Abstract
Cilia and flagella are long extensions commonly found on the surface of eukaryotic cells. In fact, most human cells have a flagellum, and failure to correctly form cilia leads to a spectrum of diseases gathered under the name ‘ciliopathies’. The cilium distal tip is where it grows and signals. Yet, out of the flagellar regions, the distal tip is probably the least intensively studied. In this review, we will summarise the current knowledge on the diverse flagellar tip structures, the dynamicity and signalling that occurs here and the proteins localising to this important cellular region.
Keywords: cilia, electron microscopy, flagella, microtubule, signalling
Introduction
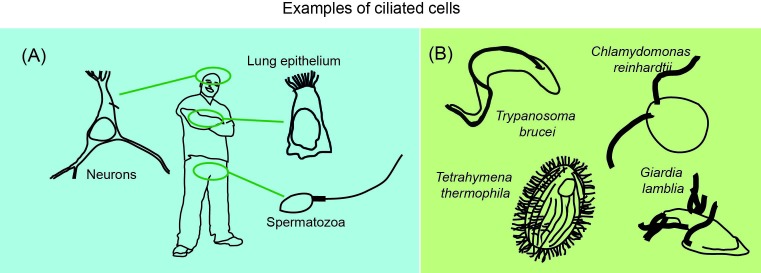
Protruding into the extracellular environment, the cilium (aka flagellum) is a microtubule-based organelle located at the cell surface [1]. Many unicellular protists as well as cells in complex multicellular organisms such as humans have a flagellum in this exposed position (Figure 1), where it is a crucial tool for cellular signalling and locomotion. The distal tip of the flagellum is the epicentre of much of its signalling and its assembly [2–7], yet we know very little of its composition and structure, especially in humans. If cilia do not form properly, the consequences are dire – a whole group of diseases called the ciliopathies [8]. What is this mysterious organelle that is so important to cellular function?
Figure 1. Examples of ciliated cells in both multicellular organisms such as humans (A) and unicellular organisms (B).
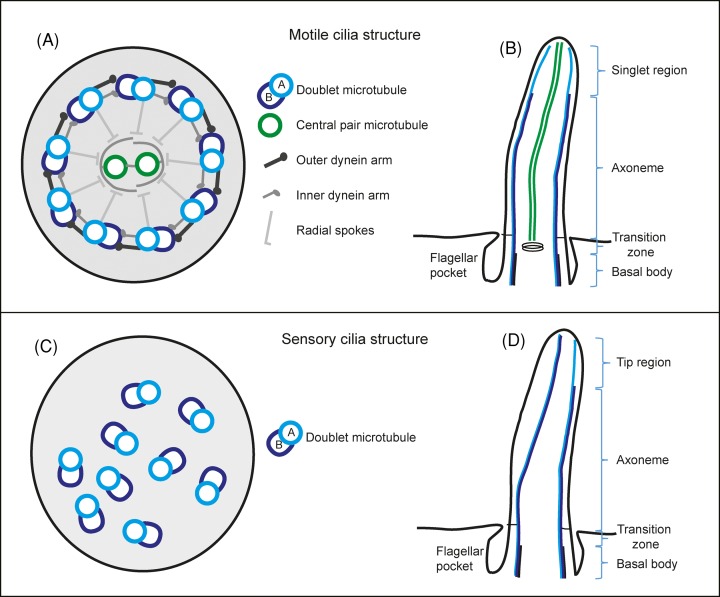
Inside the flagellar membrane there is a specialised structure composed of nine microtubule doublets arranged in a circle (Figure 2A). Each doublet microtubule consists of a complete 13 protofilament A-tubule and an incomplete 10 protofilament B-tubule [9]. Inside motile flagella there are two additional singlet microtubules in the middle, the central pair, that are thought to be involved in the regulation of the flagellar beat [10]. The beat occurs as thousands of dynein motor proteins walk on one microtubule while being attached to the neighbouring microtubule, causing it to bend. The microtubules together with the associated structural proteins are called the axoneme. The complexity of this machine is shown in proteomics studies that find that it is composed of approximately 400–1000 different proteins [11–13]. Sensory cilia usually lack dynein motors, the central pair microtubules and their associated proteins and have therefore a smaller proteome of approximately 200–400 proteins [14,15]. What fraction of the cilium proteome resides at the distal tip is currently unknown.
Figure 2. Schematic drawing of eukaryotic flagella ultrastructure.
(A) Cross-sectional view of a motile cilium seen from the proximal end containing the canonical nine doublet microtubules and two central pair microtubules. (B) A motile cilium in longitudinal view showing the different regions along its length. (C) A cross-sectional view of a sensory cilium showing the variable orientation of the nine doublet microtubules and lack of central pair microtubules. (D) A sensory cilium and its different regions along its length.
The flagellum can be further divided into multiple zones along its length (Figure 2B). Beginning in the cytoplasm, there is the proximal end of the flagellum, the basal body. The basal body has nine triplet microtubules, of which only the A- and B-tubules extend into and beyond the transition zone where usually a basal plate or other electron-dense structures are found. It is also in the transition zone that the central pair microtubules start in motile flagella. The axoneme then extends for approximately 2.5–50 μm, depending on the species and cell type (for a review on the whole flagella structure, see [16]). In this review, we will focus on the distal most tip of the flagellum, a specialised region full of cellular activity.
Structural models for distal tips of motile flagella
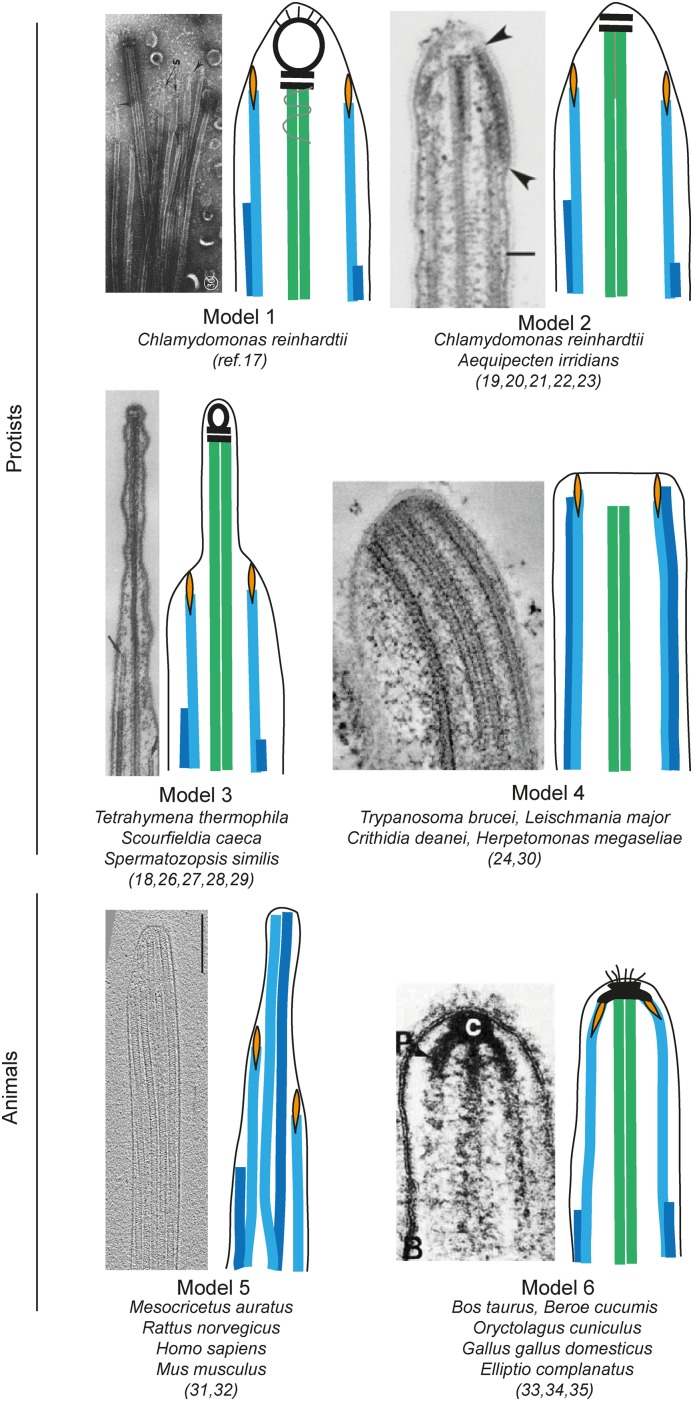
Most studies on flagellar tips focused on protists like the green alga Chlamydomonas reinhardtii, the ciliate Tetrahymena thermophila and the human parasite Trypanosoma brucei. However, the ultrastructure of flagellar tips has been investigated in many different organisms, revealing considerable differences. We sorted the reported flagella tip structures into six different models (Figure 3).
