Abstract
Background. Tetrapyrrole substrates and products of heme oxygenase are potent inhibitors of hepatitis C virus (HCV) replication. It is not clear whether this occurs through primary induction of type I interferon (IFN), inhibition of viral NS3/4A protease, or a combination of these mechanisms. We studied the antiviral actions of tetrapyrroles and their potential influence on type I IFN induction.
Methods. The effects of tetrapyrrole on NS3/4A protease activity and type I IFN induction were assessed in HCV-permissive cells, replicons, or human embryonic kidney (HEK) 293 cells transfected with NS3/4A protease. Activation of innate immune signaling was determined after transfection of double-strand surrogate nucleic acid antigens or infection with defined sequence HCV cell culture (HCVcc) RNA.
Results. Tetrapyrroles failed to directly induce IFN expression at concentrations that inhibited HCV replication and NS3/4A protease activity. However, they potently restored IFN induction after attenuation with NS3/4A protease, a process accompanied by preservation of the adapter protein, mitochondrial antiviral signaling protein, nuclear localization of IFN regulatory factor 3, and augmentation of IFN-stimulated gene products.
Conclusions. Tetrapyrroles do not directly induce IFN, but they dramatically restore type I IFN signaling pathway after attenuation with NS3/4A protease. They show immunomodulatory as well as antiprotease activity and may be useful for treatment of HCV infection.
Keywords: hepatitis C virus, HCV protease inhibitors, type I interferon, heme metalloporphyrins
Hepatitis C (HCV) is a major cause of cirrhosis and end-stage liver disease worldwide. Since discovery of the virus, interferon (IFN) α has been the cornerstone for successful antiviral therapy. The early addition of ribavirin and now the recent approval of first-generation protease inhibitors (PIs) have greatly improved treatment outcomes [1]. Nevertheless, in a sizeable percentage of patients, clinical cure is still not achieved because of weak IFN response and low tolerability of drug combinations [2, 3]. Consequently, intense research efforts have focused on development of new direct-acting anti-HCV drugs.
The HCV NS3/4A protease-helicase protein complex is a useful target for drug development [4]. The enzyme performs crucial proteolytic steps in the viral life cycle and also has multiple extraviral interactions within the host cell that enable the virus to evade host innate immune defenses and increase viral efficiency [5, 6]. NS3/4A cleaves and inactivates adapter proteins, such as mitochondrial antiviral signaling protein (MAVS) or Toll/interleukin 1 receptor domain–containing adapter-inducing IFN-β (TRIF), which allow host pattern recognition receptors (PRRs) to initiate signaling and induction of IFN-α and IFN-β responses [5, 7–9]. It is not known whether PIs significantly alter the success of antiviral therapy through counterinhibition of NS3/4A at the level of PRR adapter proteins, but further study of these interactions is important. Recent work has shown that new PIs require higher intracellular concentrations (>100 fold) in vitro to restore IFN signaling than is necessary for inhibition of polyprotein processing and viral replication [6, 10].
Heme oxygenase 1 (HO-1) is an inducible enzyme that oxidizes the metalloporphyrin, heme, to the linear tetrapyrrole, biliverdin (BV), which is then rapidly reduced to bilirubin (BR). Past work has demonstrated that precursors and products of the heme oxygenase system, including heme and BV have antiviral activities that target not only HCV but also human immunodeficiency virus (HIV) and hepatitis B virus [11]. In model systems, HCV replication and infection is inhibited with induction of HO-1 [12, 13] or incubation with HO-1–related tetrapyrroles, such as BV and heme [14]. The antiviral behavior of BV has been attributed to direct binding and inhibition of the NS3/4A protease in our work [14] and direct induction of type I IFN in another study [15]. Although heme and its metabolites have extensive interactions with inflammatory mediators and stress-response signaling pathways [16–20], it is not clear how or where these agents might influence IFN induction. Except in a single report [15], tetrapyrroles have not been reported to directly stimulate IFN pathways. Consequently, we were prompted to investigate further their potential interactions with IFN induction further.
In the present study, we investigated the effects of several tetrapyrroles on type I IFN induction, both directly and in relation to their intracellular antiprotease activities. Although these agents do not directly induce type I IFN, they do restore innate immune system signaling and IFN expression after attenuation with NS3/4A protease. These findings expand the known antiviral capabilities of natural tetrapyrroles and suggest that they represent a novel class of PIs that should be further investigated in vivo.
MATERIALS AND METHODS
See the Supplementary Material for a detailed description of our methods.
RESULTS
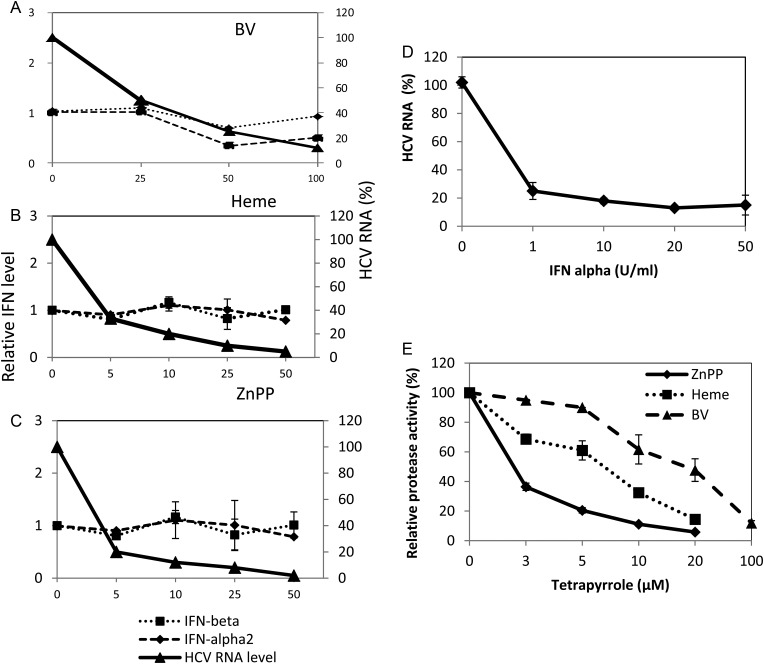
We have shown elsewhere that the tetrapyrroles BV and heme inhibit NS3/4A protease and severely attenuate HCV replication in replicons and infected hepatocytes [14]. To investigate whether tetrapyrrole antiviral activity might also be accompanied by IFN induction in replicons, we assayed IFN-α and IFN-β messenger RNA (mRNA) levels after incubation with various concentrations of BV, heme, and zinc protoporphyrin (ZnPP) (Figure 1). Although all the tetrapyrroles potently inhibited HCV replication as well as NS3/4A protease activity, they did not significantly elevate type I IFN mRNA levels (Figure 1A–D), which did not change even when >90% of viral RNA was eliminated. Similar results were obtained with full-length Con 1 replicons [21], indicating that antiviral activity was not selective for NS replicons (not shown). We also considered the possibility that these replicons might be refractory to IFN. However, addition of exogenous IFN rapidly reduced HCV replication (Figure 1E), and we have shown elsewhere that tetrapyrroles such as BV actually increase the antiviral activity of exogenous IFN-α [14].
