Abstract
Vaccination with MHC-II-restricted peptides from Apolipoprotein B (ApoB) with complete and incomplete Freund’s adjuvant (CFA/IFA) is known to protect mice from atherosclerosis. This vaccination induces antigen-specific IgG1 and IgG2c antibody responses and a robust CD4 T cell response in lymph nodes. However, CFA/IFA cannot be used in humans. To find a clinically applicable adjuvant, we tested the effect of vaccinating Apoe-deficient mice with ApoB peptide P6 (TGAYSNASSTESASY). In a broad screening experiment, Addavax, a squalene oil similar to MF59, was the only adjuvant that showed similar efficacy as CFA/IFA. This was confirmed in a confirmation experiment for both the aortic arch and whole aorta analyzed by en face analysis after atherosclerotic lesion staining. Mechanistically, restimulated peritoneal cells from mice immunized with P6 in Addavax released significant amounts of IL-10. Unlike P6 in CFA/IFA, vaccination with P6 in Addavax did not induce any detectable IgG1 or IgG2c antibodies to P6. These data suggest that squalene-based adjuvants such as MF59 are good candidate adjuvants for developing a clinically effective atherosclerosis vaccine.
Keywords: Adjuvant, Vaccine, Atherosclerosis, Apolipoprotein B-100, Self-antigen
Introduction
Atherosclerosis is an inflammatory disease of the arterial wall. Its sequelae include myocardial infarction, stroke, and peripheral artery disease [1]. These diseases are devastating, and cause disability or death in a very large number of patients each year. All together, the atherosclerosis-dependent diseases are the #1 killer worldwide [Centers for Disease Control and Prevention (CDC)] [2].
Atherosclerosis is associated with elevated levels of low-density lipoprotein (LDL) cholesterol, and is accompanied by an autoimmune response to Apolipoprotein B (ApoB), the core protein of LDL [3]. Current atherosclerosis medications like statins [4] and Proprotein convertase subtilisin/kexin type (PCSK9) inhibitors [5, 6] target the atherosclerosis-associated risk factors LDL and total cholesterol, but not the underlying autoimmune response. Therefore, the development of an effective atherosclerosis vaccine is an alluring medical technology that has broad potential to prevent atherosclerosis and atherosclerosis-dependent diseases.
Many prior vaccination studies have been performed using mouse models of atherosclerosis [7, 8], such as Apoe- and Ldlr-deficient mice. These studies discovered candidate vaccine antigens that induced atheroprotective responses. We showed that atheroprotection induced by vaccination with major histocompatibility complex (MHC) class II-restricted peptides of ApoB-100 induced mRNA for the anti-inflammatory cytokine interleukin 10 (IL-10) in the mouse aorta [9]. IL-10 is known to be anti-atherogenic [10, 11]. Recently, we showed that a similar vaccination scheme using three different MHC-II-restricted ApoB peptides induced robust production of IL-10 in peritoneal CD4 T cells [12]. Similar to other studies [13, 14], we also see induction of regulatory T cell (Treg) responses. Tregs produce several anti-inflammatory cytokines including TGF-β, IL-10 and IL-35 [15] and are known to protect against atherosclerosis [16].
In vaccine development, many different adjuvants are used. Clinically, the most common adjuvants are based on aluminum salts that work by activating the inflammasome [17] and releasing double-stranded DNA from dying cells [18]. Aluminum salts have been used as a vaccine adjuvant for more than 80 years. Recently, a squalene-based adjuvant and monophosphoryl lipid A were approved in pandemic influenza virus vaccines [19, 20] and human papilloma virus vaccines for cervical cancer [21]. In addition, several candidate vaccine adjuvants, such as Toll-like receptor ligands and STING ligands, have been tested in preclinical models of infectious diseases and in clinical trials. Many experimental atherosclerosis vaccines are administered in complete and incomplete Freund’s adjuvants (CFA and IFA) [9, 12, 22–25]. CFA is not used clinically, because it induces severe granuloma formation. Other experimental adjuvants including CpG oligodeoxynucleotide (ODN) and cholera toxin B subunit were also tested for atherosclerosis vaccines [13, 26–28].
The present study was undertaken to find a vaccine adjuvant that can be developed clinically as an adjuvant for an atherosclerosis vaccine. All studies were conducted with the same mouse ApoB peptide, P6 (TGAYSNASSTESASY). First, we screened four different adjuvants, Hydroxypropyl β-cyclodextrin (HP-β-CD), CpG ODN, Addavax and CFA/IFA (positive control). HP-β-CD is known as an excipient for hydrophobic agents and has been proposed as an influenza vaccine adjuvant [29]. CpG ODN was recently approved as a hepatitis B virus vaccine adjuvant. Addavax is a squalene-based adjuvant similar to MF59, which is FDA-approved and licensed in Europe for a seasonal influenza vaccine for elderly people [30]. Here, we report that vaccinating Apoe-deficient (Apoe−/−) mice with ApoB P6 in Addavax was as atheroprotective as P6 in CFA/IFA.
