Abstract
Olanzapine (OLZ) is an antipsychotic used in the treatment of schizophrenia, bipolar disorder, and treatment-resistant depression. Glucuronidation via the UDP-glucuronosyltransferase (UGT) family of enzymes is the major mode of OLZ metabolism and polymorphisms in these enzymes could contribute to inter-individual variability in OLZ metabolism and therapeutic response. In a screening of cell lines over-expressing individual UGTs, only UGTs 1A4 and 2B10 exhibited glucuronidation activity against OLZ. The UGT1A424Pro/48Val variant exhibited a 3.7-fold (p<0.0001) higher Vmax/KM for formation of the OLZ-10-N-glucuronide isomer 1 and a 4.3-fold (p<0.0001) higher Vmax/KM for formation of the OLZ-10-N-glucuronide isomer 2 than wild-type UGT1A424Pro/48Leu. The UGT2B1067Y variant exhibited no glucuronidation activity against OLZ. In a screening of 105 human liver microsomal (HLM) specimens, there was a 2.1- (p=0.04) and 1.6- (p=0.0017) fold increase in the rate of OLZ-10-N-glucuronide isomer 1 and OLZ-4’-N-glucuronide formation, and a 2.0-fold (p=0.02) increase in overall OLZ glucuronidation formation, in HLM with the UGT1A4 (*3/*3)/UGT2B10 (*1/*1) genotype compared to HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*1) genotype. There was a 1.9-fold (p<0.003) decrease in formation of both isomers of the OLZ-10-N-glucuronide, a 2.7-fold (p<0.0001) decrease in OLZ-4’-N-glucuronide formation, and a 2.1-fold (p=0.0002) decrease in overall OLZ-glucuronide formation in HLM with at least one UGT2B10*2 allele. In regression analysis, both the UGT1A4*3 (p<0.02) and UGT2B10*2 (p<0.002) alleles were significant predictors of formation of all OLZ-glucuronide isomers. These data suggest that the UGT1A4*3 and UGT2B10*2 alleles contribute significantly to inter-individual variability in OLZ metabolism.
Keywords: UDP-glucuronosyltransferases, UGT, olanzapine, variant alleles, UGT1A4, UGT2B10, metabolism
Introduction
Olanzapine (OLZ) is a second-generation antipsychotic that is FDA (U.S.)-approved for the treatment of schizophrenia (1), bipolar disorder (2, 3), and for treatment-resistant depression in combination with fluoxetine (1). OLZ improves negative symptoms such as conceptual organization, social interaction, and mood while decreasing positive symptoms such as delusions and paranoia (4) with a lower incidence of extrapyramidal side effects, less prolactin elevation, and improved patient compliance compared to first generation antipsychotics (5, 6). Although OLZ has a lower discontinuation rate and a greater reduction in psychopathology, (7, 8), it is associated with significant weight gain in patients (8–13), contributing to increased metabolic dysfunction, dyslipidemia, overweight/obesity, type II diabetes mellitus, heart disease, and mortality (14, 15).
There is significant inter-individual variability in OLZ clearance (16–21) and studies indicate clinical outcomes and plasma concentrations are related (17, 22). Severe weight gain and obesity are seen in a subset of patients taking OLZ (23) and the evidence suggests that there is a dose-response relationship between OLZ serum concentrations and metabolic outcomes (24–27). Acute OLZ administration results in lower basal and peak glucose levels and lower insulin response to oral glucose tolerance tests, while increasing circulating leptin (28). However, it is not clear if these mechanisms contribute to the obesity and diabetes seen in patients who take OLZ chronically.
OLZ is normally administered to patients at a dose of 8 – 64 µg a day and is excreted predominantly through the urine by the enzymatic addition of glucuronic acid by the UDP-glucuronosyltransferase (UGT) family of phase II metabolic enzymes (18, 29). Inhibition of OLZ glucuronidation in patients by co-administering OLZ with probenecid resulted in significantly elevated plasma levels of OLZ compared to OLZ administered alone (30). Published studies have shown that OLZ is extensively metabolized and the OLZ-10-N-glucuronide is the major metabolic product found in human plasma and urine, and the only metabolite identified in feces, accounting for 25% of the overall OLZ dose (18, 29, 31). Other OLZ glucuronide products have been reported in humans including the directly conjugated OLZ-4’-N-glucuronide (18, 29, 31). In addition to increasing hydrophobicity and excretion of OLZ and its metabolites, glucuronidation deactivates the compound as evidenced by decreased binding to known OLZ receptors (32). Previous in vitro studies using over-expressing baculosomes demonstrated that UGT1A4 is active against OLZ, with no activity observed for UGTs 1A1, 1A3, 1A4, 1A6, 1A9, 2B7. However, kinetic data for OLZ glucuronidation could not be established for human liver microsomes (HLM) using OLZ concentrations spanning the apparent KM of UGT1A4, suggesting that other untested UGT enzymes may also contribute to OLZ glucuronidation in vivo (33).
The goal of the present study was to characterize the glucuronidation activity of known UGT1A and 2B family enzymes against OLZ in vitro and examine the potential effects of prevalent SNPs in active UGTs on formation of individual OLZ glucuronide metabolites. The results of this study demonstrate that prevalent missense SNPs in UGTs 1A4 and 2B10 may be important in the overall metabolism of OLZ.
Materials and Methods
Chemicals and Materials.
OLZ was purchased from Toronto Chemicals (Toronto, Canada). Alamethicin, β-glucuronidase, bovine serum albumin, anti-ß-actin monoclonal antibody, and lamotrigine (LTG) were purchased from Sigma-Aldrich (St. Louis, MO). DMEM, Dulbecco’s PBS (minus calcium chloride and magnesium chloride), fetal bovine serum, penicillin-streptomycin, geneticin (G418), Platinum Pfx DNA polymerase, and the pcDNA3.1/V5-His-TOPO mammalian expression vector were all obtained from Invitrogen (Carlsbad, CA). The BCA protein assay kit was purchased from Pierce (Rockford, IL). The UGT2B7 microsomal standard (58 kDa) was purchased from BD Gentest (Woburn, MA). The goat anti-human UGT2B polyclonal antibody and the donkey anti-goat IgG antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PCR primers were purchased from Integrated DNA Technologies (Coralville, IA). All other chemicals were purchased from Fisher Scientific (Waltham, MA) unless specified otherwise.
Tissues.
The normal human liver tissue specimens used for these studies have been described previously (34, 35). Normal adjacent liver specimens were obtained from patients and quick-frozen at −80°C within 2 h post surgery. HLM were prepared through differential centrifugation as previously described (36) and stored (10–20 mg microsomal protein/mL) at −80°C. Microsomal protein concentrations were measured using the BCA assay. All protocols involving the analysis of tissue specimens were approved by the institutional review board at the Penn State College of Medicine and in accordance with assurances filed with and approved by the United States Department of Health and Human Services.
Cell lines.
The cell lines over-expressing the UGT1A and UGT2B isoforms used in this study were described previously (37–42). All UGT over-expressing cell lines were grown in DMEM to 80% confluence before preparing cell homogenates by re-suspending the cell pellet in Tris-buffered saline (25 mM Tris base, 138 mM NaCl, and 2.7 mM KCl, pH 7.4) and subjecting them to three rounds of freeze-thaw before gentle homogenization. Total homogenate protein concentrations were measured using the BCA protein assay. Homogenates were stored at −80°C in 200-μL aliquots to minimize freeze-thaws.
Western blot analysis.
UGT1A4 protein levels were determined by Western blot analysis for variant UGT1A4-overexpressing cell lines as described previously (41, 42). UGT2B10- and UGT2B10 variant -over-expressing cell homogenate protein (250–400 µg), HEK 293 homogenate protein (250 µg), and UGT2B7 standard (335 ng; BD Gentest, Woburn, MA) were adjusted to contain equal volumes of loading buffer and heated at 100°C for 10 min. Samples were run at 93 V on a 10% acrylamide gel then transferred to a polyvinylidene difluoride (PVDF) membrane for 2 h at 30 V. PVDF membranes were then probed with the goat polyclonal UGT2B antibody (1:500 dilution) for 1 h at 23°C, washed three times, followed by horseradish peroxidase-conjugated donkey anti-goat IgG (1:4500 dilution). UGT2B protein was visualized using the SuperSignal West Dura Extended Duration Substrate (ThermoScientific, Waltham, MA) and Hyblot CL autoradiography film (Denville Scientific, Metuchen, NJ).UGT2B10 protein levels were determined by Western blot analysis for wild-type and variant UGT2B10-overexpressing cell lines by densiometric analysis of X-ray film exposure (2–4min exposures) of Western blots using ImageJ software. After exposure, blots were stripped and re-probed using the β-actin antibody. Quantification of UGT2B10 was performed first by normalizing to the β-actin loading control, then by quantifying protein levels relative to known amounts of UGT2B7 loaded on the same gel.