Figure 3. Schematic drawing illustrating six different models of motile flagellar structures published to date, and in which species each structure was shown.
The A-tubules are shown in turquoise, B-tubules in blue, central pair microtubules in green, carrot-like plugs in orange and other electron-dense structures in black. Models are adjoined by original data reprinted with permission from publications: [17] for Model 1, [18] for Model 2, [19] for Model 3, [20] for Model 4 and [21] for Model 6. Cryo-EM in Model 5 is an original picture from the authors. All samples were prepared with chemical fixation or negative stain except for models 3, 4, and 5, that were shown with HPF-FS or cryo-EM too. Abbreviation: HPF-FS, high-pressure frozen and freeze substituted.
Model 1: Electron microscopy (EM) of C. reinhardtii flagellar tips on demembranated negatively stained samples showed a large capping structure, formed by two plate-like structures and an electron-dense bead at the distal ends of central pair microtubules. It was termed as central microtubule cap [17,22]. Smaller carrot-like plugs capped the A-tubules in doublet microtubules, by extending into their lumen. Distal filaments connected the plugs and the central microtubule cap to the plasma membrane of the flagellar tip in multiple spots.
Model 2: Thin sections of chemically fixed C. reinhardtii flagella revealed an approximately 400-nm long singlet region, which was characterised by the absence of radial spokes and by the gradual termination of the doublets (B-tubules terminated before the A-tubules) [23]. The bead of the central microtubule cap was not observed in images of sectioned flagellar tips and only the two plates were left in association with the central pair microtubules [18,23–26]. Instead, an electron density was observed to laterally connect the distal segments of the two central pair microtubules. Flagella of bay scallop gills also have this tip arrangement, but here distal filaments were seen to connect A-tubules to the central microtubule cap [22]. Only undefined densities were observed instead of a structured central pair cap in electron tomography (ET) of high-pressure frozen and freeze substituted (HPF-FS) growing C. reinhardtii flagellar tips and the singlet region was measured to a mere 50 nm [20]. The discrepancies in the data might be explained by differences in dynamic states of the flagellum (growing compared with mature) or in sample preparation, as it was shown that chemical fixation induced depolymerisation of microtubules in the tips of T. brucei [27]. To settle this debate, HPF-FS or cryo-EM of C. reinhardtii mature flagella needs to be performed.
Model 3: Like in model 1, a central microtubule cap, complete with plates and bead, was observed in T. thermophila with three different EM techniques [22,28,29], except that its central pair microtubules extend approximately 200 nm longer than the A-tubules in the singlet region, giving the tip a more protruding shape. The bead of the central microtubule cap was not visible in the cryo-ET reconstructions of T. thermophila flagella tips [28]. Two other species of protist ciliates shared a similar tip ultrastructure [19,30].
Model 4: Four species of kinetoplastids including T. brucei, had no singlet region giving the tip a blunt rounded shape [20,31]. The central pair microtubules terminated approximately 50 nm farther from the tip compared with the doublets and in multiple instances B-tubules extended farther than the A-tubules of the same doublet [18,27]. Cryo-ET on mature T. brucei flagella confirmed the presence of carrot-like densities that protruded into the lumen of A-tubules also in this species [20].
Model 5: The singlet region in flagella of rodent and human spermatozoa extends for several micrometres and each singlet microtubule terminates at variable distance from the tip [32,33] (Zabeo and Höög, manuscript in preparation). In these systems the A- and B-tubules of doublet microtubules can split and form two complete singlet microtubules – a ‘duplex’. In rodents, the singlets of each duplex microtubule were held together by electron-dense material at their distal tips, similar to the central pair microtubules in Model 2, and became structurally indistinguishable from the central pair itself [33]. However, the central pair could be tracked from the complete axoneme to the very tip of the flagellum, while not all duplex microtubules extended that far. Cryo-ET of human spermatozoa did not show any clear connection between singlets of the same doublet after they split apart (Zabeo and Höög, manuscript in preparation). In this study, the central pair could not be traced along the entire singlet region but in one instance, it terminated before the start of the singlet region, 7.5 µm from the flagellum tip. Densities associated with the distal ends of singlet microtubules, similar to the carrot-like plugs, were seen in chemically fixed rodent spermatozoa [33] and in cryo-ET of human spermatozoa (Zabeo and Höög, manuscript in preparation). In addition, an inner microtubule structure called TAILS, which spanned several micrometres, was discovered in the lumen of singlet, doublet and central pair microtubules in the human spermatozoon tip [32]. It was hypothesised that TAILS might prevent microtubule dynamicity or aid cell motility.
Model 6: Flagella from tracheal tissue of rabbit, chicken and bovine showed yet another tip ultrastructure, where only A-tubules and central pair microtubules extended into the singlet region and terminated at the tip by connecting to an electron-dense body that was called a ciliary cap [21]. Here, carrot-like densities seemed to anchor the distal ends of the singlet microtubules to the ciliary cap. Hair-like protrusions called the ciliary crown were found on the surface of the plasma membrane of the flagellar tip in rabbit and chicken, but not in bovine. The ciliary crown was still present after demembranation, suggesting that it might extend through the plasma membrane and be directly anchored to the ciliary cap. The A-tubules and central pair microtubules of flagella from gills of the freshwater mussel Elliptio complanatus also converge together at the tip where electron densities are associated with their distal ends, however no ciliary crown was found [34]. Convergence to electron densities at the tip after a singlet region was also seen in the extreme structure of macrocilia in the ctenophore Beroe cucumis [35].
These different structures likely mirror different flagellar functions that are specific to each cell type and possibly influence flagellar stability and motility. Dentler and LeCluyse [21] speculated that a ciliary cap might provide a stronger stroke in flagella that beats in a more viscous environment, as in tracheal epithelia, compared with flagella that beat in water. They also suggested that the ciliary crown might help with mucus translocation, since it was only found in animal duct epithelia.
