Abstract
Background and study aims Accurate diagnosis and classification of pancreatic cysts (PCs) remains a challenge. The aims of this study were to: (1) evaluate the safety and technical success of a novel microforceps for EUS-guided through-the-needle biopsy (TTNB) of PCs; and (2) assess its diagnostic yield for mucinous PCs when compared to FNA cyst fluid analysis and cytology.
Patients and methods This was a multicenter retrospective analysis of 47 patients who underwent EUS-FNA and TTNB for PCs between January 2014 and June 2017. Technical success was defined as acquisition of a specimen adequate for cytologic or histological evaluation. Cyst fluid carcinoembryonic antigen (CEA) was used to initially categorize cysts as non-mucinous (CEA < 192 ng/mL) or mucinous (CEA ≥ 192 ng/mL). Final diagnosis was based on identifiable mucinous pancreatic cystic epithelium on cytology, microforceps histology and/or surgical histology when available.
Results Forty-seven patients with PCs (mean size 30.7 mm) were included. TTNB was successfully performed in 46 of 47 (97.9 %). Technical success was significantly lower with FNA (48.9 %) compared to TTNB (85.1 %) ( P < .001). For cysts with insufficient amount of fluid for CEA (n = 19) or CEA < 192 ng/mL, the cumulative incremental diagnostic yield of a mucinous PC was significantly higher with TTNB vs. FNA (52.6 % vs 18.4 %; P = .004). TTNB alone (34.4 %) diagnosed more mucinous PCs than either CEA ≥ 192 ng/mL alone (6.3 %) or when combined with FNA cytology (9.4 %). One episode of self-limited bleeding (2.1 %) and one of pancreatitis (2.1 %) occurred.
Conclusions EUS-TTNB is safe and effective for evaluating PCs. TTNB may help increase the diagnostic yield of mucinous PCs.
Introduction
Pancreatic cysts (PCs) are being diagnosed with increasing frequency because of the pervasive use of cross-sectional imaging 1 . These lesions can be inflammatory or neoplastic, with prevalence of pancreatic cystic neoplasms (PCNs) in the general population estimated to be as high as 13.5 % 2 . Given the malignant potential of PCNs, accurate diagnosis and risk stratification are fundamental in directing the most appropriate management strategy, which includes surgical resection for those at high risk of malignant transformation.
Endoscopic ultrasound (EUS) has been shown to increase the diagnostic yield of PCNs over cross-sectional imaging 3 , and is the test of choice for select lesions with high-risk features 4 . EUS with fine-needle aspiration (FNA) for cytology and cyst fluid analysis for carcinoembryonic antigen (CEA) is routinely performed for high-risk lesions, but that approach has its limitations related to low sensitivity and specificity 4 5 6 . Hence, in an effort to improve our diagnostic accuracy, multiple adjunct modalities including advanced imaging and the use of molecular markers have garnered significant interest 7 8 9 , although their role in clinical practice is yet to be determined, with availability, reproducibility, and costs to be considered.
Previous studies have shown that targeted cyst wall sampling with the tip of the FNA needle can lead to a modest increase in diagnostic accuracy 10 11 , yet the cytological yield with EUS-FNA remains low due to the relatively small tissue sample that can be obtained via conventional EUS needles. Recently, a through-the-needle forceps device (Moray Micro Forceps, US Endoscopy, Mentor, Ohio, United States) has been introduced as a novel approach for EUS-guided tissue acquisition ( Fig. 1 ). The microforceps can be passed through the lumen of a 19-gauge FNA needle for through-the-needle tissue biopsy (TTNB). Recent reports have supported diagnosis of mucinous PCNs based on TTNB 12 13 14 . The aims of this study were to: (1) evaluate the technical success and safety of EUS-TTNB using the microforceps; and (2) assess its potential incremental diagnostic yield for mucinous PCNs when compared to standard evaluation with CEA and cytology.
Fig. 1 a.
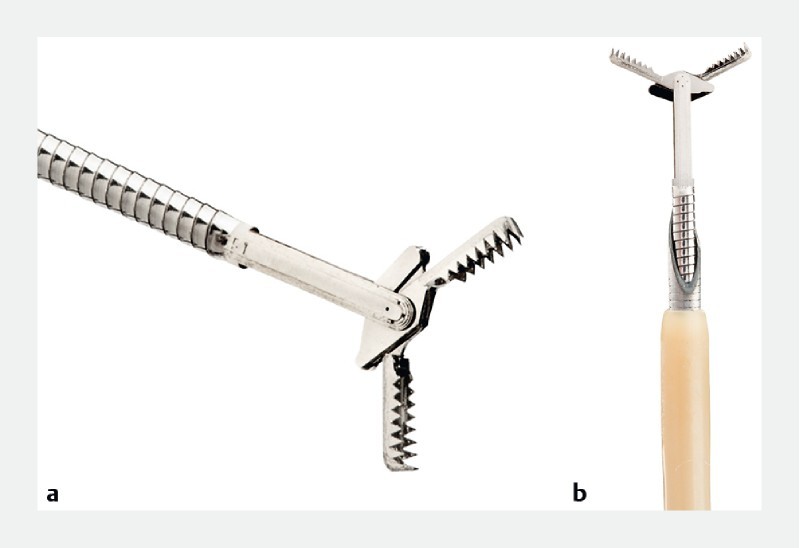
Through-the-needle forceps device with open jaws (4.3 mm) (Moray Micro Forceps, US Endoscopy, Mentor, Ohio, United States). b Through-the-needle forceps device through the bore of a 19-gauge FNA needle. Image is courtesy of US Endoscopy. Unauthorized use not permitted.