UGT genotyping.
Genotyping for UGT1A4 and UGT2B10 has been previously described for all of the HLM specimens described in this study (34, 37, 38). Briefly, UGT1A4 genotypes were determined by direct sequencing of PCR-amplified PCR products from liver genomic DNA spanning both codons 24 and 48 for UGT1A4. The same primers were used for both PCR amplification and sequencing of UGT1A4: sense, 5’-GGCTTCTGCTGAGATGGCCAG-3’, and antisense, 5’-CCTTGAGTGTAGCCCAGCGT-3’, corresponding to nucleotides located –13 to +8 and +277 to +306, respectively, relative to the UGT1A4 translation start site (Genbank accession no. NM_007120). Sequencing was performed using an ABI 3130 Capillary Sequencer at the Functional Genomics Core Facility at the Penn State College of Medicine. Restriction fragment length polymorphism (RFLP) analysis of the UGT2B10 codon 67 polymorphism (SNP 199G>T) was performed to identify individuals with the UGT2B10 codon 67 polymorphism as described previously (37). PCR amplification was performed on genomic DNA using a sense primer (5’-AAGGATGGCTCTGAAATGGACTA-3’) and an antisense primer (5’-ATGAGTAGCCAGGACTGAAGCTGT-3’) corresponding to nucleotides −4 to +19 and +535 to +512, respectively, relative to the UGT2B10 translation start site. The 539 bp PCR product was subjected to digestion with HinfI (New England Biolabs) at 37°C for 3 h. An endogenous HinfI restriction enzyme site present within this PCR-amplified fragment acted as a control for enzyme digestion. A migration pattern of 426 and 113 bp is indicative of the polymorphic SNP199T variant, whereas a migration pattern of 222, 204, and 113 bp is indicative of the wild-type SNP199G.
Glucuronidation assays.
Homogenates or HLM were incubated with alamethicin (50 μg/mg protein) for 15 min on ice similar to that described previously (41, 43). Glucuronidation assays were performed in 50 mmol/L Tris buffer (pH 7.5), 10 mmol/L MgCl2, 4 mmol/L UDP-glucuronic acid (UDPGA), and 7.8 μmol/L to 4 mmol/L of substrate at 37°C in a water bath for 2 h. HLM (20 μg of protein) or human UGT-over-expressing cell homogenate (50 μg of protein) were screened for glucuronidation activity against OLZ using 50 µM of OLZ in a 10 or 50 μL reaction, respectively. Kinetic assays were performed in 50 μL reactions using a range of 9.4–2000 µM of OLZ, and 50 μg and 1.5 mg of UGT1A4- and UGT2B10-over-expressing cell homogenate protein, respectively, or 12.5 μg of HLM protein. To test the relative contribution of UGT1A4 in overall OLZ glucuronidation, five HLM exhibiting the UGT1A4(*1/*1)/UGT2B10 (*1/*1) genotype were randomly selected and co-treated with 300 µM OLZ and 2000 µM of the UGT1A4 inhibitor LTG. Reactions were terminated by the addition of the same volume of cold acetonitrile as the initial reaction volume. Reactions were centrifuged at 13,000 g for 10 min at 4°C and supernatants were collected. Glucuronidation assays (5 μL) were analyzed for OLZ glucuronide formation using a Waters ACQUITY ultra pressure liquid chromatography (UPLC) system (Milford, MA) as previously described (34, 44) using a 100 × 2.1 mm i.d. Acquity UPLC Beth C18 column with 1.7 μm particles (Waters) and a 0.2 μm prefilter installed before the column. Elution consisted of a gradient elution starting with 87.5% buffer B (100% acetonitrile) and 17.5% buffer A [5mmol/L ammonium acetate (pH 6.0)] for 5 min, a linear gradient to 90% buffer B over 1 min and held for 2 min, then a linear gradient back to initial conditions and held for 2 min for a total run time of 10 min. The flow rate was maintained at 0.5 mL/min. The amount of glucuronide formed was determined based on the ratio of OLZ-glucuronide versus unconjugated OLZ after calculating the area under the curve for the OLZ and OLZ-glucuronide peaks using the known amount of OLZ added to each reaction as the reference. OLZ-glucuronide was confirmed by sensitivity to treatment with 1,000 U β-glucuronidase at 37°C for 12–16h as previously described (35), by treatment with 3 N HCl at 50°C for 24 h (29), and by mass spectrometry (described below). As controls, glucuronidation assays were performed using HLM as a positive control for glucuronidation activity and untransfected HEK 293 cell homogenate protein as a negative control for glucuronidation activity. Four independent experiments were performed for kinetic analysis of UGT-over-expressing cell homogenates, with all assays within each experiment performed in duplicate; two independent experiments were performed for rate determination assays and three for kinetic analysis for HLM specimens.
Mass spectrometry.
Triple-quadrupole tandem mass spectrometric detection was performed using an ACQUITY SQD (Waters Corp.) with electrospray ionization interface and an UPLC system consisting of a binary gradient pump, an auto sampler (4°C), and a column oven (40°C). UPLC was operated under the same conditions as described above for glucuronidation assays. Peaks were detected at 270 nm wavelength. The mass spectrometer operated in positive mode was set up to scan the daughter ion of m/z 312.43. The optimized mass spectrometry parameters used were as follows: capillary voltage, 0.57 kV; cone voltage, 30 V; collision energy, 15 V; source temperature, 450°C; and desolvation temperature, 140°C. Nitrogen was used as the desolvation and cone gas with a flow rate of 760 L/h. Argon was used as the collision gas at a flow rate of 0.1 mL/min. Data acquisition and analysis were performed using the MassLynx NT 4.1 software with QuanLynx program (Waters Corp.).
Statistical analysis.
Michaelis-Menten kinetic constants were determined using Prism Version 5 software (La Jolla, CA). The two-sample t-test (two-tailed) was used to compare kinetic values of glucuronide formation for the UGT1A4 and 2B10 isoforms against OLZ in cell lines and HLMs. To perform the most conservative comparison, unequal variances were assumed when comparing levels of OLZ-glucuronide formation in HLMs with wild-type alleles versus HLM with one or two polymorphic alleles. All the levels of OLZ-glucuronide formation were power transformed (using a power of 1/1.5) to make sure the data was approximately normally distributed. Analysis of OLZ glucuronidation in HLM stratified by UGT1A4 genotypes was performed only for those specimens also exhibiting the wild-type UGT2B10 (*1/*1) genotype (n=92); similarly, analysis of OLZ glucuronidation in HLM stratified by UGT2B10 genotypes was performed only for those specimens also exhibiting the wild-type UGT1A4 (*1/*1) genotype (n=95). Regression analysis of OLZ-glucuronide formation against genotype was performed (SAS Corporation, Cary, NC).
Results
Characterization of OLZ glucuronidation.
Figure 1 outlines the enzymes previously shown to be involved in OLZ metabolism and the relative urinary contribution of each pathway (45). In subjects taking OLZ, unchanged OLZ comprises ~18% of total urinary OLZ, while OLZ glucuronides comprise nearly 61% of all urinary OLZ metabolites with the 10-N-glucuronide comprising ~67% of urinary OLZ glucuronides (29, 31, 32). While UGT1A4 was shown to be active against OLZ, several UGTs were not previously screened. To better characterize the enzymes responsible for hepatic OLZ glucuronidation, glucuronidation assays were performed and OLZ glucuronides were separated by UPLC. In addition to an OLZ peak at a retention time of 6.2 min (Figure 1, panel B), incubations of OLZ with HLM yielded three peaks at 1.45 min, 1.78 min, and 3.83 min (Figure 1, panel C). While the 3.83 min peak was highly sensitive to treatment with β-glucuronidase (Figure 1D), the peaks at 1.45 and 1.78 min were less sensitive to this treatment but were extremely sensitive to treatment with 3N HCl solution (data not shown), a pattern that was previously reported for these OLZ glucuronides (29, 31). The peaks at 1.45, 1.78, and 3.83 min all demonstrated a [M+] peak at m/z 489 (the glucuronide conjugate of OLZ) by mass spectrometry (MS/MS) analysis. The peaks at 1.45 and 1.78 min showed a [M+H]+ peak at m/z 313 for OLZ after loss of the glucuronic acid moiety (molecular weight = 176 g/mol) and a m/z 432 fragment after loss of the CH2=CH-NH-CH3 methyl piperazine moiety (Figure 1, panel E). This is identical to the pattern observed for the two OLZ-10-N-glucuronide isomers reported previously (29, 31). The peak at 3.83 min (panel F) demonstrated a [M+H]+ peak at m/z 313 (OLZ) and at m/z 282 due to the loss of CH3NH2 and glucuronic acid, but no peak at m/z 423, which is characteristic of the OLZ-4’-N-glucuronide (29, 31).
