Abstract
Propulsion by slender cellular appendages called cilia and flagella is an ancient means of locomotion. Unicellular organisms evolved myriad strategies to propel themselves in fluid environments, often involving significant differences in flagella number, localisation and modes of actuation. Remarkably, these appendages are highly conserved, occurring in many complex organisms such as humans, where they may be found generating physiological flows when attached to surfaces (e.g. airway epithelial cilia), or else conferring motility to male gametes (e.g. undulations of sperm flagella). Where multiple cilia arise, their movements are often observed to be highly coordinated. Here I review the two main mechanisms for motile cilia coordination, namely, intracellular and hydrodynamic, and discuss their relative importance in different ciliary systems.
Keywords: algae, coordination, cilia, centrioles/basal bodies, hydrodynamics, swimming
Introduction
A major signature of living organisms is their ability to generate and coordinate movement. Even plants, which are often considered to have a static existence, exhibit purposeful, directed movement for growth optimisation (e.g. circumnutation) [1]. Throughout evolution, multiple divergent strategies for locomotion have arisen in land, air and sea, including swimming, crawling, galloping and flying. By far the most ancient of these is locomotion through a fluid environment (known as motility), which gave motile organisms the ability to navigate towards favourable conditions (light, nutrients), and consequently a significant selective advantage over their non-motile counterparts. Here, we focus on motility associated with surface-attached appendages known as cilia, or interchangeably flagella. This ubiquitous and evolutionarily successful organelle appears in virtually all extant branches of eukaryotes [2]. Figure 1 illustrates a number of different species spanning several orders of magnitude in size, which invariably use cilia to swim or to move fluid from one region to another. At the unicellular extreme, spermatozoa propagate bending waves from base to tip to push themselves through the fluid [3], the biflagellate green alga Chlamydomonas coordinates two flagella in a synchronous breaststroke [4–6], while larger ciliates including Paramecium and Stentor are covered in cilia which have become specialised either for efficient swimming or feeding [7]. In the ventricles of the brain, cilia generate a complex, directional transport network for precise control of substance redistribution [8] and even provides structural support [9], while in the human trachea, the coordinated sweeping of cilia clears debris and mucus up and out of the lungs over distances of tens of centimetres [10,11]. Do these diverse ciliary systems rely upon unified mechanistic principles to achieve coordinated patterns of activity?
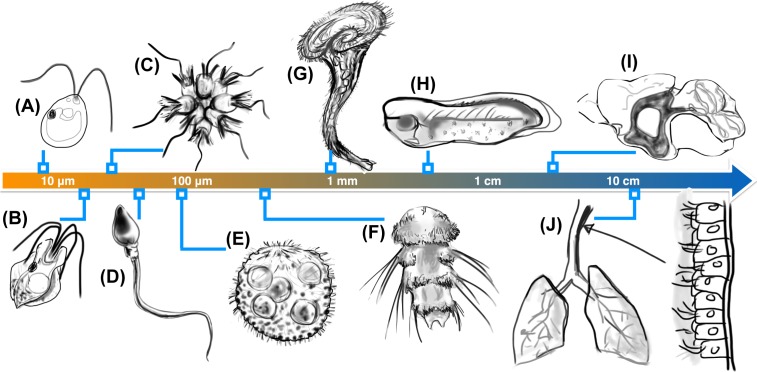
Figure 1. Unity and diversity of ciliary systems.
The same fundamental structure occurs in the tiniest of microorganisms as well as ciliated tissues, but exhibits drastic differences in number and localisation. Examples include (A) the algal biflagellate Chlamydomonas reinhardtii [4], (B) a quadriflagellate Prasinophyte alga Pyramimonas sp. [12], (C) rosette-forming choanoflagellates [13,14], (D) human sperm [15], (E) the spherical alga Volvox carteri [16,17], (F) the ciliated larvae of the marine annelid Platynereis dumerilii which have segmental multiciliated cells, and long, stiff chaetae [18], (G) the trumpet-shaped ciliate Stentor coeruleus [19], (H) ciliated epithelia of Xenopus laevis embryos [20], (I) ependymal cilia in mouse brain ventricles which direct cerebrospinal fluid flows [8] (cilia are localised to shaded region) and (J) multiciliated columnar cells in the human trachea [10,21].
The green algal flagellates, in particular, have emerged as a preferred model system not only for studying the structural biology of cilia and their relation to human ciliopathies [22–25] but also the fluid physics of cilia-driven flows [16,26–28]. Here, we focus on select species of microalgae that exhibit significant differences in size, number and spatial organisation of their flagella, and which, presumably through adaptation to various ecological niches, are able to produce a surprising diversity of swimming gaits. These species encompass single-celled organisms with a diameter of 10 μm or less, but also larger, multicellular species such as Volvox spp which are several hundreds of microns in size. As we shall see, their distinct ciliary coordination patterns could only have arisen from a delicate interplay between two very different physical mechanisms.
Swimming with cilia and flagella
The act of waving an appendage through a fluid creates a local disturbance of decaying magnitude away from the source [29]. Micron-sized organisms dwell in a regime dominated by viscous effects, in which there is zero inertial coasting, so that they must employ very different mechanisms of self-propulsion compared with larger organisms such as fish. Consequently, cilia have evolved to harness drag-based propulsion [30]. There is a surge of recent interest in mimicking the success of this design in the manufacture of artificial robotic microswimmers for biomedical applications [31].