Results
Squalene-based adjuvant induces atheroprotection
To compare the efficacy of adjuvants as part of an atherosclerosis vaccine, we used the known immunogenic MHC class II-restricted ApoB-100 peptide, ApoB978–993 (referred to in this study as P6) as a vaccine antigen. As a positive control, five week-old female Apoe−/− mice were subcutaneously immunized with P6 in CFA at week 0, followed by intraperitoneal immunization with P6 in IFA at weeks 2, 4, 6, 8, and 10 (Fig. 1A). This was similar to a vaccination scheme we used in previous experiments [9]. As a negative control, female Apoe−/− mice were immunized subcutaneously with P6 alone without adjuvant. Other groups were immunized with P6 in Addavax, HP-β-CD or CpG-ODN, all injected subcutaneously at weeks 0, 2, 4, 6, 8, and 10. Western diet (WD) was started 2 weeks after the first immunization and maintained for 10 weeks. Aortas, peritoneal cells (PECs), lymph nodes (LNs), spleen, and blood were harvested 2 weeks after the last immunization.
Figure 1. Screening of vaccine adjuvant.
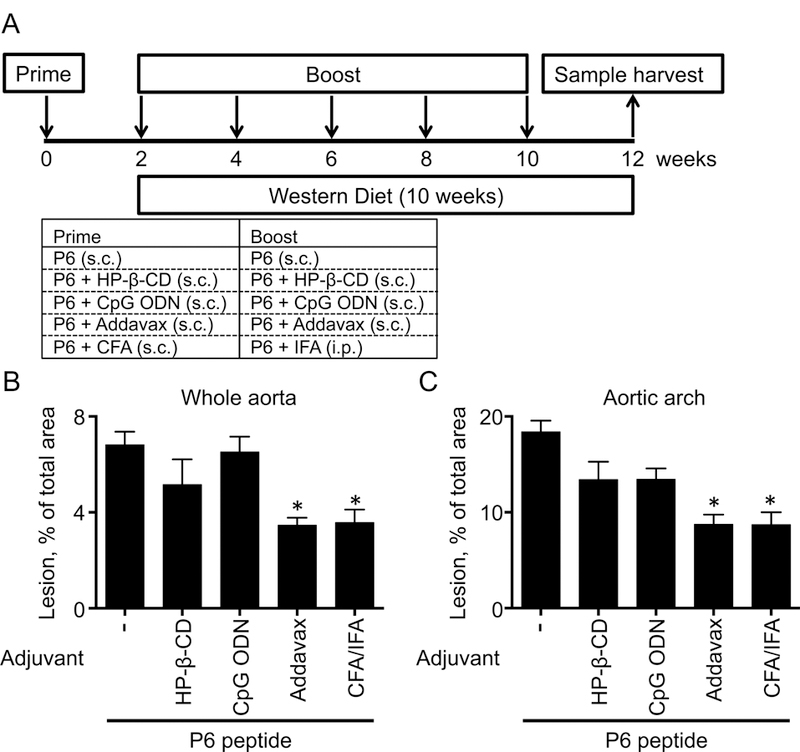
(A) Immunization schedule. Five week-old female Apoe−/− mice which were subcutaneously (s.c.) immunized with either P6 (10 μg), P6 (10 μg) + HP-β-CD (30%), P6 (10 μg) + CpG ODN (10 μg), or P6 (10 μg) + Addavax (50%) at week 0, 2, 4, 6, 8, 10. In the CFA/IFA group, five week-old Apoe−/− mice were subcutaneously immunized with P6 (50 μg) + CFA (50%) at week 0 and intraperitoneally (i.p.) immunized with P6 + IFA (50%) at 2, 4, 6, 8, and 10. WD was started 2 weeks after the first immunization (prime) and maintained for 10 weeks (during the four boost injections). Aortas, peritoneal cells, lymph nodes, spleen, and blood were harvested at 2 weeks after the last immunization. (B and C) Aortas were harvested, fixed, stained with Sudan IV, microdissected, and mounted for en face lesion measurement. Lesion area is expressed as % of total area in the whole aorta (B) or aortic arch (C). Data from single experiment from 4–5 mice per group, mean + SEM, * p<0.05 by Mann-Whitney test (vs P6 alone group).
We first assessed atherosclerotic lesion size by en-face analysis of pinned and Sudan-IV-stained aortas (Fig. 1B and 1C). The lesion area was 18% of the total area in the aortic arches of mice immunized with P6 alone. As expected, lesion area was significantly reduced by ~50% when mice were immunized with P6 in CFA/IFA. A similar reduction was observed in mice immunized with P6 in Addavax (Fig. 1B and 1C). CpG ODN and HP-β-CD were ineffective (Fig. 1B and 1C). This screening study was not powered to show statistical significance with multiple comparisons (5 groups), but suggested that Addavax might be an effective adjuvant in this setting.
Confirmation experiment
To rigorously test whether Addavax is a suitable adjuvant for an atherosclerosis vaccine, we tested Addavax against positive and negative controls. Since adjuvants can have effects on atherosclerosis even when injected without antigen [31, 32], we included a group with Addavax only (Fig. 2A). Statistical power was improved by using 9–15 mice per group for a total of 34 Apoe−/− mice. The immunization scheme was the same as in Fig. 1A (one prime, five boosts over 10 weeks). Immunization with P6 in Addavax reduced atherosclerotic lesion size in both the aortic arch and the whole aorta (Fig. 2B). Lesion size in the whole aorta was significantly reduced by 52% in mice vaccinated with P6 in Addavax (Fig. 2C) compared to the negative control (P6 alone) alone. This reduction was similar to that seen in mice vaccinated with P6 in CFA/IFA. Addavax alone (no peptide) had no effect. A similar result was obtained in the aortic arch, where lesion size was significantly reduced by 57%.
