 ± 0.21 | 0.17 ± 0.01 |
aStatistically significant difference among treatment groups (α = 0.05).
Fig. 2.
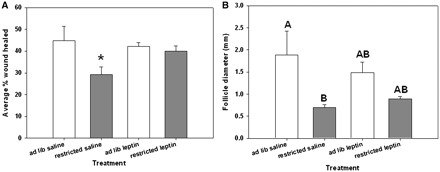
Effects of leptin treatment and food intake on wound-healing and follicular investment. (A) The effects of leptin and food intake on wound-healing in female tree lizards. Asterisk denotes a significant difference from the other treatment groups (α = 0.05). (B) The effects of leptin and food intake on follicular size in early-vitellogenic female tree lizards. Letters denote differences according to Tukey’s HSD corrected post-hoc comparisons (α = 0.05). Error bars represent 1 SE.
Discussion
As expected, reproductive animals undergoing food restriction exhibited decreased fat stores, suppressed wound-healing, and slowed follicular growth (Fig. 2). Interestingly, leptin treatment reversed the immunosuppressive effect as well as attenuated the reproductive effect. To our knowledge, the current study provides the first evidence that leptin is immunomodulatory in a reptile. This finding is also similar to that in Siberian hamsters in which leptin ameliorated both the experimental (lipectomy) and the seasonal immunosuppressive effects of decreased fat reserves on immune function (Drazen et al. 2001; Demas and Sakaria 2005). Leptin also is likely to provide an analogous signal to that found in mammals, whereby leptin, either due to food intake or to exogenous treatment, does not enhance reproduction but allows it to occur (Casanueva and Dieguez 1999; Baldelli et al. 2002; Margetic et al. 2002; Zieba et al. 2005). Therefore, the presence of leptin may communicate to each system that resources are not limited and thus allow each system to utilize resources as needed, and thereby not employ the trade-off that has been previously described (e.g., French et al. 2007b). At this juncture, it is unclear whether this endogenous signal is leptin or a leptin-like molecule. These results do suggest that even if reptiles do not generate the leptin protein, they are likely to have a functional receptor able to respond to a leptin-like ligand.
Leptin influenced food intake in the current study, but only in ad libitum females, whereas leptin-treated females consumed significantly more food than their saline-treated counterparts. Although other studies report an effect of leptin on food intake in chickens (Denbow et al. 2000) and fence lizards (chickens: Denbow et al. 2000; fence lizards: Niewiarowski et al. 2000), others do not (chickens: Bungo et al. 1999; Wistar rats: Passos et al. 2004), and still others find mixed, context-dependent effects (rats: Bojanowska and Nowak 2007; chickens: Cassy et al. 2004; rats: Kuo et al. 2005). The lack of an effect on food intake by females on restricted diets in the current study is advantageous because it supports a direct relationship between leptin and wound-healing that is not mediated via intake. Moreover, the reinstatement of wound-healing by leptin in food-restricted animals with decreased fat stores suggests that immunoregulation is modulated by a direct leptin signal rather than by the availability of the fat stores themselves.
When food restricted, leptin-treated animals can heal wounds faster and produce slightly larger follicles than do saline-treated animals undergoing food restriction. Because all these processes are resource intensive, resources must be coming from another source (French et al. 2007b). One indication is that all food-restricted animals have smaller fat stores than do ad libitum animals, suggesting that their fat stores are depleted. Additionally, there may be other physiological parameters not measured in the current study that were suppressed to provide resources for wound-healing and vitellogenesis in food-restricted leptin-treated animals, such as somatic growth or other branches of the immune system.
The results of this recent study are consistent with previous findings in mammals and support the idea that leptin, or a related peptide in reptiles, provides an endocrine signal that coordinates energetic trade-offs between reproduction and immune function. Future studies hopefully will examine multiple different components of the immune system to see whether the effects are uniform or whether specific aspects are targeted by leptin. Lastly, it is likely to observe that leptin is involved in the resource-dependent regulation of other physiological systems as well. It would be interesting to determine which systems are preferentially given access to resources or whether the permissive use of energy stores is consistent and nonspecific.