Patients and methods
Study population
This was a multicenter observational, retrospective, cohort study of consecutive patients aged ≥ 18 years with PCs who underwent EUS-TTNB at three different centers in the United States between January 1, 2014 to June 1, 2017. All patients referred for EUS-FNA with a PC large enough to accommodate the microforceps (cyst ≥ 10 mm) were included. Indications for EUS-FNA included: (1) new diagnosis of a PC; (2) interval changes in morphology on surveillance of a suspected PCN (e. g. size, mural nodule, solid component); and (3) symptoms (e. g. pancreatitis, abdominal pain, obstructive jaundice). In addition to EUS-FNA and TTNB, one patient included in the study also underwent cystoscopy and confocal laser endomicroscopy as part of her evaluation. This study was approved by the institutional review board for human research at each participating institution, with the University of Florida serving as the central coordinating center. All authors had access to the study data and reviewed and approved the final manuscript.
Endoscopic reports were obtained from prospectively maintained institutional endoscopy electronic reporting databases and subjects’ medical records were retrospectively reviewed. Data obtained from all participating centers were compiled into a central database. Informed procedural consents were obtained for all patients. None of the subjects in our analysis have been included in other prior or current separate studies.
EUS-TTNFB procedure
All EUS procedures were performed by using a curvilinear echoendoscope (GF-UCT140-AL5 or GF-UCT180; Olympus Medical Systems, Center Valley, Pennsylvania, United States) with the patients under either conscious sedation, monitored anesthesia care or general anesthesia. All endoscopic procedures were performed according to the American Society of Gastrointestinal Endoscopy (ASGE) practice guideline recommendations on antibiotic prophylaxis and management of antithrombotic agents and coagulopathy 15 16 .
The target lesion was identified under EUS and punctured under Doppler guidance with a standard 19-gauge FNA needle (EchoTip Ultra, Cook Medical, Bloomington, Indiana, United States). With the needle within the lesion, the style was removed and cyst fluid was aspirated and sent for biochemical analysis (e. g. amylase and CEA) when at least 0.5 to 1 mL was obtained. Following this, with the needle still within the cyst, the microforceps was inserted through the needle for tissue sampling. Two to three “bites” of microforceps biopsy specimens were obtained under EUS-guidance with each pass of the microforceps ( Fig. 2 ). The decision to perform two to three bites with each pass of the microforceps was based on our prior experience with this device 12 13 . Tissue acquisition was visually confirmed by presence of gross specimens on the microforceps jaws, which were then directly placed into formalin containers and sent for evaluation by surgical pathology ( Fig. 3 ). Following this, any remaining cyst fluid was aspirated through the needle and sent for cytology. FNA of the cyst wall, septations, and/or solid components (i. e. mural nodules) was then performed as previously described 11 and the specimens were sent for cytological analysis along with the previously aspirated fluid. No on-site cytopathological examination was performed. All specimens were evaluated by experienced gastrointestinal cytopathologists.
Fig. 2.
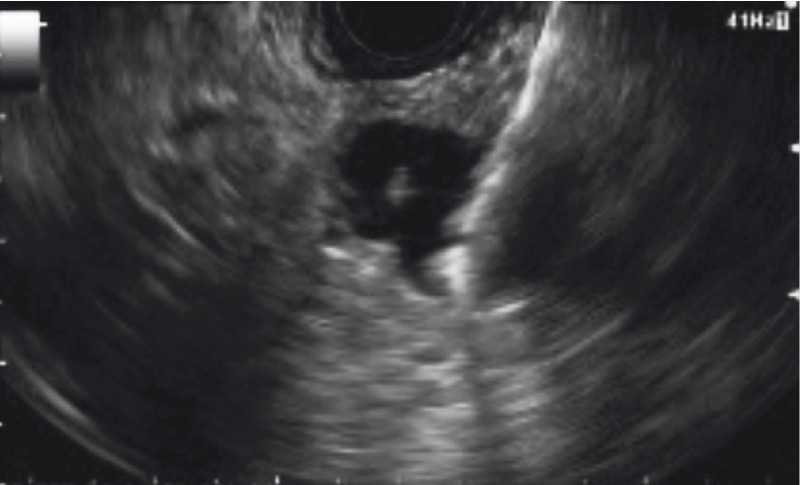
EUS-guided through-the-needle biopsy (TTNB) with the microforceps.
