Abstract
We report a case of a 41-year-old male with end-stage renal disease receiving chronic hemodialysis who was referred to this hospital because of dyspnea. He had been on a regular dialysis for 20 years due to chronic glomerulonephritis. His transthoracic echocardiography revealed severe pulmonary hypertension (PH), and cardiac catheterization confirmed this diagnosis. From clinical examination and review of the chest X-ray and computed tomography images, we thought PH was due to multifactorial mechanisms typical of hemodialysis patients. However, microscopic examination of lung tissue from autopsy specimen revealed extensive calcium deposits not only in alveolar septal wall but also in alveolar capillaries and small vessels, which had diffuse intimal thickening causing the narrowing of the lumens. These pathological findings suggest that pulmonary vascular calcification contributed to the PH in this patient.
<Learning objective: Pulmonary hypertension (PH) is prevalent and associated with mortality in patients with end-stage renal disease (ESRD). However, the pathogenesis of PH with ESRD remains uncertain. Here we report a PH case receiving long-term hemodialysis, and whose pathological findings revealed extensive calcification in small pulmonary vessels and alveolar capillaries. This case will provide evidence indicating the causative role of pulmonary calcification for the development of PH in dialysis patients.>
Keywords: Pulmonary hypertension, Pulmonary calcification, End stage renal disease, Hemodialysis
Introduction
Pulmonary hypertension (PH) is an elevation of pulmonary arterial pressure that can be the result of heart, lung, or systemic disorders. Regardless of etiology, the morbidity and mortality from long-standing PH exceed that expected from the causative condition [1].
Studies have demonstrated that end-stage renal disease (ESRD) patients receiving long-term hemodialysis often develop unexplained PH, and PH is a strong independent predictor of mortality in these patients [2]. The pathogenesis of PH in patients on hemodialysis has been known to be multi-factorial and remains to be fully determined [3]. Here, we report a male dialysis patient with severe PH whose pathological examination of autopsy lung specimens showed profound calcification in small vessels with diffuse intimal thickening as well as scattered calcified deposits surrounding alveolar capillaries and in alveolar septa. These data suggest the causative role of pulmonary vascular calcification in the development of PH in patients with chronic hemodialysis.
Case report
A 41-year-old man was referred to our hospital for cardiac evaluation for dyspnea. At the age of 10 years, he had proteinuria, but he had refused to receive treatment. At the age of 20, he underwent thoracoplasty for lung tuberculosis. At the age of 23, he suffered from dyspnea and edema due to end-stage renal insufficiency. Then he underwent chronic hemodialysis. At that time, his medications included sevelamer hydrochloride, precipitated calcium carbonate, allopurinol, and tocopherol nicotinate.
The blood pressure was 108/70 mmHg, and the pulse was 100 beats per minute. He had patent arterio-venous access on left arm, dilatation of jugular vein, and cardiac assessment revealed a large heart, and loud pulmonic component of the second heart sound with holosystolic regurgitant murmur in the tricuspid area. He had mild hepatomegaly and leg edema.
Laboratory examination revealed low hemoglobin 11.6 g/dl (normal 13.2–17.3 g/dl), elevated serum creatinine 5.3 mg/dl (normal 0.8–1.2 mg/dl), elevated brain natriuretic peptide 1194.8 pg/ml (normal 0–18.4 pg/dl), serum calcium 9.9 mg/dl (normal 8.9–10.5 mg/dl), elevated phosphorus 6.8 mg/dl (normal 2.5–4.1 mg/dl), calcium–phosphorus product 67.2 mg2/dl2, and marginally elevated serum parathyroid hormone 670 pg/ml (normal 15–65 pg/ml). There were no abnormal findings related to thrombus and collagen disease.
Arterial blood gas values were normal [arterial oxygen tension (PaO2) 82 mmHg, arterial carbon dioxide tension (PaCO2) 38 mmHg, and pH 7.44].
Electrocardiogram revealed right-axis deviation and right ventricular hypertrophy.