Figure 1. Schematic of OLZ metabolism.
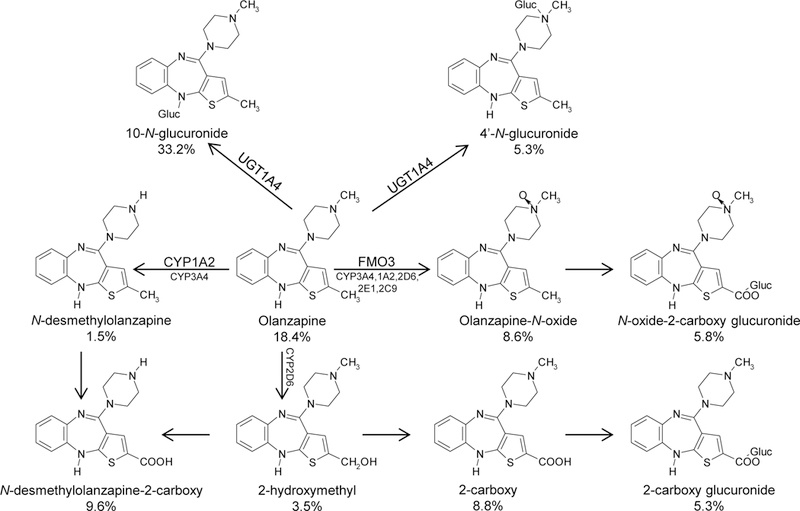
Previous studies identified the 10-N-glucuronide as the major metabolite of OLZ in urine, feces, and plasma (31). Percentages are reported OLZ metabolite recovery in urine (29). Enzymes responsible for the metabolism of OLZ are indicated, with the most active enzymes indicated with a larger font.
Previous studies of selected UGT enzymes suggested that UGT1A4 was active against OLZ. To fully characterize all of the UGTs responsible for OLZ glucuronidation, a comprehensive screening of OLZ glucuronidation activity by homogenates from HEK 293 cells over-expressing wild-type UGTs 1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B10, 2B11, 2B15 and 2B17 was performed. Two UGTs exhibited detectable levels of activity against OLZ: the hepatic UGTs 1A4 and 2B10. None of the other UGTs screened in our assays (UGTs 1A1, 1A3, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B11, 2B15 or 2B17) exhibited any glucuronidation activity against OLZ using up to 250 μg of UGT-over-expressing cell homogenate. The UPLC 3-peak pattern and retention times for UGTs 1A4 and 2B10 were identical to that observed for HLM and showed similar sensitivity to treatment with β-glucuronidase and 3N HCl solution (data not shown). Similar to that observed for OLZ glucuronides observed in incubations with HLM, the UGT1A4- and UGT2B10-generated peaks were identified as OLZ-10-N-glucuronide isomer 1 at 1.45 min, OLZ-10-N-glucuronide isomer 2 at 1.78 min, and OLZ-4’-N-glucuronide at 3.83 min as determined by UPLC/MS/MS analysis (data not shown).
Representative kinetic plots of glucuronidation rate versus substrate concentration are shown in Figure 2 for wild-type UGTs 1A4 (UGT1A424Pro48Leu; top panels) and 2B10 (UGT2B1067Asp; middle panels) as well as HLMs with wild-type UGTs 1A4 and 2B10 (bottom panels). UGT2B10 exhibited a significantly (p<0.0001) decreased binding affinity against OLZ (KM = 564 ± 15 μM) as compared to UGT1A4 (KM = 156 ± 17 μM) for the formation of OLZ-10-N-glucuronide isomer 1 and for OLZ-10-N-glucuronide isomer 2 (KM = 818 ± 23 μM vs. 206 ± 37 μM, respectively; p<0.0001; Table 1). A significantly (p=0.002) higher binding affinity was observed for UGT2B10 (KM = 457 ± 11 μM) as compared to UGT1A4 (KM = 810 ± 135 μM) when forming the OLZ-4’-N-glucuronide. The KM exhibited by HLM with wild-type UGTs 1A4 and 2B10 for both OLZ-10-N-glucuronide isomer 1 and for OLZ-10-N-glucuronide isomer 2 formation (Table 2) was between the KMs exhibited by homogenates from UGT1A4 and UGT2B10-over-expressing cells. The KM exhibited by these HLM for OLZ-4’-N-glucuronide formation was similar to that observed for homogenates from UGT2B10-over-expressing cells. The Vmax for formation of OLZ-10-N-glucuronide isomer 1 and 2 by UGT2B10 was 12- and 2.3-fold lower than for the reaction catalyzed by UGT1A4 (both p<0.0001) and 2.7-fold lower for OLZ-4’-N-glucuronide formation (p=0.002).
Figure 2. UPLC and MS/MS analysis of OLZ glucuronides formed by HLM.

Glucuronidation assays were performed using 12.5 μg HLM protein and 300 μM OLZ, and incubated at 37°C for 2 h with 4 mM UDP-glucuronic acid prior to analysis by UPLC as described in the Materials and Methods. Panel A, HLM + vehicle (1% DMSO); panel B, UGT-null HEK 293 cell homogenates + OLZ; panel C, HLM + OLZ; panel D, HLM + OLZ and 1,000 U of β-glucuronidase . Panel E, mass spectra of UPLC peaks 1 and 2; panel F, mass spectra of peak 3; Peak 1, OLZ-10-N-glucuronide isomer 1; peak 2, OLZ-10-N-glucuronide isomer 2; peak 3, OLZ-4’-N-glucuronide; peak 4, OLZ; peak 5, UDP-glucuronic acid. AU, absorbance.
Table 1.
Kinetic analysis of UGT1A4 and UGT2B10 variants against OLZ in vitro.
| OLZ-10-N-glucuronide isomer 1 | OLZ-10-N-glucuronide isomer 2 | OLZ-4’-N-glucuronide | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
UGT variant |
Vmax (pmol·min−1·µg−1) |
KM (µM) |
Vmax/KM* (nl·min−1·µg−1) |
Vmax (pmol·min−1·µg−1) |
KM (µM) |
Vmax/KM* (nl·min−1·µg−1) |
Vmax (pmol·min−1·µg−1) |
KM (µM) |
Vmax/KM* (nl·min−1·µg−1) |
| UGT1A424Pro/48Leu | 11 ± 0.8 | 156 ± 17 | 70 ± 2 | 5.5 ± 0.4 | 206 ± 37 | 30 ± 5 | 8.0 ± 1.7 | 810 ± 135 | 10 ± 4 |
| UGT1A424Thr/48Leu | 14 ± 1.9a | 584 ± 67b | 24± 0.5b,c | 4.8 ± 1.2 | 152 ± 23a | 31± 3.4c | 4.7 ± 1.3a | 358 ± 54d | 13± 4 |
| UGT1A424Pro/48Val | 17.4 ± 0.39b | 69 ± 6.5b | 260± 20b | 14.6 ± 1.4b | 110 ± 14e | 130± 6b | 3.7± 0.4e | 326 ± 33d | 10 ± 1 |
| UGT2B1067Asp | 0.92 ± 0.02b | 564 ± 15b | 2 ± 0.1b | 2.4 ± 0.07b | 818 ± 23b | 3 ± 0. 02b | 3.0 ± 0.1f | 457 ± 11f | 7 ± 0.6f |
| UGT2B1067Tyr | no activity detected | no activity detected | no activity detected | ||||||
For UGT1A4 variants, value represents KCAT (min−1), UGT2B10 protein levels was quantified relative to UGT2B7 protein standard by Western blotting as described in the Materials and Methods and kinetic data are shown as Vmax/KM.
p<0.05
p<0.0001
p<0.0001 versus corresponding value for UGT1A424Pro/48Val.
p<0.001
p<0.005
p=0.002 versus corresponding value for UGT1A424Pro/48Leu.
Table 2.