Fig. 3.
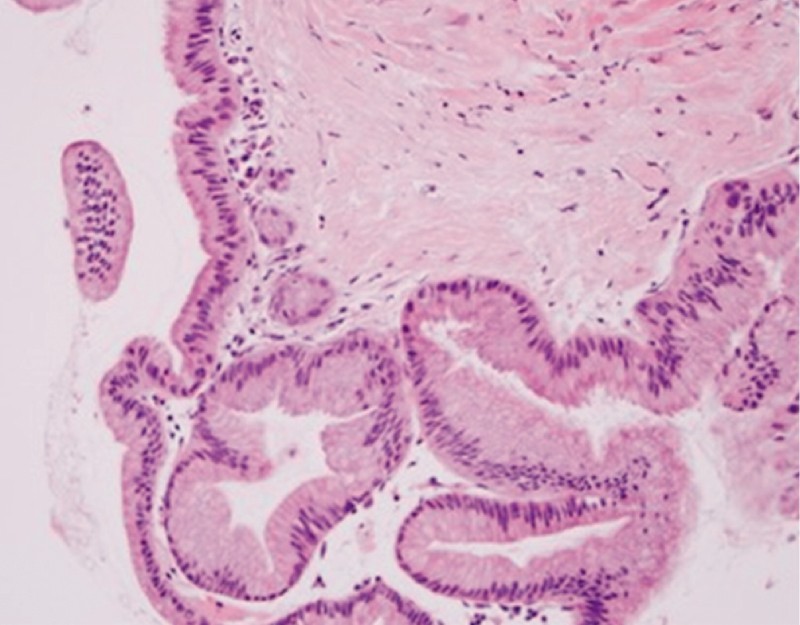
Histologic specimen obtained with TTNB of a pancreas cyst. Mucinous columnar epithelial cells (gastric subtype) of an intrapapillary mucinous neoplasm. Image courtesy of Yuxin Lu, MD; Department of Pathology, University of California, Irvine, California, United States.
Definitions
Technical success by FNA or TTNB was defined as successful tissue acquisition of a specimen adequate for cytologic or histological evaluation. Cyst fluid CEA was used to initially categorize the lesion as likely non-mucinous (CEA < 192 ng/mL) or likely mucinous (CEA ≥ 192 ng/mL) 6 . A cyst was determined to be mucinous if there was identifiable epithelium with characteristics consistent with mucinous pancreatic cystic epithelium on cytology, microforceps histology and/or surgical histology (when available). In the absence of surgical histopathology, TTNB histology, or FNA cytology, a suspected diagnosis of a mucinous cyst was based on a combination of the following information: imaging characteristics, CEA ≥ 192 ng/mL, absent history of acute/chronic pancreatitis, and stable appearance on follow-up imaging at 12 months or later. A suspected diagnosis of a pseudoscyst was based on a combination of a documented history of acute/chronic pancreatitis, cyst fluid characteristics (e. g. thin viscosity, straw, brown color), CEA < 192 ng/mL). Adverse events (AEs) were assessed based on previously established criteria by the ASGE 17 .
Study outcomes
The aims of this study were to: (1) evaluate the technical success and safety of EUS-TTNB using the microforceps for the evaluation of PCs; and (2) assess its potential incremental diagnostic yield for mucinous PCNs when compared to standard evaluation with CEA and cytology.
Statistical analysis
Summary data were expressed as the mean ± standard deviation (SD), median and range. Frequencies and percentages were calculated using basic descriptive statistics. Fisher exact test for categorical variables and the t test for continuous variables were performed when indicated. Nominal P values are reported; P values < 0.05 were considered significant. All statistical analysis was performed with the SPSS software v22 (IBM, SPSS Statistics, Armonk, New York, United States).
Results
Patients and pancreatic cyst characteristics
Forty-seven patients (female 55.3 %; mean age 66.2 years) underwent EUS-TTNB for PCs between January 2014 and June 2017 ( Table 1 ). Most cysts were located in the pancreatic head (16; 34 %), followed by the body (13; 27.7 %), tail (12; 25.5 %) and neck (6; 12.8 %). Mean PC size was 30.8 mm (range 11.6 to 110 mm). Most cysts were multilocular and septated (32; 68.1 %) and approximately one-third were seen to be in communication with the main pancreatic duct (15; 31.9 %). Presence of a mural nodule or solid component was identified in 7 (13.5 %) and 5(9.6 %), respectively.
