Abstract
The incidence of hepatitis C virus (HCV) genotype 4 infection in Egypt provides a unique opportunity to study the innate immune response to symptomatic acute HCV infection. We investigated whether plasmacytoid dendritic cells (pDCs) are activated as a result of HCV infection. We demonstrate that, even during symptomatic acute infection, circulating pDCs maintained a similar precursor frequency and resting phenotype, compared with pDCs in healthy individuals. Moreover, stimulation with a Toll-like receptor 9 agonist resulted in an intact inflammatory response. These data support the growing consensus that pDCs are not directly activated by HCV and therefore are viable targets for immunotherapy throughout HCV infection.
Hepatitis C virus (HCV) infects ∼170 million people worldwide and is a major public health risk. In particular, Egypt maintains the highest prevalence of infection, affecting an estimated 15%–20% of the population. The origin of the HCV epidemic in Egypt has been attributed to schistosomiasis treatment campaigns in the 1960s and 1970s [1]. Acute HCV infection results in clinical symptoms in only 15%–20% of patients, and as a result, little is known about the host response during the early phase of the disease [2]. As part of a hospital-based surveillance program in Cairo, Egypt, we have studied a group of individuals presenting with symptomatic acute HCV infection.
Although little is known about the initial antiviral response in humans, studies in HCV-infected chimpanzees have demonstrated that the induction of type I interferons (IFNs) correlates with spontaneous viral clearance [3]. More specifically, IFN-stimulated genes (ISGs) are upregulated in the liver of acutely infected animals [3, 4]; however, no definitive cell source of IFN has been identified. This essential role for type I IFNs in HCV clearance is supported by the use of recombinant type I IFNs in the management of patients with chronic HCV infection.
Plasmacytoid dendritic cells (pDCs) are considered to be the principal source of endogenous type I IFNs. pDCs express Toll-like receptor (TLR) 7 and TLR9, which recognize single-stranded RNA and double-stranded DNA, respectively. Upon viral infection, pDC stimulation through these receptors results in the robust production of type I IFNs. The role of pDCs in HCV infection remains controversial and has not been adequately studied during the acute phase of infection. Herein, we aimed to evaluate the innate immune response to acute HCV infection, specifically monitoring the number, phenotype, and function of circulating pDCs. We demonstrate that the pDCs in patients with acute HCV infection are similar in activation status, surface marker expression, and functionality to those of healthy donors. Thus, we propose pDCs as a viable drug target for the stimulation of endogenous type I IFNs.
Patients and methods. Twenty-one patients with symptomatic acute HCV infection were recruited from 2 “fever hospitals” in Cairo, Egypt [5]. Inclusion criteria included a minimum age of 18 years, symptomatic disease as indicated by fever or jaundice, ⩾4-fold increase in serum alanine aminotransferase (ALT) level over the upper limit of normal, and the presence of viral HCV RNA. Patients who were concurrently infected with other infectious agents were excluded. Patients were monitored at The National Hepatology and Tropical Medicine Research Institute (NHTMRI) in Cairo, Egypt. Clinical and biochemical assays were performed 1, 4, 8, 24, and 52 weeks after presentation with symptomatic acute HCV infection. Spontaneous viral clearance was defined as loss of serum HCV RNA in the absence of treatment, based on 2 consecutive negative viral RNA PCR results, as well as subsequent negative tests. Peripheral blood mononuclear cell (PBMC)-based assays were performed on the samples collected at the last time point prior to clearance, such that all patients were viremic at the time of examination. Assays performed on samples from patients failing to clear HCV were matched for time post-onset of disease. As a control population, PBMCs were obtained from healthy donors. Clinical protocols were reviewed by the Ministry of Health and Population in Egypt and the NHTMRI ethics committee; all patients gave written informed consent.
Thirty milliliters of total blood was collected from each patient, and plasma was separated by high-speed centrifugation. PBMCs were isolated using a Ficoll-Hypaque (Amersham Biosciences) solution and cryopreserved until experimentation. Surface expression of phenotypic markers was monitored by antibodies directed against the following surface antigens: BDCA2 FITC, BDCA-4 APC (Miltenyi), CD123 PeCy5, CXCR3 PE, CCR7 PeCy7 (BD Bioscience), HLA-DR APC-Cy7, and CD86 Pacific Blue (Biolegend). Appropriate isotype-matched antibodies were used to establish gating parameters. Data was collected using a FACS Canto II (BD Biosciences), and analysis was performed with FlowJo software (TreeStar).