Figure 1.
Antiprotease/antireplication activity of tetrapyrroles without interferon (IFN) induction. Nonstructural hepatitis C virus (HCV) replicon cells were treated for 48 hours with biliverdin (BV; A), heme (B), zinc protoporphyrin (ZnPP) (C), or exogenous IFN-β (D). Total RNA from cellular lysates was then assayed for both HCV RNA (A–D) and IFN-α and IFN-β messenger RNA (mRNA; A–C) with real-time reverse-transcription polymerase chain reaction, using the comparative cycle threshold method with glyceraldehyde phosphate dehydrogenase (GAPDH) as the housekeeping gene standard. For panels A–C, comparable data were obtained for INF-β promoter activation with luciferase assays. E, Inhibitory activity of each tetrapyrrole for recombinant NS3/4A protease was determined independently with fluorescence resonance energy transfer (FRET) assays, as described in the Supplementary Material. Results are shown as means ± standard errors of the mean for 6 determinations per point. Where error bars are not visible, the range is included in the size of the symbol.
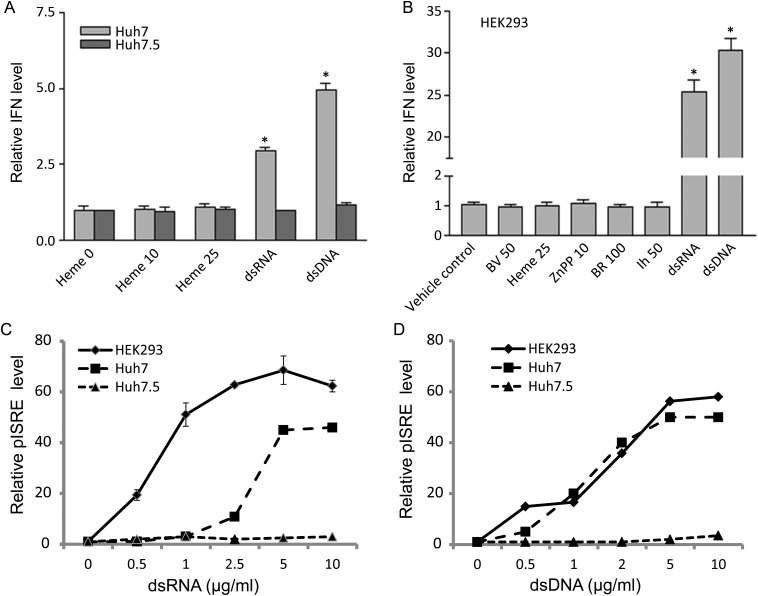
It is known that cell lines permissive for HCV, such as Huh 7.5, generally have poor type I IFN induction responses [22, 23]. Consequently, it was important to test whether cells with intact IFN induction systems, such as wild-type (wt) Huh 7 and human embryonic kidney (HEK) 293 cells, would induce IFN in response to a tetrapyrrole. No cell line tested, including wt Huh 7, the permissive clonal line Huh 7.5 (Figure 2A), and HEK 293 (Figure 2B), showed induction of IFN in response to tetrapyrroles. However, classic nucleic acid antigens, such as double-stranded RNA (dsRNA) or double-stranded DNA (dsDNA), both elicited significant IFN induction in either wt Huh 7 or HEK 293 cells (Figure 2A and B). As might be expected, the HCV-permissive Huh 7.5 cells or their replicon containing clonal lines (also see Figure 1) did not show appreciable IFN induction when transfected with similar amounts of double-stranded nucleic acids. The differences in cell line responsiveness to IFN were further confirmed with luciferase assays for IFN-stimulated response elements in response to double-stranded nucleic acid antigens (Figure 2C and D). Collectively, the findings of Figures 1 and 2 indicate that tetrapyrroles do not directly induce type I IFN and that their antiviral activity is more likely to be mediated through direct inhibition of NS/34A protease.
Figure 2.
A and B. Type I interferon (IFN) induction with double-stranded nucleic acid compared with tetrapyrrole. A, B, Huh 7 and Huh 7.5 (A) or human embryonic kidney (HEK) 293 (B) cell lines were stimulated with the indicated amounts of double-stranded nucleic acid (2 ug/ml) or tetrapyrrole (umoles/liter), together with type I IFN promoter and controls, as described in the Supplementary Material. After 24 hours, cells were lysed, and promoter activation was assayed with luciferase assay. BR, bilirubin; BV, biliverdin. C and D, HEK 293, Huh 7, or Huh 7.5 cells were transfected with double-stranded RNA (dsRNA; C) or double-stranded DNA (dsDNA; D), together with interferon stimulatory response element (pISRE) promoter and transfection control, as described in the Supplementary Material. After 24 hours, cells were lysed and promoter activation was assayed with luciferase assay. All fluorescence units are relative to controls, which received no double-stranded nucleic acid, and all points were corrected for transfection efficiency using a Ranilla construct, as described in the Supplementary Material; each point is the mean ± standard error of the mean for 6 determinations. *P < .01.