Table 1. Demographics.
| Age, mean ± SD | 66.2 ± 13.1 years |
| Sex; n (%) | |
|
21 (44.7) |
|
26 (55.3) |
| Past medical history; n (%) | |
|
4 (8.5) |
|
1 (2.1) |
|
1 (2.1) |
| Pancreas cyst size, mean (range) | 30.8 (11.6 – 110) mm |
| Cyst location; n (%) | |
|
16 (34.0) |
|
6 (12.8) |
|
13 (27.7) |
|
12 (25.5) |
| Cyst appearance on EUS; n (%) | |
|
16 (34) |
|
35 (74.5) |
|
7 (14.9) |
|
16 (34) |
|
5 (10.6) |
EUS, endoscopic ultrasound
Fluid analysis
Mean volume of cyst fluid aspirated was 6.2 mL (range 0.2 to 100 mL). An adequate amount of fluid was aspirated for CEA and amylase analysis in 28 patients (59.6 %). Median amylase and CEA levels were 265 U/L (range 11 to > 20,000 U/L) and 50.9 ng/mL (range 0.7 to 2659 ng/mL), respectively. There were 19 patient (40.4 %) with cyst fluid CEA < 192 ng/mL (median 23.6 ng/mL; range 0.7 to 144.3 ng/mL) compared to 9 patients (19.2 %) with CEA ≥ 192 ng/mL (median 327; range 206 to 2659 ng/mL). Cyst fluid for CEA analysis was not available in 19 patients (40.4 %).
EUS-FNA and TTNB procedures
Cyst puncture with a 19-gauge EUS needle was successfully performed in all 47 cases (100 %) with a median number of passes of one (range 1 – 2). Advancement and removal of the microforceps through the indwelling EUS needle was successful in 46 out of 47 cases (97.9 %). In one case, the microforceps was not used because of presence of a bloody aspirate with initial FNA needle puncture. Median number of passes with the microforceps was three (range 0 – 6). Neither needle nor microforceps malfunction was documented.
In all, there was one case of bleeding reported (2.1 %). In that case, initial cyst puncture with the EUS needle yielded a bloody aspirate; hence, TTNB was not attempted. The patient remained asymptomatic without clinical signs of bleeding. One patient (2.1 %) developed acute pancreatitis 48 hours after the procedure. Both cystoscopy (SpyGlass, Boston Scientific Corporation, Marlborough, Massachusetts, United States) and confocal laser endomicroscopy (Mauna Kea Technologies, Paris, France) were performed in this patient at the same session as the FNA/TTNB.
EUS-FNA cytology and EUS-TTNB histology
Technical success, defined as successful tissue acquisition of a specimen adequate for cytologic or histological evaluation, was significantly lower with FNA (23/47 patients; 48.9 %) compared to TTNB (40/47; 85.1 %) ( P < .001). Both cytological and histological diagnoses of cyst sampling are summarized in Table 2 . EUS-FNA cytology and EUS-TTNB histology were diagnostic of a mucinous cyst in 10 and 26 cases, respectively. Four serous cystadenomas were diagnosed on TTNB histopathology. In one patient, EUS-FNA yielded a cytological diagnosis of adenocarcinoma whereas TTNB showed only fibrous tissue. Subsequent surgical pathology in this patient confirmed a benign specimen (false-positive FNA cytology/true-negative TTNB) with no evidence of malignancy.
Table 2. Pancreas cyst cytologic and histopathologic diagnosis.
| EUS-FNA cytology | EUS-TTNB histology | |
| Inadequate specimen | ||
|
0 | 1 |
|
23 | 6 |
| Atypical cells | 2 | 0 |
| Mucinous cyst | 10 | 26 |
| Adenocarcinoma | 1 | 0 |
| Benign fibrous tissue, epithelium or glandular cells | 9 | 7 |
| Cellular debris/inflammatory cells | 2 | 2 |
| Serous cystadenoma | 0 | 4 |
EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration; EUS-TTNS, endoscopic ultrasound-guided through-the-needle biopsy
CEA analysis and incremental diagnostic yield with EUS-FNA cytology and TTNB histology
CEA analysis and incremental diagnostic yield of mucinous PCNs with FNA and TTNB are summarized in Table 3 . Of the 19 cysts without enough fluid for CEA analysis, FNA and TTNB were diagnostic of a mucinous cyst in 6 (31.6 %) and 12 (63.2 %), respectively ( P = 0.1). For cysts with CEA < 192 ng/mL, the incremental diagnostic yield of a mucinous cyst was significantly higher with TTNB (42.1 %) compared to EUS-FNA (5.3 %) ( P = 0.02). In aggregate, the cumulative additional diagnostic yield for mucinous PCNs was 52.6 % with TTNB and 18.4 % with FNA ( P = .004). The mean CEA concentration from cysts with positive TTNB was significantly higher than cysts with negative TTNB (325.1 ng/mL vs. 79.2 ng/mL; P = 0.05).