Figure 2. Addavax is as atherosclerosis vaccine adjuvant.
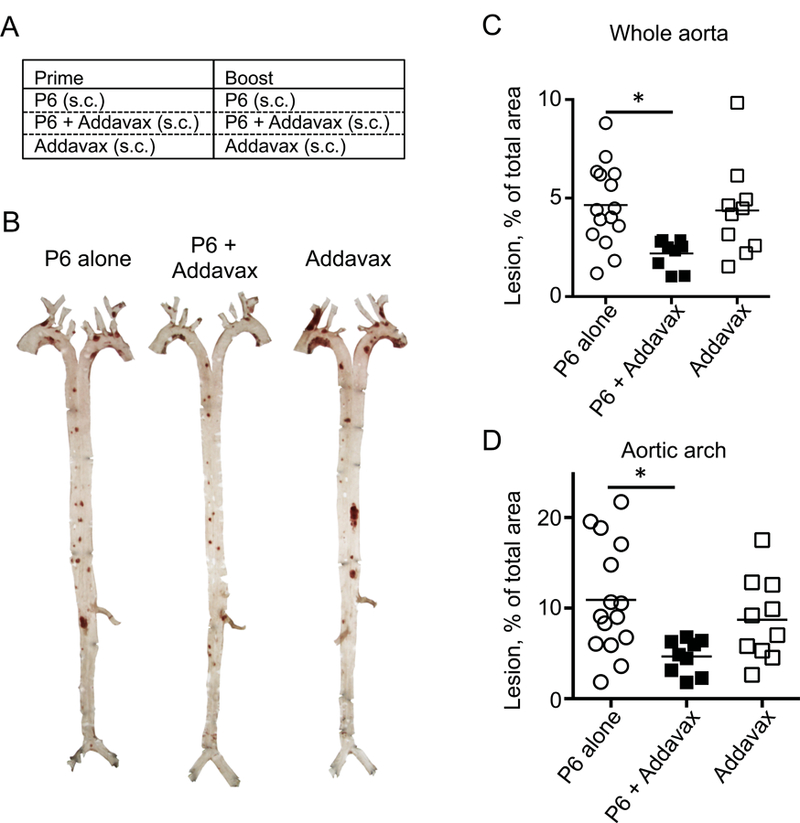
(A) Five week-old female Apoe−/− mice were immunized with either P6 (10 μg), Addavax alone (50%), or P6 (10 μg) + Addavax (50%) Immunization schedule same as in Figure 1A. (B-D) Aortas were harvested, fixed, stained with Sudan IV, microdissected, and mounted for en face lesion measurement. (B) Representative aorta microphotograph of each immunization group. Lesion area is expressed as % of total area in the whole aorta (C) or aortic arch (D). N=9–15 mice per group, mean + SEM, * p<0.05 by ANOVA followed by Dunn’s multiple comparisons test. Data are pooled from two independent experiments.
To test the long-term efficacy of vaccination with P6 peptide in Addavax, female Apoe−/− mice were immunized subcutaneously with P6 alone, P6 in Addavax, or Addavax alone at weeks 0, 2, 4, 6, 8,10, 12, 16, 20, 24, and 28. WD was started 2 weeks after the first immunization and maintained for 30 weeks until week 32. At 32 weeks, immunization with P6 in Addavax had lost its effect (Supporting Figure 1).
Restimulation of lymphocytes from P6-immunized mice induces secretion of IL-10
Atheroprotection by vaccination with MHC-II-restricted ApoB peptides is associated with induction of Tregs and anti-inflammatory IL-10 [12]. IL-2 is the main Treg-sustaining cytokine [33]. Therefore, we measured IL-10 and IL-2 by cytometric bead array (CBA) in PECs, LNs and splenocytes after restimulation with the immunogenic peptide (P6) in vitro. In P6 in Addavax-vaccinated mice, but not in mice receiving Addavax adjuvant only, restimulated PECs secreted considerable amounts of IL-10 (~150 pg/ml, Fig. 3A). However, whole LN cells failed to produce IL-10, and splenocytes secreted minor amounts of IL-10 (~50 pg/ml). Vaccination with P6 alone (no adjuvant) was sufficient to induce detectable but low (~50 pg/ml) secretion of IL-10 in PECs and splenocytes. The percentage of IL-10+ cells among bulk CD4+ T cells was not changed by vaccination with P6 in Addavax or CFA/IFA (Supporting Fig. 2).
Figure 3. Addavax boosts IL-10 production upon restimulation.