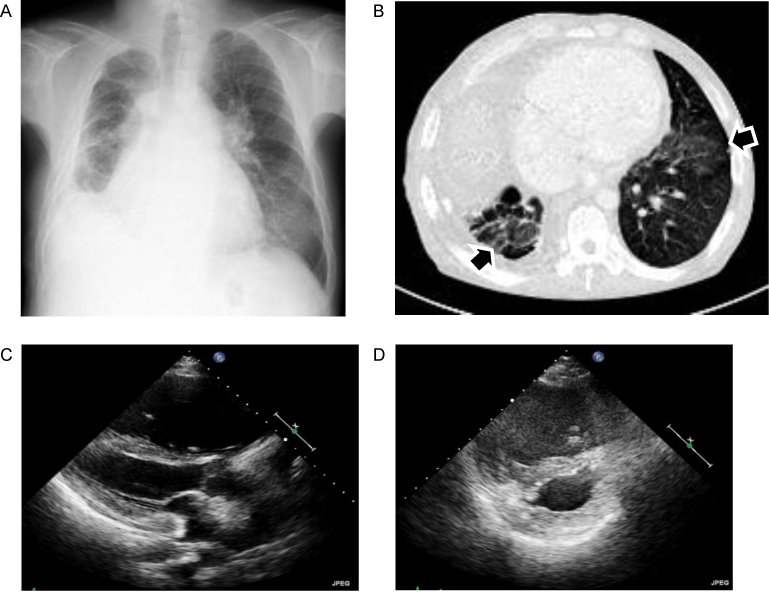
Chest X-ray showed cardiomegaly (cardiothoracic ratio of 0.70), dilatation of the pulmonary arteries, lower volume in the right lung due to post thoracoplasty, and gland-grass opacity in the bilateral lower lung (Fig. 1A).
Fig. 1.
(A) Chest X ray showed cardiomegaly, dilatation of the pulmonary arteries, lower volume in the right lung due to post thoracoplasty, and ground-grass opacity in the bilateral lower lung. (B) Chest computed tomography showed bilateral ground glass densities with interlobular thickening (arrow). (C and D) Transthoracic echocardiography, parasternal long-axis view (C) and parasternal short-axis view (D). There was massive dilatation and hypertrophy of the right ventricle, which compressed the left ventricle.
Computed tomography (CT) of the chest showed dilatation of pulmonary arteries, right heart dilatation, and bilateral ground glass densities with interlobular thickening, but did not detect thrombus in pulmonary arteries (Fig. 1B). Complete pulmonary function tests revealed mild restrictive abnormality which was consistent with post-thoracoplasty state.
Transthoracic echocardiography showed marked dilatation and hypertrophy of the right ventricle, which compressed the left ventricle (Fig. 1C and D). Doppler examination measured a tricuspid regurgitation pressure gradient of 70 mmHg and a mean pulmonary artery pressure (PAP) of 45 mmHg. Left ventricular ejection fraction was 60% and there was no evidence of left diastolic dysfunction. These findings indicated the presence of severe PH.
Right heart catheterization revealed elevated PAP, pulmonary vascular resistance, and right ventricular pressure. It also showed that cardiac output and pulmonary artery wedge pressure (PAWP) was normal (Table 1). Left heart catheterization showed significant coronary stenosis in the left anterior descending artery, and normal left ventricular contraction.
Table 1.
Hemodynamic data at cardiac catheterization.
| Pulmonary artery pressure (mean) | (mmHg) | 75/30 (47) |
| Right ventricular pressure | (mmHg) | 75/13 |
| Right atrial pressure (mean) | (mmHg) | 15 |
| Pulmonary vascular resistance | (dyn s cm−5) | 384 |
| Pulmonary artery wedge pressure | (mmHg) | 13 |
| Cardiac output | (L/min) | 6.7 |
| Cardiac index | (L/min/m2) | 3.6 |
We considered that PH derived from chronic renal failure on dialysis, because of the absence of thrombosis or embolic episodes, underlying collagen disease, and normal range of PAWP.
After percutaneous coronary intervention, he started to receive oral prostacyclin analog and endothelin-1 (ET-1) receptor antagonist to reduce PAP. But his symptoms and clinical data relevant to cardiopulmonary function had gradually deteriorated thereafter. Two years later, he died from septic shock due to pneumonia by methicillin-resistant Staphylococcus aureus. His family agreed autopsy.