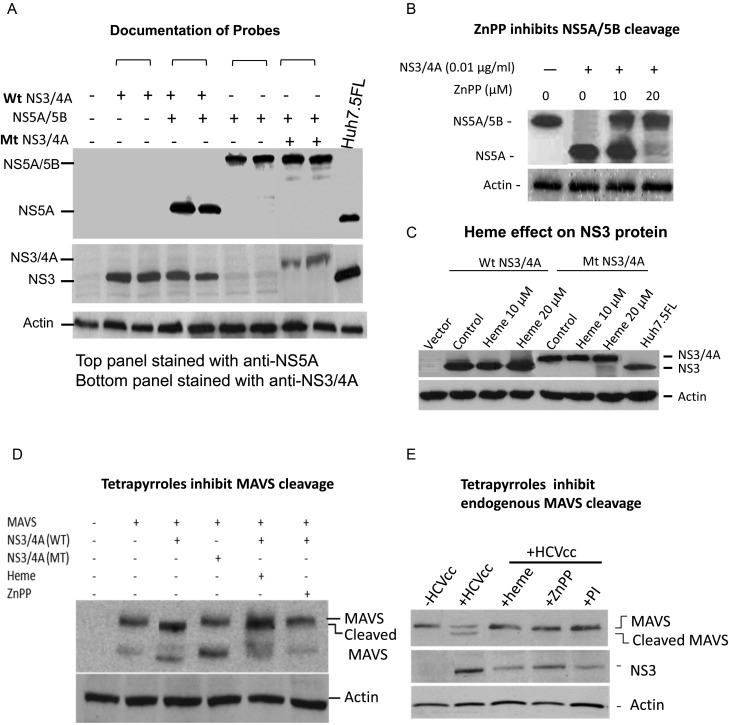
We next documented that tetrapyrroles can inhibit NS3/4A cleavage sites intracellularly. These experiments were important because tetrapyrroles and metalloporphyrins have variable solubility profiles and are extensively bound to protein and/or lipid within blood and cells. We transfected cloned sequences of tandem NS5A/5B (with conserved NS3/4A cleavage site) into HEK 293 cells, either with or without concomitant transfection of NS3/4A (Figure 3A). Expression of NS5A/5B without protease showed a high-molecular-weight product (120 K), after staining with anti-NS5A antibody probe on Western blots. However, cotransfection of NS5A/5B sequences with protease showed virtually all immunoreactive protein at the appropriate mobility for NS5A (65 K), indicating complete cleavage of the tandem protein. When these experiments were conducted in the presence of active tetrapyrroles, such as ZnPP, NS3/4A activity was clearly inhibited (Figure 3B). We also prepared an inactive mutant of NS3/4A by changing the catalytic serine (serine 139) of the enzyme's active site (serine 1165 HCV-H strain) to an inert alanine, using site-directed polymerase chain reaction. As expected, this mutant was unable to cleave NS5A/5B, and because it was also unable to cis-cleave the linked NS4A protein fragment after translation, the mutant protein migrated more heavily on sodium dodecyl sulfate gels [24–26] (Figure 3A). This probe was used as a control in the experiments below to ensure that NS3/4A was not affecting an extraviral site that does not depend on protease activity [6]. A final control experiment showed that tetrapyrroles do not indirectly affect the amount of NS3/4A protein, nor do they affect the cis processing of wt enzyme or the lack of processing of the mutant enzyme in transfected cells. Interference with these activities might account for protease inhibition (Figure 3C).
Figure 3.
Western blot (WB) analysis of NS3/4A protease, NS5A-B constructs, and mitochondrial antiviral signaling protein (MAVS). A, Documentation of probes. pcDNA3.1 vectors wild-type (wt) NS3/4A, NS5A/5B, and mutant (mt) NS3/4A were transfected into human embryonic kidney (HEK) 293 cells. After 48 hours, denatured cellular lysates were separated on sodium dodecyl sulfate gels and WB stained with antibodies to NS3/4A (middle panel) or NS5A (top panel). Cellular lysates of full-length replicon were used as appropriate controls to verify reactivity and position of authentic viral enzyme-processed NS5A. B, zinc protoporphyrin (ZnPP) inhibits NS5A/5B cleavage. HEK 293 cells were transfected with vectors containing linked NS5A/5B and NS3/4A and then incubated with ZnPP for 48 hours. Denatured cellular lysates were then assayed on WBs and stained for NS5A. At the indicated concentrations of NS3/4A vector, the enzyme was not reliably detectable on WBs. C, Heme effect on NS3 protein. HEK 293 cells were transfected with vectors containing NS3/4A wt or NS3/4A mt, then incubated with medium containing 10 or 20 µmol/L heme or control vehicle for 48 hours. Denatured protein extracts were then assayed on Western blots stained with antiNS3/4A antibody. D, Tetrapyrroles inhibit MAVS cleavage. HEK 293 cells were transfected with vectors containing full-length MAVS sequences, and/or NS3/4A sequences or controls for 24 hours. Cells were then incubated with heme (20 µmol/L) or ZnPP (10 µmol/L) for 24 hours, harvested, denatured, and assayed on WBs using anti MAVS antibody. The pattern of immunoreactive MAVS bands at about 75 and 57 kDa correspond to the MAVS pattern described by Seth et al [27]; the pattern of NS3/4A MAVS digestion, with a 75-kDa band shift to smaller species, is well described and indicated. E, Tetrapyrroles inhibit endogenous MAVS cleavage. Huh 7.5 cells were infected with J6/JFH HCV cell culture (HCVcc) RNA, as described in the Supplementary Material. After 48 hours, cells were incubated with heme (20 µmol/L), ZnPP (10 µmol/L), or protease inhibitor (AnaSpec 25346; 50 µmol/L) for 48 hours. Cells were then harvested, denatured, and assayed for endogenous MAVS on WBs using anti-MAVS antibody.