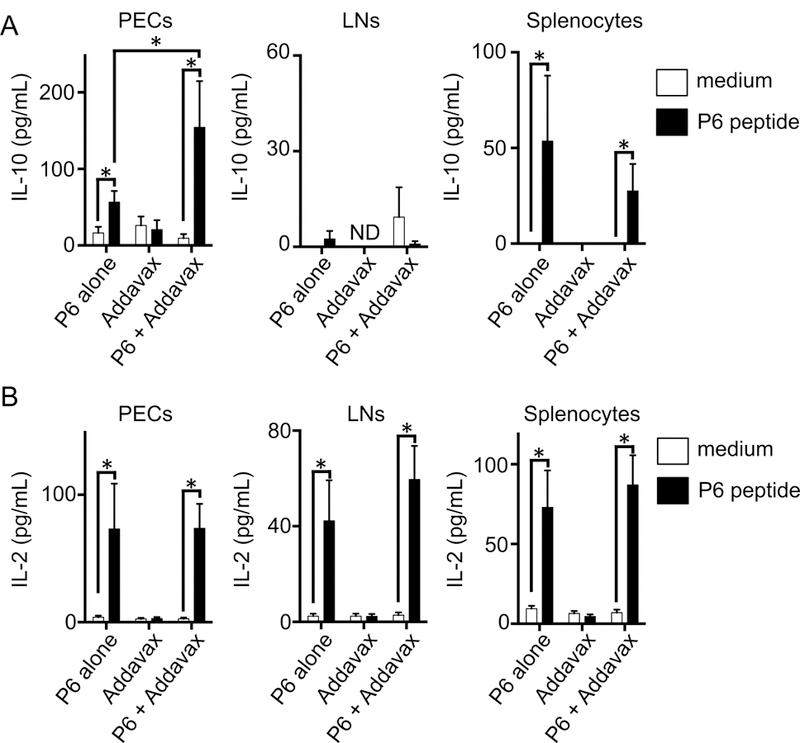
(A and B) Peritoneal cells (PECs), lymph nodes (LNs), and spleen were collected from immunized mice in Figure 2. The cells were stimulated with medium or P6 peptide (10μg/mL) for 48 hours. IL-10 (A) and IL-2 (B) concentrations were measured by cytometric bead array. N=9–11 mice per group, mean + SEM, * p<0.05 by Mann-Whitney test. Data represent one of two independent experiments with similar results.
IL-2 secretion was also induced by restimulation of PECs, LN cells and splenocytes from P6-vaccinated, but not adjuvant only control mice (Fig. 3B). Like IL-10 production, IL-2 secretion was strictly dependent on restimulation with P6. Interestingly, the amount of IL-2 secreted into the supernatant was the same whether the mice had been vaccinated with P6 in Addavax or with P6 alone. Since P6 alone is not atheroprotective, we suspect that the induction of IL-2 is not sufficient for atheroprotection.
Addavax does not induce antibodies against vaccine antigen peptide
It is known that vaccination with P6 or similar ApoB peptides in CFA/IFA induces a strong antigen peptide-specific antibody response (IgG1 and IgG2c) [9, 12]. Apoe−/− mice immunized with 10 μg P6 in CFA/IFA, but not with P6 alone, developed peptide-specific IgG1 (Fig. 4A). IgG2c antibody titers were not detectable. When immunized with a higher dose of antigenic peptide (50 μg), vaccination in CFA/IFA induced both P6-specific IgG1 and IgG2c (Fig. 4B), confirming previous results obtained with a slightly different vaccination scheme [9]. Surprisingly, the mice vaccinated with P6 in Addavax failed to elicit a P6-specific antibody response (Fig. 4). Since vaccination with P6 in Addavax is atheroprotective (Fig. 2), we conclude that a peptide-specific IgG response is not necessary to confer atheroprotection by vaccination with ApoB peptides.
Figure 4. Antigen-specific antibody responses are not required for atheroprotection.
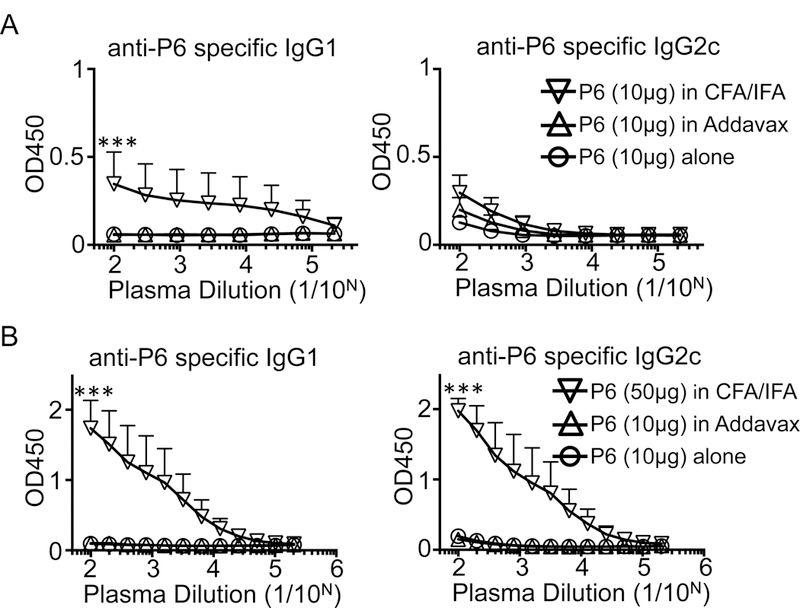
Peptide P6 was coated on 96-well plates, overlaid with diluted plasma, developed with HRP-labeled goat-anti-mouse IgG1 or IgG2c, and developed for ELISA. The plasma samples were collected from immunized mice in Figure 2 (A) or Figure 1 (B). N= 4–11 mice per group, mean + SEM, *** p<0.001 by ANOVA followed by Tukey’s multiple comparisons test. Data represent one of two independent experiments with similar results.
Discussion
Vaccination is one the most successful medical interventions. At least 27 diseases are preventable by currently available and FDA-approved vaccines. All existing vaccines target infectious and pathogen-induced diseases. As the vaccine development field is moving from empirical to systematic approaches, and new epitopes are identified that induce strong CD4 and CD8 T cell responses [34], vaccines are being developed for non-communicable diseases such as cancer [35], atherosclerosis [7], hypertension [36], Alzheimer’s disease [37], and diabetes [38]. Most recently, there has been increasing recognition of the importance of CD4 T cell responses in vaccines against non-communicable diseases such as cancer [39].