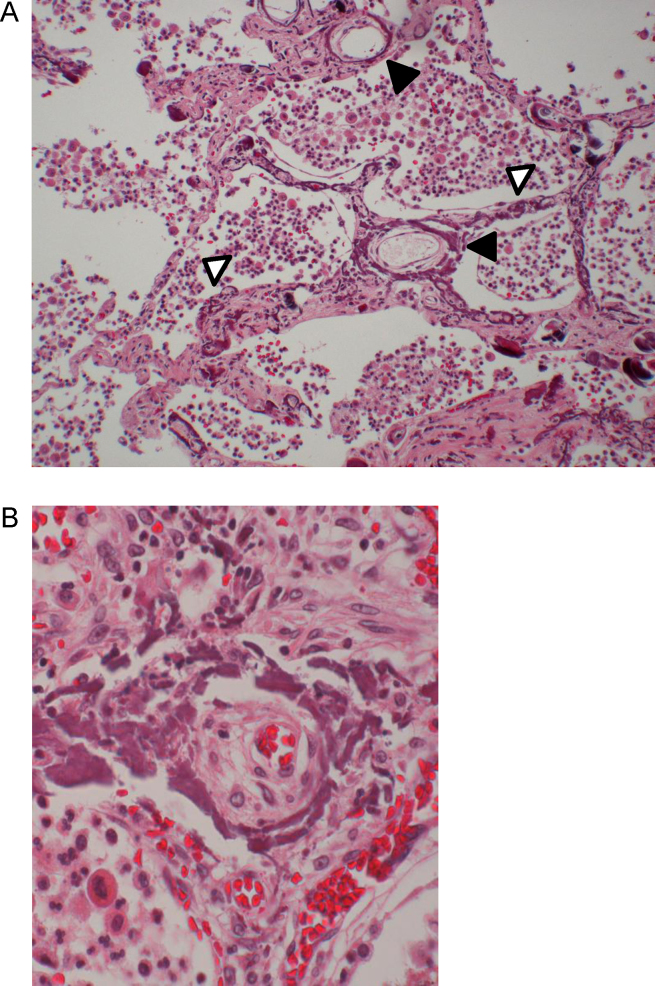
On microscopical examination of the autopsy specimen of the lung, there were broad linear calcium deposits in the alveolar septa, in addition to the scattered fine calcium deposits. Linear calcification was present in the elastic laminae and medial layers in the small vessel walls. These lesions were accompanied by intimal thickening, which caused luminal narrowing. The alveolar septa were diffusely thickened and had abundant collagen fibers in the interstitium (Fig. 2). In addition, there were calcium deposits in different organs including liver, kidney, parathyroid, and arteries. Arterial calcification consisted mainly of medial calcification. Parathyroid was swollen due to secondary parathyroidism.
Fig. 2.
Pathological findings of the lung autopsy specimen. Fine calcific deposits along vessel walls (black arrows) and alveolar septa with interstitial fibrosis (white arrows). Inflammatory cells occupy alveolar space [hematoxylin and eosin (H&E), 100×]. Thick circumferential calcific deposits in vessel wall with severely narrowing of the lumen due to intimal thickening (H&E, 400×).
These pathological findings support the hypothesis that the progression of PH was attributed to the metastatic pulmonary calcification in this patient. Imaging of chest radiograph and CT scan revealing a ground glass opacification may suggest vascular and interstitial calcification rather than pulmonary edema that is commonly associated with PH in patients with chronic kidney disease.
Discussion
In the present patient, on clinical examination including right heart catheterization and chest CT, we excluded any apparent cause of the severe PH. On pathological examination of autopsied pulmonary specimens, we deemed that pulmonary calcification may play a major role in PH, because extensive calcium deposits were detected in the pulmonary arterial walls and in thickened interstitium surrounding capillaries. In addition, there were many vessels with narrowing of the lumen. These features resemble those observed in patients with pulmonary arterial hypertension, such as “plexiform lesion”. Although it is unclear whether this finding of intimal thickening is attributed to the reaction derived from pulmonary calcification, a previous pathological study showed that severe cases had linear calcification of the accompanied by loose intimal fibrosis with narrowing of the vessel lumens [4]. However, there have been no reports that related pathological findings of the lung with PH in hemodialysis patients. This case supports our hypothesis that pulmonary calcification is likely to be important as one of the multiple etiologies of PH.
