Abstract
We report a case of infective endocarditis complicated with left ventricular pseudoaneurysm originating from the posterior annulus of the prosthetic mitral valve in a 56-year-old woman. Despite prolonged antibiotic treatment, transesophageal echocardiography (TEE) showed partial detachment of the prosthesis from the posterior mitral annulus. Three-dimensional rotational computed tomography clearly demonstrated a pseudoaneurysm toward the posterolateral portion of the mitral prosthetic valve, which was not evident by TEE. Valve replacement and repair of the pseudoaneurysm were performed 83 days after initiation of antibiotic therapy. Left ventricular pseudoaneurysm is a rare but serious complication of mitral prosthetic valve endocarditis. It requires prompt diagnosis and early surgical intervention.
<Learning objective: We present a case of infective endocarditis (IE) complicated with left ventricular pseudoaneurysm originating from the prosthetic mitral valve. Repeated transesophageal echocardiography is recommended for all IE patients when perivalvular extension is suspected. Electrocardiography-gated three-dimensional-computed tomography is useful for detection and evaluation of pseudoaneurysm, especially in planning surgical procedures.>
Keywords: Prosthetic valve endocarditis, Left ventricular pseudoaneurysm, Mitral valve prosthesis, Electrocardiography-gated computed tomography
Introduction
Pseudoaneurysm of the left ventricle is a rare complication of mitral valve infective endocarditis (IE), although perivalvular cavities or pseudoaneurysm complicating aortic valve IE have been frequently reported [1]. In this report, we describe a case of pseudoaneurysm of the left ventricular posterior wall, which developed as a complication of mitral prosthetic IE. This complication of endocarditis is associated with high rates of morbidity and mortality, especially for those in whom surgery is not performed.
Case report
A 56-year-old woman was admitted to our hospital for evaluation of persistent low-grade fever lasting for 10 days. Past history included rheumatic mitral valvular disease requiring mitral valve replacement (Hall–Kaster single tilting-disc prosthesis) 26 years previously and chronic renal failure requiring hemodialysis for 13 years. A recent echocardiogram revealed progression of aortic stenosis, regurgitation, and decreased left ventricular function (ejection fraction: 30%), but the prosthetic mitral valve functioned well. After admission, blood tests revealed Streptococcus salivarius in culture, and transesophageal echocardiography (TEE) revealed 11-mm diameter vegetation attached to the mitral prosthesis (E-component 1A). No evidence of prosthetic valve detachment or paravalvular leakage was found initially. The detected S. salivarius was ampicillin-sensitive (minimal inhibitory concentration = 0.064 μg/mL), and we started intravenous ampicillin 2 g twice a day.
On the 12th day after admission, contrast-enhanced computed tomography (CT) revealed a perivalvular cavity located in the posterior atrioventricular groove (Fig. 1A), previously there had been no cavity formation with CT scanned about 1 year previously. The cavity was poorly enhanced with contrast dye and was considered to be an abscess cavity. Coronary angiography was performed to rule out the possibility of aneurysm originating from the left circumflex artery or coronary sinus vein, but no communication between the cavity and the coronary arteries or veins was detected. Early surgical intervention was recommended, but the patient refused. On the 47th hospital day, she developed low-grade fever and dysarthria, and contrast-enhanced head CT revealed mycotic aneurysm and brain abscesses (Fig. 2A and D). Electrocardiography (ECG)-gated three-dimensional (3D) CT with a 64-slice CT scan, which was performed on the 48th day, revealed that the cavity had developed as a pseudoaneurysm originating from the posterior annulus of the mitral prosthesis (Fig. 1B–D). As the pseudoaneurysm had grown rapidly, surgical intervention was once again strongly recommended. However, the patient refused after being informed of possible complications resulting from surgery. On the 70th day, she developed low-grade fever again and C-reactive protein had increased to 30 mg/dl, having been under 2.0 mg/dl after 4 weeks of antibiotic treatment, and repeated TEE, on the 76th day, showed that the mitral prosthesis was partly detached from the posterior annulus, causing perivalvular leakage (E-component 1B). At this point, the patient accepted all the possible risks and agreed to undergo surgical intervention.