Self-propelling bodies in the viscous (so-called low Reynolds number) regime experience no net forces or torques, so that motion is completely specified by their shape kinematics. There are two main considerations for generating net propulsion, first, a cilium’s characteristic slender shape ensures drag-anisotropy when moving through the fluid, second, microorganisms actively prescribe a time-varying distribution of bending moments along the cilium to generate a cyclical, but importantly non-reciprocal sequence of shape changes – in other words a stroke. A typical ciliary beat consists of a power stroke, in which the long axis is perpendicular to the direction of motion, and a recovery stroke, in which it is much more curved, and aligned with the direction of motion. Eukaryotic cilia and flagella are distinctive in their capacity to propagate large-amplitude bending waves of activity. Indeed, the observation of slow amplitude decay first led Machin [32] to postulate the existence of active force generating components distributed along the entire length of the filament, before the first experiments were conducted showing these putative components to be dyneins (from the Greek for ‘force’) residing inside the axoneme. The precise mechanism by which distributed dynein activity leads to emergence of ciliary beating is still not fully understood, and remains a highly active field of research [33–40].
Individual as well as groups of cilia and flagella show a remarkable sensitivity and mutability to extracellular as well as intracellular perturbations. For multiple cilia, different propulsion modes are produced by careful modulation of the phase difference between the periodic strokes of neighbouring cilia: zero phase difference for the biflagellate breaststroke, or a fixed, non-zero phase difference for metachronal waves in ciliary arrays.
Centrioles in the algal flagellar apparatus
Across diverse evolutionary phyla, a high degree of conservation pertains not only to the cilium itself but also to the centrioles to which these microtubule-based organelles are attached [41,42]. Centrioles are present in many unicellular eukaryotes but lost in most land plants [43], and have been studied extensively in several model species including Chlamydomonas reinhardtii, Drosophila melanogaster, Caenorhabditis elegans and human cell lines [44–46], and it is to C. reinhardtii in particular that we owe much of our knowledge of centriole assembly and the microtubular composition of cilia and flagella [25,47]. Centrioles also serve as the structural basis of centrosomes, which in turn organise spindles during cell division and regulate cytokinesis [48]. Centrosomal duplication is tightly coupled to the cell-cycle [49–51], and in mammalian cells are intimately involved in cell proliferation, migration and determining polarity [52,53]. Mature centrioles, in this context then known as basal bodies, can dock to the plasma membrane (Figure 2) where they are responsible for templating sensory or primary cilia for signal transduction, or motile flagella for cell motility.
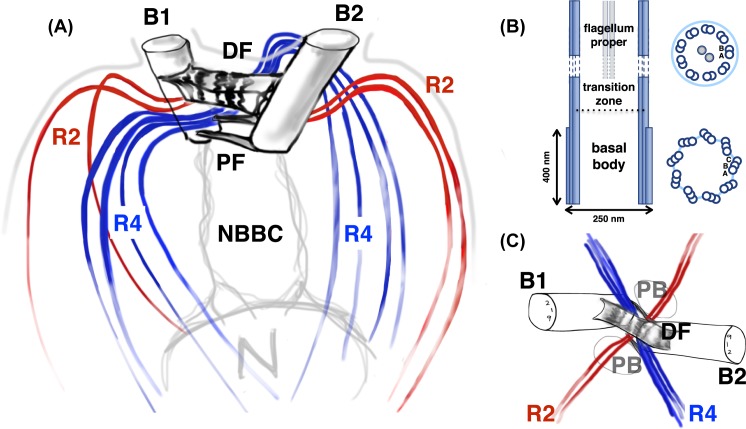
Figure 2. The Chlamydomonas flagellar apparatus.
(A) Schematic showing the cytoskeletal architecture of basal bodies (B1,2), microtubular roots (two-membered rootlets R2 and four-membered rootlets R4), and fibrous/contractile connections (NBBCs to the nucleus, proximal and distal striated fibres PF and DF). (B) Longitudinal section of a flagellum showing a structural change from triplet microtubules to doublets, two characteristic cross-sections are shown: one through the basal body and the second through flagellum proper. (C) Top view, highlighting radial symmetries in the flagellar apparatus, the locations of the two PB, the cruciate arrangement of microtubule bundles and the DF connecting specific microtubule doublets in the mature basal bodies (B1,2),
Eukaryotic centrioles/basal bodies are cylindrical, have a chiral arrangement of triplet microtubules (termed A, B, C) which become doublets extending into the axoneme proper, giving rise to a distinctive nine-fold symmetry. In the flagellar apparatus of C. reinhardtii, individual triplets also exhibit a gradual longitudinal twist going from the basal to distal end [54]. C. reinhardtii flagella are approx. 12 μm long and 250 nm wide, and have a ubiquitous structure comprising nine doublet microtubules encircling a central pair, which consists of two microtubules (Figure 2B). Basal bodies associated with such ‘9 + 2’ axonemes are thought to be the ancestral form present in the common unicellular ancestor of eukaryotes [44]. The C tubules terminate in the transition zone, which gates protein entry into the axoneme, while A and B tubules terminate in the distal part of the flagellum [55]. The peripheral doublets are transiently linked by tens of thousands of dynein motors, regulatory components and other complexes in 96 nm repeating units [56], and beating occurs through the distributed activity of these various dynein isoforms [57]. Signalling and mechanical interactions via the central pair/radial spokes are also thought to be involved in axonemal beat modulation [40,58].
Algal basal bodies are particularly important for organising the cytoskeleton and for organellar placement [59,60]. In interphase, C. reinhardtii cells have two mature flagella-bearing basal bodies (∼400 nm) and two nascent/pro-basal bodies (PB, ∼86 nm), anchored in a fixed orientation (Figure 2C). Unlike metazoan centrosomes where mother and daughter centrioles are oriented perpendicularly [41], in C. reinhardtii they assume a V-shape [61,62], to facilitate breaststroke swimming. Two types of fibrous structures are present in the flagellar apparatus [63,64]: microtubular roots, and contractile, centrin-based fibres. Each mature basal body has a four-membered and a two-membered microtubule rootlet, containing acetylated α-tubulin, which are assembled to form a cruciate pattern characteristic of green algal flagellates. The four-membered rootlets demarcate the cleavage furrow, and determine the placement of a de novo assembled eyespot (photosensor) near the daughter basal body. Each cell has a unique eyespot, thereby breaking bilateral symmetry. A large distal striated fibre (DF) connects the two mature basal bodies just below the transition zone. Fibrous bundles called nuclear basal body connectors (NBBCs) connect the basal bodies to the nucleus [65]. The precise functions of many of these accessory structures remain to be further elucidated [66,67].