PBMCs were stimulated with 5 μg/mL CpG-2216 (Eurogentec) or media alone. Cell culture supernatants were collected 20 h after stimulation, and chemokine and cytokine expression levels were measured by Luminex xMAP assay (Biosource). At least 100 bead events were acquired for each analyte measured. Where applicable, Mann-Whitney U tests were performed to assess statistical significance.
Results. Twenty-one patients were identified who displayed symptoms of acute HCV infection. Type I IFNs are thought to be important for clearance of HCV infection during the acute phase of disease [3]. Using samples obtained from our acute HCV genotype 4 cohort, we aimed to investigate the expression of IFN and ISGs at early time points after infection. For this analysis, we analyzed RNA from 13 symptomatic patients with acute HCV infection, and we recruited 10 uninfected individuals as negative control subjects. Using reverse-transcription quantitative PCR, we investigated the transcript levels of IFNα2 , IFNβ1 , and the ISGs CXCL10 and MxA in patient PBMCs. Although IFNβ1 and IFNα2 were undetectable in cells isolated from individuals with acute infection, we did observe elevated levels of CXCL10 and MxA messenger RNA (mRNA) in a subset of patients with acute HCV infection, compared with levels in healthy donors (data not shown). ISG expression suggests that type I IFNs are being produced, despite little to no detection of IFNα/β mRNA. One possible explanation is that type I IFNs are being produced but at low levels and by a rare cell population, such that the signal is undetectable when total mRNA from whole PBMCs is assessed. We therefore hypothesized that pDCs are responsible for IFN expression and the induction of ISG expression in a subset of patients.
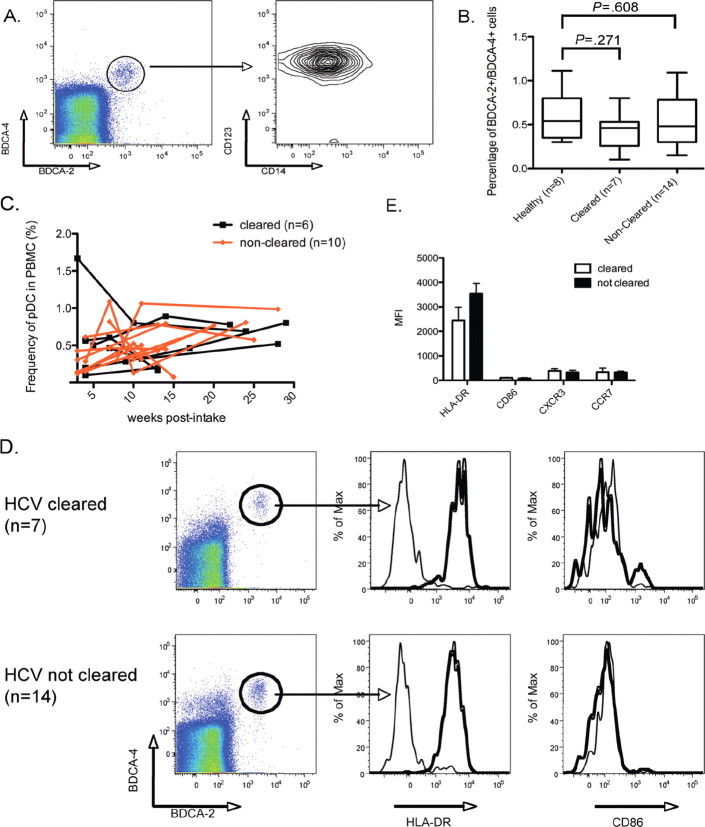
To explore this question, we examined the frequency and surface phenotype of circulating pDCs, as activation is expected to induce upregulation of CD86, HLA-DR, and CCR7, with the latter mediating migration to the lymph organs. As such, HCV stimulation of pDCs would lead to altered surface markers and a reduction in the number of circulating cells. pDC populations were identified on the basis of the co-expression of BDCA-2, BDCA-4, and CD123, in the absence of CD14 (Figure 1 A and 1 B). No statistically significant differences were observed in the frequency of circulating pDCs between patients with acute HCV infection and healthy donors (cleared vs healthy, P=.271 ; noncleared vs healthy, P= .608) (Figure 1 B). In addition, no correlations between the number of pDCs detected in our patient populations and serum ALT levels were observed (data not shown). Kinetic analysis demonstrated no significant association between the clearance of HCV and the percentage of circulating pDCs over time (Figure 1 C ; mixed model regression analysis, P= .629).