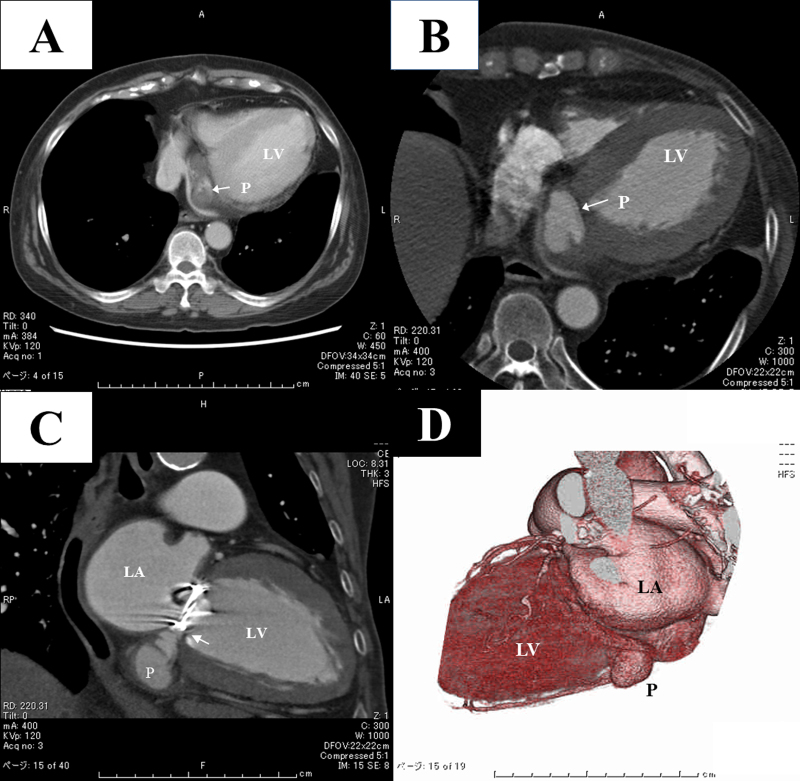
Fig. 1.
(A) Non-electrocardiography (ECG)-gated contrast-enhanced computed tomography (CT) on day 12 showing a mass adjacent to the posterior mitral annulus. The cavity was poorly enhanced at this time. (B–D) An ECG-gated CT performed with a 64-slice CT scanner on day 48 clearly showing that the cavity was the pseudoaneurysm originating from the posterior annulus of the mitral prosthesis. Clear communication of the pseudoaneurysm and left ventricle was identified (white arrow in C). Ao, aorta; LA, left atrium; LV, left ventricle; P, pseudoaneurysm.
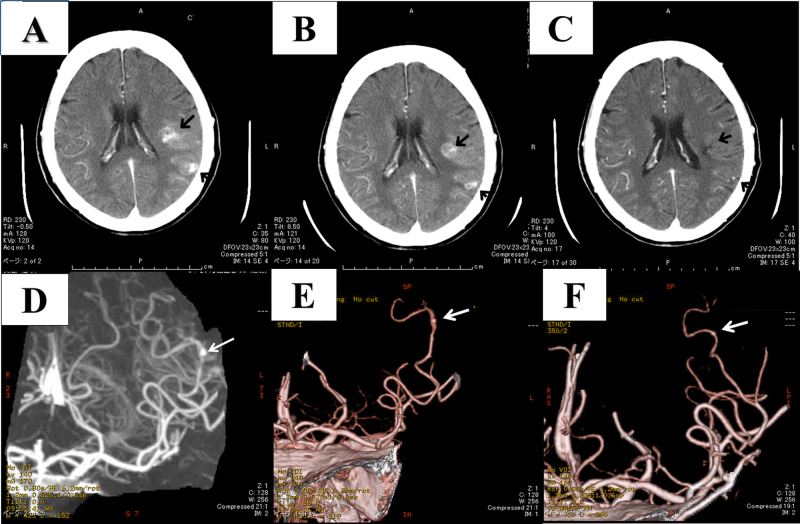
Fig. 2.
Serial contrast-enhanced brain computed tomography (CT) and CT angiographic images obtained on days 47 (A, D), 75 (B, E), and 122 (C, F). Note that the mycotic aneurysm reduced in size (white arrows) and the brain abscesses (black arrows) improved after surgery.