More than one cilium – a question of coordination
Given all of the above, how do cilia and flagella cooperate to optimise the movement of fluid given physical constraints such as placement of basal bodies within a precisely defined cytoskeletal architecture? In the human ciliopathy known as primary ciliary dyskinesia (PCD), mutations were first identified in ciliary ultrastructure, but variants of the syndrome have also been found that are due entirely to disorientation of the cilia [68]. Likewise, in unicellular protists such as Tetrahymena or Paramecium the main function of the ciliate cortex is to order basal bodies and nucleate cilia for motility. In all these cases, maintaining correct ciliary orientation and coordination is crucial. Neighbouring cilia which inhabit a shared fluid environment must interact hydrodynamically due to their physical proximity, but cilia may also constrained intracellularly, which of these two (possibly antagonistic) contributions dominate can only be ascertained on a case-by-case basis.
Proof of hydrodynamic interactions
The colonial alga Volvox (Figure 1E) holds clues to the ancient evolutionary origins of multicellularity [69]; spheroids have two cell types, large germ cells in the interior, and thousands of flagellated somatic cells adorning the surface are oriented with their basal bodies directed away from the A-P axis to produce large-scale flows and directed swimming [70]. Individual flagellated somatic cells, when isolated from their parental colonies and placed in pairs with different relative beat orientations on separate micropipettes, can synchronize their beating purely as a result of interactions through the fluid [71]. Pairs of cells with parallel stroke orientations exhibited in-phase synchrony, but anti-phase synchrony when the power strokes faced away from each other (Figure 3A,B). Moreover, the decay of synchronization with increasing distance of separation was shown to be functionally consistent with hydrodynamic predictions [71]. Theoretical models can also account for the emergence of phase synchrony and even large-scale metachronal waves of ciliary activity, consistent with experimental observations [17,72,73]. A corollary of this is that flagellar beating must be compliant: mechanical forces and hydrodynamic loading can alter the engagement of axonemal dyneins, thereby changing the beating waveform and frequency. The physics of these non-local, feedback-driven phenomena is currently under further investigation [74–77]. More recently, hydrodynamic stresses have even been suggested as a mechanism for ultrafast and efficient cell–cell communication in the protist Spirostomum ambiguum [78].
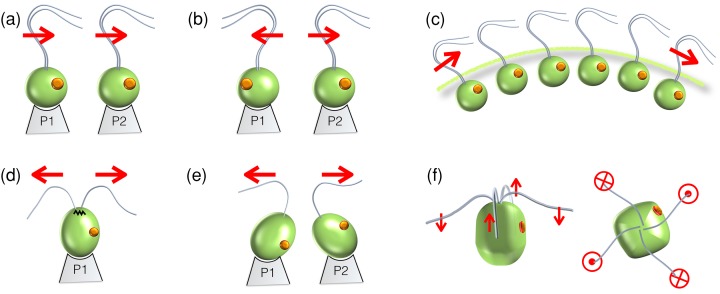
Figure 3. Experimental versus natural configurations of algal flagella.
In all cases, the conspicuous orange dots represent algal eyespots (rudimentary photoreceptors), whose positioning is related to the developmental age of the flagella. A pair of V. carteri somatic cells held on nearby micropipettes, interacting hydrodynamically, exhibits either in-phase synchrony (A) or anti-phase synchrony (B) depending on their relative orientation. (C) In vivo, arrays of these cells coordinate metachronal waves in the Volvox colony. By contrast, the in-phase breaststroke of C. reinhardtii (D) cannot be reproduced in pairs of wildtype cells that have been rendered uniflagellate (E), implicating an internal (possibly spring-like) coupling provided by the distal striated fibre. Arrows indicate power stroke directions (A–E). (F) In a different species (see also Figure 1B), a quadriflagellate beat pattern (aka trot) is observed, comprising two pairs of breaststrokes displaced temporally by 1/2 beat cycle.
Proof of intracellular control
Contrary to the case of Volvox, the in-phase synchronous breaststroke of Chlamydomonas (Figure 3D–F) could not be reconciled with the same hydrodynamic theory (see also [79]). Instead, can fibrous connections in the algal flagellar apparatus, which not only lie in the plane of flagellar beating, but are even found attached to each basal body at specific numbered microtubule doublets, provide additional intracellular coupling [61,65,80]? Recent experiments examining the motility phenotypes of vfl-3 mutants – which have a variable number of full-length, fully motile flagella (0 ≤ N ≤ 5), suggest this is indeed the case [12]. In this mutant, flagella are also found in aberrant positions and orientations [81], but importantly are missing or defective in the distal striated fibre (recall Figure 2). No in-phase synchronous breaststrokes were observed, indicating that internal coupling must have been necessary to coordinate the biflagellate breaststroke of wildtype cells. Indeed, flagella in certain configurations (e.g. triplets) exhibited hydrodynamic synchronisation in the absence of these intracellular connections (Figure 4A).
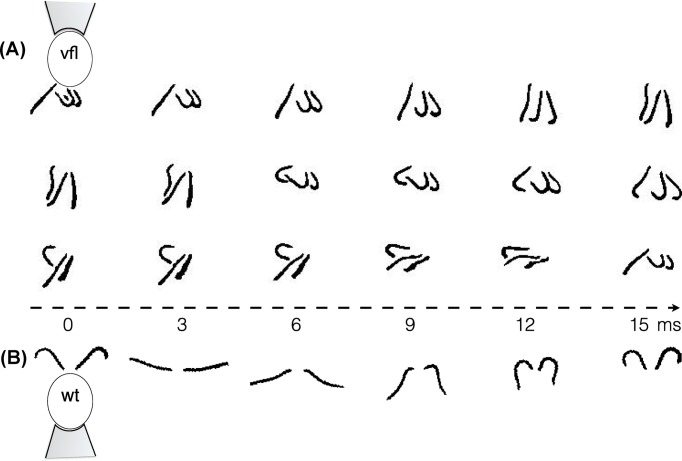
Figure 4. Aberrant flagellar coordination patterns in a basal-coupling mutant.