Overall, these results suggest that leptin serves as a proximate endocrine signal of available energy, attenuating the previously observed immunosuppression. These results further emphasize the context-dependent nature of physiological trade-offs; they are dynamic, adjusting to current environmental conditions, including energetic signals (e.g., leptin).
Integrating measurements of leptin into disease ecology
The central goal in disease ecology is to predict the dynamics of an infectious agent within a population of hosts. The mathematical workhorse used to predict disease dynamics is known as the “SIR” model in which individuals are classified by pathogen status as either susceptible, infectious or recovered (aka resistant). The SIR model consists of sets of linked differential equations for each category with the simple model consisting of three categories and two rates: beta, the infection rate and gamma, the recovery rate (Kermack and McKendrick 1927). When there is a high level of heterogeneity among individuals within a category, such as gender differences in susceptibility, independent modeling of the subcategories within a classification (i.e., male versus female susceptible individuals) are required to more accurately predict the dynamics of disease.
The potential exists for hormonal measurements, such as leptin, to inform disease ecology models about the heterogeneity of individuals within a category and their ability to respond to a pathogenic challenge. As discussed above, leptin is clearly an indicator of an animal’s energy reserves and the immune response is energetically costly, particularly during the acute phases (Derting and Compton 2003; Klasing 2007). Furthermore, the immune response can be impaired in animals with limited energy reserves (Lochmiller and Deerenberg 2000; Demas et al. 2003; Demas 2004; French et al. 2007b). Leptin levels have been shown to vary from 3- to 7-fold across a number of vertebrates (Zieba et al. 2005). Thus, an understanding of the variability in leptin levels across and within the categories of a SIR model may be useful in determining whether categories should be further subdivided to better predict an animal’s degree of susceptibility, infectiousness or resistance.
Consider the following hypothetical example of how leptin may be informative about the prospective disease status of a host in a simplified SI model in which there are only susceptible and infected individuals and no resistant individuals. A population of mice differs 7-fold in leptin levels across individuals. Leptin levels within susceptible individuals in the population are at the high end of the range and vary by >5-fold. Susceptible individuals at the highest end of the leptin range have the greatest energy reserves and, thus, may be able to mount the strongest innate immune response towards pathogens attempting to establish infections. These individuals with high-leptin levels may be significantly less susceptible than other individuals in this category that have lower energy reserves (Fig. 3). Extending this hypothetical example to the infected category, these individuals vary 4 × in leptin levels, but are at the lower end of the leptin range for the population. Infected individuals that have the lowest leptin levels are expected to have the weakest immune response (Fig. 3). Thus, these low-leptin individuals could represent the most infectious individuals in the population, i.e., the “superspreaders” (Hudson et al. 2008). In contrast, infected individuals with high-leptin levels may be able to clear the pathogen more rapidly, and thus these individuals would have a much shorter infectious stage. From a modeling perspective, it may be more realistic to separately model the high-leptin and low-leptin groups within each category, since in this example, they are assumed to differ in their susceptibility and infectiousness as a function of their leptin levels.
Fig. 3.
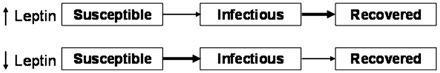
Incorporating leptin into SIR models. Individuals with higher fat stores and thus elevated leptin levels (top panel) have enhanced immune responses, are less susceptible and therefore less likely to become infectious (indicated by the thin arrow). Similarly, infectious animals with high leptin are more likely to recover quickly due to more robust immune defense and thus there would be a smaller window of time during which to transmit a pathogen to conspecifics (indicated by the thick arrow). Alternatively, animals with lower fat reserves and leptin levels are more likely to be susceptible and a larger number of these individuals will become infectious (indicated by the thick arrow). These same animals, once infectious, are also less likely to recover due to reduced immune defenses (indicated by the thin arrow).