PH is relatively common among patients receiving long-term hemodialysis, and an independent predictor for cardiovascular mortality [2], [4]. The causes of PH in hemodialysis patients are multifactorial, as described in the Proceedings of the 4th World Symposium on Pulmonary Hypertension held in 2008 at Dana Point, California, USA [3]. Those include: (1) the increased pulmonary circulation due to elevated cardiac output resulting from the arterio-venous access, concomitant anemia and fluid overload; (2) hormonal and metabolic derangement associated with ESRD which might lead to vasoconstriction of pulmonary vessels and increased pulmonary vascular resistance; (3) endothelial dysfunction with decreased NO production and accumulation of ET-1 which could reduce the capacity of pulmonary vasoconstriction. In addition, an increased stiffness of the pulmonary vessels due to pulmonary vascular calcification is postulated as a possible mechanism of pulmonary hypertension in animal experimental chronic kidney disease models [5].
What might be the most plausible explanation for extensive calcification in this patient? It is becoming clear that vascular calcification is a highly regulated process involving cell activity and specific protein synthesis such as bone formation [6]. Among many factors contributing to vascular calcification, our patient had marked hyperphosphatemia, hypercalcemia, an elevated calcium-phosphate product, and severe secondary hyperparathyroidism, whose intact parathyroid hormone values were exceeding 600 pg/mL. Thus, we propose that mineral derangement may play a predominant role in the development of mineralization of vascular smooth muscle cells (SMC) and pericytes in alveolar capillary wall.
Is the vascular calcification related to intimal thickening in pulmonary small vessels? Increased attention has focused on the role of inflammation in the development of atherosclerosis and vascular calcification [7], [8]. Thus, it is possible that both vascular calcification and atherosclerosis are inflammation-dependent processes. Although the information on the impact of uremia on acceleration of intimal thickening in small vessels is limited, contribution of inflammation to intimal thickening is likely to be distinct from that to medial vascular calcification [9]. Future work will be obviously necessary to determine the role of hyperphosphatemia and secondary hyperparathyroidism in vascular SMC proliferation in uremic patients.
In conclusion, we report a case of a hemodialysis patient, who showed severe PH with extensive calcification in the pulmonary small vessels and alveolar interstitium surrounding capillaries. We propose that small pulmonary vessel and capillary wall calcification is a possible mechanism of PH in uremic patients with otherwise unexplained PH.
References
- 1.Archer S., Rich S. Primary pulmonary hypertension: a vascular biology and translational research “work in progress”. Circulation. 2000;102:2781–2791. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- 2.Yigla M., Nakhoul F., Sabag A., Tov N., Gorevich B., Abassi Z., Reisner S.A. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577–1582. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G., Robbins I.M., Beghetti M., Channick R.N., Delcroix M., Denton C.P., Elliott C.G., Gaine S.P., Gladwin M.T., Jing Z.C., Krowka M.J., Langleben D., Nakanishi N., Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl.):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Conger J.D., Hammond W.S., Alfrey A.C., Contiguglia S.R., Stanford R.E., Huffer W.E. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med. 1975;83:330–336. doi: 10.7326/0003-4819-83-3-330. [DOI] [PubMed] [Google Scholar]
- 5.Akmal M., Barndt R.R., Ansari A.N., Mohler J.G., Massry S.G. Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int. 1995;47:158–163. doi: 10.1038/ki.1995.18. [DOI] [PubMed] [Google Scholar]
- 6.Moe S.M., Chen N.X. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;17(95):560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa E., Nahrendorf M., Figueiredo J.L., Swirski F.K., Shtatland T., Kohler R.H., Jaffer F.A., Aikawa M., Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P., Heimburger O., Jogestrand T., Karnell A., Samuelsson A. Does persistent infection with Chlamydia pneumoniae increase the risk of atherosclerosis in chronic renal failure? Kidney Int. 1999;55:2531–2532. doi: 10.1046/j.1523-1755.1999.00499.x. [DOI] [PubMed] [Google Scholar]
- 9.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]