Flagellar waveforms and dynamics are tracked in a tri-flagellate vfl-3 mutant (A) and wildtype C. reinhardtii (B). (Cell body/pipette not shown on subsequent image frames.) The distal striated fibre is missing or defective in vfl-3 [81]. Consequently, coordination between the three flagella reverts to hydrodynamic interactions, in which the pair beating with power strokes in the same direction tends to synchronize in in-phase, but in anti-phase with respect to the third singlet flagellum. The wildtype cell on the other hand, maintains an in-phase synchronous breaststroke. (For further examples, see [12].)
Notably, it has been predicted in multiple theoretical models that changes in the amount of sliding between microtubule doublets at the flagellar base or other boundary conditions can effect global changes in the beating dynamics in a non-trivial manner [36,40,82,83]. Such considerations may yet help to explain observations of a novel (biflagellate) anti-phase gait in another C. reinhardtii mutant known as ptx1 [83,84]. More generally, elaborate networks of intracellular structures are associated with the basal bodies of other species of algal flagellates including quadri- and octoflagellates, revealing a surprising diversity of gaits involving precise phase relationships between the flagella [12]. Further investigation into the morphology and function of these fibrous structures may yield fresh insights into the evolution of the algal flagellar apparatus and the origins of active, biochemical gait-control.
The generational age of basal bodies
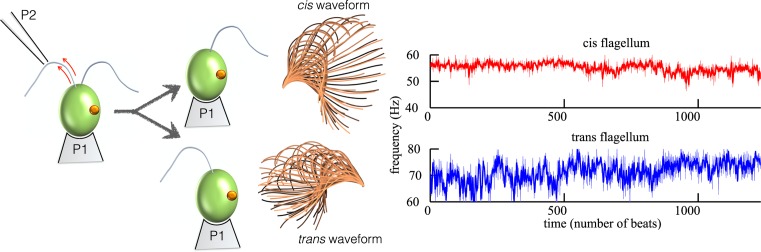
For many simple eukaryotes, cilia and flagella are used simultaneously for sensing and motility, which leads to one further complication. Organisms must be able to produce asymmetric behavioural changes in response to environmental cues: in addition to directed swimming, they must be able to turn. This is of particular importance in phototaxis – motility with respect to light, where they adjust to photostimuli by altering the beating of cilia/flagella [85,86]. In C. reinhardtii, this is accomplished through asymmetric actuation of the two flagella [87], which are termed cis or trans depending on relative distances from the unique eyespot (Figure 5). Both flagella insert into the apical region of the cell and are of equal length, and have no visible ultrastructural or morphological differences. An inherent centriolar asymmetry exists however, rooted in the difference in generational age of the cis versus trans basal body; at each round of cell division, the cis basal body is always formed anew yet the trans is inherented from the parent cell. This sequence of semi-conservative basal body duplication forms the basis of a greater, cell-wide asymmetry [88,89].
Figure 5. Revealing intrinsic differences between the two C.reinhardtii flagella.
The two flagella are similar but not identical. In vivo micromanipulation and microsurgery revealed a differential sensitivity to deflagellation-induced intracellular calcium elevation – here inferred indirectly by measuring ciliary beat frequencies in the two flagella of the same cell, after successive rounds of deflagellation and regrowth. The stereotypical trans-flagellum beating mode is markedly faster, has an attenuated waveform, and exhibits greater frequency variations than the cis.
At a behavioural level, evidence of differential Ca2+ sensitivities in the two C. reinhardtii flagella first emerged in demembranated cell models placed in buffers with varying concentrations of calcium [90,91], as well as in early micropipette experiments [4]. More recently, high-speed imaging was used in combination with micromanipulation and microsurgery in live cells to decouple the intrinsic beating dynamics of one flagellum from its partner, in which individual cis- or trans-flagella were carefully removed from each biflagellate cell by induced self-scission (Figure 5), allowing sufficient time for flagellar regeneration between the successive amputations [6]. Attributing these in vivo flagellar responses to deflagellation-induced calcium elevation [92], isolated cis-flagella were found to beat with a lower mean frequency as well as lower frequency noise [93] than isolated trans-flagella. Biochemical explanations for these differences remain elusive, but entry of signalling proteins into a basal body may well depend on its generational age [35]. In the normal wildtype breaststroke (a putative lower calcium state), intrinsic cis–trans frequency and amplitude differences are presumed sufficiently suppressed [91] to enable intracellular fibres to lock the two flagella into synchrony.
Conclusions
For many eukaryotes, the importance of the centriole/basal body complex is well established. Highly conserved basal body and axonemal architectures are thought to have originated in unicellular organisms that adopted the very first proto-cilium for motility [44,94]. The algal flagellates display a great diversity in form yet conservatism in function, emphasising their value in helping us disentangle the ancient evolutionary and phylogenetic origins of the flagellar apparatus. By performing comparative studies across different species spanning extremes in size and complexity, causal relationships between ultrastructure and motile behaviour were deduced, uncovering two distinct mechanisms for achieving robust beat coordination that are exemplified by dominance of hydrodynamic interactions in the colonial Volvox, and of intracellular coupling in the unicellular Chlamydomonas.