Kinetic analysis of OLZ glucuronidation in HLMs of varying UGT1A4 and UGT2B10 genotypes.
| OLZ-10-N-glucuronide isomer 1 | OLZ-10-N-glucuronide isomer 2 | OLZ-4’-N-glucuronide | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
HLM genotype |
Vmax (pmol·min−1·mg−1) |
KM (µM) |
Vmax/KM (µl·min−1·mg−1) |
Vmax (pmol·min−1·mg−1) |
KM (µM) |
Vmax/KM (µl·min−1·mg−1) |
Vmax (pmol·min−1·mg−1) |
KM (µM) |
Vmax/KM (µl·min−1·mg−1) |
| UGT1A4(*1/*1)/ UGT2B10(*1/*1)* |
94.0 ± 14 | 352 ± 59 | 0.28 ± 0.08 | 63 ± 23 | 311 ± 27 | 0.2 ± 0.07 | 82 ± 20 | 317 ± 39 | 0.26 ± 0.07 |
| UGT1A4(*1/*3)/ UGT2B10(*1/*1)* |
160 ± 14a | 328 ± 33 | 0.50 ± 0.08b | 73 ± 17 | 239 ± 22b | 0.31 ± 0.08 | 70 ± 24d | 263 ± 59 | 0.26 ± 0.04e |
| UGT1A4(*3/*3)/ UGT2B10(*1/*1)* |
188 ± 47b | 268 ± 41 | 0.70 ± 0.07c | 106 ± 16 | 263 ± 86 | 0.42 ± 0.08 | 156 ± 18b | 337 ± 37 | 0.46 ± 0.01c |
| UGT2B10(*1/*2)/ UGT1A4(*1/*1)** |
56 ± 23d | 302 ± 50 | 0.19 ± 0.08f | 30 ± 14e | 196 ± 54b | 0.16 ± 0.09 | 47 ± 20e | 602 ± 57a,e | 0.08 ± 0.03b,g |
| UGT2B10(*2/*2)/ UGT1A4(*1/*1)** |
69 | 273 | 0.25 | 41.5 | 271 | 0.15 | 37 | 796 | 0.047 |
Three HLM with the UGT1A4 (*1/*1) genotype, three HLM with the UGT1A4 (*1/*3) genotype, and two HLM with the UGT1A4 (*3/*3) genotype were examined in this analysis. Only HLM with the UGT2B10 (*1/*1) genotype was used to compare varying UGT1A4 genotypes. HLMs of each genotype were chosen at random.
Three HLM with the UGT2B10 (*1/*2) genotype and the single HLM with the UGT2B10 (*2/*2) genotype were examined in this analysis. Only HLM with the UGT1A4 (*1/*1) genotype were used to compare varying UGT2B10 genotypes.
p<0.005
p<0.05
p<0.01 versus the corresponding value for HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*1) genotype.
p<0.05
p<0.01
p<0.005
p<1 versus the corresponding value for HLM with the UGT1A4 (*3/*3)/UGT2B10 (*1/*1) genotype.
Analysis of OLZ glucuronidation by hepatic UGT variants.
UGT1A4 is a hepatic enzyme that exhibits prevalent functional missense polymorphisms (41, 46, 47). HEK 293 cell lines over-expressing the UGT1A424Thr/48Leu and UGT1A424Pro/48Val variants were described previously (34, 35, 37, 40, 43, 47). After normalization of UGT1A4 variant protein levels in UGT1A4-over-expressing cell homogenates by Western blot analysis, no significant difference in Vmax/KM for OLZ-4’-N-glucuronide formation was observed between the UGT1A424Pro/48Val variant and either of the other two UGT1A4 isoforms (Table 1). The Vmax/KM of the UGT1A424Pro/48Val variant was 3.7- (p<0.0001) and 11- (p<0.0001) fold higher than that observed for wild-type UGT1A424Pro/48Leu and the UGT1A424Thr/48Leu variant, respectively, for formation of OLZ-10-N-glucuronide isomer 1, and 4.3- (p<0.0001) and 4.2- (p<0.0001) fold higher, respectively, for OLZ-10-N-glucuronide isomer 2 formation (Table 1). While a significant (p<0.0001) 3-fold lower Vmax/KM was observed for the UGT1A424Thr/48Leu variant versus the wild-type UGT1A424Pro/48Leu for formation of OLZ-10-N-glucuronide isomer 1, no difference was observed for OLZ-10-N-glucuronide isomer 2 or the OLZ-4’-N-glucuronide.
Similar to that described for UGT1A4, a prevalent missense polymorphism also exists for UGT2B10 at codon 67. A HEK 293 cell line over-expressing the UGT2B1067Tyr variant has been described previously (37, 43). UGT2B10 protein expression in the UGT2B10-over-expressing cell lines was analyzed by Western blot analysis (Figure 3). As a UGT2B10 protein standard was not available, the purchased UGT2B7 protein standard (reported to run at 58 kDa; personal communication, BD Gentest technical support) was used to quantify UGT2B10 protein. Western blot analysis demonstrated similar levels of UGT2B10 expression in the UGT2B10 wild-type and codon 67 variant-over-expressing cell lines, which were in agreement with UGT2B10 mRNA levels determined by real-time PCR as previously published for these cell lines (37, 38). Unlike the activity observed for the wild-type UGT2B1067Asp, no glucuronidation activity was observed for the UGT2B1067Tyr variant against OLZ (Table 1).
Figure 3. Kinetic curves for wild-type UGT1A424Pro/48Leu- and UGT2B1067Asp-over-expressing cell homogenates and wild-type HLM against OLZ.

Representative kinetic curves were performed as described in the Materials and Methods using OLZ concentrations of 9.4, 18.8, 37.6, 75, 150, 300, 400, 600, 800, 1000, 1200, 1600, and 2000 μM for cell lines and 62.5, 125, 250, 500, 1000, and 2000 µM for HLM. Wild-type HLM were from subjects exhibiting the UGT1A4(*1/*1)/UGT2B10(*1/*1) genotype.
Analysis of OLZ glucuronidation by HLM stratified by UGT1A4 or UGT2B10 genotypes.
The prevalence of the UGT1A424Thr/48Leu, UGT1A424Pro/48Val and UGT2B1067Tyr variants is ~8%, 9%, and 10% in Caucasians, respectively (37, 43, 46, 47). To explore a possible in vivo relationship between OLZ glucuronidation and the UGT1A4 and UGT2B10 polymorphisms, a series of 105 HLM were examined for their glucuronidation activity against OLZ. The rate of OLZ-glucuronide formation was determined by UPLC using 300 μM OLZ, a concentration within the linear range of kinetic analysis for HLM against OLZ. Comparing HLM homozygous for the wild-type UGT2B1067Asp allele [UGT2B10 (*1/*1) genotype (n=92)], no significant difference in formation of any OLZ glucuronide was observed when comparing those homozygous for the UGT1A424Thr/48Leu allele [UGT1A4 (*2/*2) genotype; n=2] to HLM homozygous for the UGT1A424Pro/48Leu allele [UGT1A4 (*1/*1) genotype; n=73], even after excluding HLM with one or more UGT1A4*3 (UGT1A424Pro/48Val) alleles (results not shown). Therefore, for further analysis of the UGT1A4*3 and UGT2B1067Tyr (UGT2B10*2) alleles, HLM were not sub-stratified based on the UGT1A4*2 allele.
For HLM homozygous for the wild-type UGT2B1067Asp (*1/*1) genotype, there was a significant 2.1- (p=0.04) and 1.6- (p=0.0017) fold increase in formation of the OLZ-10-N-glucuronide isomer 1 and the OLZ-4’-N-glucuronide, respectively (Figure 4, panels A and C) and a significant (p=0.02) 2.0-fold increase in overall OLZ glucuronidation activity (panel D) in HLM with the UGT1A4 (*3/*3) genotype (n=2) as compared with HLM with the UGT1A4 (*1/*1) genotype (n=82). While a 2.2-fold increase in OLZ-10-N-glucuronide isomer 2 formation was observed for HLM with the UGT1A4 (*3/*3) genotype versus HLM with the UGT1A4 (*1/*1) genotype, this difference was not significant (p=0.09). Regression analysis showed that the UGT1A4 *3 allele is a significant predictor for formation of the OLZ-10-N-glucuronide isomer 1 (p=0.0047), the OLZ-10-N-glucuronide isomer 2 (p=0.0001), the OLZ-4’-N-glucuronide (p=0.02), and overall OLZ glucuronidation (p=0.0013). For each UGT1A4 *3 allele, product formation increased by 3.0, 3.3, 1.2, and 7.9 pmol·min−1·mg protein−1 for OLZ-10-N-glucuronide isomer 1, OLZ-10-N-glucuronide isomer 2, OLZ-4’-N-glucuronide, and overall glucuronidation, respectively, representing a 16, 25, 10, and 15% increase in glucuronidation for each UGT1A4 *3 allele compared to the mean glucuronide-product formation by wild-type samples, respectively.