We acknowledge that the above example is speculative. However, data on leptin levels and pathogen status of wild individuals are consistent with this conjuncture. For example, leptin signaling confers resistance to gut amoebas in mice (Guo et al. 2010). Mice lacking a leptin receptor had far greater infection rates and higher mortality than did wild-type mice. Leptin levels also appear to be predictive of infectiousness. In a study of sheep with helminth infections, animals with higher leptin levels had fewer, smaller worms, and a lower fecal egg count (Valderrabano et al. 2006). Thus, these individuals are less likely to be infectious than sheep with lower leptin levels. A caveat in this scenario is that some parasites potentially can manipulate leptin levels and thereby increase their own fitness (e.g., Zaralis et al. 2008). In such cases, the individuals with the highest leptin levels may in fact be the most infectious (Zaralis et al. 2008). Clearly, more research is needed to establish the nature of the relationship between leptin levels and disease status. Nonetheless, this provides one example of how hormonal measures such as leptin, as well as other metabolically-relevant hormones, hold promise for providing critical information to hone models of disease dynamics.
Funding
The authors thank the National Science Foundation (DEB-1042525) for “Bridging the Gap Between Ecoimmunology and Disease Ecology” symposium grant to S.S.F. and the divisions of Comparative Endocrinology and Ecology and Evolution in the Society for Integrative and Comparative Biology for the grant (EF 0326999) to M.D.D.
Acknowledgments
We would like to thank Dale DeNardo and Michael Moore for help with all aspects of the lizard case study. We also thank Natalyia Emmert for help with injections, photographing, and feeding, and Christy Strand for help in the field collecting animals. Leptin was kindly donated by the National Hormone and Pituitary Agency (NIADKK-NIH, Bethesda, MD, USA). We thank the divisions of Comparative Endocrinology and Ecology and Evolution in the Society for Integrative and Comparative Biology.
References
- Alonso-Alvarez C, Bertrand S, Sorci G. Energetic reserves, leptin and testosterone: a refinement of the immunocompetence handicap hypothesis. Biol Lett. 2007;3:271–4. doi: 10.1098/rsbl.2007.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli R, Dieguez C, Casanueva FF. The role of leptin in reproduction: experimental and clinical aspects. Ann Med. 2002;34:5–18. doi: 10.1080/078538902317338599. [DOI] [PubMed] [Google Scholar]
- Bojanowska E, Nowak A. Interactions between leptin and exendin-4, a glucagon-like peptide-1 agonist, in the regulation of food intake in the rat. J Physiol Pharmacol. 2007;58:349–60. [PubMed] [Google Scholar]
- Boorse G, Libbon J. Genomic characterization of two leptin genes and a leptin receptor gene in the Green Anole, Anolis carolinensis. Integr Comp Biol. 2010;50:e198–319. [Google Scholar]
- Bungo T, Shimojo M, Masuda Y, Tachibanab T, Tanaka SJ, Sugahara K, Furuse M. Intracerebroventricular administration of mouse leptin does not reduce food intake in the chicken. Brain Res. 1999;817:196–8. doi: 10.1016/s0006-8993(98)01223-2. [DOI] [PubMed] [Google Scholar]
- Burness G, Armstrong C, Fee T, Tilman-Schindel E. Is there an energetic-based trade-off between thermoregulation and the acute phase response in zebra finches? J Exp Biol. 2010;213:1386–94. doi: 10.1242/jeb.027011. [DOI] [PubMed] [Google Scholar]
- Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol. 1999;20:317–63. doi: 10.1006/frne.1999.0187. [DOI] [PubMed] [Google Scholar]
- Cassy S, Picard M, Crochet S, Derouet M, Keisler DH, Taouis M. Peripheral leptin effect on food intake in young chickens is influenced by age and strain. Domest Anim Endocrinol. 2004;27:51–61. doi: 10.1016/j.domaniend.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Deen CM, Hutchison VH. Effects of lipopolysaccharide and acclimation temperature on induced behavioral fever in juvenile Iguana iguana. J Therm Biol. 2001;26:55–63. doi: 10.1016/s0306-4565(00)00026-7. [DOI] [PubMed] [Google Scholar]