In metazoa however, or even in the larger ciliates, causality is not usually so clear-cut and additional mechanisms must also be considered [95]. In Platynereis larvae for example (Figure 1F), ciliary coordination is closely gated by neural activity [18]. In Tetrahymena, striated kinetodesmal fibres help maintain basal body orientation and resist hydrodynamic stresses produced by ciliary beating [96], while in the mouse trachea, the apical cytoskeleton was found to be necessary for correct basal body alignment [97]. Conversely, ciliary flows are implicated in the establishment of order: ciliary patterning in the larval skin of Xenopus is a two-stage process in which basal bodies are first oriented relative to the tissue axis but subsequently refined through feedback by extracellular fluid flows [98], in mouse brain ventricles however, basal bodies begin randomly docked, before a coupling between hydrodynamic forces and intracellular Planar Cell Polarity signalling sets the correct orientation [99]. With these more confounding contexts in mind, further study of flagellar coordination in algae and other protists will help reveal with greater clarity, the physics behind this complex interplay between flows, mechanical coupling and intracellular signalling.
Summary
Motile cilia and flagella occur in diverse biological systems.
Multiple cilia can interact intracellularly as well as hydrodynamically.
Passive hydrodynamic interactions can drive the beating of nearby filaments into synchrony or metachrony, e.g. in ciliary arrays.
In unicellular algae with only a few front-mounted flagella, additional coupling is instead provided by contractile fibres, implicating an essential role of the flagellar apparatus for the coordination and control of appendages.
Acknowledgments
I thank Raymond E. Goldstein, Marco Polin, Douglas R. Brumley and Kyriacos C. Leptos for collaborations and support, and other former colleagues from the Goldstein Lab at DAMTP, University of Cambridge, whose work have been discussed here. I am grateful to Gáspár Jékely for a critical reading of the manuscript.
Abbreviations
- DF
distal fibre
- NBBC
nuclear basal body connector
- PF
proximal fibre
- PB
pro-basal bodies
Funding
This work was supported by a startup grant from the Living Systems Institute, University of Exeter, and a Springboard Award from the Academy of Medical Sciences.
Competing Interests
The author declares that there are no competing interests associated with the manuscript.
References
- 1.Darwin C.R. (1875) The Movements and Habits of Climbing Plants, John Murray, London [Google Scholar]
- 2.Mitchell D.R. (2007) The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv. Exp. Med. Biol. 607, 130–140 10.1007/978-0-387-74021-8_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishimoto K., Gadelha H., Gaffney E.A., Smith D.J. and Kirkman-Brown J. (2017) Coarse-graining the fluid flow around a human sperm. Phys. Rev. Lett. 118, 124501 10.1103/PhysRevLett.118.124501 [DOI] [PubMed] [Google Scholar]
- 4.Ruffer U. and Nultsch W. (1987) Comparison of the beating of cis-flagella and trans-flagella of Chlamydomonas cells held on micropipettes. Cell Motil. Cytoskeleton 7, 87–93 10.1002/cm.970070111 [DOI] [PubMed] [Google Scholar]
- 5.Goldstein R.E., Polin M. and Tuval I. (2009) Noise and synchronization in pairs of beating eukaryotic flagella. Phys. Rev. Lett. 103, 168103 10.1103/PhysRevLett.103.168103 [DOI] [PubMed] [Google Scholar]
- 6.Wan K.Y., Leptos K.C. and Goldstein R.E. (2014) Lag, lock, sync, slip: the many ‘phases’ of coupled flagella. J. R. Soc. Interface 11, 20131160 10.1098/rsif.2013.1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang S. K.Y. and Marshall W.F. (2017) Self-repairing cells: how single cells heal membrane ruptures and restore lost structures. Science 356, 1022–1025 10.1126/science.aam6496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faubel R., Westendorf C., Bodenschatz E. and Eichele G. (2016) Cilia-based flow network in the brain ventricles. Science 353, 176–178 10.1126/science.aae0450 [DOI] [PubMed] [Google Scholar]
- 9.Mahuzier A., Shihavuddin A., Fournier C., Lansade P., Faucourt M., Menezes N.. et al. (2018) Ependymal cilia beating induces an actin network to protect centrioles against shear stress. Nat. Commun. 9, 2279 10.1038/s41467-018-04676-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith D.J., Gaffney E.A. and Blake J.R. (2008) Modelling mucociliary clearance. Respir. Physiol. Neurobiol. 163, 178–188 10.1016/j.resp.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 11.Leopold P.L., O’Mahony M.J., Lian X.J., Tilley A.E., Harvey B.G. and Crystal R.G. (2009) Smoking is associated with shortened airway cilia. PLoS One 4, e8157 10.1371/journal.pone.0008157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan K.Y. and Goldstein R.E. (2016) Coordinated beating of algal flagella is mediated by basal coupling. Proc. Natl. Acad. Sci. USA 113, E2784–E2793 10.1073/pnas.1518527113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alegado R.A., Brown L.W., Cao S.G., Dermenjian R.K., Zuzow R., Fairclough S.R.. et al. (2012) A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals. Elife 1, UNSP e00013 10.7554/eLife.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkegaard J.B., Marron A.O. and Goldstein R.E. (2016) Motility of colonial choanoflagellates and the statistics of aggregate random walkers. Phys. Rev. Lett. 116, 038102 10.1103/PhysRevLett.116.038102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaffney E.A., Gadelha H., Smith D.J., Blake J.R. and Kirkman-Brown J.C. (2011) Mammalian sperm motility: observation and theory. Annu. Rev. Fluid Mech. 43, 501–528 10.1146/annurev-fluid-121108-145442 [DOI] [Google Scholar]