Figure 4. Western blot analysis of UGTB10-over-expressing cell lines.
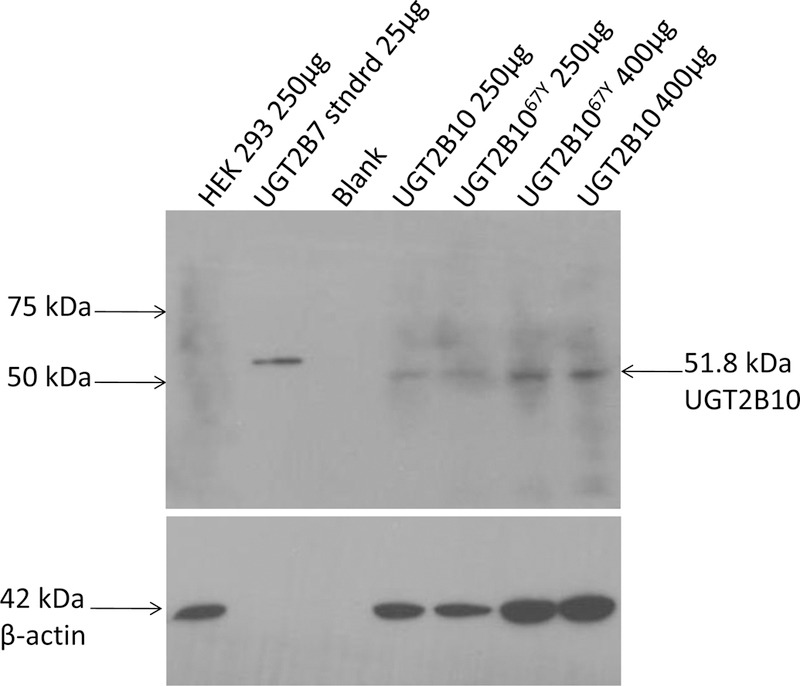
PVDF membrane was probed with 1:500 UGT2B antibody for 1 h at 23°C followed by donkey anti-goat IgG conjugated to horseradish peroxidase (1:4500) for 45 min at 23°C. Lane 1, HEK 293 (250 µg); lane 2, UGT2B7 protein standard (335 ng); lane 3, blank; lane 4, UGT2B10-over-expressing cell line (250 µg); lane 5, UGT2B1067Y-over-expressing cell line (250 µg); lane 6, UGT2B1067Y-over-expressing cell line (400 µg); lane 7, UGT2B10-over-expressing cell line (400 µg).
For HLM with the wild-type UGT1A4 (*1/*1) genotype (n=95), there was only a single HLM with the UGT2B10 (*2/*2) genotype. Therefore, HLM with the UGT2B10 (*1/*2) (n=12) or (*2/*2) (n=1) genotypes were combined into one group for analysis of OLZ glucuronidation activity stratified by UGT2B10 genotype. There was a significant 1.9-fold decrease in both OLZ-10-N-glucuronide isomer 1 (p=0.0016; Figure 4, panel E) and isomer 2 (p=0.0013; panel F) formation, a significant 2.7-fold decrease in OLZ-4’-N-glucuronide formation (p<0.0001; panel G), and a significant 2.1-fold decrease in overall OLZ glucuronidation (p=0.0002; panel H) in UGT1A4 (*1/*1) HLMs with at least one UGT2B10*2 allele as compared to UGT1A4 (*1/*1) HLMs with the UGT2B10 (*1/*1) genotype. The single HLM with a combined UGT1A4 (*1/*1)/UGT2B10 (*2/*2) genotype exhibited a 2.6-, 2.1-, and 4.1-fold decrease in the formation of OLZ-10-N-glucuronide isomer 1, OLZ-10-N-glucuronide isomer 2, and OLZ-4’-N-glucuronide, respectively, as compared to UGT1A4 (*1/*1) HLMs with the UGT2B10 (*1/*1) genotype (results not shown), suggesting a trend towards decreasing OLZ glucuronidation activity with increasing numbers of the UGT2B10*2 allele. Regression analysis showed that the UGT2B10*2 allele was a significant predictor for formation of the OLZ-10-N-glucuronide isomer 1 (p=0.002), the OLZ-10-N-glucuronide isomer 2 (p=0.0019), the OLZ-4’-N-glucuronide (p<0.0001), and overall OLZ glucuronidation (p<0.0001). For each UGT2B10 *2 allele, product formation decreased by 4.1, 2.0, 4.3, and 10.5 pmol·min−1·mg protein−1 for OLZ-10-N-glucuronide isomer 1, OLZ-10-N-glucuronide isomer 2, OLZ-4’-N-glucuronide, and overall glucuronidation, respectively, representing a 20, 20, 50, and 30% decrease in glucuronide formation for each UGT2B10 *2 allele compared to the mean glucuronide-product formed by wild-type samples, respectively. There was insufficient power to examine the effect of combined UGT1A4 and UGT2B10 genotypes on OLZ glucuronidation in this series of HLM specimens.
To better assess the relative contribution of UGT1A4 to OLZ glucuronidation, HLM were co-incubated with 300 µM OLZ and 2000 µM of the UGT1A4-specific inhibitor, LTG (33, 48). Overall OLZ glucuronidation was inhibited by 12 + 3.4% in UGT1A4 (*1/*1)/UGT2B10 (*1/*1) HLM incubated with LTG (data not shown). Using assay conditions (25 µM OLZ and 2000 µM LTG) similar to those of previous studies (33), overall OLZ glucuronidation was inhibited by 41% in homogenates from UGT1A4-over-expressing cells (data not shown).
Results of kinetic analysis of HLM with varying UGT1A4 and UGT2B10 genotypes are shown in Table 2. For HLM stratified by UGT1A4 genotypes, there was a significant 1.8- (p<0.05) and 2.5- (p<0.01) fold increased Vmax/KM for formation of the OLZ-10-N-glucuronide isomer 1 in UGT2B10 (*1/*1) HLM with the UGT1A4 (*1/*3) and UGT1A4 (*3/*3) genotypes, respectively, as compared to UGT2B10 (*1/*1) HLM with the UGT1A4 (*1/*1) genotype. This was manifested primarily via an increase in Vmax, with HLM with the UGT1A4 (*1/*3)/UGT2B10 (*1/*1) and UGT1A4 (*3/*3)/UGT2B10 (*1/*1) genotypes exhibiting a significant 1.7- (p<0.005) and 2.0- (p<0.05) fold higher Vmax, respectively, for OLZ-10-N-glucuronide isomer 1. There was a significant trend of increasing activity as measured by Vmax/KM with increasing numbers of the UGT1A4*3 allele for formation of the OLZ-10-N-glucuronide isomer 1 (p<0.0001). For formation of the OLZ-10-N-glucuronide isomer 2, there was a significant (p<0.01) trend of increasing Vmax/KM with increasing numbers of the UGT1A4*3 allele (Table 2). For the OLZ-4’-N-glucuronide, HLM with the UGT1A4 (*3/*3)/UGT2B10 (*1/*1) genotype exhibited a 1.8-fold higher Vmax/KM (p<0.01), manifested primarily via a 1.9-fold higher Vmax (p<0.05), as compared to HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*1) genotype (Table 2). A significant (p=0.0075) trend of increasing Vmax/KM for HLM with increasing numbers of the UGT1A4*3 allele was also observed.
The effect of UGT2B10 genotype on HLM glucuronidation activities was strongest for the OLZ-4’-N-glucuronide. There was a significant 3.3-fold lower Vmax/KM (p<0.05) for formation of the OLZ-4’-N-glucuronide in HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*2) genotype as compared to HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*1) genotype, an effect that was manifested by primarily by a significant increase in KM (p<0.005; Table 2). A significant (p<0.001) 5.8-fold lower Vmax/KM was observed for HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*2) genotype when compared to HLM with the UGT1A4 (*3/*3)/UGT2B10 (*1/*1) genotype. While statistical analysis could not be performed for comparisons with the single HLM with the UGT1A4 (*1/*1)/UGT2B10 (*2/*2) genotype, this HLM exhibited a 1.7-, 5.5- and 9.8-fold lower Vmax/KM for formation of the OLZ-4’-N-glucuronide as compared to HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*2), UGT1A4 (*1/*1)/UGT2B10 (*1/*1) and UGT1A4 (*3/*3)/UGT2B10 (*1/*1) genotypes, respectively.