Sensory cilia tip ultrastructure
In contrast with motile cilia, sensory cilia are generally characterised by a 9 + 0 axoneme which lacks central pair microtubules as well as inner and outer dynein arms. Consequently, sensory cilia are immotile and instead function like a cellular antenna to transduce optical, chemical, osmotic or mechanosensory signals [36]. Despite a resurgence of interest in the vertebrate primary cilium, the majority of information about its ultrastructure is from when chemical fixation techniques was the convention rather than the modern HPF-FS or cryo-EM [37–39]. Although microtubule doublets begin arranged in a circular symmetry in sensory cilia, similar to motile cilia, this 9 + 0 arrangement is soon broken as one or two doublet microtubules folds in to form an 8 +1 or 7 + 2 pattern [37,40] which has been referred to as a 9 variable (9v) axoneme [41]. Near the tip, the arrangement of microtubules becomes more disordered, several doublet microtubules become singlets, and both doublet and singlet microtubules terminate as the cilium tapers resulting in a reduced number of microtubules that reach the tip (Figure 4A-B) [18,41].
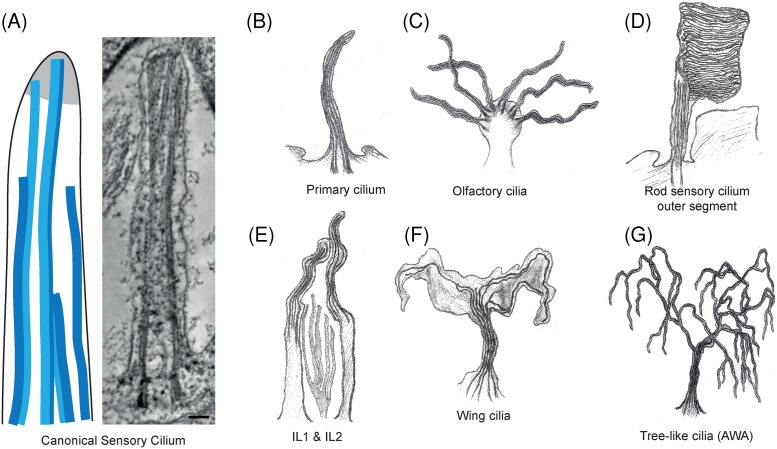
Figure 4. Divergence in the structure of sensory cilia tips.
(A) A schematic drawing of a canonical sensory cilia tip, showing that both singlet and doublet microtubules can reach the distal most flagellar membrane. An electron density is found in the distal tip of some, but not all, sensory cilia. A tomographic slice from a 3D reconstruction of the entire length of the Leishmania mexicana amastigote flagellum. (Scale bar=100 nm). (B-F) A gallery of the extreme modifications to structure that can be found in sensory cilia. The top row can be found in humans and animals whereas the bottom row are found in C. elegans neuronal sensory cilia.
However, many sensory cilia have adopted extreme structures deviating from this theme as depicted in Figure 4. The axoneme of human olfactory cilia (Figure 4C) contains nine outer doublet microtubules with a variable number (2–4) of inner singlet microtubules [42]. Doublets are lost distally and the shaft tapers as microtubules terminate and only two or three singlet microtubules exist at the tip. Amphid channel cilia in the neurons of C. elegans also have between one and seven inner singlet microtubules composed of eleven protofilaments rather than the normal thirteen [43,44]. Near the tip, only singlet microtubules exist and the cilium tapers.
The outer segment of rod sensory cilium in the eye contains a normal axonemal structure but the cilium is expanded to contain thousands of lamellar disk-shaped membranes containing rhodopsin and other proteins involved in phototransduction (Figure 4D) [45]. These disks decrease in size in the distal region of the flagellum, and are shed at the tip to be exocytosed by pigmented epithelial cells [46]. The eyes of the tardigrade Milnesium tardigradum also contain large branching, arborescent cilia that extend into their optic cavity [47].
Several other sensory neuronal cilia have been described using 3D EM of HPF-FS C. elegans worms, which have widely divergent axonemal and tip structures [44]. The function of such diversity is still largely unknown, but they may reflect specific needs for sensorial signalling, such as increasing the surface of receptor organelles. The tips of cilia in inner labia (IL1 and IL2) neurons contain four to six disorganised singlet microtubules, and curve sharply inwards before terminating. IL2 curves back outwards again at the tip (Figure 4E). Wing cilia flatten to form a large wing-like structure containing sparse singlet microtubules in their distal segments (Figure 4F). Successive and complex branching is observed in tree-like cilia, which can contain as many as 80 branches in which the nine microtubule doublets segregate haphazardly (Figure 4G). In the cilia of outer labia neurons (OLL and OLQ) and IL1, electron-dense material is detected at the tips. Furthermore, microtubules arranged in a square around a central filament comprise the tip of OLQ. Electron-dense material is also found surrounding microtubules at the cilium tip of the mechanosensory CEP neuron, which widens to form a cluster of many singlet microtubules that nucleate in this region. These recent studies have demonstrated that sensory cilia vary considerably both between and within organisms, and the power of modern EM is to visualise these complex structures in great detail.
Is the distal tip a dynamic structure?
The flagellum tip in C. reinhardtii is constantly growing and shrinking as seen by the incorporation of tagged tubulin at the axonemal microtubule plus ends [48]. This has led to a general understanding that eukaryotic flagella tips are dynamic structures. But how common is this dynamicity at the flagellum tip really? Neither T. brucei [49] nor mouse spermatozoa [50] have dynamic flagellar distal tips once they are fully grown. This was shown as their motile flagella remains the same length in the absence of intraflagellar transport (IFT), that delivers new building blocks to the tip [51]. It is interesting to note that dynamicity is not observed in flagellar models without a capping structure, like in T. brucei or mouse spermatozoa, while it exists in C. reinhardtii’s capped flagella. However, data from a larger number of models need to be recorded before a correlation can be induced. The lack of dynamicity in spermatozoa end pieces agrees well with the hypothesis that the recently discovered TAILS complex is a microtubule stabiliser that prevents normal dynamic instability in the end piece in humans [32]. In contrast, C. elegans sensory cilia appear to maintain a dynamicity after formation, as it displays fluorescence recovery after photobleaching of tubulin-YFP [52] and the genes associated with ciliary assembly continue to be expressed even in adult worms [53]. Therefore, it appears that there are cases of both dynamic and non-dynamic distal tips in both motile and sensory cilia, making generalisations difficult.
Proteins residing at the cilium tip
Compared with the axoneme and the basal body, relatively little is known about the biochemical composition of ciliary tips. Recently, over 20 proteins that reside in ciliary tips have been identified and we have gathered them into a list (Table 1). These are involved in functions including, but not limited to, ciliary dynamics, contribution to the tip ultrastructure, regulation of IFT events and signalling.