- 16.Solari C.A., Drescher K., Ganguly S., Kessler J.O., Michod R.E. and Goldstein R.E. (2011) Flagellar phenotypic plasticity in volvocalean algae correlates with Péclet number. J. R. Soc. Interface 8, 1409–1417 10.1098/rsif.2011.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brumley D.R., Polin M., Pedley T.J. and Goldstein R.E. (2015) Metachronal waves in the flagellar beating of Volvox and their hydrodynamic origin. J. R. Soc. Interface 12, 20141358 10.1098/rsif.2014.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veraszto C., Ueda N., Bezares-Calderon L.A., Panzera A., Williams E.A., Shahidi R.. et al. (2017) Ciliomotor circuitry underlying whole-body coordination of ciliary activity in the Platynereis larva. Elife 6, e26000 10.7554/eLife.26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slabodnick M.M. and Marshall W.F. (2014) Stentor coeruleus. Curr. Biol. 24, R783–R784 10.1016/j.cub.2014.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks E.R. and Wallingford J.B. (2014) Multiciliated cells. Curr. Biol. 24, R973–R982 10.1016/j.cub.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sears P.R., Thompson K., Knowles M.R. and Davis C.W. (2013) Human airway ciliary dynamics. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L170–L183 10.1152/ajplung.00105.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witman G.B., Rosenbaum J.L., Berliner J. and Carlson K. (1972) Chlamydomonas flagella. 1. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J. Cell Biol. 54, 507–539 10.1083/jcb.54.3.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pazour G.J., Dickert B.L., Vucica Y., Seeley E.S., Rosenbaum J.L., Witman G.B.. et al. (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 10.1083/jcb.151.3.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechtreck K.F., Johnson E.C., Sakai T., Cochran D., Ballif B.A., Rush J.. et al. (2009) The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 187, 1117–1132 10.1083/jcb.200909183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silflow C.D. and Lefebvre P.A. (2001) Assembly and motility of eukaryotic cilia and flagella. Lessons from Chlamydomonas reinhardtii. Plant Physiol. 127, 1500–1507 10.1104/pp.010807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill N.A. and Bees M.A. (2002) Taylor dispersion of gyrotactic swimming micro-organisms in a linear flow. Phys. Fluids 14, 2598–2605 10.1063/1.1458003 [DOI] [Google Scholar]
- 27.Pedley T.J. and Kessler J.O., Hydrodynamic phenomena in suspensions of swimming microorganisms. Annu. Rev. Fluid Mech. 24, 313–358 10.1146/annurev.fl.24.010192.001525 [DOI] [Google Scholar]
- 28.Goldstein R.E. (2015) Green algae as model organisms for biological fluid dynamics. Annu. Rev. Fluid Mech. 47, 343–375 10.1146/annurev-fluid-010313-141426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drescher K., Goldstein R.E., Michel N., Polin M. and Tuval I. (2010) Direct measurement of the flow field around swimming microorganisms. Phys. Rev. Lett. 105, 168101 10.1103/PhysRevLett.105.168101 [DOI] [PubMed] [Google Scholar]
- 30.Lauga E. and Powers T.R. (2009) The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 72, 096601 10.1088/0034-4885/72/9/096601 [DOI] [Google Scholar]
- 31.Nelson B.J., Kaliakatsos I.K. and Abbott J.J. (2010) Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 12, 55–85 10.1146/annurev-bioeng-010510-103409 [DOI] [PubMed] [Google Scholar]
- 32.Machin K.E. (1958) Wave propagation along flagella. J. Exp. Biol. 35, 796–806 [Google Scholar]
- 33.Edwards B. F.L., Wheeler R.J., Barker A.R., Moreira-Leite F.F., Gull K. and Sunter J.D. (2018) Direction of flagellum beat propagation is controlled by proximal/distal outer dynein arm asymmetry. Proc. Natl. Acad. Sci. USA 115, E7341–E7350 10.1073/pnas.1805827115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han J.H. and Peskin C.S. (2018) Spontaneous oscillation and fluid–structure interaction of cilia. Proc. Natl. Acad. Sci. USA 115, 4417–4422 10.1073/pnas.1712042115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa T. (2017) Axoneme structure from motile cilia. Cold Spring Harb. Perspect. Biol. 9, a028076 10.1101/cshperspect.a028076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oriola D., Gadelha H. and Casademunt J. (2017) Nonlinear amplitude dynamics in flagellar beating. R. Soc. Open Sci. 4, 160698 10.1098/rsos.160698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sartori P., Geyer V.F., Scholich A., Julicher F. and Howard J. (2016) Dynamic curvature regulation accounts for the symmetric and asymmetric beats of Chlamydomonas flagella. Elife 5, e13258 10.7554/eLife.13258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Canio G., Lauga E. and Goldstein R.E. (2017) Spontaneous oscillations of elastic filaments induced by molecular motors. J. R. Soc. Interface 14, 20170491 10.1098/rsif.2017.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayly P.V. and Dutcher S.K. (2016) Steady dynein forces induce flutter instability and propagating waves in mathematical models of flagella. J. R. Soc. Interface 13, 20160523 10.1098/rsif.2016.0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu T.C. and Bayly P.V. (2018) Finite element models of flagella with sliding radial spokes and interdoublet links exhibit propagating waves under steady dynein loading. Cytoskeleton 75, 185–200 10.1002/cm.21432 [DOI] [PubMed] [Google Scholar]
- 41.Azimzadeh J. and Marshall W.F. (2010) Building the centriole. Curr. Biol. 20, R816–R825 10.1016/j.cub.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho-Santos Z., Azimzadeh J., Pereira-Leal J.B. and Bettencourt-Dias M. (2011) Tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 194, 165–175 10.1083/jcb.201011152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodges M.E., Scheumann N., Wickstead B., Langdale J.A. and Gull K. (2010) Reconstructing the evolutionary history of the centriole from protein components. J. Cell Sci. 123, 1407–1413 10.1242/jcs.064873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta A. and Kitagawa D. (2018) Ultrastructural diversity between centrioles of eukaryotes. J. Biochem. (Tokyo) 164, 1–8 10.1093/jb/mvy031 [DOI] [PubMed] [Google Scholar]