NS3/4A is also known to cleave and allow further degradation of the adapter protein MAVS, thus interrupting a crucial link between nucleic acid detection in the cytoplasm with signaling for induction of type I IFN [6]. Transfection of full-length MAVS sequences into HEK 293 cells showed expression of a 75 KD exogenous immunoreactive protein with smaller bands at 57 kDa, as described by Seth et al [27]. The pattern showed cleavage of the 75-kDa band to a slightly smaller fragment with wt but not mutant NS34A (Figure 3D). However, incubation of MAVS and wt NS3/4A transfected cells with heme or ZnPP showed that MAVS cleavage was inhibited (Figure 3D). Finally, we also evaluated endogenous MAVS expression in permissive Huh 7.5 cells after infection with clonal J6/JFH infectious HCV. Overall, 75-kDa expression of MAVS was decreased after infection, with appearance of a cleaved MAVS fragment (Figure 3E). MAVS cleavage and degradation were clearly inhibited with heme, ZnPP, or the known PI Anaspec 25346 (Figure 3E). Collectively, these findings show that tetrapyrroles can inhibit cleavage of both host cytoplasmic NS3/4A target sites such as the MAVS adapter protein, as well as genuine viral target sites, such as the NS5A-NS5B junction.
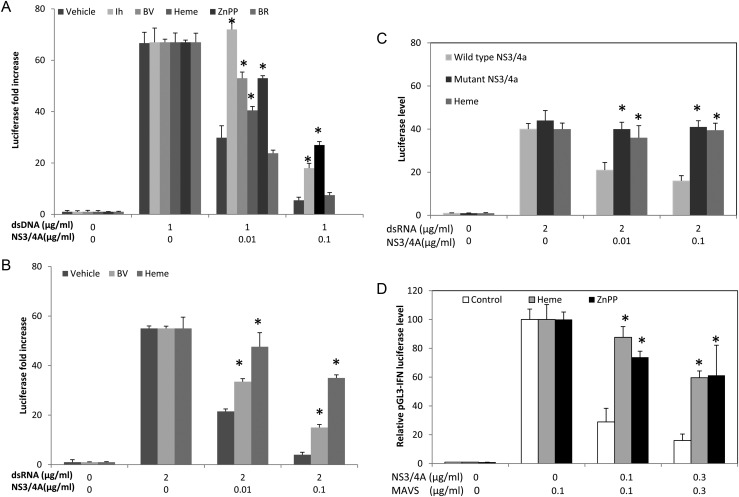
We next investigated whether tetrapyrroles are capable of restoring type I IFN induction after attenuation with NS3/4A. IFN induction was initiated with dsRNA or dsDNA surrogate antigens, which both can use MAVS adapter and signaling for IFN regulatory factor (IRF) 3 activation and inhibition with NS3/4A [22]. Transfection of double-stranded nucleic acid into HEK 293 cells showed vigorous IFN induction, but this was markedly reduced when cells also received NS3/4A (Figure 4). Biliverdin, heme, and ZnPP, as well as the PI (Anaspec 25346), significantly restored induction after protease; depending on protease and substrate concentrations, restoration was nearly complete in some cases (Figure 4A and B). However, in another experiment, we investigated whether a tetrapyrrole with only weak antiprotease activity, such as BR (Kd > 300 µm) [14], could restore IFN induction; BR was ineffective in counteracting the effects of NS3/4A protease (Figure 4A).
Figure 4.
Restoration of interferon (IFN) promoter activation with tetrapyrrole after interference with NS3/4A. A and B, Human embryonic kidney (HEK) 293 cells were transfected with double-stranded DNA (dsDNA; A) or RNA (dsRNA; B) antigens, together with vectors containing IFN-β luciferase promoter (pGL3-IFN-ß) and NS3/4A sequences or controls for 24 hours. Cells were then incubated with heme (20 µmol/L), Zinc protoporphyrin (ZnPP) (20 µmol/L), biliverdin (BV; 50 µmol/L), or bilirubin (BR; 100 µmol/L) for 24 hours. Cellular lysates were assayed for IFN-β promoter activation using luciferase assay. C, HEK 293 cells were transfected with dsRNA together with vectors containing IFN-β luciferase promoter, NS3/4A sequences (either wild-type or mutant protease), or control vectors. Cells were then incubated with heme (20 µmol/L) or control vehicle for 24 hours, and then cellular lysates were assayed for IFN-β promoter activation using luciferase assay. D, HEK 293 cells were transfected with plasmid vector containing mitochondrial antiviral signaling protein (MAVS) sequences, together with vectors containing IFN-β luciferase promoter, NS3/4A sequences, or empty vector controls. Cells were then incubated with heme (20 µmol/L), ZnPP (10 µmol/L), or control vehicle for 24 hours, and cellular lysates then assayed for IFN-β promoter activation using luciferase assay. In all panels, each point represents the overall mean ± standard error of the mean for 6 determinations per point, with 2 culture wells per point and 3 determinations per culture well. *P < .01.
As expected, further experiments with mutant NS3/4A failed to show attenuation of type I IFN induction as compared to wt NS3/4A (Figure 4C). Furthermore, transfection of mutant enzyme and incubation with heme did not augment type I IFN induction, indicating that heme can rescue IFN induction only after inhibition with a competent protease (Figure 4C). Finally, MAVS, a known potent inducer of IFN-β [27], was markedly inhibited by cotransfection of NS3/4A into HEK 293 cells (Figure 4D); however, inhibition was nearly completely reversed by incubation with heme or ZnPP (Figure 4D).