During surgery, a detachment of mitral prosthesis and a defect of myocardium were observed along the posterior part of the mitral prosthesis, communicating with the pseudoaneurysm. The defect was closed with a Gore-Tex patch from inside the left ventricle. The mitral prosthesis was replaced by a 27-mm St. Jude bi-leaflet mitral prosthesis, and the degenerated and stenotic aortic valve was replaced with a 17-mm St. Jude Regent aortic prosthesis. The antibiotics were continued for 4 weeks postoperatively, and the patient was discharged after 18 weeks of hospitalization. CT evaluation of the brain prior to discharge revealed that the aneurysm had decreased in size and the brain abscesses had improved (Fig. 2B, C, E, F). At 4-year follow-up, the patient was classified as New York Heart Association functional class I with no evidence of any neurological deficit.
Discussion
Left ventricular pseudoaneurysm is commonly caused by myocardial infarction. A review of 290 cases of left ventricular pseudoaneurysm showed that myocardial infarction was present in more than half of the cases; infection was responsible for this condition in only 13% of cases [2]. Left ventricular pseudoaneurysm is commonly located in the posterolateral wall, in contrast to true left ventricular aneurysm which typically forms in the anterior or apical wall. In the present case, the pseudoaneurysm originated from the posterior annulus of the prosthetic mitral valve as a complication of IE in the prosthetic mitral valve.
Periannular extension of IE has been reported to occur in 19–40% of cases of native valve endocarditis and in 55–94% of cases of prosthetic valve endocarditis (PVE). A strong association between aortic valve infection and perivalvular complications has been reported [3]. Periannular extension of PVE sometimes results in periannular ring abscesses or pseudoaneurysm formation and cause dehiscence of the prosthesis. Although the frequency of formation of periannular ring abscesses and pseudoaneurysm is unclear, these conditions are associated with high mortality and the need for surgical intervention [4].
We have two hypotheses for formation of the left ventricular pseudoaneurysm. Bacterial invasion into the annulus of the prosthetic valve leads to tissue destruction, necrosis, and abscess formation. Spontaneous drainage of the abscess into the aorta or left ventricle then results in formation of the pseudoaneurysm. Alternatively, infection may cause weakening of the wall, predisposing to dissection and cavity formation, and finally resulting in a pseudoaneurysm. In our case, the initial CT scan demonstrated relatively poor contrast enhancement, but a later scan revealed clear communication between the cavity and left ventricle, and strong enhancement of the cavity with contrast dye was observed (Fig. 1). We speculate that the initial abscess cavity spontaneously drained into the left ventricle, resulting in formation of the pseudoaneurysm.
The microbiological profile in patients with periannular complications has varied from study to study. Although some studies found no statistical differences regarding frequency of etiologic agents responsible for these complications, Graupner et al. identified Staphylococcus as the most common microorganism implicated in periannular destruction [3], [5]. In the present case, a progressive large pseudoaneurysm rapidly developed, infected by Streptococcus species as a complication of mitral PVE.
TEE has advantages over transthoracic examination in evaluation of PVE, especially in confirmation of perivalvular invasion [6]. In our case, initial TEE only demonstrated vegetations attached to the mitral valve leaflets, later it revealed detached mitral prosthesis. In the present case, TEE could not detect a flow draining into the pseudoaneurysm clearly, however it was useful in detecting the valve detachment, and confirming the indications for surgical intervention.
ECG-gated 3D-CT is another useful imaging modality for evaluation of perivalvular invasion. Exact anatomic delineation is possible with this imaging modality. Rotational 3D-CT shows the spatial relationship between the pseudoaneurysm and other cardiovascular structures, which is of some benefit in preoperative assessment. ECG-gated 3D-CT is less sensitive than TEE in detecting signs of valvular dehiscence, but it may potentially be superior in detecting pseudoaneurysm associated with PVE [7]. These multiple diagnostic techniques may need to be used as complementary to each other for giving an accurate guidance to the surgeon.