- 45.Mirvis M., Stearns T. and Nelson W.J. (2018) Cilium structure, assembly, and disassembly regulated by the cytoskeleton. Biochem. J. 475, 2329–2353 10.1042/BCJ20170453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nigg E.A. and Raff J.W. (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 10.1016/j.cell.2009.10.036 [DOI] [PubMed] [Google Scholar]
- 47.Satir P. and Christensen S.T. (2007) Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69, 377–400 10.1146/annurev.physiol.69.040705.141236 [DOI] [PubMed] [Google Scholar]
- 48.Yubuki N. and Leander B.S. (2013) Evolution of microtubule organizing centers across the tree of eukaryotes. Plant J. 75, 230–244 10.1111/tpj.12145 [DOI] [PubMed] [Google Scholar]
- 49.Stearns T. (2001) Centrosome duplication: a centriolar pas de deux. Cell 105, 417–420 10.1016/S0092-8674(01)00366-X [DOI] [PubMed] [Google Scholar]
- 50.Bornens M. (2012) The centrosome in cells and organisms. Science 335, 422–426 10.1126/science.1209037 [DOI] [PubMed] [Google Scholar]
- 51.Nabais N., Gomes Pereira S. and Bettencourt-Dias M. (2018) Noncanonical biogenesis of centrioles and basal bodies. Cold Spring Harb. Perspect. Biol. [DOI] [PubMed] [Google Scholar]
- 52.Dawe H.R., Farr H. and Gull K. (2007) Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell Sci. 120, 7–15 10.1242/jcs.03305 [DOI] [PubMed] [Google Scholar]
- 53.Fu J.Y., Hagan I.M. and Glover D.M. (2015) The centrosome and its duplication cycle. Cold Spring Harb. Perspect. Biol. 7, a015800 10.1101/cshperspect.a015800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S., Fernandez J.J., Marshall W.F. and Agard D.A. (2012) Three-dimensional structure of basal body triplet revealed by electron cryo-tomography. EMBO J. 31, 552–562 10.1038/emboj.2011.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Toole E.T., Giddings T.H., McIntosh J.R. and Dutcher S.K. (2003) Three-dimensional organization of basal bodies from wild-type and delta-tubulin deletion strains of Chlamydomonas reinhardtii. Mol. Biol. Cell 14, 2999–3012 10.1091/mbc.e02-11-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oda T., Yanagisawa H., Kamiya R. and Kikkawa M. (2014) A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 346, 857–860 10.1126/science.1260214 [DOI] [PubMed] [Google Scholar]
- 57.Lin J.F. and Nicastro D. (2018) Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 360, 396 10.1126/science.aar1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barber C.F., Heuser T., Carbajal-Gonzalez B.I., Botchkarev V.V. and Nicastro D. (2012) Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol. Biol. Cell 23, 111–120 10.1091/mbc.e11-08-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dutcher S.K. (2003) Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic 4, 443–451 10.1034/j.1600-0854.2003.00104.x [DOI] [PubMed] [Google Scholar]
- 60.Feldman J.L., Geimer S. and Marshall W.F. (2007) The mother centriole plays an instructive role in defining cell geometry. PLoS Biol. 5, e149 10.1371/journal.pbio.0050149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ringo D.L. (1967) Flagellar motion and fine structure of flagellar apparatus in Chlamydomonas. J. Cell Biol. 33, 543–+ 10.1083/jcb.33.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Toole E.T. and Dutcher S.K. (2014) Site-specific basal body duplication in Chlamydomonas. Cytoskeleton 71, 108–118 10.1002/cm.21155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melkonian M. (1980) Ultrastructural aspects of basal body associated fibrous structures in green-algae – a critical-review. Biosystems 12, 85–104 10.1016/0303-2647(80)90040-4 [DOI] [PubMed] [Google Scholar]
- 64.O’Kelly C.J. and Floyd G.L. (1983) Flagellar apparatus absolute orientations and the phylogeny of the green algae. Biosystems 16, 227–251 10.1016/0303-2647(83)90007-2 [DOI] [PubMed] [Google Scholar]
- 65.Geimer S. and Melkonian M. (2004) The ultrastructure of the Chlamydomonas reinhardtii basal apparatus: identification of an early marker of radial asymmetry inherent in the basal body. J. Cell Sci. 117, 2663–2674 10.1242/jcs.01120 [DOI] [PubMed] [Google Scholar]
- 66.Salisbury J.L. (1989) Centrin and the algal flagellar apparatus. J. Phycol. 25, 201–206 10.1111/j.1529-8817.1989.tb00114.x [DOI] [Google Scholar]
- 67.Wingfield J.L. and Lechtreck K.F. (2018) Chlamydomonas basal bodies as flagella organizing centers. Cells 7, 79 10.3390/cells7070079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rayner C. F.J., Rutman A., Dewar A., Greenstone M.A., Cole P.J. and Wilson R. (1996) Ciliary disorientation alone as a cause of primary ciliary dyskinesia syndrome. Am. J. Respir. Crit. Care Med. 153, 1123–1129 10.1164/ajrccm.153.3.8630555 [DOI] [PubMed] [Google Scholar]
- 69.Herron M.D. (2016) Origins of multicellular complexity: Volvox and the volvocine algae. Mol. Ecol. 25, 1213–1223 10.1111/mec.13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirk D.L. (1998) Volvox, Cambridge University Press [Google Scholar]
- 71.Brumley D.R., Wan K.Y., Polin M. and Goldstein R.E. (2014) Flagellar synchronization through direct hydrodynamic interactions. Elife 3, e02750 10.7554/eLife.02750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feriani L., Juenet M., Fowler C.J., Bruot N., Chioccioli M., Holland S.M., Bryant C.E., Cicuta P. (2017) Assessing the Collective Dynamics of Motile Cilia in Cultures of Human Airway Cells by Multiscale DDM. Biophys. J. 113, 109–119, 10.1016/j.bpj.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo H.L., Fauci L., Shelley M., Kanso E. (2018) Bistability in the synchronization of actuated microfilaments. J. Fluid Mechanics 836, 304–323 10.1017/jfm.2017.816 [DOI] [Google Scholar]