As a group, tetrapyrroles have signaling contacts with cellular proliferation and apoptosis pathways [16, 28]. Consequently, a confounding effect of tetrapyrrole on these pathways had to be considered as an alternative explanation for the restoration of IFN induction. We have shown elsewhere that BV and BR do not influence cellular growth or viability at working concentrations of ≤100 µmol/L in our system [14]. Routine assays for the effects of heme and ZnPP on these parameters were performed using trypan blue exclusion and cell growth studies (Supplementary Figure 1). Heme had no effect on viability or growth at concentrations of ≤20 µmol/L (Supplementary Figure 1A). However, ZnPP caused 10% and 40% growth reduction at 10 and 20 µmol/L, respectively without significant changes in cellular viability (Supplementary Figure 1B). Although a proliferation or apoptotic confounding effect of ZnPP on IFN induction and/or restoration is possible, these can be eliminated for heme and BV. Most importantly, all 3 tetrapyrroles showed the same effects on IFN restoration.
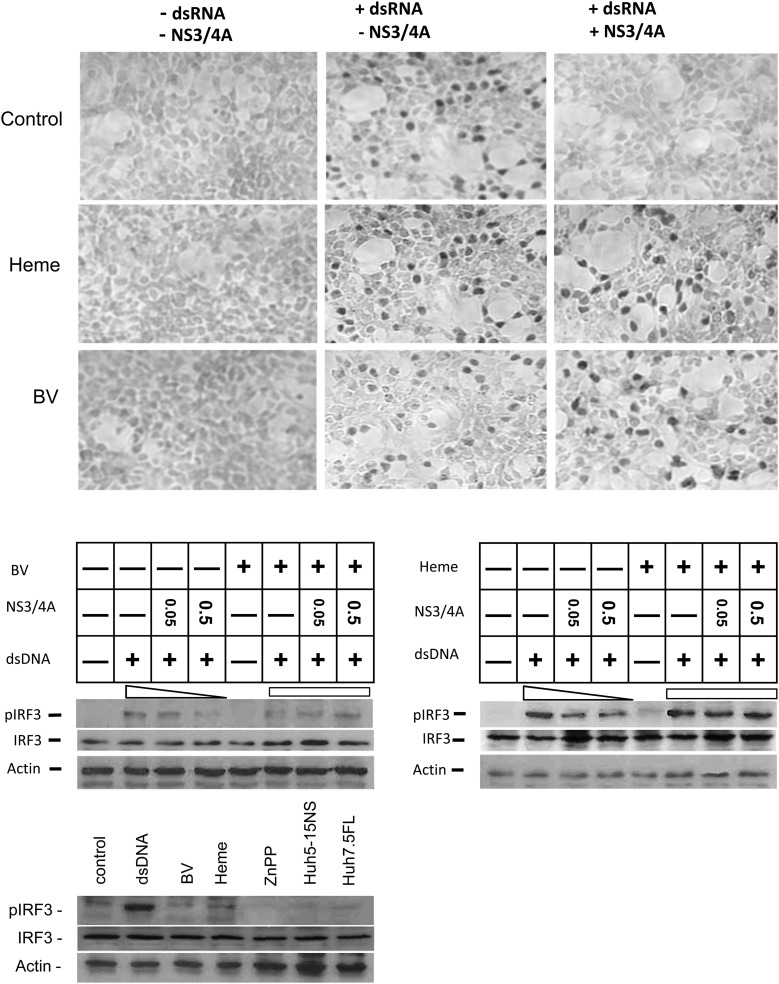
Toll-like receptor (TLR) 3 and retinoic acid–inducible gene I (RIG-I) signaling pathways depend on specific activation of transcriptional factors IRF-3 and IRF-7, with phosphorylation, nuclear translocation, and increased transcription of type I IFN [29]. Consequently, restoration of IFN signaling with tetrapyrrole after protease antagonism should be accompanied by IRF-3 activation and translocation into the nucleus. This hypothesis was tested in the experiments shown in Figure 5. Cells transfected with dsRNA or dsDNA (not shown) had increased nuclear localization of IRF-3 (Figure 5, immunohistochemistry panel), which was nearly eliminated if cells were cotransfected with NS3/4A. However, incubation of protease transfected cells with heme or BV resulted in recovery of IRF-3 nuclear translocation.
Figure 5.
Biliverdin (BV) or heme can restore interferon (IFN) regulatory factor (IRF) 3 activation after inhibition of IFN signaling with NS3/4A. Human embryonic kidney (HEK) 293 cells were transfected with double-stranded nucleic acid antigens, NS3/4A-containing vectors, or controls. After 24 hours, cells were incubated with heme, BV, or Zinc protoporphyrin (ZnPP) for 48 hours. Upper panels show cells stained immunohistochemically for phosphorylated IRF-3, as described in the Supplementary Material. Lower panels show Western blot analysis for phosphorylated IRF-3 (pIRF-3), performed on cellular lysates with specific antibody. Note that BV or heme can restore the loss of IRF-3 phosphorylation and nuclear translocation that is caused by NS3/4A. Abbreviations: dsDNA, double-stranded DNA; dsRNA, double-stranded RNA; FL, full length; NS, non-structural.
Similar experiments were performed in which IRF-3 activation was directly measured with specific antibody for phosphorylated IRF-3 on Western blots. These confirmed that NS3/4A inhibition of IRF-3 activation could be significantly reversed with the tetrapyrroles (Figure 5; Western blot, middle panels). Here again, controls that received tetrapyrrole without protease showed little activation of IRF-3 in HEK 293 or replicon cells, indicating that tetrapyrroles have little direct ability to induce IRF-3 activation. These findings support the hypothesis that tetrapyrroles primarily behave as antiviral agents through antagonism of NS3/4A protease and not through direct type I IFN induction.