Similar to traditional vaccines against pathogens, non-communicable disease vaccines need to induce and modulate the human immune system. One of the major differences between traditional and non-communicable disease vaccines is the origin of the vaccine antigen. Traditional vaccine antigens are derived from the pathogen and elicit pathogen-specific immune responses, while vaccine antigens modulating non-communicable diseases are self-antigens [40]. Some non-communicable diseases vaccines including atherosclerosis vaccines may induce antigen-specific immune tolerance.
The theoretical basis for vaccines to MHC-II-restricted self-peptides is based on the observation that negative selection is incomplete [41, 42] and autoimmunity is controlled by peripheral tolerance [43, 44]. Negative selection in the thymus is so ineffective that most CD8 T cells recognizing self-peptides in MHC-I are not eliminated [45]. Instead, autoimmunity is largely prevented by Tregs and other regulatory T cells like Tr1 cells [46, 47]. These findings suggest that inducing antigen-specific Treg and Tr1 responses by vaccination with self-peptides can strengthen peripheral tolerance and exert beneficial effects.
Our group and others have already discovered vaccine antigen and epitope candidates for atherosclerosis vaccines [9, 48, 49]. Immunization with P6 was associated with increased mRNA for IL-10 in mouse aortas [9], and vaccination with three other ApoB peptides, p101, 102 and 103, induced IL-10-producing CD4 T cells in the peritoneal cavity of vaccinated mice [12]. In addition, we recently found that antigen-specific CD4+ T cells in the peritoneal cavity were increased and the majority of these antigen-specific CD4+ T cells were Tregs after immunization with another ApoB-derived peptide (P18) [50]. These results support our finding that the peritoneal cavity is a relevant location where vaccination-induced atheroprotective CD4 T cells reside.
To induce effective antigen-specific immune responses against self-antigens, self-peptides must be combined with vaccine adjuvants that can effectively trigger innate immune responses. Indeed, almost all licensed vaccines contain adjuvants, either endogenously (as part of the killed or attenuated pathogen) or exogenously. Therefore, optimization of vaccine formulation and especially vaccine adjuvants is essential for clinical vaccine development.
Previously, most atherosclerosis vaccine studies used aluminum salt adjuvant or Freund’s adjuvant to enhance vaccine efficacy. However, aluminum salt and CFA/IFA have a strong antigen-independent anti-atherosclerotic effect that can overshadow antigen-specific effects [32, 51], calling into question the specificity of the vaccination. Here, we show that mice vaccinated mice with the ApoB peptide P6 in Addavax are as effectively protected from atherosclerosis as mice vaccinated with P6 in CFA/IFA (Fig. 1 and 2). In addition, Addavax alone or P6 peptide alone did not induce atheroprotective responses (Fig. 2 and Supporting Figure 3). These results suggest that both antigen and Addavax administration is required to exert vaccine efficacy.
To begin to address long-term vaccine efficacy using P6 in Addavax immunization, we tested a longer period (WD for 30 weeks). After P6 in Addavax immunization, we could not see significant differences in atherosclerotic lesions compared with P6 alone and Addavax alone (Supporting Figure 1). In this experiment, the mice had around 30% atherosclerotic lesions in the whole aorta. We think that 30 weeks WD feeding induced an overwhelming atherosclerosis burden caused by a clinically unrealistic total cholesterol of more than 1000 mg/dl.
An effective atherosclerosis vaccine is thought to induce anti-inflammatory responses such as Tregs and IL-10 production. Indeed, P6 in Addavax significantly induced IL-10 production from peritoneal cells after restimulation by P6 peptide (Fig. 3). Interestingly, P6 in Addavax did not induce IFN-γ or IL-17A production, known pro-atherogenic cytokines [52, 53] (data not shown). These results strongly suggest that Addavax has potential to induce antigen-specific tolerance by mechanisms including antigen-specific IL-10 production without undesirable pro-atherogenic inflammatory responses that have been observed with other vaccination schemes and adoptive transfers [54]. The present study adds further support to the hypothesis that vaccination-induced antigen-specific IL-10 production may contribute to atheroprotection.
We also show that unlike P6 in CFA/IFA, P6 in Addavax does not induce a P6-specific IgG antibody response (Fig. 4). Based on multiple animal studies suggesting that antibodies to ApoB might be atheroprotective [22, 55], a clinical trial [56] (GLACIER, clinicaltrials.gov #NCT01258907) was initiated in which a monoclonal antibody to oxidized LDL was given. Atherosclerosis was assessed by PET imaging after injection of FDG glucose, a marker for myeloid cell accumulation. This trial showed no evidence of efficacy. Our data and the results of this clinical trial are consistent with the hypothesis that atheroprotection in response to vaccination is not mediated by antigen-specific antibodies.
Squalene-based adjuvant such as MF59 and AS03 have been already approved as influenza vaccine adjuvants. MF59 induces ATP release from cells at the injection site [57]. Indeed, non-hydrolysable ATP can act as a vaccine adjuvant, resulting in enhancement of antigen-specific antibody responses [58]. ATP may also activate Tregs and enhance their suppressive ability [59], although other studies showed that ATP can inhibit the suppressive ability and stability of Tregs [60]. More recently, Maji et al., showed that released ATP from apoptotic Tregs is converted to immunosuppressive adenosine [61]. These observations support that ATP release by Addavax administration might contribute to the mechanisms of atheroprotective immune responses.