While significant alterations in OLZ-10-N-glucuronide isomer 1 kinetics were not observed when comparing UGT1A4 (*1/*1)/UGT2B10 (*1/*2) versus UGT1A4 (*1/*1)/UGT2B10 (*1/*1) HLM, a significant (p<0.005) 3.7-fold decrease in Vmax/KM was observed when comparing UGT1A4 (*1/*1)/UGT2B10 (*1/*2) versus UGT1A4 (*3/*3)/UGT2B10 (*1/*1) HLM. A similar pattern was observed for OLZ-10-N-glucuronide isomer 2, with HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*2) genotype exhibiting a near-significant (p=0.057) 2.6-fold lower Vmax/KM as compared to HLM with the UGT1A4 (*3/*3)/UGT2B10 (*1/*1) genotype. In both cases these differences were primarily due to significant (p≤0.02) decreases in Vmax.
Discussion
Similar to that observed in previous studies, three primary OLZ-glucuronide products were observed in HLM – OLZ-4’-N-glucuronide and two isomers of OLZ-10-N-glucuronide (29, 31). Previous studies had shown that UGT1A4 is active against OLZ (33). However, only selected human UGTs (1A1, 1A3, 1A4, 1A6, 1A9, 2B7 and 2B15) over-expressed in baculovirus-infected insect cells were examined in these studies (33). Using UGT-over-expressing HEK 293 cell homogenates, all of the known human UGT1A and UGT2B enzymes except UGTs 1A5 and 2B28 were screened for activity against OLZ in the present study. In addition to UGT1A4, UGT2B10 exhibited glucuronidation activity against OLZ. The 3.6- and 4-fold larger KM observed for UGT2B10 versus UGT1A4 for OLZ-10-N-glucuronide isomer 1 and OLZ-10-N-glucuronide isomer 2, respectively, and the 12- and 2.3-fold lower Vmax suggests that UGT1A4 is more important in their formation than UGT2B10. However, previous studies have demonstrated that UGT2B10 exhibits a 1.5 – 4.8-fold higher level of expression than UGT1A4 in human liver (49–51), suggesting that both enzymes may play an important role in OLZ-10-N-glucuronide formation in vivo. A combined effect of both UGT1A4 and UGT2B10 on OLZ-10-N-glucuronide formation is further suggested by the fact that the KM for both isomers of OLZ-10-N-glucuronide of HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*1) genotype was in between that observed in cell lines over-expressing UGT1A4 and UGT2B10 in vitro.
For the OLZ-4’-N-glucuronide, the KM for HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*1) genotype was similar to that observed in vitro for the UGT2B10-over-expressing cell line. In addition, UGT1A4 (*1/*1) HLM with one ‘knock-out’ UGT2B10*2 allele exhibited a 2.7-fold decrease in rate of OLZ-4’-N-glucuronide formation and a >3.2-fold decrease in Vmax/KM as compared to UGT1A4 (*1/*1)/UGT2B10 (*1/*1) HLM. This decrease was even greater for the single UGT1A4 (*1/*1) HLM specimen exhibiting the UGT2B10 (*2/*2) genotype, with UGT1A4-dependent activity accounting for <50% of OLZ-10-N-glucuronide isomers and <20% of OLZ-4’-N-glucuronide formation when comparing the kinetics of this HLM specimen versus that observed for HLM with the UGT1A4 (*1/*1)/UGT2B10 (*1/*1) genotype. UGT2B10 exhibited a ~2-fold decrease in KM and a similar Vmax/KM as compared to UGT1A4 for the OLZ-4’-N-glucuronide. Given the higher level of expression of UGT2B10 versus UGT1A4 in liver (49–51), these data are consistent with the larger effect observed for the UGT2B10*2 allele on the rate of OLZ-4’-N-glucuronide formation and OLZ-4’-N-glucuronide kinetic parameters than that observed for either isomer of the OLZ-10-N-glucuronide and suggest that UGT2B10 is the most active hepatic UGT in terms of OLZ-4’-N-glucuronide formation. The importance of UGT2B10 in OLZ glucuronidation was also implicated in experiments using the UGT1A4-specific inhibitor, LTG, to inhibit UGT1A4 activity in HLM, with LTG inhibiting OLZ glucuronidation by 12%. While these data support an important role for UGT2B10 in the glucuronidation of OLZ, it is also likely that, unlike that observed for UGT1A4-over-expressing baculosomes (33), LTG may not be the best inhibitor of OLZ in the cell line system utilized in the present study since excess LTG inhibited OLZ glucuronidation by only 41% in UGT1A4-over-expressing cell homogenates.
The present study is the first to examine the functional importance of missense SNPs in relevant UGTs on OLZ in vitro. HEK 293 cell homogenates over-expressing the UGT1A424Pro/48Val variant exhibited significantly higher levels of OLZ-10-N-glucuronide formation (both isomers 1 and 2). While a discernable effect on OLZ-4’-N-glucuronide formation was not similarly observed in vitro in UGT1A4-over-expressing cell homogenates, there was a significant trend towards increased formation of all three OLZ glucuronides both in terms of rate of formation as well as in kinetic parameters in HLM with increasing numbers of the UGT1A4*3 allele. While cell homogenates over-expressing the UGT1A424Thr/48Leu variant exhibited a significant decrease in OLZ-10-N-glucuronide isomer 1 formation compared with wild-type UGT1A424Pro/48Leu, no effect on the formation of other OLZ glucuronides were observed in vitro and no association was observed between the formation of any of the three OLZ glucuronides and the UGT1A4*2 allele in HLM. These data are consistent with results from a recent study demonstrating a lower level of plasma OLZ in subjects with the more active detoxifying UGT1A448Val-encoding *3 allele (19). Together, these data suggest that the UGT1A4 Leu48Val polymorphism is a prevalent UGT1A4 missense SNP that may be linked to altered hepatic OLZ glucuronidation activity.
Cell lines over-expressing the UGT2B1067Tyr variant exhibited no glucuronidation activity against OLZ. This is similar to that observed for this polymorphism against a number of UGT2B10 substrates including nicotine, cotinine and tobacco-specific nitrosamines and is consistent with this variant being a functional ‘knock-out’ of UGT2B10 enzyme activity (37, 43, 49, 52–54). This lack of in vitro activity against OLZ is consistent with the observed association between the UGT2B10*2 allele and significant decreases in OLZ glucuronide formation in HLM.
When examining combined genotypes, the effect of the UGT2B10*2 allele was most profound when comparing UGT2B10 (*1/*2)/UGT1A4 (*1/*1) HLM versus UGT2B10 (*1/*1)/UGT1A4 (*3/*3) HLM – a 2.7–3-fold decrease in Vmax/KM was observed for both isomers of the OLZ-10-N-glucuronide and a nearly 6-fold decrease in Vmax/KM was observed for the OLZ-4’-N-glucuronide. In all cases, the single HLM exhibiting the UGT2B10 (*2/*2) genotype exhibited even lower activity. These data strongly implicate both the UGT1A4 Leu48Pro and UGT2B10 Asp67Tyr polymorphisms as modifiers of hepatic OLZ glucuronidation activity.
These polymorphisms likely contribute to the wide inter-individual variability seen in OLZ plasma levels (16, 18–21). It is possible that individuals with these polymorphisms could be at a higher risk of OLZ treatment failure or development of side effects, most notably weight gain and metabolic dysfunction. Other studies have examined the effects of polymorphisms, enzyme induction, or enzyme inhibition of CYP1A2, responsible for formation of the 4’-N-desmethylolanzapine metabolic product (18, 55, 56). However, as 4’-N-desmethylolanzapine comprises only 1.5% of urine metabolites (29) and is a lesser plasma metabolite, the impact of variations in CYP1A2 are likely less significant than variations in the OLZ glucuronidation pathway in terms of overall OLZ clearance and metabolism.
In conclusion, the data presented in this study suggests that the UGT1A448Val and UGT2B1067Tyr variants significantly alter OLZ glucuronidation in vitro and could be important in determining inter-individual differences in overall OLZ metabolism in vivo. Other factors such as drug-induced UGT-gene expression, age, and gender also contribute to inter-individual differences in drug metabolism. Therapeutic drug monitoring (TDM) is a costly method routinely used to ensure patient serum drug levels are within the therapeutic window and accounts for all causes of differences in drug metabolism. However, TDM cannot provide information regarding which therapies would be more efficacious and result in less adverse effects for a given patient before starting a therapy. The results presented in this paper could lead to new screening strategies to help determine dosing and patient response to OLZ before starting treatment of schizophrenia, bipolar disorder, or treatment-resistant depression.