Table 3. CEA analysis and incremental diagnostic yield of EUS-FNA cytology and EUS-microforceps histology.
| Cysts; n (%) | Median CEA (ng/mL) | Positive FNA cytology (incremental yield%) | Positive TTNB histology (incremental yield %) | P value | |
| CEA not available | 19 | N/A | 6 (31.6 %) | 12 (63.2 %) | .10 |
| CEA < 192 ng/mL | 19 | 23.6 | 1 (5.3 %) | 8 (42.1 %) | .02 |
| CEA > 192 ng/mL | 9 | 327 | 3 (n/a) | 6 (n/a) | N/A |
CEA, carcinoembryonic antigen; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration
Pathological specimen analysis and final diagnosis
Eight (17 %) patients underwent surgical resection ( Table 4 ). The CEA level was either not available (due to insufficient cyst fluid) or < 192 ng/mL in all the PCNs that underwent surgery. Surgical pathology was positive for an intrapapillary mucinous cystic neoplasm (IPMN) in seven cases, of which EUS-FNA and TTNB were diagnostic in two (28.6 %) and 6 (85.7 %) respectively. One patient (Case 6) with no high-risk features on imaging and scant cellularity on EUS-FNA underwent surgical resection based on TTNB findings alone (mucinous lesion with advanced dysplasia). A diagnosis of adenocarcinoma based on EUS-FNA but negative on TTNB (benign fibrous tissue) was later confirmed to be benign on surgical specimen.
Table 4. Surgical pathology as compared to FNA cytology and TTNB histology in 8 patients.
| Case | CEA (ng/mL) | High-risk features on imaging | EUS-FNA | EUS-microforceps | Surgical pathology |
| 1 | NA | Cyst ≥ 3 cm | Mucinous-type epithelium | IPMN (no subtype) | Branch-duct IPMN with low grade dysplasia |
| 2 | NA | Mural nodule | Mucinous-type epithelium with moderate dysplasia | IPMN (intestinal subtype) with moderate dysplasia | Main-duct IPMN with low grade dysplasia; intestinal subtype |
| 3 | 2.2 | None | Adenocarcinoma | Fibrous tissue | Benign specimen |
| 4 | NA | Cyst ≥ 3 cm and mural nodule | No malignant cells identified, abundant mucin | IPMN | Branch-duct IPMN (gastric subtype) with low grade dysplasia |
| 5 | 34.7 | None | Scant cellularity | IPMN with high-grade dysplasia | IPMN with focal high-grade dysplasia |
| 6 | NA | Mural nodule | Scant cellularity | Atrophic glands and fibrotic stroma | IPMN with low to moderate dysplasia |
| 7 | 1.2 | Cyst ≥ 3 cm | Scant cellularity | IPMN | IPMN with low grade dysplasia (intestinal and pancreaticobiliary subtype) |
| 8 | NA | None | Suspicious cells | IPMN with high-grade dysplasia | IPMN with high-grade dysplasia |
CEA, carcinoembryonic antigen; EUS, endoscopic ultrasound; FNA, fine-needle aspiration; IPMN, intraductal papillary mucinous neoplasm
Fig. 4 shows how a diagnosis of a mucinous lesion was reached in 32 out of the 47 pancreas cysts. Mucinous cysts were diagnosed more often by TTNB alone (11;34.3 %) than by CEA > 192 ng/mL alone (2;6.3 %) ( P = 0.01) or a combination of CEA > 192 ng/mL and FNA cytology (3;9.4 %) ( P = 0.03). One patient had a diagnosis of a mucinous cyst based on surgery alone.
Fig. 4.

Diagnosis of mucinous cysts.
The final and suspected diagnoses are depicted in Fig. 5 . The final diagnosis was confirmed in eight patients by surgical histopathology and 26 by TTNB histology. Nine patients were suspected to have a pseudocyst based on a combination of the following: cyst fluid characteristics (e. g. low viscosity, straw or brownish fluid), benign fibrous tissue and/or inflammatory cells on FNA cytology or TTNB histology, documented acute pancreatitis (n = 5), and/or history of chronic pancreatitis (n = 3) based on imaging studies. Two patients were suspected to have mucinous cysts based on imaging characteristics, CEA ≥ 192 ng/mL, absence of documented acute pancreatitis, and stable cyst appearance on repeat computed tomography scan at 12 and 15 months, respectively. In two patients, a clinical/histopathological diagnosis was not reached. Neither of these patients had a prior history of acute/chronic pancreatitis and one of them had showed stable cyst appearance at repeat imaging at 18 months.
Fig. 5.