- Demas GE. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm Behav. 2004;45:173–80. doi: 10.1016/j.yhbeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol- Regul Integr Comp Physiol. 1997;42:R1631–7. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Demas GE, Drazen DL, Nelson RJ. Reductions in total body fat decrease humoral immunity. Proc R Soc Lond Ser B-Biol Sci. 2003;270:905–11. doi: 10.1098/rspb.2003.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Greives TJ, Chester EM, French SS. The energetics of immunity: Mechanisms mediating trade-offs in eco-immunology. In: Nelson RJ, Demas GE, editors. Eco-Immunology. New York, NY: Oxford University Press; 2011. [Google Scholar]
- Demas GE, Sakaria S. Leptin regulates energetic tradeoffs between body fat and humoural immunity. Proc R Soc Lond Ser B-Biol Sci. 2005;272:1845–50. doi: 10.1098/rspb.2005.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denbow DM, Meade S, Robertson A, McMurtry JP, Richards M, Ashwell C. Leptin-induced decrease in food intake in chickens. Physiol Behav. 2000;69:359–62. doi: 10.1016/s0031-9384(99)00258-9. [DOI] [PubMed] [Google Scholar]
- Derting TL, Compton S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus) Physiol Biochem Zool. 2003;76:744–52. doi: 10.1086/375662. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Demas GE, Nelson RJ. Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus) Endocrinology. 2001;142:2768–75. doi: 10.1210/endo.142.7.8271. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Kriegsfeld LJ, Schneider JE, Nelson RJ. Leptin, but not immune function, is linked to reproductive responsiveness to photoperiod. Am J Physiol- Regul Integr Comp Physiol. 2000;278:R1401–7. doi: 10.1152/ajpregu.2000.278.6.R1401. [DOI] [PubMed] [Google Scholar]
- Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav. 2001;74:703–8. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Leptin: Nourishment for the immune system. Eur J Immunol. 2006;36:3101–4. doi: 10.1002/eji.200636770. [DOI] [PubMed] [Google Scholar]
- French SS, DeNardo DF, Moore MC. Trade-Offs between the reproductive and immune systems: Facultative responses to resources or obligate responses to reproduction? Am Nat. 2007;170:79–89. doi: 10.1086/518569. [DOI] [PubMed] [Google Scholar]
- French SS, DeNardo DF, Moore MC. Trade-offs between the reproductive and immune systems: facultative responses to resources or obligate responses to reproduction? Am Nat. 2007;170:79–89. doi: 10.1086/518569. [DOI] [PubMed] [Google Scholar]
- French SS, Greives TJ, Zysling DA, Chester EM, Demas GE. Leptin increases maternal investment. Proc R Soc Lond Ser B-Biol Sci. 2009;276:4003–11. doi: 10.1098/rspb.2009.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SS, Johnston GIH, Moore MC. Immune activity suppresses reproduction in food-limited female tree lizards (Urosaurus ornatus) Funct Ecol. 2007;21:1115–22. [Google Scholar]
- French SS, Matt KS, Moore MC. The effects of stress on wound healing in male tree lizards (Urosaurus ornatus) Gen Comp Endocrinol. 2006;145:128–32. doi: 10.1016/j.ygcen.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Gaspar-López E, Casabiell J, Estevez J, Landete-Castillejos T, De La Cruz L, Gallego L, García A. Seasonal changes in plasma leptin concentration related to antler cycle in Iberian red deer stags. J Comp Physiol (B) 2009;179:617–22. doi: 10.1007/s00360-009-0343-7. [DOI] [PubMed] [Google Scholar]
- Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC, Jr, Myers MG, Jr, Duggal P, Houpt ER, Petri WA. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 2010 doi: 10.1038/mi.2010.76. published online (doi: 10.1038/mi.2010.76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson P, Perkins S, Cattadori I. The emergence of wildlife disease and the application to ecology. In: Ostfeld R, Keesing F, Eviner V, editors. Infectious Disease Ecology. Princeton: Princeton University Press; 2008. pp. 347–67. [Google Scholar]
- Johnson RM, Johnson TM, Londraville RL. Evidence for leptin expression in fishes. J Exp Zool. 2000;286:718–24. doi: 10.1002/(sici)1097-010x(20000601)286:7<718::aid-jez6>3.0.co;2-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermack W, McKendrick A. A contribution to the mathematical theory of epidemics. Proc R Soc Lond Ser B-Biol Sci. 1927;115:700–21. [Google Scholar]
- Klasing K. Nutritional modulation of resistance to infectious diseases. Poult Sci. 1998;77:1119–25. doi: 10.1093/ps/77.8.1119. [DOI] [PubMed] [Google Scholar]
- Klasing KC. Nutrition and the immune system. Br Poult Sci. 2007;48:525–37. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Kordonowy LL, McMurtry JP, Williams TD. Variation in plasma leptin-like immunoreactivity in free-living European starlings (Sturnus vulgaris) Gen Comp Endocrinol. 2010;166:47–53. doi: 10.1016/j.ygcen.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Kuo AY, Cline MA, Werner E, Siegel PB, Denbow DM. Leptin effects on food and water intake in lines of chickens selected for high or low body weight. Physiol Behav. 2005;84:459–64. doi: 10.1016/j.physbeh.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Uji S, Suzuki T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides. 2005;26:745–50. doi: 10.1016/j.peptides.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Lin Y-H, Shiau S-Y. Dietary lipid requirement of grouper, Epinephelus malabaricus, and effects on immune responses. Aquaculture. 2003;225:243–50. [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Lõhmus M, Björklund M. Leptin affects life history decisions in a passerine bird: A Field Experiment. PLoS ONE. 2009;4:e4602. doi: 10.1371/journal.pone.0004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lõhmus M, Olin M, Sundström LF, Troedsson MHT, Molitor TW, El Halawani M. Leptin increases T-cell immune response in birds. Gen Comp Endocrinol. 2004;139:245–50. doi: 10.1016/j.ygcen.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Londraville RL, Duvall CS. Murine leptin injections increase intracellular fatty acid-binding protein in green sunfish (Lepomis cyanellus) Gen Comp Endocrinol. 2002;129:56–62. doi: 10.1016/s0016-6480(02)00510-5. [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes. 2002;26:1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. 1996;71:511–48. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ. Seasonal patterns of stress, immune function, and disease. New York: Cambridge University Press; 2002. [Google Scholar]
- Niewiarowski PH, Balk ML, Londraville RL. Phenotypic effects of leptin in an ectotherm: A new tool to study the evolution of life histories and endothermy? J Exp Biol. 2000;203:295–300. doi: 10.1242/jeb.203.2.295. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Granbom M, RÂberg L. Does the strength of an immune response reflect its energetic cost? J Avian Biol. 2007;38:488–94. [Google Scholar]
- Norgan NG. The beneficial effects of body fat and adipose tissue in humans. Int J Obes Relat Metab Disord. 1997;21:738–46. doi: 10.1038/sj.ijo.0800473. [DOI] [PubMed] [Google Scholar]
- Norris K, Evans MR. Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol. 2000;11:19–26. [Google Scholar]
- Ots I, Kerimov AB, Ivankina EV, Ilyina TA, Horak P. Immune challenge affects basal metabolic activity in wintering great tits. Proc R Soc Lond Ser B-Biol Sci. 2001;268:1175–81. doi: 10.1098/rspb.2001.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos MCF, Vicente LL, Lisboa PC, de Moura EG. Absence of anorectic effect to acute peripheral leptin treatment in adult rats whose mothers were malnourished during lactation. Horm Metab Res. 2004;36:625–9. doi: 10.1055/s-2004-825927. [DOI] [PubMed] [Google Scholar]