Figure 5. HLM activity stratified by UGT genotypes.
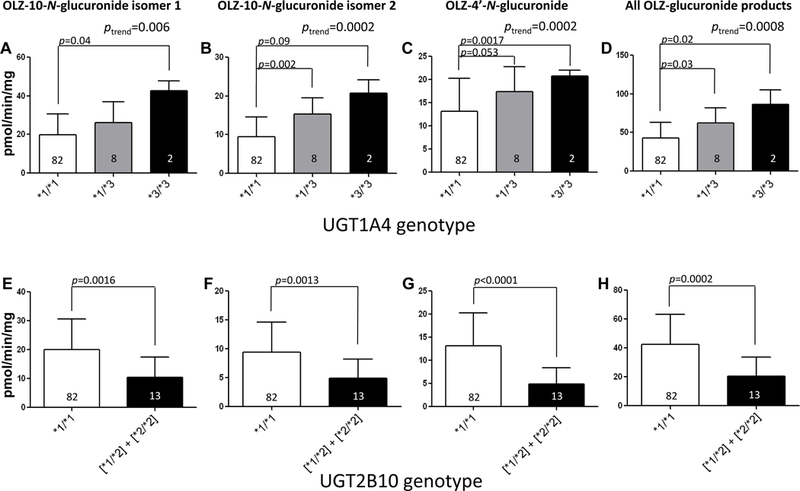
Shown are the levels of OLZ-10-N-glucuronide isomer 1, OLZ-10-N-glucuronide isomer 2, and OLZ-4’-N-glucuronide formation versus UGT1A4 or UGT2B10 genotypes in HLM. Glucuronidation activity assays were performed using 300 μM OLZ and 12.5 µg of HLM protein, and OLZ glucuronides were detected and separated by UPLC as described in the Materials and Methods. Using genomic DNA from the same liver specimens for which HLMs were prepared, UGT1A4 and UGT2B10 genotypes were determined using DNA sequencing and RFLP analysis, respectively. Panels A-D, OLZ glucuronidation in HLM stratified by UGT1A4 genotypes; panels E-H, OLZ glucuronidation in HLM stratified by UGT2B10 genotypes. Panel A and E, OLZ-10-N-glucuronide isomer 1 formation; panels B and F, OLZ-10-N-glucuronide isomer 2 formation; panels C and G, OLZ-4’-N-glucuronide formation; panels D and H, all OLZ-glucuronide product formation. Analysis of OLZ glucuronidation in HLM stratified by UGT1A4 genotypes was performed only for those specimens also exhibiting the wild-type UGT2B10 (*1/*1) genotype (n=92); similarly, analysis of OLZ glucuronidation in HLM stratified by UGT2B10 genotypes was performed only for those specimens also exhibiting the wild-type UGT1A4 (*1/*1) genotype (n=95).
Acknowledgements
We thank the Biostatistics Core Facility of the Penn State Cancer Institute for invaluable biostatistical support, and Nino Giambroni for technical support with mass spectrometry.
Funding for this study was provided in part by a grant from the American Diabetes Association grant # 7–08-CST-01
Footnotes
This material is original research, has not been previously published and has not been submitted for publication elsewhere while under consideration
Conflict of Interest Statement: None of the authors have conflict of interests to report
References
- 1.FDA. Electronic Orange Book 2011. 6/10/2009 [cited 2009 3/10/2009]; Available from: http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm
- 2.Katz R NDA 21–520. Indianapolis: Food and Drug Administration; 2003 December 24th, 2003.
- 3.Katz R NDA 20–592 / S-019. Indianapolis: Food and Drug Administration; 2004 Jan 14th, 2004.
- 4.Rosenheck R, Perlick D, Bingham S, Liu-Mares W, Collins J, Warren S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. Jama 2003. November 26;290(20):2693–702. [DOI] [PubMed] [Google Scholar]
- 5.Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 2008. March 29;371(9618):1085–97. [DOI] [PubMed] [Google Scholar]
- 6.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. American Journal of Psychiatry 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 7.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005. September 22;353(12):1209–23. [DOI] [PubMed] [Google Scholar]
- 8.Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Duggan L, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2010;3(CD006654). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gothelf DFB, Singer P, Kairi M, Phillip M, Zigel L et al. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry 2002; 159:1055–7. [DOI] [PubMed] [Google Scholar]
- 10.Kinon BJ BB, Gilmore JA, Tollefson GD. Longterm olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry 2001;62:92–100. [PubMed] [Google Scholar]
- 11.Wirshing DA WW, Kysar L, Berisford MA, Goldstein D, Pashdag J et al. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 1999;60:358–63. [PubMed] [Google Scholar]
- 12.Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res 2009. May;110(1–3):103–10. [DOI] [PubMed] [Google Scholar]
- 13.Rosenheck RA, Davis S, Covell N, Essock S, Swartz M, Stroup S, et al. Does switching to a new antipsychotic improve outcomes? Data from the CATIE Trial. Schizophr Res 2009. January;107(1):22–9. [DOI] [PubMed] [Google Scholar]
- 14.Monteleone P, Martiadis V, Maj M. Management of schizophrenia with obesity, metabolic, and endocrinological disorders. Psychiatr Clin North Am 2009. December;32(4):775–94. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Boutouyrie P. Arterial stiffness and stroke in hypertension: therapeutic implications for stroke prevention. CNS Drugs 2005;19(1):1–11. [DOI] [PubMed] [Google Scholar]
- 16.Wu TH, Chiu CC, Shen WW, Lin FW, Wang LH, Chen HY, et al. Pharmacokinetics of olanzapine in Chinese male schizophrenic patients with various smoking behaviors. Prog Neuropsychopharmacol Biol Psychiatry 2008. December 12;32(8):1889–93. [DOI] [PubMed] [Google Scholar]
- 17.Mauri MC, Volonteri LS, Colasanti A, Fiorentini A, De Gaspari IF, Bareggi SR. Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet 2007;46(5):359–88. [DOI] [PubMed] [Google Scholar]
- 18.Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine: Pharmacokinetic and Pharmacodynamic Profile. Clinical Pharmacokinetics 1999;37:177–93. [DOI] [PubMed] [Google Scholar]
- 19.Ghotbi R, Mannheimer B, Aklillu E, Suda A, Bertilsson L, Eliasson E, et al. Carriers of the UGT1A4 142T>G gene variant are predisposed to reduced olanzapine exposure—an impact similar to male gender or smoking in schizophrenic patients. European Journal of Clinical Pharmacology 2010. May;66(5):465–74. [DOI] [PubMed] [Google Scholar]
- 20.Bergemann N, Frick A, Parzer P, Kopitz J. Olanzapine plasma concentration, average daily dose, and interaction with co-medication in schizophrenic patients. pharmacopsychiatry 2004. March;37(2):63–8. [DOI] [PubMed] [Google Scholar]
- 21.Citrome L, Stauffer VL, Chen L, Kinon BJ, Kurtz DL, Jacobson JG, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: An analysis of correlations with efficacy, weight gain, and prolactin concentration. Journal of Clinical Psychopharmacology 2009. June;29(3):278–83. [DOI] [PubMed] [Google Scholar]
- 22.Nozawa M, Ohnuma T, Matsubara Y, Sakai Y, Hatano T, Hanzawa R, et al. The relationship between the response of clinical symptoms and plasma olanzapine concentration, based on pharmacogenetics: Juntendo University Schizophrenia Projects (JUSP). Ther Drug Monit 2008. February;30(1):35–40. [DOI] [PubMed] [Google Scholar]
- 23.Bottai T, Quintin P, Perrin E. Antipsychotics and the risk of diabetes: a general data review. European Psychiatry 2005. December;20(Suppl 4):S349–S57. [DOI] [PubMed] [Google Scholar]
- 24.Baldessarini RJTF. Drugs and the treatment of psychiatric disorders 10th ed. New York: McGraw-Hill Medical Publishing Division; 2001. [Google Scholar]
- 25.Toren PRS, Laor N, Weizman A. Benefit-risk assessment of atypical antipsychotics in the treatment of schizophrenia and comorbid disorders in children and adolescents. Drug Saf 2004;27:1135–56. [DOI] [PubMed] [Google Scholar]
- 26.Baptista TBS. Are leptin and cytokines involved in body weight gain during treatment with antipsychotic drugs? Can J Psychiatry 2002;47:742–9. [DOI] [PubMed] [Google Scholar]
- 27.Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry 2010. July;70(7):1041–50. [DOI] [PubMed] [Google Scholar]
- 28.Albaugh VL, Henry CR, Bello NT, Hajnal A, Lynch SL, Halle B, et al. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity 2006;14:36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassahun K, Mattiuz E, Nyhart E Jr., Obermeyer B, Gillespie T, Murphy A, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos 1997. January 1, 1997;25(1):81–93. [PubMed] [Google Scholar]