- 74.Elfring G.J. and Lauga E. (2009) Hydrodynamic phase locking of swimming microorganisms. Phys. Rev. Lett. 103, 088101 10.1103/PhysRevLett.103.088101 [DOI] [PubMed] [Google Scholar]
- 75.Klindt G.S., Ruloff C., Wagner C. and Friedrich B.M. (2016) Load response of the flagellar beat. Phys. Rev. Lett. 117, 258101 10.1103/PhysRevLett.117.258101 [DOI] [PubMed] [Google Scholar]
- 76.Man Y., Koens L. and Lauga E. (2016) Hydrodynamic interactions between nearby slender filaments. Epl 116, 24002 10.1209/0295-5075/116/24002 [DOI] [Google Scholar]
- 77.Goldstein R.E., Lauga E., Pesci A.I. and Proctor M. R.E. (2016) Elastohydrodynamic synchronization of adjacent beating flagella. Phys. Rev. Fluids 1, 073201 10.1103/PhysRevFluids.1.073201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathijssen A., Culver J., Saad Bhamla M. and Prakash M. (2018) Collective intercellular communication through ultra-fast hydrodynamic trigger waves. bioRxiv URL https://www.biorxiv.org/content/biorxiv/early/2018/09/26/428573.full.pdf [DOI] [PubMed] [Google Scholar]
- 79.Quaranta G., Aubin-Tam M.E. and Tam D. (2015) Hydrodynamics versus intracellular coupling in the synchronization of eukaryotic flagella. Phys. Rev. Lett. 115, 238101 10.1103/PhysRevLett.115.238101 [DOI] [PubMed] [Google Scholar]
- 80.Salisbury J.L. (1988) The lost neuromotor apparatus of Chlamydomonas – rediscovered. J. Protozool. 35, 574–577 10.1111/j.1550-7408.1988.tb06128.x [DOI] [PubMed] [Google Scholar]
- 81.Wright R.L., Chojnacki B. and Jarvik J.W. (1983) Abnormal basal-body number, location, and orientation in a striated fiber-defective mutant of Chlamydomonas reinhardtii. J. Cell Biol. 96, 1697–1707 10.1083/jcb.96.6.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riedel-Kruse I.H., Hilfinger A., Howard J. and Julicher F. (2007) How molecular motors shape the flagellar beat. HFSP J. 1, 192–208 10.2976/1.2773861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klindt G.S., Ruloff C., Wagner C. and Friedrich B.M. (2017) In-phase and anti-phase flagellar synchronization by waveform compliance and basal coupling. New J. Phys. 19, 113052 10.1088/1367-2630/aa9031 [DOI] [Google Scholar]
- 84.Leptos K.C., Wan K.Y., Polin M., Tuval I., Pesci A.I. and Goldstein R.E. (2013) Antiphase synchronization in a flagellar-dominance mutant of Chlamydomonas. Phys. Rev. Lett. 111, 158101 10.1103/PhysRevLett.111.158101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harz H. and Hegemann P. (1991) Rhodopsin-regulated calcium currents in Chlamydomonas. Nature 351, 489–491 10.1038/351489a0 [DOI] [Google Scholar]
- 86.Jekely G., Colombelli J., Hausen H., Guy K., Stelzer E., Nedelec F.. et al. (2008) Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399 10.1038/nature07590 [DOI] [PubMed] [Google Scholar]
- 87.Bennett R.R. and Golestanian R. (2015) A steering mechanism for phototaxis in Chlamydomonas. J. R. Soc. Interface 12, 20141164 10.1098/rsif.2014.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marshall W.F. (2012) Centriole asymmetry determines algal cell geometry. Curr. Opin. Plant Biol. 15, 632–637 10.1016/j.pbi.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pearson C.G. (2014) Choosing sides – asymmetric centriole and basal body assembly. J. Cell Sci. 127, 2803–2810 10.1242/jcs.151761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bessen M., Fay R.B. and Witman G.B. (1980) Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J. Cell Biol. 86, 446–455 10.1083/jcb.86.2.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamiya R. and Witman G.B. (1984) Submicromolar levels of calcium control the balance of beating between the 2 flagella in demembranated models of Chlamydomonas. J. Cell Biol. 98, 97–107 10.1083/jcb.98.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wheeler G.L. and Brownlee C. (2008) Rapid spatiotemporal patterning of cytosolic Ca2+ underlies flagellar excision in Chlamydomonas reinhardtii. Plant J. 53, 401–413 10.1111/j.1365-313X.2007.03349.x [DOI] [PubMed] [Google Scholar]
- 93.Wan K.Y. and Goldstein R.E. (2014) Rhythmicity, recurrence, and recovery of flagellar beating. Phys. Rev. Lett. 113, 238103 10.1103/PhysRevLett.113.238103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Satir P., Mitchell D.R. and Jekely G. (2008) How did the cilium evolve? Curr. Top. Dev. Biol. 85, 63–82 10.1016/S0070-2153(08)00803-X [DOI] [PubMed] [Google Scholar]
- 95.Wallingford J.B. (2010) Planar cell polarity signaling, cilia and polarized ciliary beating. Curr. Opin. Cell Biol. 22, 597–604 10.1016/j.ceb.2010.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galati D.F., Bonney S., Kronenberg Z., Clarissa C., Yandell M., Elde N.C.. et al. (2014) DisAp-dependent striated fiber elongation is required to organize ciliary arrays. J. Cell Biol. 207, 705–715 10.1083/jcb.201409123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herawati E., Taniguchi D., Kanoh H., Tateishi K., Ishihara S. and Tsukita S. (2016) Multiciliated cell basal bodies align in stereotypical patterns coordinated by the apical cytoskeleton. J. Cell Biol. 214, 571–586 10.1083/jcb.201601023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitchell B., Jacobs R., Li J., Chien S. and Kintner C. (2007) A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 447, 97–U8 10.1038/nature05771 [DOI] [PubMed] [Google Scholar]
- 99.Guirao B., Meunier A., Mortaud S., Aguilar A., Corsi J.M., Strehl L.. et al. (2010) Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341–U86 [DOI] [PubMed] [Google Scholar]