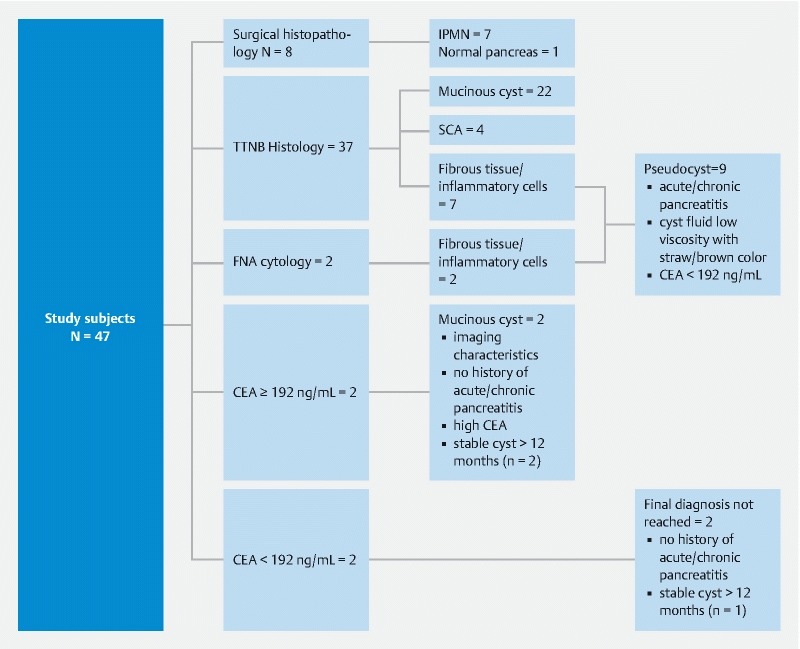
Assessment of final and suspected diagnoses in study participants.
Discussion
Diagnosis and management of PCNs is challenging. Accurate diagnosis and risk stratification of mucinous PCNs is essential to direct the most appropriate management strategy. In this study, we demonstrated that EUS-guided TTNB of pancreatic cysts with a novel microforceps was safe, associated with a high rate of technical success, and provided a substantial incremental yield in diagnosis of mucinous PCNs when compared with CEA fluid analysis or cytology.
Tissue biopsy remains the gold standard for obtaining an accurate histopathological diagnosis throughout the gastrointestinal tract, but until now, it was not feasible to obtain in PCs. Although EUS-FNA of PCs has an adequate safety profile, the diagnostic yield has been suboptimal due to the limited tissue sample that can be procured 18 19 20 . Previous studies have shown that use of a cytology brush can further improve the cytological diagnostic yield, but this practice has been abandoned because of the high rate of AEs 21 22 . The new microforceps is a novel through-the-needle device that allows targeted tissue acquisition under EUS guidance. Data on the performance of the microforceps for the evaluation of PCs are limited. In this large multicenter study, we demonstrated that TTNB with the microforceps was completed in nearly all of the cases (98 %), irrespective of cyst size or location within the pancreas (e. g. head, body, tail). Furthermore, TTNB was safe, with only one case of intracystic bleeding following EUS-FNA needle insertion; which did not require additional interventions. While there was one case of mild acute pancreatitis documented, other confounding factors in this patient included use of cystoscopy and confocal laser endomicroscopy during the same procedure. Our data corroborate results from recent studies demonstrating high technical feasibility (98 %-100 %) with the microforceps 23 24 25 . Similarly, these studies demonstrated a very low AE rate (0 %-12.5 %) with TTNB, with self-limited intracystic hemorrhage being the most common 23 24 25 . Future prospective, controlled trials are necessary to corroborate the safety profile of this device and to help establish the optimal number of biopsies that should be performed to enhance diagnostic yield without incurring in additional risks to the patient.
Imaging alone has not been shown to reliably identify the underlying pathology in PCNs with a high degree of accuracy 6 26 27 28 . Hence, EUS-FNA with cyst fluid analysis is routinely performed in clinical practice during workup of PCs. A cyst fluid CEA cutoff of 192 ng/mL has been commonly accepted for differentiating mucinous from non-mucinous cysts 6 . Limitations of CEA include: (1) the need to acquire at least 0.5 mL of cyst fluid for CEA analysis; and (2) its relatively low sensitivity (73 %) 6 . In our study, CEA could not be tested in 40.4 % of the patients due to an insufficient cyst fluid sample, which is common in smaller and/or highly viscous cysts. Of these, twice as many lesions were diagnosed as mucinous by TTNB histopathology (63.2 %) vs. FNA cytology (31.6 %), although not found to be statistically significant ( P = 0.1). For the 19 patients in our series with a CEA < 192 ng/mL, TTNB histopathology diagnosed an additional eight (42.1 %) mucinous cysts compared to only one (5.3 %) with FNA cytology ( P = 0.002). TTNB not only increased the diagnostic yield of mucinous lesions in patients in whom CEA was not available or less than the cutoff of 192 ng/mL, but TTNB alone diagnosed more mucinous cysts than either cyst fluid alone or when combined with FNA cytology. Hence, our results suggest that TTNB can potentially enhance the diagnostic yield of mucinous lesions and should be considered in addition to standard EUS-FNA and cyst fluid analysis during evaluation of PCNs.
In our study, four cases of serous cystadenoma were diagnosed with TTNB, all of which had a non-diagnostic FNA. In spite of the small sample size, these findings have important direct clinical implications as a definitive diagnosis of SCA and exclusion of a mucinous lesion avoids unnecessary testing and reduces health-care related expenditures and patient/physician uncertainty.