Table 1. Protein residents of the flagellum tip identified by immunofluorescence or immunogold labelling in various organisms.
| Protein | Involved In | Tb | Cr | Hs | Mm | Ce | Dm | Gi | Tt | Lm | Xl | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EB1 | Dynamics, IFT regulation |  |
 |
[54–58] | ||||||||
| EB3 | Dynamics |  |
 |
[55,59] | ||||||||
| CDPK1 | IFT regulation |  |
[60] | |||||||||
| FLA8 (Kif3B) | IFT regulation |  |
[60] | |||||||||
| Kif19A | Dynamics |  |
[61,62] | |||||||||
| Kinesin 13 | Dynamics |  |
 |
 |
[63,64,58] | |||||||
| Kif7 | Dynamics, signalling |  |
[65–67] | |||||||||
| Kif17 (OSM-3) | IFT motor |  |
[68] | |||||||||
| CEP104 (CrFAP256, TbACS3) | Ciliogenesis |  |
 |
 |
[25,69,70] | |||||||
| PRMT1 | Dynamics |  |
[71] | |||||||||
| PRMT3 | Dynamics |  |
[71] | |||||||||
| PRMT5 | Dynamics |  |
[71] | |||||||||
| PRMT10 | Dynamics |  |
[71] | |||||||||
| Agglutinin | Tip adhesion |  |
[72] | |||||||||
| ACS1 | Structure |  |
[70] | |||||||||
| ACS2 | Structure |  |
[70] | |||||||||
| FCP1 | Structure |  |
[70] | |||||||||
| FCP4 (TbKin15) | Structure |  |
[70] | |||||||||
| Sentan | Structure |  |
 |
[73] | ||||||||
| Amo | Sperm activation |  |
[74,75] | |||||||||
| NOMPC | Signalling |  |
[76] | |||||||||
| Gli2 | Signalling |  |
[67,77,78] | |||||||||
| Gli3 | Signalling |  |
[67,77,78] | |||||||||
| Sufu | Signalling |  |
[65,78] | |||||||||
| DCDC2C | Unknown |  |
[12] | |||||||||
| Tb927.1.2710 | Unknown |  |
[70] | |||||||||
| FLAM8 | Unknown |  |
[11] | |||||||||
| CALP1.3 | Unknown |  |
[79] | |||||||||
| SKCRP7.2 | Unknown |  |
[79] |
Blue cross sections of a cilium indicate presence in motile cilia, green indicates presence in sensory cilia. Abbreviations: Ce, Caenorhabditis elegans; Cr, Chlamydomonas reinhardtii; Dm, Drosophila melanogaster; EB, end binding protein; Gi, Giardia intestinalis; Hs, Homo sapiens; Lm, Leishmania major; Mm, Mus musculus; Sufu, suppressor of fused; Tb, Trypanosoma brucei; Tt, Tetrahymena thermophila; Xl, Xenopus laevis.
Proteins involved in regulating ciliary dynamics
End binding proteins (EBs) are a widely conserved family of proteins involved in regulating microtubule dynamics, localising mainly to the dynamic plus ends of microtubules throughout the cell [80]. EB1 also localises to the tips of microtubules in C. reinhardtii cilia, and human EB3 has been found at the tips of primary cilia in bronchial epithelial cells [54,55]. The mammalian protein CEP104 (homologue in C. reinhardtii, FAP256) is localised to the cilium tip in an EB-dependent manner [69] and appears to play a vital role in ciliogenesis. CEP104 relocates from the distal ends of the centriole to the ciliary tips as ciliogenesis begins [25]. Cells depleted of CEP104/FAP256 display complete ciliary loss or short, blunt cilia, due to doublet microtubules of the same length as the central pair microtubules [25]. Furthermore, CEP104/FAP256 contains a tubulin-binding TOG domain present in other proteins that regulate microtubule dynamics [69].
Various members of the kinesin superfamily (Kinesin-13, Kif7, Kif19A) regulate ciliary length at the tips by affecting microtubule polymerisation dynamics, and consequently cells deficient in certain KIFs display abnormally long and unstable cilia [61,63–65]. In C. reinhardtii however, kinesin-13 appears to be absent from flagellar tips but functions to depolymerise cytoplasmic microtubules in order to generate a pool of tubulin subunits during flagellar regeneration. In addition to these known microtubule-associated proteins, four protein arginine methyltransferases localise to the tip of C. reinhardtii cilia where they play a role in regulating resorption of the flagellum [71].
IFT turnaround is regulated at the tip
Anterograde IFT trains deliver building blocks to the ciliary assembly site in the distal tip. There the IFT trains are reloaded with different cargo and then moved in the retrograde direction – towards the cell body. Therefore, the ciliary tip is an important site of IFT regulation and components of IFT trains will transiently localise to this area (reviewed by Taschner and Lorentzen [81]). Here we will focus on the few tip resident proteins that have been found to be involved in IFT transport. In C. elegans, many cilia contain extended singlet zones where kinesin-2 (which carries IFT cargo the whole length of flagella in C. reinhardtii) disassociates. Instead, the motor protein OSM-3 functions to extend the singlet zone by carrying IFT along singlet microtubules to the tip [68]. In C. reinhardtii, the kinase CDPK1 (CrCDPK1) relocates from the proximal cilium to the tip where it regulates IFT turnaround during flagellar assembly. CrCDPK1 is thought to phosphorylate FLA8, another protein that accumulates at the tip of the growing flagellum, which then causes dissociation of anterograde IFT-B train from its kinesin-2 motor [60]. Furthermore, IFT172, a component of IFT-B, is required for transition between anterograde and retrograde IFT and has been suggested to do so by interaction with EB1 upon arrival at the tip [82,56]. Further details of the molecular mechanisms underpinning IFT turnaround will be exciting advancements in years to come.
Together, our current knowledge of the cilium tip proteome suggests that the protein composition of the flagellum tip is variable between species. It remains to be seen if a core set of proteins emerge as conserved cilium-tip residents as additional proteomics data and localizstions become available in the future. One such promising advancement is the whole proteome of T. brucei being localised in the TrypTag project [83]. With that we will have a first complete list of proteins that localise to the flagellum tip in one species.
The primary cilium tip and signalling
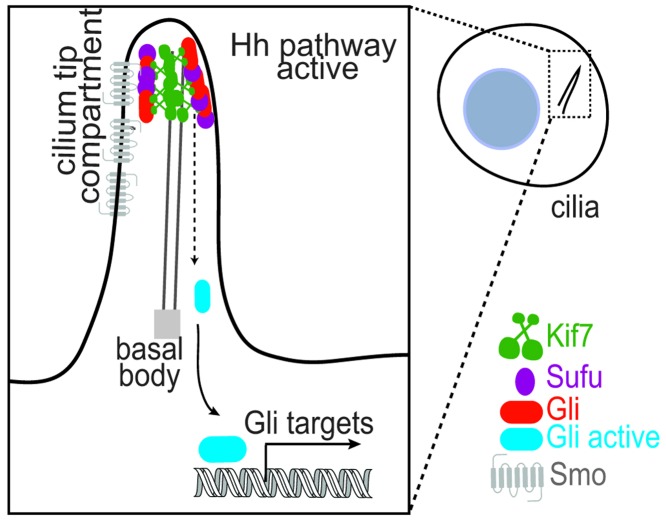
In recent years, the primary cilium has emerged as a major hub for signal transduction pathways including Hedgehog (Hh), Wnt and PDGFRα signalling [4]. Perhaps the best-characterised signalling pathway that critically depends on the primary cilium is the vertebrate Hh signal transduction, which is disrupted in a number of tumors and important for embryonic development and adult tissue homoeostasis [5,6]. It is observed that the axoneme-associated protein complex of the Hh pathway, which is composed of the kinesin Kif7, the Gli transcription factors and their negative regulator Suppressor of fused (Sufu), localises to a submicron region at the distal tip of the primary cilium upon pathway activation (Figure 5) [65,66,67,77]. In current models, it is suggested that Gli is activated at the cilium tip, and subsequently transported to the nucleus for gene activation [84]. These observations raise some fundamental questions: What is the mechanism by which Kif7, Gli and Sufu proteins localise to the cilium tip upon pathway activation? What is the functional consequence of concentrating these proteins at the cilium tip?