In addition to discovering that Addavax (similar to MF59) is an effective adjuvant for an MHC-II restricted ApoB peptide vaccine against atherosclerosis, we found that an IgG antibody response is not required for atheroprotection. The present study does not fully address the mechanism of vaccine-induced atheroprotection, but suggests that antigen-specific CD4 T cell responses are involved. The identification of Addavax as a benign and clinically translatable adjuvant is an important step in moving atherosclerosis vaccination development forward.
Materials and Methods
Mice
Five-weeks old Apoe-deficient female mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were maintained under specific pathogen-free conditions. All animal studies were approved by the local Institutional Animal Care and Use Committee.
Reagents
ApoB978–993 (P6): TGAYSNASSTESASY peptide was purchased from A&A Labs (San Diego, CA). CFA and IFA were purchased from SIGMA (St. Louis, MO). CpG ODN (ODN 2006) and Addavax were purchased from Invivogen (San Diego, CA). HRP-labeled goat anti-mouse IgG1 and IgG2c were purchased from SouthernBiotech (Brimingham, AL). Substrate buffer (TMB Microwell Peroxidase Substrate Systems) for ELISA was purchased from KPL (Gaithersburg, MD)
Immunization
Apoe-deficient mice were subcutaneously immunized with P6 peptide (10 μg or 50 μg) in CFA (50%) at week 0 and then intraperitoneally immunized with P6 peptide (10 μg or 50 μg) in IFA (50%) at week 2, 4, 6, 8, and 10. For other groups, the mice were subcutaneously immunized with P6 peptide (10 μg) only, P6 peptide (10 μg) with HP-β-CD (30%), P6 peptide (10 μg) with CpG ODN (10 μg), or P6 peptide (10 μg) in Addavax (50%) at week 0, 2, 4, 6, 8, and 10. Western diet started from week 2 for 10 weeks. Two weeks after final immunization, peritoneal cells, lymph nodes, aorta, spleen, and blood were collected.
Atherosclerosis quantification
To measure en face lesion area in aorta, the whole aorta was collected from immunized mice and then incubated in 4% paraformaldehyde/PBS. Atherosclerotic plaques were stained by Sudan IV. Quantification of lesion area in whole aorta and aortic arch was performed with ImagePro software.
Cytometric Bead Array (CBA) for antigen-specific cytokine production
Single cell suspension of PECs, LNs, and spleen were plated at a concentration of 1×107 cells/ml. Cells were stimulated with P6 peptide (10 μg/ml) for 48 h. Supernatants were subjected to CBA (BD Biosciences; San Diego, CA) for IL-10 and IL-2.
Intracellular staining assay for cytokines
Single cell suspension of PECs and LNs were stained with anti-CD45 (30-F11: eBioscience; San Diego, CA), anti-CD4 (RM4–5: Biolegend; San Diego, CA), and anti-TCRβ (H57–597: eBioscience) antibodies. Subsequently, the cells were stimulated with cell stimulation cocktail (eBioscience) and monensin (eBioscience) for 5 hours. After stimulation, the cells were stained with Ghost Dye UV450 (TONBO) to identify dead cells. After fixation and permeabilization by IC Fixation Buffer (eBioscience), intracellular cytokines were stained by anti-IFN-γ (XMG1.2: Biolegend), and anti-IL-10 (JES5–16E3: eBioscience) antibodies. Cytokine+ CD4+ T cells numbers were analyzed by flow cytometry.
ELISA
P6 peptide-specific antibody titers were determined by ELISA. In brief, flat-bottom high binding 96 well plates were coated with P6 peptide (10 μg/mL) in bicarbonate buffer at 4 °C. Plates were blocked with PBS containing 1% BSA for 60 min at room temperature (RT). After three times washing with PBST, plates were incubated with diluted plasma at RT for 120 min. Plates were washed three times with PBST and incubated with HRP-labeled goat anti-mouse IgG1 or IgG2c at RT for 90 min. After three times washing with PBST, substrate buffer was added and incubated at RT for 10 min. Subsequently, 1N H2SO4 was added to stop the colorimetric reaction. OD 450 nm was measured by spectrophotometer.
Statistical Analysis
Statistical significance (P < 0.05) between groups was determined using Mann–Whitney U test or ANOVA.
Supplementary Material
Acknowledgements
We thank members of K.L.’s laboratory for valuable comments and assistance. This work was supported by a grant from the National Institutes of Health R01 HL121697, P01 HL088093, and P01 HL136275 to K.L.
Abbreviations
- ApoB-100
Apolipoprotein B-100
- CFA
complete Freund’s adjuvant
- IFA
incomplete Freund’s adjuvant
- IFN
interferon
- FoxP3
forkhead box P3
- IL
interleukin
- LDL
low-density lipoprotein
- MHC
major histocompatibility complex
- PMA
phorbol 12-myristate 13-acetate
- Th
T helper type
- Treg
regulatory T cell
- HP-β-CD
hydroxypropyl-β-cyclodextrin
- CpG ODN
CpG oligodeoxynucleotide
- WD
western diet
Footnotes
Conflict of Interest Disclosures
None.