In our study, eight patients underwent surgical resection, of whom seven were diagnosed with an intrapapillary mucinous neoplasm (IPMN). There was no false-positive TTNB histology, suggesting that a positive TTNB histology is highly specific. Furthermore, in one patient, TTNB histology alone altered the treatment course. The patient’s incidental pancreas cyst did not exhibit any high-risk features (e. g. ≥ 3 cm, dilated main pancreatic duct, mural nodule/solid component) and FNA cytology was inadequate (scant cellularity). However, TTNB histology showed the cyst to be an IPMN with high-grade dysplasia, later confirmed on the surgical specimen. While our knowledge of PCNs has continued to evolve over the past decades, these findings underscore the need to have additional parameters, beyond the currently accepted “worrisome” or “high-risk” features, to help guide our management strategy. Indeed, a recent retrospective study by Pergolini et al demonstrated that cyst size > 1.5 cm at long-term follow-up was independently associated with a higher risk of malignancy in patients with branch-duct IPMN 29 . In all, it is clear that ongoing data are necessary to further clarify the natural history and prognosis of PCNs.
We acknowledge the limitations of this study, which should be taken into consideration when interpreting our results. The study was performed at tertiary care academic centers and results may not be generalizable. Given the retrospective nature of this study, the indication for EUS-FNA and/or TTNB could not be readily elucidated in many cases based on the endoscopy report or electronic chart review. This in turn may have led to the potential for inclusion bias as some cysts without apparent high-risk features (i. e. dilated main duct, associated solid mass, mural nodule) underwent EUS-FNA and TTNB. Furthermore, we acknowledge that potential outcomes, including post-procedural AEs, may have been underestimated if these were not reported or captured in the electronic system. The main limitation of our study was lack of surgical pathology for most of the patients (39 /47;82.9 %). Hence, in most cases, final diagnosis of the non-surgical patients was based on cytology (FNA), histology (TTNB), CEA level, imaging, and clinical history. In daily practice, only the minority of PCNs will undergo resection. Hence, using surgery as the reference standard is not without its limitations, particularly selection bias. While our study precludes estimation of the sensitivity and accuracy of TTNB, this practice is consistent with the fact that most cysts are followed conservatively and not referred for surgery 4 30 . We also recognize the possibility that variations in the diagnostic yield with FNA and TTNB reported in our study may stem from differences in the analysis (cytology vs. histology), and perhaps, not exclusively based on the type technique used for tissue acquisition. As such, these differences may have been disproportional as a histological specimen often contains more information than a cytological sample. Nonetheless, our results further underscore the ability of TTNB to successfully procure an adequate tissue specimen for histological assessment, which is not feasible with the current standard FNA needles. Lastly, while this is the largest case series on EUS-guided TTNB of PCs, the relatively small sample size could have precluded detection of potentially meaningful differences in outcomes.
Conclusion
In summary, this study demonstrated that EUS-guided TTNB of PCs was safe and associated with a high rate of technical success. TTNB is a viable adjunctive tissue acquisition method that may help increase the diagnostic yield of mucinous PCNs, particularly in patients in whom cyst fluid analysis is inconclusive. Future prospective studies are needed to further validate our initial findings.
Footnotes
Competing interests None
References
- 1.Stark A, Donahue T R, Reber H A et al. Pancreatic Cyst Disease. JAMA. 2016;315:1882–1893. doi: 10.1001/jama.2016.4690. [DOI] [PubMed] [Google Scholar]
- 2.Lee K S, Sekhar A, Rofsky N M et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 3.Khashab M A, Kim K, Lennon A M et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/RMI for prediction of cystic neoplasms. Pancreas. 2013;42:717–721. doi: 10.1097/MPA.0b013e3182883a91. [DOI] [PubMed] [Google Scholar]
- 4.Vege S S, Ziring B, Jain R et al. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology. 2015;148:819–822. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Alkaade S, Chahla E, Levy M. Role of endoscopic ultrasound-guided fine-needle aspiration, cytology, viscosity, and carcinoembryonic antigen in pancreatic cyst fluid. Endosc Ultrasound. 2015;4:299–303. doi: 10.4103/2303-9027.170417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brugge W R, Lewandrowski K, Lee-Lewandrowski E et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Hata T, Dal Molin M, Hong S M et al. Predicting the grade of dysplasia of pancreatic cystic neoplasms using cyst fluid DNA methylation markers. Clin Cancer Res. 2017;23:3935–3944. doi: 10.1158/1078-0432.CCR-16-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishna S G, Brugge W R, Dewitt J M et al. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos) Gastrointest Endosc. 2017;86:644–654. doi: 10.1016/j.gie.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Karia K, Waxman I, Konda V J et al. Needle-based confocal endomicroscopy for pancreatic cysts: the current agreement in interpretation. Gastrointest Endosc. 2016;83:924–927. doi: 10.1016/j.gie.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 10.Rogart J N, Loren D E, Singu B S et al. Cyst wall puncture and aspiration during EUS-guided Fine Needle Aspiration may increase the diagnostic yield of mucinous cysts of the pancreas. J Clin Gastroenterol. 2011;45:164–169. doi: 10.1097/MCG.0b013e3181eed6d2. [DOI] [PubMed] [Google Scholar]