Figure 5. Schematic representation shows the localisation of the kinesin Kif7, the transcription factor Gli and its repressor Sufu to the distal cilium-tip upon Hh pathway activation.
Gli is activated at the cilium tip and subsequently translocates to the nucleus.
Initial clues to these questions come from studies on Kif7, a conserved protein of the Hh signalling pathway [85]. Mutations in this ciliary kinesin are associated with a number of ciliopathies [85]. In the absence of Kif7, Gli can enter the cilium but is not concentrated at the cilium tip [65,77]. These initial findings suggested that Kif7 might be a plus-end directed transport motor that transports Gli to the cilium tip. However, in vitro studies indicate that Kif7 is a non-motile motor that autonomously recognises the plus-ends of microtubules and suppresses microtubule polymerisation rates [65,86]. Consistent with these observations, the knockdown of Kif7 results in an over-elongated cilium with defective architecture [65]. Together, these cell biological and biochemical studies have led to the proposal that Kif7 plays a role in sculpting the cilium tip-architecture to establish a single ‘compartment’ for the proper recruitment and regulation of Gli and Sufu. Currently the mechanism by which Kif7 organises such a platform at the distal cilium tip is unknown. Furthermore, whether Kif7 plays additional roles in Gli activation besides localising it to the cilium tip is unclear. How membrane-associated proteins modulate the specification of a tip compartment at the distal end of the axoneme remains an open question. Finally, it remains to be elucidated if the distal cilium tip acts as a compartment for biochemical reactions underlying other cilium-dependent signalling pathways.
Signalling in motile cilia
Primary cilia are well known for possessing sensorial and signalling functions in almost all animals [87]. However, sensorial functions of both mechanoreceptive and chemoreceptive nature have been reported for motile cilia in mammals [88–92] and protists [93,94], as reviewed previously [95]. Most of these functions have not been specifically associated with the cilium tip, but rather with the whole organelle. In some instances, primary cilium tips have been observed making direct contact with a nearby cell or organelle, which might underlie signalling processes as well [96].
The tip of motile cilia is reported to have signalling functions in C. reinhardtii mating. Solter and Gibor [94] first observed that deflagellated cells could not initiate mating. Agglutinins, long and mating type-specific glycoproteins [97], allow mating gametes to come in contact. Within seconds, a process termed as flagellar tip activation [98] occurs: densities accumulate at the flagellar tip, between the outer doublet microtubules and the plasma membrane, and the microtubules in the singlet region polymerise. This causes the tip to swell and increase in length by approximately 30% of its original size. Both in C. reinhardtii and C. eugametos, agglutinins accumulate at the flagellum tip [99–101] and likely contribute to its swelling. The flagellar tip activation was shown to rise cAMP levels [102] and activate protein tyrosine kinase activity [103].
Motile cilia are also known to release extracellular vesicles from the plasma membrane (ectosomes), which can deliver signals or biomolecules to target cells [104]. A recent review in this journal covered this topic extensively [105]. In C. reinhardtii the release of ectosomes localises at the flagellum tip [100,106].
Conclusion
The eukaryotic flagellum distal tip is the place of ciliary growth and a hub for cellular signalling. In this review we show that the motile flagella distal tip structures described to date could be sorted into six different models. Primary cilia also show a wide array of tip structures, showing a widespread evolutionary adaptability of this region and a difficulty to generalise from research on a few model organisms only. In the past 15 years, 29 protein residents of the flagellar tip have been identified in different organisms, but a complete flagellar tip proteome from any one species is still missing. Knowing all the structure and components found in this region would greatly increase our understanding of the processes of ciliogenesis and signalling emanating from the flagellum tip.
Summary
Both motile and primary cilia have a wide diversity of structures at the distal tip.
A list of the proteins known to reside at the distal tip is presented.
The distal tip is a hub of cellular signalling both in motile and primary cilia.
Acknowledgments
We thank Dimitra Panagaki for contributing with beautiful artwork of primary cilia structures and Per Widlund for providing comments on the manuscript.
Abbreviations
- EB
end binding protein
- EM
electron microscopy
- ET
electron tomography
- Hh
Hedgehog
- HPF-FS
high-pressure frozen and freeze substituted
- IFT
intraflagellar transport
- KIF
kinesin superfamily protein
- OSM-3
osmotic avoidance abnormal protein 3
- PDGFR
platelet-derived growth factor
- Sufu
suppressor of fused
- TAILS
tail axoneme intra-lumenal spiral
- Wnt
Wingless/integrated
Funding
This work was supported by the Swedish Research Council Young Investigator Grant [grant number 2015-05427 (to J.L.H.)]; the Pew Charitable Trust (to R.S.) and the Smith Family Foundation (to R.S.).