The appropriate treatment for PVE is still a matter of debate. Both medical treatment alone and combined medical/surgical therapy have been advocated. No randomized trial has been conducted, mainly because of the heterogeneity of patient populations [8]. In our opinion, in cases with annular abscess or pseudoaneurysm, surgery is required to prevent spontaneous rupture and eradicate infection. However, higher postoperative mortality and morbidity rates have been reported in patients with periannular complications than in those with uncomplicated active endocarditis [9].
In addition to double valve replacement, patch closure of the orifice of the pseudoaneurysm was performed in the case reported here to prevent rupture. This technique was reported by Aoyagi et al. [10]. Follow-up treatment was successfully managed conservatively, including 4 weeks of postoperative antibiotic treatment with no worsening of cerebral complications.
In summary, we present a case of IE complicated with left ventricular pseudoaneurysm originating from the prosthetic mitral valve. Repeated TEE is recommended for all IE patients when perivalvular extension is suspected. ECG-gated 3D-CT is useful for detection and evaluation of pseudoaneurysm, especially in planning surgical procedures.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2013.03.004.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Baumgartner F.J., Omari B.O., Robertson J.M., Nerson R.J., Pandya A., Milliken J.C. Annular abscesses in surgical endocarditis: anatomic, clinical, and operative features. Ann Thorac Surg. 2000;70:442–447. doi: 10.1016/s0003-4975(00)01363-1. [DOI] [PubMed] [Google Scholar]
- 2.Frances C., Romero A., Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. 1998;32:557–561. doi: 10.1016/s0735-1097(98)00290-3. [DOI] [PubMed] [Google Scholar]
- 3.Graupner C., Vilacosta I., SanRomán J., Ronderos R., Sarriá C., Fernández C., Mújica R., Sanz O., Sanmartín J.V., Pinto A.G. Periannular extension of infective endocarditis. J Am Coll Cardiol. 2002;39:1204–1211. doi: 10.1016/s0735-1097(02)01747-3. [DOI] [PubMed] [Google Scholar]
- 4.Habib G., Thuny F., Avierinos J.F. Prosthetic valve endocarditis: current approach and therapeutic options. Prog Cardiovasc Dis. 2008;50:274–281. doi: 10.1016/j.pcad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 5.San Román J.A., Vilacosta I., Sarriá C., de la Fuente L., Sanz O., Vega J.L., Ronderos R., González Pinto A., Jesús Rollán M., Graupner C., Batlle E., Lahulla F., Stoermann W., Portis M., Fernández-Avilés F. Clinical course, microbiologic profile, and diagnosis of periannular complications in prosthetic valve endocarditis. Am J Cardiol. 1999;83:1075–1079. doi: 10.1016/s0002-9149(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 6.Hanai M., Hashimoto K., Mashiko K., Sasaki T., Sakamoto Y., Shiratori K., Tanaka K., Yoshitake M., Naganuma H., Shinohara G. Active infective endocarditis: management and risk analysis of hospital death from 24 years’ experience. Circ J. 2008;72:2062–2068. doi: 10.1253/circj.cj-08-0224. [DOI] [PubMed] [Google Scholar]
- 7.Fagman E., Perrotta S., Bech-Hanssen O., Flinck A., Lamm C., Olaison L., Svensson G. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol. 2012;22:2407–2414. doi: 10.1007/s00330-012-2491-5. [DOI] [PubMed] [Google Scholar]
- 8.Hill E.E., Herregods M.C., Vanderschueren S., Claus P., Peetermans W.E., Herijgers P. Management of prosthetic valve infective endocarditis. Am J Cardiol. 2008;101:1174–1178. doi: 10.1016/j.amjcard.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 9.David T.E., Regesta T., Gavra G., Armstrong S., Maganti M.D. Surgical treatment of paravalvular abscess: long-term results. Eur J Cardiothorac Surg. 2007;31:43–48. doi: 10.1016/j.ejcts.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Aoyagi S., Fukunaga S., Oryoji A., Kosuga K., Kanaya S., Ouchida M., Kuwano K., Sakamoto T. Reconstruction of the mitral annulus with porcine pericardium: report of a case with mitral annular disruption due to staphylococcal endocarditis. Jpn Circ J. 1996;60:258–261. doi: 10.1253/jcj.60.258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.