References
- 1.Gregersen I, Holm S, Dahl TB, Halvorsen B and Aukrust P, A focus on inflammation as a major risk factor for atherosclerotic cardiovascular diseases. Expert Rev Cardiovasc Ther 2016. 14: 391–403. [DOI] [PubMed] [Google Scholar]
- 2.Heron M, Deaths: Leading Causes for 2014. Natl Vital Stat Rep 2016. 65: 1–96. [PubMed] [Google Scholar]
- 3.Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY et al. , Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol 2003. 23: 872–878. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q and Liao JK, Pleiotropic effects of statins. - Basic research and clinical perspectives. Circ J 2010. 74: 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES et al. , Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015. 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 6.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM et al. , Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015. 372: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 7.Kimura T, Tse K, Sette A and Ley K, Vaccination to modulate atherosclerosis. Autoimmunity 2015. 48: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita T, Sasaki N, Kasahara K and Hirata K, Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol 2015. 66: 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Tse K, Gonen A, Sidney J, Ouyang H, Witztum JL, Sette A, Tse H et al. , Atheroprotective Vaccination with MHC-II Restricted Peptides from ApoB-100. Front Immunol 2013. 4: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, Curtiss LK et al. , Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ Res 2002. 90: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 11.Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, Groux H, Tedgui A et al. , Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 2004. 24: 1474–1478. [DOI] [PubMed] [Google Scholar]
- 12.Kimura T, Tse K, McArdle S, Gerhardt T, Miller J, Mikulski Z, Sidney J et al. , Atheroprotective vaccination with MHC-II-restricted ApoB peptides induces peritoneal IL-10-producing CD4 T cells. Am J Physiol Heart Circ Physiol 2017. 312: H781–H790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredrikson GN, Soderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK and Nilsson J, Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol 2003. 23: 879–884. [DOI] [PubMed] [Google Scholar]
- 14.Wigren M, Kolbus D, Duner P, Ljungcrantz I, Soderberg I, Bjorkbacka H, Fredrikson GN et al. , Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J Intern Med 2011. 269: 546–556. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Yamaguchi T, Nomura T and Ono M, Regulatory T cells and immune tolerance. Cell 2008. 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 16.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R et al. , Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 2006. 12: 178–180. [DOI] [PubMed] [Google Scholar]
- 17.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS and Flavell RA, Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008. 453: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P et al. , DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med 2011. 17: 996–1002. [DOI] [PubMed] [Google Scholar]
- 19.McElhaney JE, Beran J, Devaster JM, Esen M, Launay O, Leroux-Roels G, Ruiz-Palacios GM et al. , AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis 2013. 13: 485–496. [DOI] [PubMed] [Google Scholar]
- 20.Black S, Safety and effectiveness of MF-59 adjuvanted influenza vaccines in children and adults. Vaccine 2015. 33 Suppl 2: B3–5. [DOI] [PubMed] [Google Scholar]
- 21.McKeage K and Romanowski B, AS04-adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix(R)): a review of its use in the prevention of premalignant cervical lesions and cervical cancer causally related to certain oncogenic HPV types. Drugs 2011. 71: 465–488. [DOI] [PubMed] [Google Scholar]
- 22.Palinski W, Miller E and Witztum JL, Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A 1995. 92: 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freigang S, Horkko S, Miller E, Witztum JL and Palinski W, Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol 1998. 18: 1972–1982. [DOI] [PubMed] [Google Scholar]
- 24.Rittershaus CW, Miller DP, Thomas LJ, Picard MD, Honan CM, Emmett CD, Pettey CL et al. , Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol 2000. 20: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Caligiuri G, Hamsten A, Lefvert AK and Hansson GK, LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol 2001. 21: 108–114. [DOI] [PubMed] [Google Scholar]
- 26.Thomas LJ, Hammond RA, Forsberg EM, Geoghegan-Barek KM, Karalius BH, Marsh HC Jr. and Rittershaus CW, Co-administration of a CpG adjuvant (VaxImmune, CPG 7909) with CETP vaccines increased immunogenicity in rabbits and mice. Hum Vaccin 2009. 5: 79–84. [DOI] [PubMed] [Google Scholar]
- 27.Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, Ketelhuth DF et al. , Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol 2010. 30: 946–952. [DOI] [PubMed] [Google Scholar]
- 28.Salazar-Gonzalez JA, Rosales-Mendoza S, Romero-Maldonado A, Monreal-Escalante E, Uresti-Rivera EE and Banuelos-Hernandez B, Production of a plant-derived immunogenic protein targeting ApoB100 and CETP: toward a plant-based atherosclerosis vaccine. Mol Biotechnol 2014. 56: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 29.Onishi M, Ozasa K, Kobiyama K, Ohata K, Kitano M, Taniguchi K, Homma T et al. , Hydroxypropyl-beta-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J Immunol 2015. 194: 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai TF, Fluad(R)-MF59(R)-Adjuvanted Influenza Vaccine in Older Adults. Infect Chemother 2013. 45: 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Robertson AK, Rudling M, Parini P and Hansson GK, Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res 2005. 96: 427–434. [DOI] [PubMed] [Google Scholar]