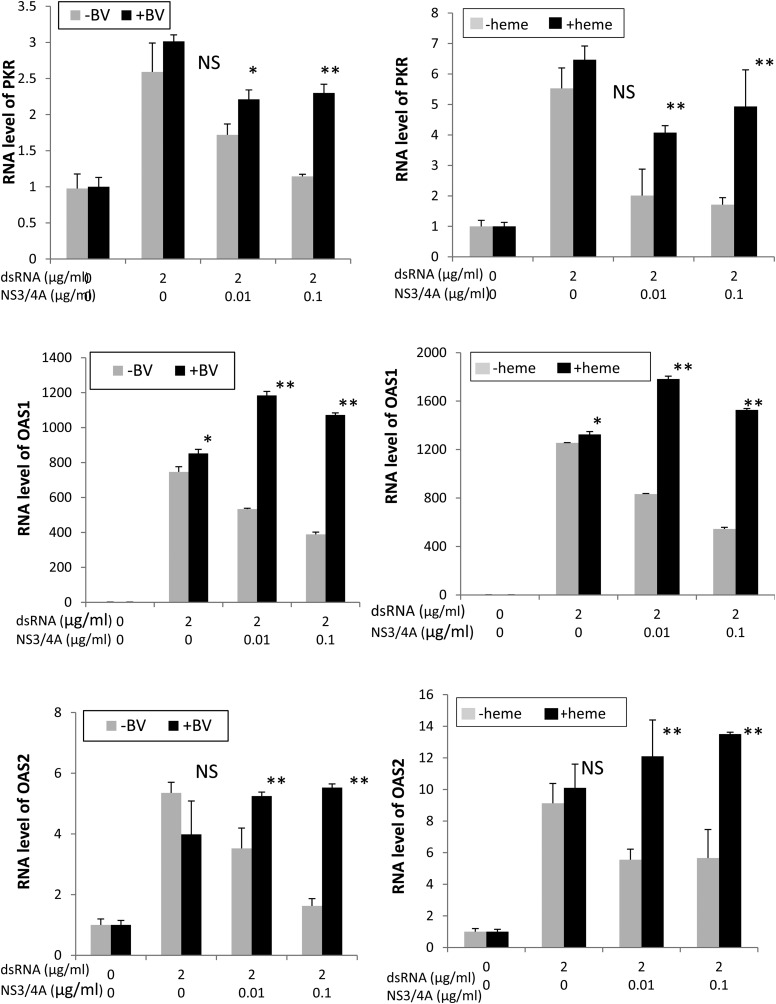
Finally, we studied whether restoration of IFN signaling with tetrapyrrole was accompanied by increased transcription of IFN-stimulated response genes (ISRGs) (Figure 6). Neither BV nor heme was active in inducing ISRG mRNA when incubated with cells alone. However, stimulation of cells with double-stranded nucleic acid led to increased induction of ISRG mRNA, which was markedly attenuated with NS3/4A. Similar to the IFN induction findings noted earlier, the antagonism of NS3/4A was easily reversed with either BV or heme, which significantly restored and occasionally augmented ISRG expression in every case. Note also that tetrapyrroles did not elevate ISRGs without double-stranded nucleic acid stimulation, indicating that they do not directly stimulate ISRGs through non-IFN signaling mechanisms.
Figure 6.
Restoration of interferon gene transcription with biliverdin (BV) or heme. Human embryonic kidney (HEK) 293 cells were transfected with double-stranded RNA (dsRNA) antigens and vectors containing protease sequences or controls. Cells were then incubated with heme (20 µmol/L), BV (50 µmol/L), or control vehicle for 24 hours. RNA was prepared from cellular lysates and assayed for 2″-5′ oligoadenylate synthetase 1 (OAS1), activated protein kinase (PKR) and 2′-5′ oligoadenylate synthetase 2 (OAS2) messenger RNA, as described in the Supplementary Material. *P < .05; **P < .01; Abbreviation: NS, not significant.
DISCUSSION
The clinical need for new direct-acting antiviral agents against HCV has spurred intense basic and translational science research efforts. It is now established that the precursors and catalytic products of the HO-1 system can behave as direct-acting anti-HCV agents. Iron can inhibit HCV RNA polymerase (NS5B) through competitive binding at the enzyme's divalent cation binding site [30, 31]. Induction or overexpression of HO-1 in replicons was shown to arrest viral replication [12, 13]. More recently, BV was demonstrated to be a potent direct inhibitor of NS3/4A protease, whereas its reduction product, BR, and most other linear tetrapyrroles were shown to have weak antiprotease and antiviral activity [14]. Furthermore, porphyrin ring tetrapyrroles, such as heme, ZnPP, tin protoporphyrin (SnPP), and cobalt protoporphyrin (CoPP) also inhibit NS3/4A protease, resulting in potent antiviral activity in vitro [32].
The NS3/4A protease is an important target for antiviral drugs, not only because it is essential for HCV polyprotein processing but also because it has extraviral proteolytic activities that inhibit innate immune recognition systems of the host cell. Viruses are sensed by intra- and extracellular PRRs that activate nuclear signaling for type I IFN transcription, thus facilitating an antiviral state [7]. Signaling occurs primarily through the RIG-I, melanoma differentiation–associated gene 5 (MDA5), and TLR systems. The NS3/4A protease cleaves ≥2 cellular adapter proteins of these pathways, MAVS and TRIF [5, 8, 9, 33, 34], thus attenuating host innate immune responses and IFN induction. The extraviral activities of NS3/4A are thought to help the virus evade host clearance mechanisms and enhance viral fitness [6].