- Pond CM. Interactions between adipose tissue and the immune system. Proc Nutr Soc. 1996;55:111–26. doi: 10.1079/pns19960014. [DOI] [PubMed] [Google Scholar]
- Putti R, Varricchio E, Gay F, Elena C, Paolucci M. Leptin effects on testis and epididymis in the lizard Podarcis sicula, during summer regression. Gen Comp Endocrinol. 2009;160:168–75. doi: 10.1016/j.ygcen.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med. 2001;79:8–20. doi: 10.1007/s001090100198. [DOI] [PubMed] [Google Scholar]
- Rousseau K, Atcha Z, Cagampang FRA, Le Rouzic P, Stirland JA, Ivanov TR, Ebling FJP, Klingenspor M, Loudon ASI. Photoperiodic regulation of leptin resistance in the seasonally breeding siberian hamster (Phodopus sungorus) Endocrinology. 2002;143:3083–95. doi: 10.1210/endo.143.8.8967. [DOI] [PubMed] [Google Scholar]
- Sciarrillo R, Virgilio F, De Falco M, Laforgia V, Varano L, Paolucci M. Localization and role of leptin in the thyroid gland of the lizard Podarcis sicula (Reptilia, Lacertidae) J Exp Zool Part A. 2005;303A:628–34. doi: 10.1002/jez.a.196. [DOI] [PubMed] [Google Scholar]
- Sharp PJ, Dunn IC, Waddington D, Boswell T. Chicken leptin. Gen Comp Endocrinol. 2008;158:2–4. doi: 10.1016/j.ygcen.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–21. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Sherman E, Baldwin L, Fernandez G, Deurell E. Fever and thermal tolerance in the toad Bufo marinus. J Therm Biol. 1991;16:297–301. [Google Scholar]
- Simon J, Rideau N, Taouis M. Reply to viewpoints by PJ Sharp, IC Dunn, D Waddington and T Boswell [Chicken Leptin. General and Comparative Endocrinology, 158, 2–4 (2008)] Gen Comp Endocrinol. 2009;161:159. doi: 10.1016/s0016-6480(09)00093-8. [DOI] [PubMed] [Google Scholar]
- Spurlock ME, Frank GR, Willis GM, Kuske JL, Cornelius SG. Effect of dietary energy source and immunological challenge on growth performance and immunological variables in growing pigs. J Anim Sci. 1997;75:720–6. doi: 10.2527/1997.753720x. [DOI] [PubMed] [Google Scholar]
- Svensson E, Raberg L, Koch C, Hasselquist D. Energetic stress, immunosuppression and the costs of an antibody response. Funct Ecol. 1998;12:912–9. [Google Scholar]
- Taouis M, Chen J, Daviaud C, Dupont J, Derouet M, Simon J. Cloning the chicken leptin gene. Gene. 1998;208:239–42. doi: 10.1016/s0378-1119(97)00670-7. [DOI] [PubMed] [Google Scholar]
- Valderrabano J, Gomez-Rincon C, Uriarte J. Effect of nutritional status and fat reserves on the periparturient immune response to Haemonchus contortus infection in sheep. Vet Parasitol. 2006;141:122–31. doi: 10.1016/j.vetpar.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Volkoff H, Canosa LF, Unniappan S, Cerdá-Reverter JM, Bernier NJ, Kelly SP, Peter RE. Neuropeptides and the control of food intake in fish. Gen Comp Endocrinol. 2005;142:3–19. doi: 10.1016/j.ygcen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Volkoff H, Joy Eykelbosh A, Ector Peter R. Role of leptin in the control of feeding of goldfish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res. 2003;972:90–109. doi: 10.1016/s0006-8993(03)02507-1. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–83. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- Zaralis K, Tolkamp BJ, Houdijk JGM, Wylie ARG, Kyriazakis I. Changes in food intake and circulating leptin due to gastrointestinal parasitism in lambs of two breeds. J Anim Sci. 2008;86:1891–903. doi: 10.2527/jas.2007-0698. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zieba DA, Amstalden M, Williams GL. Regulatory roles of leptin in reproduction and metabolism: a comparative review. Domest Anim Endocrinol. 2005;29:166–85. doi: 10.1016/j.domaniend.2005.02.019. [DOI] [PubMed] [Google Scholar]