- 32.Wigren M, Bengtsson D, Duner P, Olofsson K, Bjorkbacka H, Bengtsson E, Fredrikson GN et al. , Atheroprotective effects of Alum are associated with capture of oxidized LDL antigens and activation of regulatory T cells. Circ Res 2009. 104: e62–70. [DOI] [PubMed] [Google Scholar]
- 33.Setoguchi R, Hori S, Takahashi T and Sakaguchi S, Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 2005. 201: 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vita R, Overton JA, Greenbaum JA, Ponomarenko J, Clark JD, Cantrell JR, Wheeler DK et al. , The immune epitope database (IEDB) 3.0. Nucleic Acids Res 2015. 43: D405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N et al. , Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 2014. 11: 509–524. [DOI] [PubMed] [Google Scholar]
- 36.Ambuhl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, Nief V et al. , A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens 2007. 25: 63–72. [DOI] [PubMed] [Google Scholar]
- 37.Lambracht-Washington D and Rosenberg RN, Advances in the development of vaccines for Alzheimer’s disease. Discov Med 2013. 15: 319–326. [PMC free article] [PubMed] [Google Scholar]
- 38.Spohn G, Schori C, Keller I, Sladko K, Sina C, Guler R, Schwarz K et al. , Preclinical efficacy and safety of an anti-IL-1beta vaccine for the treatment of type 2 diabetes. Mol Ther Methods Clin Dev 2014. 1: 14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, Boegel S et al. , Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015. 520: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachmann MF and Whitehead P, Active immunotherapy for chronic diseases. Vaccine 2013. 31: 1777–1784. [DOI] [PubMed] [Google Scholar]
- 41.Robey E and Fowlkes BJ, Selective events in T cell development. Annu Rev Immunol 1994. 12: 675–705. [DOI] [PubMed] [Google Scholar]
- 42.Hogquist KA and Jameson SC, The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol 2014. 15: 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MG et al. , Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol 2005. 116: 1106–1115. [DOI] [PubMed] [Google Scholar]
- 44.Ochs HD, Gambineri E and Torgerson TR, IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res 2007. 38: 112–121. [DOI] [PubMed] [Google Scholar]
- 45.Yu W, Jiang N, Ebert PJ, Kidd BA, Muller S, Lund PJ, Juang J et al. , Clonal Deletion Prunes but Does Not Eliminate Self-Specific alphabeta CD8(+) T Lymphocytes. Immunity 2015. 42: 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckner JH, Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 2010. 10: 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pot C, Apetoh L and Kuchroo VK, Type 1 regulatory T cells (Tr1) in autoimmunity. Semin Immunol 2011. 23: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chyu KY, Zhao X, Reyes OS, Babbidge SM, Dimayuga PC, Yano J, Cercek B et al. , Immunization using an Apo B-100 related epitope reduces atherosclerosis and plaque inflammation in hypercholesterolemic apo E (−/−) mice. Biochem Biophys Res Commun 2005. 338: 1982–1989. [DOI] [PubMed] [Google Scholar]
- 49.Lu X, Xia M, Endresz V, Faludi I, Szabo A, Gonczol E, Mundkur L et al. , Impact of multiple antigenic epitopes from ApoB100, hHSP60 and Chlamydophila pneumoniae on atherosclerotic lesion development in Apob(tm2Sgy)Ldlr(tm1Her)J mice. Atherosclerosis 2012. 225: 56–68. [DOI] [PubMed] [Google Scholar]
- 50.Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D et al. , Regulatory CD4(+) T Cells Recognize MHC-II-Restricted Peptide Epitopes of Apolipoprotein B. Circulation 2018. [DOI] [PMC free article] [PubMed]
- 51.Khallou-Laschet J, Tupin E, Caligiuri G, Poirier B, Thieblemont N, Gaston AT, Vandaele M et al. , Atheroprotective effect of adjuvants in apolipoprotein E knockout mice. Atherosclerosis 2006. 184: 330–341. [DOI] [PubMed] [Google Scholar]
- 52.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR and Schindler C, IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest 1997. 99: 2752–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y et al. , Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol 2009. 183: 8167–8175. [DOI] [PubMed] [Google Scholar]
- 54.Shaw MK, Tse KY, Zhao X, Welch K, Eitzman DT, Thipparthi RR, Montgomery PC et al. , T-Cells Specific for a Self-Peptide of ApoB-100 Exacerbate Aortic Atheroma in Murine Atherosclerosis. Front Immunol 2017. 8: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, Chou MY, Pattison J et al. , Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol 2011. 58: 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehrer-Graiwer J, Singh P, Abdelbaky A, Vucic E, Korsgren M, Baruch A, Fredrickson J et al. , FDG-PET imaging for oxidized LDL in stable atherosclerotic disease: a phase II study of safety, tolerability, and anti-inflammatory activity. JACC Cardiovasc Imaging 2015. 8: 493–494. [DOI] [PubMed] [Google Scholar]
- 57.Vono M, Taccone M, Caccin P, Gallotta M, Donvito G, Falzoni S, Palmieri E et al. , The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci U S A 2013. 110: 21095–21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuo K, Nishiuma S, Hasegawa Y, Kawabata F, Kitahata K and Nakayama T, Vaccination with Antigen Combined with alphabeta-ATP as a Vaccine Adjuvant Enhances Antigen-Specific Antibody Production via Dendritic Cell Activation. Biol Pharm Bull 2016. 39: 1073–1076. [DOI] [PubMed] [Google Scholar]
- 59.Ring S, Enk AH and Mahnke K, ATP activates regulatory T Cells in vivo during contact hypersensitivity reactions. J Immunol 2010. 184: 3408–3416. [DOI] [PubMed] [Google Scholar]
- 60.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C et al. , ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal 2011. 4: ra12. [DOI] [PubMed] [Google Scholar]
- 61.Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L et al. , Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol 2017. 18: 1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