The primary goal of our work was to further characterize the antiviral activity of BV and related tetrapyrroles. Lehmann et al [15] recently reported that BV can induce type I IFN in nonstructural replicons, but the mechanism was not shown. In the present study, we could not demonstrate direct induction of type I IFN by any tetrapyrrole under conditions in which exposure to classic dsRNA or DNA antigens led to vigorous IFN induction. This was evident not only for nonstructural replicons similar to those used by Lehmann et al [15] (Huh 5.15 nonstructural subgenomic replicons [35]) but also for wt HEK 293 cells, which are a common cellular model for immune activation and are known to be highly responsive to type I IFN induction. Although clonal variations of replicons may explain some of the differences in findings, it is also possible that extended treatment of replicons with tetrapyrroles may lead to enough recovery of signaling pathways to allow IFN induction because of replicon RNA. On the other hand, HCV-permissive variants of Huh 7 cells, such as Huh 7.5, lack parts of both TLR3 and RIG-I sensing systems, resulting in weak IFN induction profiles in replicons regardless of stimulus [36, 37]. Potentially, tetrapyrroles could also influence downstream signaling for IFN induction, but this seems doubtful, because we observed no activation or nuclear translocation of IRF-3, nor production of effector ISRG with tetrapyrrole alone (Figures 5 and 6 respectively). Although our findings do not rule out a direct mechanism for IFN induction by tetrapyrroles, they do show that these agents are capable of inhibiting both viral and host protease targets intracellularly, which are important considerations for further development of new antiviral agents.
Although the tetrapyrroles did not directly induce type I IFN, they significantly restored IFN signaling in cells after attenuation with NS3/4A protease. Restoration of signaling with tetrapyrrole was further documented with enhanced IRF-3 activation, increased IRF-3 nuclear localization, and concomitant recovery of ISRG expression. The increased ISRG expression with tetrapyrrole after double-stranded nucleic acid induction also supports previous data showing that BV enhances exogenous IFN antiviral activity in replicon cells [14]. Additional controls using a mutant NS3/4A protease, which did not inhibit IFN signaling, had no effect on IFN induction even if present with tetrapyrrole. These experiments ensured that tetrapyrroles were not interacting with NS3/4A in a nonantiprotease manner that could conceivably lead to increased type I IFN induction. Overall, these findings show that tetrapyrroles can interact with host NS3/4A targets and positively influence host immune recognition and signaling pathways. These are favorable characteristics for potential therapeutic applications of tetrapyrroles which also offer antioxidative, antiinflammatory, and other proposed immunotherapeutic benefits [38].
It is interesting that restoration of IFN signaling was observed with tetrapyrrole concentrations quite close to those necessary for inhibition of NS3/4A protease and HCV replication in replicons. In contrast, other antiprotease agents rescued IFN induction only at 100X the antiviral EC50 for protease inhibition [10]. This was also seen, but to a lesser extent with the positive control PI used here, AnaSpec 25346 (EC50 = 5 µmol/L vs IFN restoration = 50 µmol/L, Figure 4B). While the therapeutic possibilities of tetrapyrroles require further study, the present findings suggest a potential advantage for use of tetrapyrroles as compared to other classes of PIs.
Both dsDNA and dsRNA were used interchangeably as generic nucleic acid antigens in this study, and both showed equivalent data. Sensing and signal transduction of both nucleic acids have remarkable similarities in humans, inasmuch as they use the IRF-3 pathway and dsDNA induction is also inhibited with NS3/4A protease, probably through MAVS cleavage occurring in human cells [22]. In the RIG-I/MAVS pathway, some DNA is probably sensed through the generation of a RNA polymerase III transcript [39, 40]. Nevertheless, the ability of tetrapyrroles to restore IFN induction of dsDNA signaling suggests potential use with DNA viruses. It should be pointed out that heme and substituted heme derivatives generated early interest as therapeutic agents for the DNA virus hepatitis B virus and retroviruses, such as HIV [11]. Earlier experiments demonstrated heme inhibition of HIV reverse transcriptase and enhancement of the antiviral activity of drugs, such as zidovudine [41, 42]; BV, BR, and boron-substituted porphyrins have also been shown to specifically inhibit HIV protease [43, 44]. Recently, heme was also shown to reduce circulating HIV in humanized nonobese diabetic mice with severe combined immunodeficiency carrying infected human peripheral blood mononuclear cells [45]. The latter findings are important, because they document that heme antiviral activity can be established in vivo.
In summary, our findings indicate that the anti-HCV effects of tetrapyrroles can be closely linked to their antiprotease activities and that major effects on type I IFN induction most likely occur through restoration of innate immune system signaling. The added benefit of restoring IFN induction enhances the overall antiviral capabilities of these compounds and suggests that their further development into therapeutic agents for HCV treatment be pursued.
Supplementary Material
Notes
Author contributions. Z. Z. was responsible for acquisition of data, analysis, manuscript editing, and statistics. M. M. M. was responsible for acquisition of data and technical support. W. N. S. was responsible for study funding, design, data interpretation, writing the text, and intellectual content.