- 11.Hong S S, Loren D E, Rogart J N et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc. 2012;75:775–782. doi: 10.1016/j.gie.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Samarasena J B, Nakai Y, Shinoura S et al. EUS-guided, through-the-needle forceps biopsy: a novel tissue acquisition technique. Gastrointest Endosc. 2015;81:225–226. doi: 10.1016/j.gie.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Coman R M, Schlachterman A, Esnakula A K et al. EUS-guided, through-the-needle forceps: clenching down the diagnosis. Gastrointest Endosc. 2016;84:372–373. doi: 10.1016/j.gie.2016.03.785. [DOI] [PubMed] [Google Scholar]
- 14.Pham K D, Engjom T, Gjelberg Kollesete H et al. Diagnosis of a mucinous pancreatic cyst and resection of an intracystic nodule using a novel through-the-needle micro forceps. Endoscopy. 2016;48 01:E125–126. doi: 10.1055/s-0042-105437. [DOI] [PubMed] [Google Scholar]
- 15.Attili F, Pagliari D, Rimbas M et al. Endoscopic ultrasound-guided histological diagnosis of a mucinous non-neoplastic pancreatic cyst using a specially designed through-the-needle microforceps. Endoscopy. 2016;48 01:E188–189. doi: 10.1055/s-0042-108194. [DOI] [PubMed] [Google Scholar]
- 16.Huelsen A X, Cooper C X, Saad N X et al. Endoscopic ultrasound-guided, through-the-needle forceps biopsy in the assessment of an incidental large pancreatic cystic lesion with prior inconclusive fine-needle aspiration. Endoscopy. 2017;49:E109–E110. doi: 10.1055/s-0043-100217. [DOI] [PubMed] [Google Scholar]
- 17.Cotton P B, Eisen G M, Aabakken L et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:450–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Yoon W J, Brugge W R. The safety of endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions. Endosc Ultrasound. 2015;4:289–292. doi: 10.4103/2303-9027.170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Jian F, Zhu J et al. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Dig Endosc. 2017;29:667–675. doi: 10.1111/den.12851. [DOI] [PubMed] [Google Scholar]
- 20.Barresi L, Tarantino I, Traina M et al. Endoscopic ultrasound-guided fine needle aspiration and biopsy using a 22-gauge needle with side fenestration in pancreatic cystic lesions. Dig Liver Dis. 2014;46:45–50. doi: 10.1016/j.dld.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Van der Waaij L A, van Dullemen H M, Porte R J. Cyst fluid analysis in the differential diagnosis of cystic pancreatic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383–389. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 22.Frossard J L, Amouyal P, Amouyal G et al. Performance of endosonography-guide fine needle aspiration and biopsy in the diagnosis of cystic pancreatic lesions. Am J Gastroenterol. 2003;98:1516–1524. doi: 10.1111/j.1572-0241.2003.07530.x. [DOI] [PubMed] [Google Scholar]
- 23.Mittal C, Obuch J C, Hammad H et al. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video) Gastrointest Endosc. 2018;87:1263–1269. doi: 10.1016/j.gie.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Basar O, Yuksel O, Yang D et al. Feasibility and safety of micro-forceps biopsy in the diagnosis of pancreatic cysts. Gastrointest Endosc. 2018;88:79–86. doi: 10.1016/j.gie.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Barresi L, Crino S F, Fabbri Cet al. Endoscopic Ultrasound-Through-the-Needle Biopsy in Pancreatic Cystic Lesions: A Multicenter Study Dig Endosc 2018 10.1111/den.13197[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26.Correa-Gallego C, Ferrone C R, Thayer S P et al. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2008;8:236–251. doi: 10.1159/000243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho C S, Russ A J, Loeffler A G et al. Preoperative classification of pancreatic cystic neoplasms: the clinical significance of diagnostic inaccuracy. Ann Surg Oncol. 2013;20:3112–3119. doi: 10.1245/s10434-013-2986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Chiaro M, Segersvard R, Pozzi Mucelli R et al. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann Surg Oncol. 2014;21:1539–1544. doi: 10.1245/s10434-013-3465-9. [DOI] [PubMed] [Google Scholar]
- 29.Pergolini I, Sahora K, Ferrone C R et al. Long-term risk of pancreatic malignancy in patients with branch duct intraductal papillary mucinous neoplasm in a referral center. Gastroenterology. 2017;153:1284–1294. doi: 10.1053/j.gastro.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Fernandez-del Castillo C, Adsay V et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]