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Khan S. and Scholey J.M. (2018) Assembly, functions and evolution of archaella, flagella and cilia. Curr. Biol. 28, R278–R292 10.1016/j.cub.2018.01.085 [DOI] [PubMed] [Google Scholar]
- 2.Sherwin T. (1987) Distinct localization and cell cycle dependence of COOH terminally tyrosinolated alpha-tubulin in the microtubules of Trypanosoma brucei brucei. J. Cell Biol. 104, 439–446 10.1083/jcb.104.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherwin T. and Gull K. (1989) Visualization of detyrosination along single microtubules reveals novel mechanisms of assembly during cytoskeletal duplication in trypanosomes. Cell 57, 211–221 10.1016/0092-8674(89)90959-8 [DOI] [PubMed] [Google Scholar]
- 4.Johnson K.A. (1992) Polarity of flagellar assembly in Chlamydomonas. J. Cell Biol. 119, 1605–1611 10.1083/jcb.119.6.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheway G., Nazlamova L. and Hancock J.T. (2018) Signaling through the primary cilium. Front Cell Dev Biol. 6, 8 10.3389/fcell.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briscoe J. and Therond P.P. (2013) The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416–429 10.1038/nrm3598 [DOI] [PubMed] [Google Scholar]
- 7.Goetz S.C. and Anderson K.V. (2010) The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331–344 10.1038/nrg2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter J.F. and Leroux M.R. (2017) Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533–547 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicastro D., Fu X., Heuser T., Tso A., Porter M.E. and Linck R.W. (2011) Cryo-electron tomography reveals conserved features of doublet microtubules in flagella. Proc. Natl. Acad. Sci. U.S.A. 108, E845–E853 10.1073/pnas.1106178108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loreng T.D. and Smith E.F. (2017) The central apparatus of cilia and eukaryotic flagella. Cold Spring Harb. Perspect Biol. 9, a028118 10.1101/cshperspect.a028118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subota I., Julkowska D., Vincensini L., Reeg N., Buisson J., Blisnick T.. et al. (2014) Proteomic analysis of intact flagella of procyclic Trypanosoma brucei cells identifies novel flagellar proteins with unique sub-localization and dynamics. Mol. Cell. Proteomics 13, 1769–1786 10.1074/mcp.M113.033357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jumeau F., Chalmel F., Fernandez-Gomez F.-J., Carpentier C., Obriot H., Tardivel M.. et al. (2017) Defining the human sperm microtubulome: an integrated genomics approach. Biol. Reprod. 96, 93–106 [DOI] [PubMed] [Google Scholar]
- 13.Pazour G.J., Agrin N., Leszyk J. and Witman G.B. (2005) Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170, 103–113 10.1083/jcb.200504008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer U., Küller A., Daiber P.C., Neudorf I., Warnken U., Schnölzer M.. et al. (2009) The proteome of rat olfactory sensory cilia. Proteomics 9, 322–334 10.1002/pmic.200800149 [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa H., Thompson J., Yates J.R. and Marshall W.F. (2012) Proteomic analysis of mammalian primary cilia. Curr. Biol. 22, 414–419 10.1016/j.cub.2012.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisch C. and Dupuis-Williams P. (2011) Ultrastructure of cilia and flagella - back to the future ! Biol Cell. 103, 249–270 10.1042/BC20100139 [DOI] [PubMed] [Google Scholar]
- 17.Dentler W.L. and Rosenbaum J.L. (1977) Flagellar elongation and shortening in Chlamydomonas. III. structures attached to the tips of flagellar microtubules and their relationship to the directionality of flagellar microtubule assembly. J. Cell Biol. 74, 747–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogowski M., Scholz D. and Geimer S. (2013) Electron microscopy of flagella, primary cilia, and intraflagellar transport in flat-embedded cells. Methods Enzymol., 524, 243–263, 10.1016/B978-0-12-397945-2.00014-7 [DOI] [PubMed] [Google Scholar]
- 19.Melkonian M. and Preisig H.R. (1982) Twist of central pair microtubules in the flagellum of the green flagellate Scourfieldia caeca. Cell Biol. Int. Rep. 6, 269–277 10.1016/0309-1651(82)90079-0 [DOI] [PubMed] [Google Scholar]
- 20.Höög J.L., Lacomble S., O’Toole E.T., Hoenger A., McIntosh J.R. and Gull K. (2014) Modes of flagellar assembly in Chlamydomonas reinhardtii and Trypanosoma brucei. eLife., 3, e01479 10.7554/eLife.01479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dentler W.L. and LeCluyse E.L. (1982) The effects of structures attached to the tips of tracheal ciliary microtubules on the nucleation of microtubule assembly in vitro. Cell Motil. 2, 13–18 10.1002/cm.970020705 [DOI] [PubMed] [Google Scholar]
- 22.Dentler W.L. (1980) Structures linking the tips of ciliary and flagellar microtubules to the membrane. J. Cell Sci. 42, 207–220 [DOI] [PubMed] [Google Scholar]
- 23.Ringo D.L. (1967) Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J. Cell Biol. 33, 543–571 10.1083/jcb.33.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dentler W. (2005) Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J. Cell Biol. 170, 649–659 10.1083/jcb.200412021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satish Tammana T.V., Tammana D., Diener D.R. and Rosenbaum J. (2013) Centrosomal protein CEP104 (Chlamydomonas FAP256) moves to the ciliary tip during ciliary assembly. J. Cell Sci. 126, 5018–5029 10.1242/jcs.133439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vannuccini E., Paccagnini E., Cantele F., Gentile M., Dini D., Fino F.. et al. (2016) Two classes of short intraflagellar transport train with different 3D structures are present in Chlamydomonas flagella. J. Cell Sci. 129, 2064–2074 10.1242/jcs.183244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höög J.L., Lacomble S., Bouchet-Marquis C., Briggs L., Park K., Hoenger A.. et al. (2016) 3D architecture of the Trypanosoma brucei flagella connector, a mobile transmembrane junction. PLoS Negl. Trop Dis. 10, e0004312 10.1371/journal.pntd.0004312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dentler W.L. (1984) Attachment of the cap to the central microtubules of Tetrahymena cilia. J. Cell Sci. 66, 167–173 [DOI] [PubMed] [Google Scholar]
- 29.Reynolds M.J., Phetruen T., Fisher R.L., Chen K., Pentecost B.T., Gomez G.. et al. (2018) The developmental process of the growing motile ciliary tip region. Sci. Rep. 8, 7977 10.1038/s41598-018-26111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melkonian M. and Preisig H.R. (1984) Ultrastructure of the flagellar apparatus in the green flagellate Spermatozopsis similis. Plant Syst. Evol. 146, 145–162 10.1007/BF00989542 [DOI] [Google Scholar]
- 31.Woolley D., Gadelha C. and Gull K. (2006) Evidence for a sliding-resistance at the tip of the trypanosome flagellum. Cell Motil. Cytoskeleton 63, 741–746 10.1002/cm.20159 [DOI] [PubMed] [Google Scholar]
- 32.Zabeo D., Heumann J.M., Schwartz C.L., Suzuki-Shinjo A., Morgan G., Widlund P.O.. et al. (2018) A lumenal interrupted helix in human sperm tail microtubules. Sci. Rep., 8 10.1038/s41598-018-21165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolley D.M. and Nickels S.N. (1985) Microtubule termination patterns in mammalian sperm flagella. J. Ultrastruct. Res. 90, 221–234 10.1016/S0022-5320(85)80001-0 [DOI] [PubMed] [Google Scholar]
- 34.Satir P. (1968) Studies on cilia. Further studies on the cilium tip and a “sliding filament” model of ciliary motility. J. Cell Biol. 39, 77–94 10.1083/jcb.39.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamm S.L. and Tamm S. (1985) Visualization of changes in ciliary tip configuration caused by sliding displacement of microtubules in macrocilia of the ctenophore Beroe. J. Cell Sci. 79, 161–179 [DOI] [PubMed] [Google Scholar]