Financial support. This work was supported by the US Department of Veterans Affairs (merit review grant to W. N. S.), the University of Iowa Biological Sciences Funding Program (W. N. S.), the American Cancer Society Seed Award (seed award to Z. Z.), and the Doriann Foundation for Hepatitis Research, University of Iowa (W. N. S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429–38. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–9. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firpi RJ, Nelson DR. Current and future hepatitis C therapies. Arch Med Res. 2007;38:678–90. doi: 10.1016/j.arcmed.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Soriano V, Peters MG, Zeuzem S. New therapies for hepatitis C virus infection. Clin Infect Dis. 2009;48:313–20. doi: 10.1086/595848. [DOI] [PubMed] [Google Scholar]
- 5.Bode JG, Brenndorfer ED, Haussinger D. Subversion of innate host antiviral strategies by the hepatitis C virus. Arch Biochem Biophys. 2007;462:254–65. doi: 10.1016/j.abb.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J Biol Chem. 2010;285:22741–7. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalliolias GD, Ivashkiv LB. Overview of the biology of type I interferons. Arthritis Res Ther. 2010;12(Suppl 1):S1. doi: 10.1186/ar2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li K, Foy E, Ferreon JC, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005;102:2992–7. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meylan E, Curran J, Hofmann K, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–72. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 10.Liang YQ, Ishida H, Lenz O, et al. Antiviral suppression vs restoration of RIG-I signaling by hepatitis C protease and polymerase inhibitors. Gastroenterology. 2008;135:1710–8. doi: 10.1053/j.gastro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt WN, Mathahs MM, Zhu Z. Heme and HO-1 inhibition of HCV, HBV, and HIV. Front Pharmacol. 2012;3:129. doi: 10.3389/fphar.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shan Y, Zheng J, Lambrecht RW, Bonkovsky HL. Reciprocal effects of micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology. 2007;133:1166–74. doi: 10.1053/j.gastro.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Z, Wilson AT, Mathahs MM, et al. Heme oxygenase-1 suppresses hepatitis C virus replication and increases resistance of hepatocytes to oxidant injury. Hepatology. 2008;48:1430–9. doi: 10.1002/hep.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Z, Wilson AT, Luxon BA, et al. Biliverdin inhibits hepatitis C virus nonstructural 3/4A protease activity: mechanism for the antiviral effects of heme oxygenase? Hepatology. 2010;52:1897–905. doi: 10.1002/hep.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmann E, El-Tantawy WH, Ocker M, et al. The heme oxygenase 1 product biliverdin interferes with hepatitis C virus replication by increasing antiviral interferon response. Hepatology. 2010;51:398–404. doi: 10.1002/hep.23339. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs PE, Maines MD. Biliverdin inhibits activation of NF-kappaB: reversal of inhibition by human biliverdin reductase. Int J Cancer. 2007;121:2567–74. doi: 10.1002/ijc.22978. [DOI] [PubMed] [Google Scholar]
- 17.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30:129–37. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Scott JR, Chin BY, Bilban MH, Otterbein LE. Restoring homeostasis: is heme oxygenase-1 ready for the clinic? Trends Pharmacol Sci. 2007;28:200–5. doi: 10.1016/j.tips.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med. 2009;206:1167–79. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayan V, Mueller S, Baumgart-Vogt E, Immenschuh S. Heme oxygenase-1 as a therapeutic target in inflammatory disorders of the gastrointestinal tract. World J Gastroenterol. 2010;16:3112–9. doi: 10.3748/wjg.v16.i25.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–14. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng G, Zhong J, Chung J, Chisari FV. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc Natl Acad Sci U S A. 2007;104:9035–40. doi: 10.1073/pnas.0703285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stumper R, Loo YM, Foy E, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–99. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartenschlager R, Ahlbornlaake L, Mous J, Jacobsen H. Kinetic and structural-analyses of hepatitis-C virus polyprotein processing. J Virol. 1994;68:5045–55. doi: 10.1128/jvi.68.8.5045-5055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Failla C, Tomei L, Defrancesco R. Both Ns3 and Ns4a are required for proteolytic processing of hepatitis-C virus nonstructural proteins. J Virol. 1994;68:3753–60. doi: 10.1128/jvi.68.6.3753-3760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin C, Thomson JA, Rice CM. A central region in the hepatitis-C virus Ns4a protein allows formation of an active Ns3-Ns4a serine proteinase complex in-vivo and in-vitro. J Virol. 1995;69:4373–80. doi: 10.1128/jvi.69.7.4373-4380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seth RB, Sun LJ, Ea CK, Chen ZJJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappa B and IRF3. Cell. 2005;122:669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 28.La P, Fernando AP, Wang Z, et al. Zinc protoporphyrin regulates cyclin D1 expression independent of heme oxygenase inhibition. J Biol Chem. 2009;284:36302–11. doi: 10.1074/jbc.M109.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Fillebeen C, Muckenthaler M, Andricipoulos B, et al. Expression of the subgenomic hepatitis C virus replicon alters iron homeostasis in Huh7 cells. J Hepatol. 2007;47:12–22. doi: 10.1016/j.jhep.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 31.Fillebeen C, Rivas-Estilla AM, Bisaillon M, et al. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C Virus. J Biol Chem. 2005;280:9049–57. doi: 10.1074/jbc.M412687200. [DOI] [PubMed] [Google Scholar]
- 32.Zhu ZW, Mathahs MM, Schmidt WN. Biliverdin and heme restore type I interferon expression in cells expressing Hcv Ns3/4a protease. Hepatology. 2011;54:541a. [Google Scholar]
- 33.Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–45. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 34.Kumar H, Kawai T, Kato H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohmann V, Korner F, Koch JO, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–3. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 36.Li K, Chen ZH, Kato N, Gale M, Lemon SM. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J Biol Chem. 2005;280:16739–47. doi: 10.1074/jbc.M414139200. [DOI] [PubMed] [Google Scholar]
- 37.Sumpter R, Jr, Loo YM, Foy E, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–99. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 39.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nature Immunol. 2009;10:1065–72. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu YH, MacMillan JB, Chen ZJJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–91. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Argyris EG, Vanderkooi JM, Venkateswaran PS, Kay BK, Paterson Y. The connection domain is implicated in metalloporphyrin binding and inhibition of HIV reverse transcriptase. J Biol Chem. 1999;274:1549–56. doi: 10.1074/jbc.274.3.1549. [DOI] [PubMed] [Google Scholar]
- 42.Levere RD, Gong YF, Kappas A, Bucher DJ, Wormser GP, Abraham NG. Heme inhibits human immunodeficiency virus 1 replication in cell cultures and enhances the antiviral effect of zidovudine. Proc Natl Acad Sci U S A. 1991;88:1756–9. doi: 10.1073/pnas.88.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McPhee F, Caldera PS, Bemis GW, McDonagh AF, Kuntz ID, Craik CS. Bile pigments as HIV-1 protease inhibitors and their effects on HIV-1 viral maturation and infectivity in vitro. Biochem J. 1996;320:681–6. doi: 10.1042/bj3200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decamp DL, Babe LM, Salto R, et al. Specific-inhibition of HIV-1 protease by boronated porphyrins. J Med Chem. 1992;35:3426–8. doi: 10.1021/jm00096a020. [DOI] [PubMed] [Google Scholar]
- 45.Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol. 2006;176:4252–7. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.