We next compared the phenotype of the pDC populations in patients with acute HCV infection to that of the pDC population in healthy donors (Figure 1 D). We observed no induction of CD86 or CCR7, low levels of CXCR3, and intermediate expression of HLA-DR, suggesting a resting pDC phenotype. Between our 3 groups (patients with acute HCV infection who ultimately clear their infection, patients with acute HCV infection who develop persistent infection, and healthy donors), no statistically significant differences were observed (Figure 1 E). The resting phenotype in infected individuals indicates 2 distinct possibilities: that these cells are not activated by HCV, or that pDCs are somehow rendered nonfunctional by HCV and thus unable to undergo cellular activation.
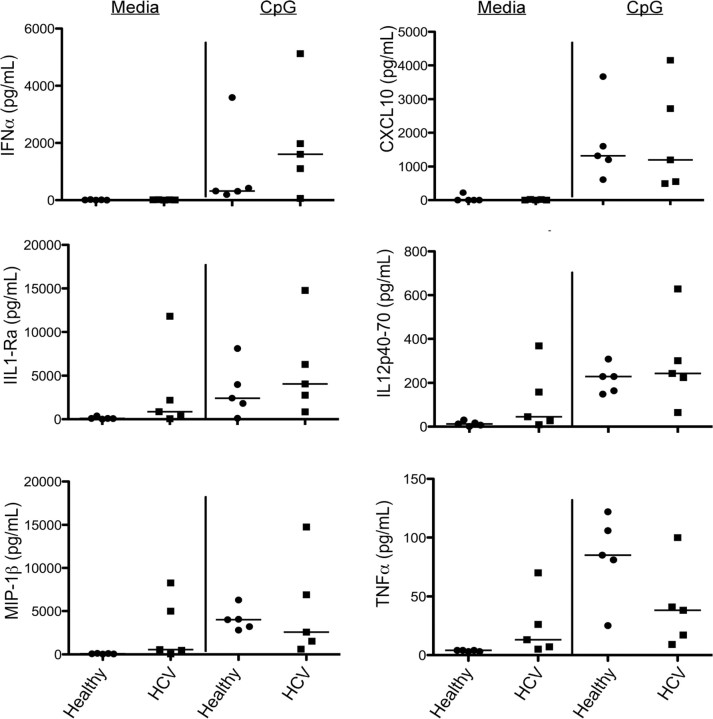
To distinguish between these 2 scenarios, we examined the functional capacity of pDCs isolated from patients with acute HCV infection by assessing their ability to respond to a TLR9 agonist. Freshly isolated PBMCs from viremic patients with HCV infection or healthy volunteers were stimulated overnight with 5 μg/mL CpG-2216, and culture supernatant was collected at 20 h and analyzed by Luminex xMAP assay. All pDCs were capable of producing robust levels of IFNα. Indeed, pDCs have been shown to express 19 different type I IFNs, accounting for ∼60% of their transcriptional activity [6]. As such, pDC research has primarily focused on their ability to produce IFNs; however, we have reported that pDCs secrete significant amounts of other inflammatory molecules, as well as acting indirectly to activate other cell types in the context of chronic HCV infection [7]. Thus, we also measured analytes that are part of the pDC inflammatory network and report that, similar to healthy donors, patients with acute HCV infection have an intact inflammatory response to TLR9 agonists (Figure 2). Together, our data indicate that pDCs are functional in the context of acute HCV genotype 4 infection and, contrary to our initial hypothesis, suggest that circulating pDCs are not the source of endogenous IFN during the acute phase of disease.
Figure 2.
TLR9 stimulation induces expression of proinflammatory molecules. Peripheral blood mononuclear cells from patients with acute hepatitis C virus (HCV) infection and from healthy donors were cultured with media or 5 μg/mL CpG. Cell culture supernatants were collected 20 h after stimulation and analyzed by Luminex xMAP assay. Each point indicates a single patient. Black lines indicate the median value for each patient population. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
HCV viremia reaches high titers within 1 week after infection, and transcriptional profiling of liver biopsies indicate induction of ISGs [3, 4]. Similarly, in chronic HCV patients, endogenous type I IFN remains elevated [8]. However, it remains unclear which cell types are producing type I IFN. Some studies have demonstrated a role for RIG-I in sensing HCV RNA [9]; however, recent data demonstrating that the HCV protein NS3–4A is capable of cleaving Cardif would suggest that the signaling pathways of intracellular host sensors for viral RNA are not active in infected cells [10, 11]. Furthermore, NS3–4A has been shown to inhibit IRF-3 phosphorylation, thus indicating that other host sensors (eg, TLR3) are inactive in infected hepatocytes [12]. In trying to account for the in vivo evidence of IFNα/β production, we investigated the role of circulating pDCs.