- 36.Falk N., Lösl M., Schröder N. and Gießl A. (2015) Specialized cilia in mammalian sensory systems. Cells 4, 500–519 10.3390/cells4030500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahl H.A. (1963) Fine structure of cilia in rat cerebral cortex. Z. Für. Zellforsch Mikrosk Anat. 60, 369–386 10.1007/BF00336612 [DOI] [PubMed] [Google Scholar]
- 38.Allen R.A. (1965) Isolated cilia in inner retinal neurons and in retinal pigment epithelium. J. Ultrastruct. Res. 12, 730–747 10.1016/S0022-5320(65)80058-2 [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M. and Kataoka K. (1986) Electron microscopic observation of the primary cilium in the pancreatic islets. Arch. Histol. Jpn. 49, 449–457 [DOI] [PubMed] [Google Scholar]
- 40.Wilsman N.J., Farnum C.E. and Reed-Aksamit D.K. (1980) Incidence and morphology of equine and murine chondrocytic cilia. Anat. Rec. 197, 355–361 10.1002/ar.1091970309 [DOI] [PubMed] [Google Scholar]
- 41.Gluenz E., Höög J.L., Smith A.E., Dawe H.R., Shaw M.K. and Gull K. (2010) Beyond 9+0: noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 24, 3117–3121 10.1096/fj.09-151381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran D.T., Rowley J.C., Jafek B.W. and Lovell M.A. (1982) The fine structure of the olfactory mucosa in man. J. Neurocytol. 11, 721–746 10.1007/BF01153516 [DOI] [PubMed] [Google Scholar]
- 43.Perkins L.A., Hedgecock E.M., Thomson J.N. and Culotti J.G. (1986) Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456–487 10.1016/0012-1606(86)90314-3 [DOI] [PubMed] [Google Scholar]
- 44.Doroquez D.B., Berciu C., Anderson J.R., Sengupta P. and Nicastro D. (2014) A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. eLife, 3, e01948 10.7554/eLife.01948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilliam J.C., Chang J.T., Sandoval I.M., Zhang Y., Li T., Pittler S.J.. et al. (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151, 1029–1041 10.1016/j.cell.2012.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickell S., Park PS-H, Baumeister W. and Palczewski K. (2007) Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J. Cell Biol. 177, 917–925 10.1083/jcb.200612010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greven H. (2007) Comments on the eyes of tardigrades. Arthropod Struct. Dev. 36, 401–407 10.1016/j.asd.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 48.Marshall W.F. and Rosenbaum J.L. (2001) Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 155, 405–414 10.1083/jcb.200106141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fort C., Bonnefoy S., Kohl L. and Bastin P. (2016) Intraflagellar transport is required for the maintenance of the trypanosome flagellum composition but not its length. J. Cell Sci. 129, 3026–3041 10.1242/jcs.188227 [DOI] [PubMed] [Google Scholar]
- 50.San Agustin J.T., Pazour G.J. and Witman G.B. (2015) Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol. Biol. Cell 26, 4358–4372 10.1091/mbc.e15-08-0578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozminski K.G., Johnson K.A., Forscher P. and Rosenbaum J.L. (1993) A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. U.S.A. 90, 5519–5523 10.1073/pnas.90.12.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao L., Thein M., Brust-Mascher I., Civelekoglu-Scholey G., Lu Y., Acar S.. et al. (2011) Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat. Cell Biol. 13, 790–798 10.1038/ncb2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujiwara M., Ishihara T. and Katsura I. (1999) A novel WD40 protein, CHE-2, acts cell-autonomously in the formation of C. elegans sensory cilia. Dev. Camb. Engl. 126, 4839–4848 [DOI] [PubMed] [Google Scholar]
- 54.Harris J.A., Liu Y., Yang P., Kner P. and Lechtreck K.F. (2016) Single-particle imaging reveals intraflagellar transport–independent transport and accumulation of EB1 in Chlamydomonas flagella. Mol. Biol. Cell 27, 295–307 10.1091/mbc.e15-08-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schroder J.M., Larsen J., Komarova Y., Akhmanova A., Thorsteinsson R.I., Grigoriev I.. et al. (2011) EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J. Cell Sci. 124, 2539–2551 10.1242/jcs.085852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pedersen L.B., Miller M.S., Geimer S., Leitch J.M., Rosenbaum J.L. and Cole D.G. (2005) Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr. Biol. 15, 262–266 10.1016/j.cub.2005.01.037 [DOI] [PubMed] [Google Scholar]
- 57.Pedersen L.B., Geimer S., Sloboda R.D. and Rosenbaum J.L. (2003) The Microtubule Plus End-Tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr. Biol. 13, 1969–1974 10.1016/j.cub.2003.10.058 [DOI] [PubMed] [Google Scholar]
- 58.Dawson S.C., Sagolla M.S., Mancuso J.J., Woessner D.J., House S.A., Fritz-Laylin L.. et al. (2007) Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot. Cell 6, 2354–2364 10.1128/EC.00128-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brooks E.R. and Wallingford J.B. (2012) Control of vertebrate intraflagellar transport by the planar cell polarity effector Fuz. J. Cell Biol. 198, 37–45 10.1083/jcb.201204072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang Y., Pang Y., Wu Q., Hu Z., Han X., Xu Y.. et al. (2014) FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Dev. Cell 30, 585–597 10.1016/j.devcel.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 61.Niwa S., Nakajima K., Miki H., Minato Y., Wang D. and Hirokawa N. (2012) KIF19A is a microtubule-depolymerizing kinesin for ciliary length control. Dev. Cell 23, 1167–1175 10.1016/j.devcel.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 62.Wang D., Nitta R., Morikawa M., Yajima H., Inoue S., Shigematsu H.. et al. (2016) Motility and microtubule depolymerization mechanisms of the Kinesin-8 motor, KIF19A. eLife 5, e18101 10.7554/eLife.18101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaineau C., Tessier M., Dubessay P., Tasse L., Crobu L., Pagès M.. et al. (2007) A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr. Biol. 17, 778–782 10.1016/j.cub.2007.03.048 [DOI] [PubMed] [Google Scholar]
- 64.Chan K.Y. and Ersfeld K. (2010) The role of the Kinesin-13 family protein TbKif13-2 in flagellar length control of Trypanosoma brucei. Mol. Biochem. Parasitol. 174, 137–140 10.1016/j.molbiopara.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He M., Subramanian R., Bangs F., Omelchenko T., Liem K.F. Jr, Kapoor T.M.. et al. (2014) The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat. Cell Biol. 16, 663–672 10.1038/ncb2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liem K.F.J., He M., Ocbina P.J.R. and Anderson K.V. (2009) Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 13377–13382 10.1073/pnas.0906944106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Endoh-Yamagami S., Evangelista M., Wilson D., Wen X., Theunissen J.-W., Phamluong K.. et al. (2009) The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr. Biol. 19, 1320–1326 10.1016/j.cub.2009.06.046 [DOI] [PubMed] [Google Scholar]
- 68.Evans J.E., Snow J.J., Gunnarson A.L., Ou G., Stahlberg H., McDonald K.L.. et al. (2006) Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J. Cell Biol. 172, 663–669 10.1083/jcb.200509115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rezabkova L., Kraatz S.H.W., Akhmanova A., Steinmetz M.O. and Kammerer R.A. (2016) Biophysical and structural characterization of the centriolar protein Cep104 interaction network. J. Biol. Chem. 291, 18496–18504 10.1074/jbc.M116.739771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varga V., Moreira-Leite F., Portman N. and Gull K. (2017) Protein diversity in discrete structures at the distal tip of the trypanosome flagellum. Proc. Natl. Acad. Sci. U.S.A. 114, E6546–55 10.1073/pnas.1703553114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizuno K. and Sloboda R.D. (2017) Protein arginine methyltransferases interact with intraflagellar transport particles and change location during flagellar growth and resorption. Mol. Biol. Cell 28, 1208–1222 10.1091/mbc.e16-11-0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodenough U.W. (1993) Tipping of flagellar agglutinins by gametes of Chlamydomonas reinhardtii. Cell Motil. Cytoskeleton 25, 179–189 10.1002/cm.970250207 [DOI] [PubMed] [Google Scholar]