- 30.Markowitz JS, Devane CL, Liston HL, Boulton DW, Risch SC. The effects of probenecid on the disposition of risperidone and olanzapine in healthy volunteers. Clin Pharmacol Ther 2002. January;71(1):30–8. [DOI] [PubMed] [Google Scholar]
- 31.Kassahun K, Mattiuz E, Franklin R, Gillespie T. Olanzapine 10-N-glucuronide. A tertiary N-glucuronide unique to humans Drug Metab Dispos 1998;26(9):848–55. [PubMed] [Google Scholar]
- 32.Calligaro DO, Fairhurst J, Hotten TM, Moore NA, Tupper DE. The synthesis and biological activity of some known and putative metabolites of the atypical antipsychotic agent olanzapine. Bioorganic and Medicinal Chemistry Letters 1997;7(1):25–30. [Google Scholar]
- 33.Linnet K Glucuronidation of olanzapine by cDNA-expressed human UDP-glucuronosyltransferases and human liver microsomes. Hum Psychopharmacol 2002. July;17(5):233–8. [DOI] [PubMed] [Google Scholar]
- 34.Blevins-Primeau AS, Sun D, Chen G, Sharma AK, Gallagher CJ, Amin S, et al. Functional significance of UDP-glucuronosyltransferase variants in the metabolism of active tamoxifen metabolites. Cancer Res 2009 March 1, 2009;69(5):1892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiener D, Doerge DR, Fang JL, Upadhyaya P, Lazarus P. Characterization of N-glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human liver: importance of UDP-glucuronosyltransferase 1A4. Drug Metab Dispos 2004. January;32(1):72–9. [DOI] [PubMed] [Google Scholar]
- 36.Coughtrie MW, Burchell B, Bend JR. A general assay for UDPglucuronosyltransferase activity using polar amino-cyano stationary phase HPLC and UDP[U-14C]glucuronic acid. Anal Biochem 1986. November;159(1):198–205. [DOI] [PubMed] [Google Scholar]
- 37.Chen G, Dellinger RW, Gallagher CJ, Sun D, Lazarus P. Identification of a prevalent functional missense polymorphism in the UGT2B10 gene and its association with UGT2B10 inactivation against tobacco-specific nitrosamines. Pharmacogenetics and Genomics 2008;18(3):181–91. [DOI] [PubMed] [Google Scholar]
- 38.Chen G, Dellinger RW, Sun D, Spratt TE, Lazarus P. Glucuronidation of tobacco-specific nitrosamines by UGT2B10. Drug Metab Dispos 2008 May 1, 2008;36(5):824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren Q, Murphy SE, Zheng Z, Lazarus P. O-Glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1- (3-pyridyl)-1-butanol (NNAL) by human UDP-glucuronosyltransferases 2B7 and 1A9. Drug Metab Dispos 2000. November;28(11):1352–60. [PubMed] [Google Scholar]
- 40.Dellinger RW, Fang JL, Chen G, Weinberg R, Lazarus P. Importance of UDP-glucuronosyltransferase 1A10 (UGT1A10) in the detoxification of polycyclic aromatic hydrocarbons: decreased glucuronidative activity of the UGT1A10 139Lys isoform. Drug Metabolism and Disposition 2006 June 2006;34(6):943–9. [DOI] [PubMed] [Google Scholar]
- 41.Sun D, Chen G, Dellinger RW, Duncan K, Fang JL, Lazarus P. Characterization of tamoxifen and 4-hydroxytamoxifen glucuronidation by human UGT1A4 variants. Breast Cancer Res 2006;8(4):R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazarus P, Blevins Primeau AS, Zheng Y, Sun D. Potential role of UGT pharmacogenetics in cancer treatment and prevention: Focus on tamoxifen. Ann N Y Acad Sci 2009. February;1155:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, Blevins-Primeau AS, Dellinger RW, Muscat JE, Lazarus P. Glucuronidation of Nicotine and Cotinine by UGT2B10: Loss of Function by the UGT2B10 Codon 67 (Asp>Tyr) Polymorphism. Cancer Research 2007 October 1, 2007;67(19):9024–9. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher CJ, Muscat JE, Hicks AN, Zheng Y, Dyer A-M, Chase GA, et al. The UDP-Glucuronosyltransferase 2B17 Gene Deletion Polymorphism: Sex-Specific Association with Urinary 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol Glucuronidation Phenotype and Risk for Lung Cancer. Cancer Epidemiol Biomarkers Prev 2007 April 1, 2007;16(4):823–8. [DOI] [PubMed] [Google Scholar]
- 45.Devane CL, Markowitz JS. Antipsychotics et al. ed. Ambler: Lippencott Williams & Wilkins; 2000. [Google Scholar]
- 46.Ehmer U, Vogel A, Schütte JK, Krone B, Manns MP, Strassburg CP. Variation of hepatic glucuronidation: Novel functional polymorphisms of the UDP-glucuronosyltransferase UGT1A4. Hepatology 2004;39(4):970–7. [DOI] [PubMed] [Google Scholar]
- 47.Wiener D, Fang JL, Dossett N, Lazarus P. Correlation between UDP-glucuronosyltransferase genotypes and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone glucuronidation phenotype in human liver microsomes. Cancer Res 2004. February 1;64(3):1190–6. [DOI] [PubMed] [Google Scholar]
- 48.Rowland A, Elliot DJ, Williams JA, Mackenzie PI, Dickinson RG, Miners JO. In Vitro Characterization of Lamotrigine N2-glucuronidation and the lamotrigine-valproic acid interaction. Drug Metabolism and Disposition 2006 June 1, 2006;34(6):1055–62. [DOI] [PubMed] [Google Scholar]
- 49.Kaivosaari S, Toivonen Pi, Hesse LM, Koskinen M, Court MH, Finel M. Nicotine glucuronidation and the human UDP-glucuronosyltransferase UGT2B10. Molecular Pharmacology 2007 September 2007;72(3):761–8. [DOI] [PubMed] [Google Scholar]
- 50.Izukawa T, Nakajima M, Fujiwara R, Yamanaka H, Fukami T, Takamiya M, et al. Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metabolism and Disposition 2009 August 2009;37(8):1759–68. [DOI] [PubMed] [Google Scholar]
- 51.Itaaho K, Mackenzie PI, Ikushiro S-i, Miners JO, Finel M. The configuration of the 17-hydroxy group variably influences the glucuronidation of β-estradiol and epiestradiol by human UDP-glucuronosyltransferases. Drug Metabolism and Disposition 2008 November 2008;36(11):2307–15. [DOI] [PubMed] [Google Scholar]
- 52.Chen G, Giambrone NE, Dluzen DF, Muscat JE, Berg A, Gallagher CJ, et al. Glucuronidation Genotypes and Nicotine Metabolic Phenotypes: Importance of Functional UGT2B10 and UGT2B17 Polymorphisms. Cancer Research October 1, 2010;70(19):7543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berg JZ, Mason J, Boettcher AJ, Hatsukami DK, Murphy SE. Nicotine Metabolism in African Americans and European Americans: Variation in Glucuronidation by Ethnicity and UGT2B10 Haplotype. Journal of Pharmacology and Experimental Therapeutics January 1, 2010;332(1):202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berg JZ, von Weymarn LB, Thompson EA, Wickham KM, Weisensel NA, Hatsukami DK, et al. UGT2B10 Genotype Influences Nicotine Glucuronidation, Oxidation, and Consumption. Cancer Epidemiology Biomarkers & Prevention June 1, 2010;19(6):1423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laika B, Leucht S, Heres S, Schneider H, Steimer W. Pharmacogenetics and olanzapine treatment: CYP1A2*1F and serotonergic polymorphisms influence therapeutic outcome. Pharmacogenomics 2010. February;10(1):20–9. [DOI] [PubMed] [Google Scholar]
- 56.Carrillo JA, Herraiz A, Ramos SI, Gervasini G, Vizcaino S, Benitez J. Role of the smoking-induced cytochrome P450 (CYP)1A2 and polymorphic CYP2D6 in steady-state concentration of olanzapine. J Clin Psychoparmacology 2003. April;23(2):119–27. [DOI] [PubMed] [Google Scholar]