Our recent work has provided evidence that pDCs are phenotypically and functionally normal in chronic HCV infection [7, 13, 14]. We now extend these conclusions to acute HCV infection and report that the multiple cytokine loops by which pDCs contribute to the initiation of an inflammatory response are intact [7]. Specifically, we report the induction of (1) molecules secreted by the pDC itself and independent of IFN (eg, TNFα); (2) molecules secreted by the pDC and amplified by autocrine IFN (eg, CXCL10 and MIP1β); and (3) molecules not produced by pDCs but triggered by paracrine IFN (eg, IL1RA and IL12p70). One caveat for our study is that we were restricted to the study of circulating pDC populations, because liver biopsies are difficult to obtain from patients with acute HCV infection, and therefore we cannot discount that pDCs in the inflamed liver may be involved in the inflammatory response to HCV infection. Additionally, our results are in direct contrast to recent work by Shiina and Rehermann [15], which demonstrated that pDC functions may be selectively inhibited by HCVcc (genotype 2) in vitro. However, the extent to which these studies reflect natural HCV infection and in vivo mechanisms of viral immune evasion remains unknown.
In sum, our study demonstrates that circulating pDCs in patients infected with symptomatic acute HCV genotype 4 are similar to those of healthy donors. It remains unclear whether these conclusions can be applied to other HCV genotypes. Therefore, we propose that, in the HCV genotype 4-infected patient population, pDCs are a viable drug target for the stimulation of endogenous type I IFN production, which may promote viral clearance in those patients who fail to achieve spontaneous clearance.
Figure 1.
Plasmacytoid dendritic cell (pDC) phenotype and enumeration in acute hepatitis C virus (HCV) infection. A, pDCs are identified on the basis of their expression of BDCA-2, BDCA-4, CD123, and the absence of CD14. B, The percentage of pDCs are plotted for healthy donors (np 8), patients who cleared HCV infection (np 7), and patients developing persistent HCV infection (np 14). Mann-Whitney statistical analysis was used to perform 2-way comparisons for significance. C, Kinentic analysis of pDC frequency in patients with acute HCV genotype 4 infection. Black lines indicate patients who spontaneously cleared HCV (np 6); red lines indicate patients who became persistently infected with HCV (np 10). D, Cytometric analysis of pDC activation markers in patients who have cleared HCV infection and patients who have not cleared HCV infection: HLADR and CD86. Gray histograms indicate staining with an isotype control E, The expression of surface markers, as measured by mean fluorescence intensity (MFI), is shown for patients who cleared HCV infection (white bars) and who did not clear HCV infection (black bars). Error bars indicate 1 standard deviation from the mean. PBMCs, Peripheral blood mononuclear cells.
Footnotes
Potential conflicts of interest: none reported.
Financial support: “ Agence Nationale de Recherches sur le SIDA et les hépatites virales” (ANRS 12135 and ANRS 12122), the European Research Council (MLA), and the Pasteur Foundation (MEL).
References
- 1.Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 2.Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008;372:321–332. doi: 10.1016/S0140-6736(08)61116-2. [DOI] [PubMed] [Google Scholar]
- 3.Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75:7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su AI, Pezacki JP, Wodicka L, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Gaafary MM, Rekacewicz C, Abdel-Rahman AG, et al. Surveillance of acute hepatitis C in Cairo, Egypt. J Med Virol. 2005;76:520–525. doi: 10.1002/jmv.20392. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 7.Decalf J, Fernandes S, Longman R, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007;204:2423–2437. doi: 10.1084/jem.20070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihm S, Frese M, Meier V, et al. Interferon type I gene expression in chronic hepatitis C. Lab Invest. 2004;84:1148–1159. doi: 10.1038/labinvest.3700135. [DOI] [PubMed] [Google Scholar]
- 9.Foy E, Li K, Sumpter R Jr, et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci U S A. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci U S A. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meylan E, Curran J, Hofmann K, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 12.Foy E, Li K, Wang C, et al. Regulation of interferon regulatory factor3 by the hepatitis C virus serine protease. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 13.Longman RS, Talal AH, Jacobson IM, Albert ML Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–1029. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 14.Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 15.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–395. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]