- 73.Kubo A., Yuba-Kubo A., Tsukita S., Tsukita S. and Amagai M. (2008) Sentan: a novel specific component of the apical structure of vertebrate motile cilia. Mol. Biol. Cell 19, 5338–5346 10.1091/mbc.e08-07-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watnick T.J., Jin Y., Matunis E., Kernan M.J. and Montell C. (2003) A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr. Biol. 13, 2179–2184 10.1016/j.cub.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 75.Köttgen M., Hofherr A., Li W., Chu K., Cook S., Montell C.. et al. (2011) Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS ONE 6, e20031 10.1371/journal.pone.0020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J., Moon S., Cha Y. and Chung Y.D. (2010) Drosophila TRPN (= NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE 5, e11012 10.1371/journal.pone.0011012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y.C., Couzens A.L., Deshwar A.R., McBroom-Cerajewski L.D.B., Zhang X., Puviindran V.. et al. (2014) The PPFIA1-PP2A protein complex promotes trafficking of Kif7 to the ciliary tip and Hedgehog signaling. Sci. Signal. 7, ra117 10.1126/scisignal.2005608 [DOI] [PubMed] [Google Scholar]
- 78.Haycraft C.J., Banizs B., Aydin-Son Y., Zhang Q., Michaud E.J. and Yoder B.K. (2005) Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein Polaris for processing and function. PLoS Genet. 1, e53 10.1371/journal.pgen.0010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W., Apagyi K., McLeavy L. and Ersfeld K. (2010) Expression and cellular localisation of calpain-like proteins in Trypanosoma brucei. Mol. Biochem. Parasitol. 169, 20–26 10.1016/j.molbiopara.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 80.Akhmanova A. and Steinmetz M.O. (2015) Control of microtubule organization and dynamics: two ends in the limelight. Nat. Rev. Mol. Cell Biol. 16, 711–726 10.1038/nrm4084 [DOI] [PubMed] [Google Scholar]
- 81.Taschner M. and Lorentzen E. (2016) The intraflagellar transport machinery. Cold Spring Harb. Perspect. Biol. 8, a028092 10.1101/cshperspect.a028092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsao C.-C. and Gorovsky M.A. (2008) Different effects of Tetrahymena IFT172 domains on anterograde and retrograde intraflagellar transport. Mol. Biol. Cell 19, 1450–1461 10.1091/mbc.e07-05-0403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dean S., Sunter J.D. and Wheeler R.J. (2017) TrypTag.org: a trypanosome genome-wide protein localisation resource. Trends Parasitol. 33, 80–82 10.1016/j.pt.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bangs F. and Anderson K.V. (2017) Primary cilia and mammalian Hedgehog signaling. Cold Spring Harb. Perspect. Biol. 9, 10.1101/cshperspect.a028175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He M., Agbu S. and Anderson K.V. (2017) Microtubule motors drive hedgehog signaling in primary cilia. Trends Cell Biol. 27, 110–125 10.1016/j.tcb.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yue Y., Blasius T.L., Zhang S., Jariwala S., Walker B., Grant B.J.. et al. (2018) Altered chemomechanical coupling causes impaired motility of the kinesin-4 motors KIF27 and KIF7. J. Cell Biol. 217, 1319–1334 10.1083/jcb.201708179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sengupta P. (2017) Cilia and sensory signaling: the journey from “animalcules” to human disease. PLoS Biol. 15, e2002240 10.1371/journal.pbio.2002240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shah A.S., Ben-Shahar Y., Moninger T.O., Kline J.N. and Welsh M.J. (2009) Motile cilia of human airway epithelia are chemosensory. Science 325, 1131–1134 10.1126/science.1173869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson N.T., Villalón M., Royce F.H., Hard R. and Verdugo P. (1991) Autoregulation of beat frequency in respiratory ciliated cells: demonstration by viscous loading. Am. Rev. Respir. Dis. 144, 1091–1094 10.1164/ajrccm/144.5.1091 [DOI] [PubMed] [Google Scholar]
- 90.Lorenzo I.M., Liedtke W., Sanderson M.J. and Valverde M.A. (2008) TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 105, 12611–12616 10.1073/pnas.0803970105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Callaghan C.L., Sikand K., Rutman A. and Hirst R.A. (2008) The effect of viscous loading on brain ependymal cilia. Neurosci. Lett. 439, 56–60 10.1016/j.neulet.2008.04.095 [DOI] [PubMed] [Google Scholar]
- 92.Sanderson M.J. and Dirksen E.R. (1986) Mechanosensitivity of cultured ciliated cells from the mammalian respiratory tract: implications for the regulation of mucociliary transport. Proc. Natl. Acad. Sci. U.S.A. 83, 7302–7306 10.1073/pnas.83.19.7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ogura A. and Takahashi K. (1976) Artificial deciliation causes loss of calcium-dependent responses in Paramecium. Nature 264, 170–172 10.1038/264170a0 [DOI] [PubMed] [Google Scholar]
- 94.Solter K.M. and Gibor A. (1977) Evidence for role of flagella as sensory transducers in mating of Chlamydomonas reinhardi. Nature 265, 444–445 10.1038/265444a0 [DOI] [PubMed] [Google Scholar]
- 95.Bloodgood R.A. (2010) Sensory reception is an attribute of both primary cilia and motile cilia. J. Cell Sci. 123, 505–509 10.1242/jcs.066308 [DOI] [PubMed] [Google Scholar]
- 96.Gluenz E., Ginger M.L. and McKean P.G. (2010) Flagellum assembly and function during the Leishmania life cycle. Curr. Opin. Microbiol. 13, 473–479 10.1016/j.mib.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 97.Goodenough U.W. (1985) Structure of the Chlamydomonas agglutinin and related flagellar surface proteins in vitro and in situ. J. Cell Biol 101, 924–941 10.1083/jcb.101.3.924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mesland D.A. (1980) Flagellar tip activation stimulated by membrane adhesions in Chlamydomonas gametes. J. Cell Biol. 84, 599–617 10.1083/jcb.84.3.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Demetsmets R., Tomson A.M., Stegwee D. and Ende van den H. (1990) Cell-cell coordination in conjugating Chlamydomonas gametes. Protoplasma 155, 188–199 10.1007/BF01322628 [DOI] [Google Scholar]
- 100.Goodenough U.W. and Jurivich D. (1978) Tipping and mating-structure activation induced in Chlamydomonas gametes by flagellar membrane antisera. J. Cell Biol. 79, 680–693 10.1083/jcb.79.3.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Homan W., Sigon C., van den Briel W., Wagter R., de Nobel H., Mesland D.. et al. (1987) Transport of membrane receptors and the mechanics of sexual cell fusion in Chlamydomonas eugametos. FEBS Lett. 215, 323–326 10.1016/0014-5793(87)80170-9 [DOI] [Google Scholar]
- 102.Pasquale S.M. (1987) Cyclic AMP functions as a primary sexual signal in gametes of Chlamydomonas reinhardtii. J. Cell Biol. 105, 2279–2292 10.1083/jcb.105.5.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Q. and Snell W.J. (2003) Flagellar adhesion between mating type plus and mating type minus gametes activates a flagellar protein-tyrosine kinase during fertilization in Chlamydomonas. J. Biol. Chem. 278, 32936–32942 10.1074/jbc.M303261200 [DOI] [PubMed] [Google Scholar]
- 104.Wood C.R. and Rosenbaum J.L. (2015) Ciliary ectosomes: transmissions from the cell’s antenna. Trends Cell Biol. 25, 276–285 10.1016/j.tcb.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J. and Barr M.M. (2018) Cell–cell communication via ciliary extracellular vesicles: clues from model systems. Essays Biochem. 62, 205–213 10.1042/EBC20170085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wood C.R., Huang K., Diener D.R. and Rosenbaum J.L. (2013) The cilium secretes bioactive ectosomes. Curr. Biol. 23, 906–911 10.1016/j.cub.2013.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]